Abstract
Poultry meat and eggs contaminated with Salmonella enterica serovar Enteritidis or Salmonella enterica serovar Typhimurium are common sources of acute gastroenteritis in humans. However, the exact nature of the immune mechanisms protective against Salmonella infection in chickens has not been characterized at the molecular level. In the present study, bacterial colonization, development of pathological lesions, and proinflammatory cytokine and chemokine gene expression were investigated in the liver, spleen, jejunum, ileum, and cecal tonsils in newly hatched chickens 6, 12, 24, and 48 h after oral infection with Salmonella serovar Typhimurium. Very high bacterial counts were found in the ileum and cecal contents throughout the experiment, whereas Salmonella started to appear in the liver only from 24 h postinfection. Large numbers of heterophils, equivalent to neutrophils in mammals, and inflammatory edema could be seen in the lamina propria of the intestinal villi and in the liver. Interleukin 8 (IL-8), K60 (a CXC chemokine), macrophage inflammatory protein 1 β, and IL-1β levels were significantly upregulated in the intestinal tissues and in the livers of the infected birds. However, the spleens of the infected birds show little or no change in the expression levels of these cytokines and chemokines. Increased expression of the proinflammatory cytokines and chemokines (up to several hundred-fold) correlated with the presence of inflammatory signs in those tissues. This is the first description of in vivo expression of chemokines and proinflammatory cytokines in response to oral infection with Salmonella in newly hatched chickens.
Salmonella enterica is one of the major causes of food-borne bacterial gastroenteritis worldwide. Up to 30,000 and an estimated 1.4 million cases of human salmonellosis are reported each year in the United Kingdom and United States, respectively (26, 19). Furthermore, with approximately 550 deaths annually, Salmonella-induced enterocolitis is the single most common cause of death from food-borne illnesses associated with viruses, parasites, or bacteria in the United States (19). The majority of these cases are caused by Salmonella enterica serovar Enteritidis or Salmonella enterica serovar Typhimurium and are often associated with consumption of infected poultry meat or eggs (21, 25). Both serovars are capable of causing severe systemic disease in newly hatched chicks but colonize the gastrointestinal tracts of chickens more than 3 days old without disease (2, 6, 7). This may lead to long-term carriage in the gut, leading to fecal shedding and horizontal transmission within the flock and to contamination of meat, principally through fecal contamination at slaughter (6, 7).
Following oral infection in mammals, with the notable exception of mice, where a systemic typhoid-like disease occurs, Salmonella serovar Typhimurium invades the gut epithelium, resulting in damage, inflammation, and fluid secretion, leading to enteritis and diarrhea (reviewed in references 27 and 29). In vitro and in vivo studies with calf models have shown the key roles played by the Salmonella pathogenicity island 1 (SPI1) type three secretion system (TTSS) in enteropathogenesis. As part of the infection process, proinflammatory cytokines and chemokines, in particular the CXC chemokines interleukin 8 (IL-8) and GRO-α, are elicited by SPI1-secreted effector proteins, (29). This results in the recruitment of neutrophils to the site of infection, leading to inflammation and damage. The molecular mechanisms of Salmonella infection in the chicken intestine and internal organs and the host immune response are not well characterized. Although not as pronounced as in mammals, infection with Salmonella serovar Typhimurium leads to some diarrhea and intestinal lesions in young chickens (2) and to an influx of heterophils into the gut accompanied by inflammation and damage to villi, but this is not seen with the avian-specific serovar Salmonella enterica serovar Pullorum (4). Heterophils are the avian equivalent of mammalian neutrophils and play a key role in protecting chickens from the development of systemic disease following infection with Salmonella serovar Enteritidis by largely restricting the bacteria to the gut (11, 12). However, there are no descriptions, as yet, of the role of cytokines or chemokines during in vivo Salmonella infections of the chicken, though Salmonella infection in an avian in vitro epithelial model has indicated that invasion with both Salmonella serovar Typhimurium and Salmonella serovar Enteritidis induce production of the proinflammatory cytokine IL-6, whereas invasion with the avian-specific serovar Salmonella enterica serovar Gallinarum did not induce IL-6 (9). Therefore, an increased understanding of immune and pathological mechanisms at molecular and cellular levels is of paramount importance in order to develop novel strategies to improve current control measures against colonization by food-poisoning Salmonella in broiler (meat producing) and egg-laying chickens. As part of this, an understanding of the role of cytokines and chemokines in both pathogenesis and the initiation of the immune response is vital.
In comparison to the case with human or mouse, the detection of avian cytokines and chemokines has been hampered by the lack of specific antibodies and reliable bioassays. Recent progress in cloning avian cytokines and chemokines has led to the analysis of proinflammatory cytokine and chemokine profiles during various diseases in chickens (15, 10) through the use of reverse transcription (RT)-PCR-based assays. Chicken orthologues of the proinflammatory cytokines IL-1β (28), IL-6 (22), the C chemokine lymphotactin (GenBank accession no. AJ242790), the CC chemokines macrophage inflammatory protein 1 beta (MIP-1β) (5) and K203 (23), and the CXC chemokines IL-8 (8) and K60 (23) have been cloned. A number of chemokine receptors have also been identified, i.e., CXCR1 (16), CXCR4 (17), and CRL1 (3). Although cytokines are highly pleiotropic and exert a wide range of effects on a number of cell types, chemokines are more restricted in their functions and act primarily as chemoattractants. Chemokines are a group of small, structurally related molecules that regulate cell trafficking of various types of leukocytes (30). Chemokines have been divided into subfamilies on the basis of the arrangement of the N-terminal cysteine residues, CXC and CC, depending on whether the first two cysteine residues have an amino acid between them (CXC) or are adjacent to each other (CC). In general, CXC chemokines are chemoattractant for polymorphonuclear cells, whereas CC and C chemokines are chemoattractant for macrophages and lymphocytes, respectively (1, 18, 29, 30). In this study proinflammatory cytokine and chemokine mRNA profiles were investigated by quantitative real time RT-PCR during Salmonella serovar Typhimurium infection of young chicks to begin to determine their potential role in pathogenesis and activation of the avian immune system.
MATERIALS AND METHODS
Experimental animals.
Forty 1-day-old specified-pathogen-free Rhode Island Red chickens were obtained from the Poultry Production Unit, Institute for Animal Health. Birds were reared in wire cages at 30°C and allowed ad libitum access to water and a vegetable protein-based diet (Special Diet Services, Witham, United Kingdom).
Bacterial strains.
A spontaneous nalidixic acid-resistant mutant of Salmonella serovar Typhimurium phage type 14 strain F98 was used in the experimental infection. Salmonella serovar Typhimurium F98 is an invasive strain that has been well described for virulence in young chicks of less than 3 days of age and persistent colonization of the gastrointestinal tract of older birds (2, 24). Bacteria were maintained as glycerol stocks at −70°C and grown in Luria-Bertani (LB) broth (Difco, West Molesey, United Kingdom) at 37°C in an orbital shaking incubator at 150 rpm.
Experimental infection.
Chicks were divided into two groups. The first group was infected with 108 CFU of Salmonella serovar Typhimurium F98 in 0.1 ml of LB broth. The second control group was mock infected with 0.1 ml of LB broth. Five birds from each group were killed at 6, 12, 24, and 48 h postinfection (p.i.) for postmortem analysis. At each time point, tissue samples of liver, spleen, jejunum, ileum, and cecal tonsils were collected aseptically into liquid nitrogen for total RNA extraction and for histology. Liver and cecal contents were taken for bacterial enumeration. Bacterial culture and enumeration was performed as previously described (24). Briefly, homogenized samples were plated out onto selective Brilliant Green agar (Difco) containing 20 μg of sodium nalidixate/ml and 1 μg of novobiocin/ml. Plates were incubated at 37°C for 24 h before enumeration of the colonies. For histological examination, tissue samples were fixed in 4% buffered formalin, embedded in paraffin wax, and then cut as 4-μm sections. Sections were stained with hematoxylin and eosin as previously described (24).
Quantitative real-time RT-PCR.
Total RNA was prepared from snap-frozen samples by using the RNeasy Mini kit (Qiagen, Crawley, United Kingdom), following the manufacturer's instructions. Purified RNA was eluted in 50 μl of RNase-free water and stored at −70°C until use. For both cytokine or chemokine mRNA and 28S rRNA-specific amplification, primers and probes were designed using the Primer Express software program (PE Applied Biosystems, Foster City, Calif.). Details of the probes and primers are given in Table 1. All cytokine and chemokine probes were designed, from the sequence of the relevant genes, to lie across intron-exon boundaries. All probes were labeled with the fluorescent reporter dye 5-carboxyfluorescein at the 5′ end and the quencher N,N,N,N′-tetramethyl-6-carboxyrhodamine (TAMRA) at the 3′ end. RT-PCR was performed using the Reverse Transcriptase qPCR Master Mix RT-PCR kit (Eurogentec, Seraing, Belgium). Amplification and detection of specific products were performed using the ABI PRISM 7700 sequence detection system (PE Applied Biosystems) with the following cycle profile: one cycle at 50°C for 2 min, 96°C for 5 min, 60°C for 30 min, and 95°C for 5 min, and 40 cycles at 94°C for 20 s and 59°C for 1 min.
TABLE 1.
Real-time quantitative RT-PCR probes and primers
| Target | Probe or primera | Sequence | Accession no.b |
|---|---|---|---|
| 28S | Probe | 5′-(FAM)-AGGACCGCTACGGACCTCCACCA-(TAMRA)-3′ | X59733 |
| F | 5′-GGCGAAGCCAGAGGAAACT-3′ | ||
| R | 5′-GACGACCGATTTGCACGTC-3′ | ||
| IL-1β | Probe | 5′-(FAM)-CCACACTGCAGCTGGAGGAAGCC-(TAMRA)-3′ | AJ245728 |
| F | 5′-GCTCTACATGTCGTGTGTGATGAG-3′ | ||
| R | 5′-TGTCGATGTCCCGCATGA-3′ | ||
| IL-6 | Probe | 5′-(FAM)-AGGAGAAATGCCTGACGAAGCTCTCCA-(TAMRA)-3′ | AJ250838 |
| F | 5′-GCTCGCCGGCTTCGA-3′ | ||
| R | 5′-GGTAGGTCTGAAAGGCGAACAG-3′ | ||
| IL-8 | Probe | 5′-(FAM)-TCTTTACCAGCGTCCTACCTTGCGACA-(TAMRA)-3′ | AJ009800 |
| F | 5′-GCCCTCCTCCTGGTTTCAG-3′ | ||
| R | 5′-TGGCACCGCAGCTCATT-3′ | ||
| K60 | Probe | 5′-(FAM)-CCACATTCTTGCAGTGAGGTCCGCT-(TAMRA)-3′ | AF277660 |
| F | 5′-CCAGTGCATAGAGACTCATTCCAAA-3′ | ||
| R | 5′-TGCCATCTTTCAGAGTAGCTATGACT-3′ | ||
| MIP-1β | Probe | 5′-(FAM)-ACACAACACCAGCATGAGGGCACTG-(TAMRA)-3′ | AJ243034 |
| F | 5′-GGCAGACTACTACGAGACCAACAG-3′ | ||
| R | 5′-ACGGCCCTTCCTGGTGAT-3′ | ||
| Lymphotactin | Probe | 5′-(FAM)-CGCTTCATCTTCTGCCGTGTGCAG-(TAMRA)-3′ | AJ242790 |
| F | 5′-CATAGTCTGGCTTGGCGTCTT-3′ | ||
| R | 5′-GCGCATTGACTGACTTGCA-3′ | ||
| CXCR1 | Probe | 5′-(FAM)-CCTGCCCCTCCTGGTCATGCTTTAC-(TAMRA)-3′ | AF227961 |
| F | 5′-CGCAGACCTTCGGCTTTG-3′ | ||
| R | 5′-GGGTGTGCACGGTGACTTC-3′ | ||
| CXCR4 | Probe | 5′-(FAM)-ACGCCTTCCTGGGTGCCAAGTTC-(TAMRA)-3′ | AF294794 |
| F | 5′-TGCTGCCTCAATCCAATTCTT-3′ | ||
| R | 5′-CAAGGCATTTTGTGCTGATGTT-3′ |
F, forward; R, reverse.
GenBank sequence accession number.
Quantification was based on the increased fluorescence detected by the ABI PRISM 7700 sequence detection system due to hydrolysis of the target-specific probes by the 5′ nuclease activity of the rTth DNA polymerase during PCR amplification. The passive reference dye 6-carboxy-χ-rhodamine, which is not involved in amplification, was used to correct for fluorescent fluctuations, resulting from changes in the reaction conditions, for normalization of the reporter signal. Results are expressed in terms of the threshold cycle value (Ct), the cycle at which the change in the reporter dye passes a significance threshold. In this work, the threshold values of the change in the reporter dye are given in Table 2 for all reactions described.
TABLE 2.
Standard curve data from real-time quantitative RT-PCR of total RNA extracted from liver, spleen, jejunum, ileum, or cecal tonsils from control and Salmonella serovar Typhimurium-infected chickens
| Target | ΔRna | Log dilutions | Ct valuesb | R2c | Slope |
|---|---|---|---|---|---|
| 28S | 0.03 | 10−1-10−6 | 5-14 | 0.988 | 3.01 |
| IL-1β | 0.03 | 10−1-10−6 | 16-29 | 0.994 | 3.33 |
| IL-6 | 0.03 | 10−1-10−5 | 24-33 | 0.988 | 3.29 |
| IL-8 | 0.03 | 10−1-10−5 | 14-30 | 0.988 | 3.43 |
| K60 | 0.03 | 10−1-10−6 | 17-31 | 0.991 | 3.51 |
| MIP-1β | 0.03 | 10−1-10−6 | 15-27 | 0.995 | 2.96 |
| Lymphotactin | 0.03 | 10−1-10−6 | 17-32 | 0.995 | 3.20 |
| CXCR1 | 0.03 | 10−1-10−6 | 26-34 | 0.991 | 3.05 |
| CXCR4 | 0.03 | 10−1-10−6 | 14-29 | 0.990 | 3.24 |
ΔRn, change in the reporter dye.
Ct, threshold cycle value (the cycle at which the change in the reporter dye levels detected passes the Rn).
R2, coefficient of regression.
Standard curves for the cytokine, chemokine, and 28S rRNA-specific reactions were generated as previously described (9, 20). Each RT-PCR experiment contained triplicate no-template controls and test samples and a log10 dilution series of standard RNA. In these studies RNA from either lipopolysaccharide-stimulated chicken macrophage-like cells (HD-11) or COS-7 cells transiently transfected with respective cytokine and chemokine constructs were used as standard RNA. Each experiment was performed in triplicate, with replicates performed on different days. Regression analysis of the mean values of six replicate RT-PCRs for the log10 diluted RNA was used to generate standard curves. To account for the variation in sampling and RNA preparations, the Ct values for the cytokine or chemokine-specific product for each sample were standardized using the Ct value for the 28S rRNA product for the same sample from the reaction run simultaneously. To normalize RNA levels between samples within an experiment, the mean Ct value for the 28S rRNA-specific product was calculated by pooling values from all samples in that experiment. Sample-to-sample variations in 28S rRNA Ct values of the experimental mean were calculated. Using slopes of the respective cytokine/chemokine and 28S rRNA log10 dilution series regression lines, the difference in input total RNA, as represented by the 28S rRNA, was then used to adjust cytokine or chemokine-specific Ct values as previously described in detail (20). The mean Ct values of different tissues from infected birds were compared with the Ct values from respective age-matched control birds using the Student t test.
RESULTS
Experimental infection.
Following infection, Salmonella bacteria were detected in the cecal contents of the infected group at 6 h p.i. (Table 3). Higher counts, up to 109 CFU/g, were seen at 12, 24, and 48 h p.i. Salmonella bacteria were initially found in the livers of four out of five infected birds at 24 h p.i. and of all birds at 48 h p.i. At 48 h p.i., the chicks displayed gross pathology consistent with Salmonella serovar Typhimurium infection of young chicks, including diarrhea, bloody cecal contents, hepatosplenomegaly, and early signs of anorexia. Microscopically, the villi of the ileum and cecal tonsils of the infected birds were thickened, and larger numbers of heterophils were accumulated in the lamina propria of those villi from 24 h p.i. (Fig. 1A). The epithelia were disrupted in several birds that were heavily infected with Salmonella serovar Typhimurium towards the end of the experiment (48 h p.i.). In the liver, focal lesions filled with heterophils, as well as large numbers of scattered heterophils, were found only 48 h p.i. (Fig. 1B).
TABLE 3.
Tissue distribution of Salmonella serovar Typhimurium following oral infectiona
| Tissue | Mean log10 CFU of Salmonella serovar Typhimurium F98/g of tissue (SEM) at h p.i.:
|
|||
|---|---|---|---|---|
| 6 | 12 | 24 | 48 | |
| Liver | 1.55 (0.38) | 1.30 (0.20) | 2.77 (0.13) | 4.46 (0.19) |
| Ileum | 6.16 (0.37) | 4.54 (0.62) | 3.53 (0.40) | 5.87 (0.39) |
| Cecal contents | 8.74 (0.23) | 8.34 (0.46) | 8.82 (0.14) | 9.88 (0.25) |
Oral infection with 108 CFU of day-old Rhode Island Red chickens. Viable counts are mean values from five animals at each time point.
FIG. 1.
Histopathology of the ileum (A) and liver (B) of chickens infected with Salmonella serovar Typhimurium. Paraffin-embedded sections from the control (1) and at 48 h p.i. (2) were conventionally stained with hematoxylin and eosin. Note the heterophil extravasation, thickening of the villi, and increased vascular permeability leading to edema in the ileal villi. The presence of small foci filled with heterophils (circled) and of scattered heterophils was evident in the liver 48 h p.i.
Quantification of proinflammatory cytokine and chemokine mRNA expression.
There was a linear relationship between the amount of input RNA and the Ct values for the various reactions (Table 2). Regression analysis of the Ct values generated by the log10 dilution series gave R2 values for all reactions in excess of 0.97 (Table 2). The increase in cycles per log10 decrease in input RNA for each specific reaction, as calculated from the slope of the respective regression line, is given in Table 2. Standardization does not dramatically alter the distribution of the results as a whole.
Gastrointestinal proinflammatory cytokine and chemokine responses following infection.
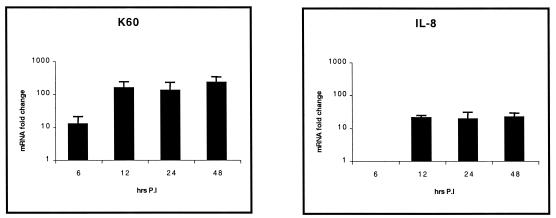
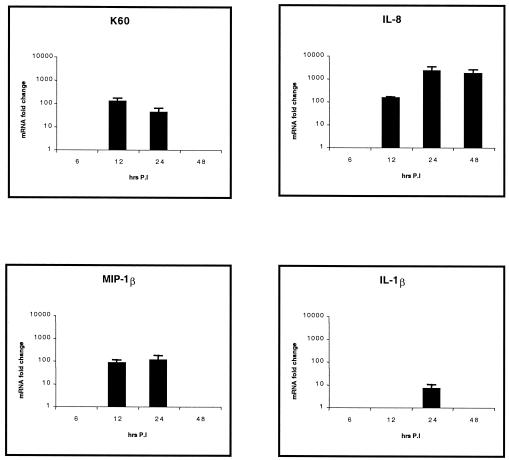
Statistically significant (P < 0.05) increased expression of the CXC chemokines K60 (up to 123-fold increase above control levels) at all time points examined and IL-8 (up to 21-fold increase) from 12 h p.i. was found in jejuna from infected birds compared to that for the uninfected controls (Fig. 2). However, IL-1β and MIP-1β levels were not different in the jejuna of both infected and mock-infected birds (data not shown). K60 levels were significantly greater at all time points in the ilea (Fig. 3) of infected birds (up to 310-fold increase; P < 0.05). Increased expression of IL-8 (up to 106-fold increase; P < 0.01) and IL-1β (up to 27-fold increase; P < 0.05) was found from 12 h p.i. onwards. MIP-1β expression was biphasic, since significant levels could be seen 12 and 48 h p.i. (up to 48-fold increase; P < 0.05). In the cecal tonsils (Fig. 4), expression levels of K60 (up to 173-fold increase; P < 0.01), IL-8 (up to 85-fold increase; P < 0.01), and IL-1β (up to 300-fold increase; P < 0.05) were significantly higher in infected birds at all time points examined, while the expression pattern of MIP-1β was similar to that in the ileum. The expression levels of lymphotactin, CXCR1, CXCR4, and IL-6 in intestinal samples were not statistically significantly different from the control birds at most of the time points after Salmonella serovar Typhimurium infection (data not shown). The data are summarized in Fig. 2 to 4.
FIG. 2.
Quantification of chemokine and proinflammatory cytokine mRNA extracted from jejuna of chickens after experimental infection with Salmonella serovar Typhimurium. Only statistically significant (P < 0.05) results are expressed as fold changes in chemokine mRNA levels for infected birds compared to those for age-matched, mock-infected controls. The error bars show standard error for triplicate samples from five birds in two separate experiments.
FIG. 3.
Quantification of chemokine and proinflammatory cytokine mRNA extracted from ilea of chickens after Salmonella serovar Typhimurium experimental infection. Only statistically significant (P < 0.05) results are expressed as fold changes in chemokine mRNA levels for infected birds compared to those for age-matched, mock-infected controls. The error bars show standard error for triplicate samples from five birds in two separate experiments.
FIG. 4.
Quantification of chemokine and proinflammatory cytokine mRNA extracted from cecal tonsils of chickens after experimental infection with Salmonella serovar Typhimurium. Only statistically significant (P < 0.05) results are expressed as fold changes in chemokine mRNA levels for infected birds compared to those for age-matched, mock-infected controls. The error bars show standard error for triplicate samples from five birds in two separate experiments.
Systemic proinflammatory cytokine and chemokine expression following infection.
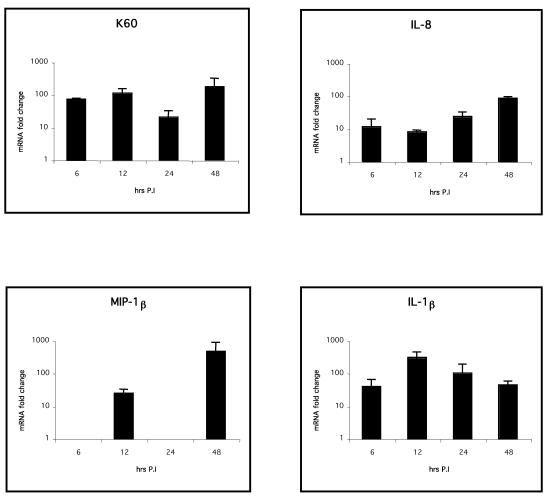
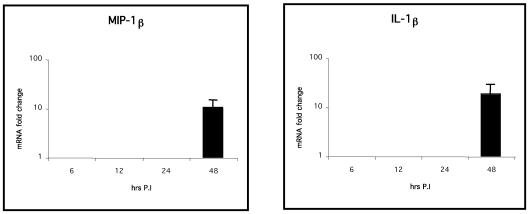
Expression of the CXC chemokines K60 and IL-8 (up to 100- and 2,000-fold increases, respectively; P < 0.01) and the CC chemokine MIP-1β (up to 104-fold increase; P < 0.05) was significantly greater in livers (Fig. 5) of infected birds from 12 h p.i., whereas IL-1β expression was significantly higher only at 24 h p.i. than for the uninfected controls (P < 0.05, Fig. 5). The expression levels of IL-6, lymphotactin, CXCR1, and CXCR4 in the liver or spleen were not significantly different from those for the control birds (data not shown). In the spleen (Fig. 6), IL-1β, IL-8, and MIP-1β expression levels were significantly greater (P < 0.05) only 48 h p.i., indicating that the inflammatory response in the spleen may occur later than that in the liver. Spleens always had significantly higher basal levels of cytokine and chemokine mRNA, except for IL-6, than livers in uninfected birds (P < 0.05; data not shown).
FIG. 5.
Quantification of chemokine and proinflammatory cytokine mRNA extracted from livers of chickens after experimental infection with Salmonella serovar Typhimurium. Only statistically significant (P < 0.05) results are expressed as fold changes in chemokine mRNA levels for infected birds compared to those for age-matched, mock-infected controls. The error bars show standard error for triplicate samples from five birds in two separate experiments.
FIG. 6.
Quantification of chemokine and proinflammatory cytokine mRNA extracted from spleens of chickens after experimental infection with Salmonella serovar Typhimurium. Only statistically significant (P < 0.05) results are expressed as fold changes in chemokine mRNA levels for infected birds compared to those for age-matched, mock-infected controls. The error bars show standard error for triplicate samples from five birds in two separate experiments.
DISCUSSION
Chemokine and cytokine responses in Salmonella infections of chickens have, as yet, been poorly described. In this study we have determined responses in young chicks following infection with Salmonella serovar Typhimurium, which normally results in a severe systemic infection following invasion and inflammation at systemic sites. In mammals, intestinal invasion by Salmonella serovar Typhimurium induces the production of CXC chemokines, including IL-8 and GRO-α, in in vitro and in vivo models (27, 29). The production of these chemokines results in an influx of polymorphonuclear cells, leading to inflammation and damage (27, 29). In this study we show that early following Salmonella serovar Typhimurium infection of the chick, the CXC chemokines IL-8 and K60 are produced in the gut (Fig. 2 and 3). The early expression of these chemokines correlates with the presence of bacteria in the gut (Table 3), and the subsequent inflammation and pathology seen in the intestines and ceca are consistent with an influx of polymorphonuclear heterophils to these sites, although it should be remembered that mRNA expression may not correlate with production of the active protein. Henderson et al. (4) showed that Salmonella serovar Typhimurium infection results in a pronounced heterophil influx to the intestinal and cecal lamina propria and subsequent pathology. Our histopathological data also confirmed the previous reports (4). The findings here suggest that induction of CXC chemokines early in intestinal infection may play a key role in the initiation of this inflammation. These findings suggest that the response to Salmonella serovar Typhimurium in the chicken is similar to that in mammalian models (29). It seems likely that the SPI1 type three secretion system is involved in this initiation and that this infection model is suitable for further studies on the role of secreted Salmonella effector proteins in the chicken. It is not yet known, however, whether these responses are found in older birds, where heterophils are thought to play a key role in protection against disease in Salmonella infection with relatively little gut-associated pathology (13, 14). Responses in the liver and spleen are less rapid than in the gut, though little or no Salmonella was detected in these sites until 24 h p.i. It is notable that chemokine expression (K60, IL-8, and MIP-1β) was first detectable at 12 h p.i., prior to the detection of bacteria in the liver. The response in the liver is more rapid than in the spleen. In both organs the expression of CXC and CC chemokines along with the proinflammatory cytokine IL-1β is indicative of an early inflammatory response, which presumably drives the subsequent early stages of hepatosplenomegaly. The low levels of IL-6 expression in both the gut and visceral organs are perhaps a little surprising. IL-6 is usually indicative of the initiation of an acute-phase response and is produced following infection with Salmonella serovar Typhimurium in an in vitro avian cell culture model (9). However, it is possible that the early time points in this study are prior to expression of this cytokine following infection in vivo.
In this study we have shown that day-old chickens express a number of proinflammatory cytokines and chemokines in both the gut and systemic sites following Salmonella serovar Typhimurium infection. The rapid expression of CXC chemokines in the intestines following infection is similar to that found during the enteropathogenic response in mammals infected with Salmonella serovar Typhimurium (27, 29). The responses in both gut and systemic sites are generally consistent with subsequent pathological changes. Further studies will be valuable in understanding both the pathogenesis of avian salmonellosis and how immune responses are initiated. Moreover, this in vivo experimental model provides the opportunity to investigate Salmonella virulence factors involved in invasion and initiation of inflammation in the actual host environment, since the typical pathogenesis of enteritis, such as vascular permeability, edema, and cellular influx, are difficult to reproduce using in vitro models.
Acknowledgments
We thank Fabrice Laurent of INRA, Paris, France, and Phillip M. Murphy of NIH, Bethesda, Maryland, for kindly providing plasmids containing some chicken chemokine gene fragments. We also thank Garry Evans and Andy Day at the Sygen International Group plc, Department of Pathology, University of Cambridge, Cambridge, United Kingdom, for allowing G.S.K.W. to use the Sequence Detection System, Lisa Rothwell (IAH) for help and advice to G.S.K.W. in Taqman assay development, and Fred Heath of CVS, University of Cambridge, for advice on statistical analysis.
This work was supported by the Biotechnology and Biological Sciences Research Council, grant 8/BFP11365.
Editor: F. C. Fang
REFERENCES
- 1.Baggiolini, M., B. Dewald, and B. Moser. 1994. Interleukin-8 and related chemotactic cytokines-CXC and CC chemokines. Adv. Immunol. 55:97-179. [PubMed] [Google Scholar]
- 2.Barrow, P. A., M. B. Huggins, M. A. Lovell, and J. M. Simpson. 1987. Observations of the pathogenesis of experimental Salmonella typhimurium infection in chickens. Res. Vet. Sci. 42:194-199. [PubMed] [Google Scholar]
- 3.Gupta, S. K., K. Pillarisetti, S. L. Gray, and J. M. Stadel. 1998. Molecular cloning of a novel chemokine receptor-like gene from early stage chicken embryos. Biochem. Mol. Biol. Int. 44:673-681. [DOI] [PubMed] [Google Scholar]
- 4.Henderson, S. C., D. I. Bounous, and M. D. Lee. 1999. Early events in the pathogenesis of avian salmonellosis. Infect. Immun. 67:3580-3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes, S., and N. Bumstead. 1999. Mapping of the gene encoding a chicken homologue of the mammalian chemokine SCYA4. Anim. Genet. 30:404. [DOI] [PubMed] [Google Scholar]
- 6.Humphrey, T. J., A. Baskerville, H. Chart, and B. Rowe. 1989. Infection of egg laying hens with S. enteritidis PT 4 by oral inoculation. Vet. Rec. 125:531-532. [DOI] [PubMed] [Google Scholar]
- 7.Humphrey, T. J. 1999. Contamination of meat and eggs with Salmonella serotype Enteritidis, p. 183-192. In A. M. Saeed (ed.), Salmonella enterica serotype Enteritidis in humans and animals. Iowa State University Press, Ames, Iowa.
- 8.Kaiser, P., S. Hughes, and N. Bumstead. 1999. The chicken 9E3/CEF4 CXC chemokine is the avian orthologue of IL8 and maps to chicken chromosome 4 syntenic with genes flanking the mammalian chemokine cluster. Immunogenetics 49:673-684. [DOI] [PubMed] [Google Scholar]
- 9.Kaiser, P., L. Rothwell, E. E. Galyov, P. A. Barrow, J. Burnside, and P. Wigley. 2000. Differential cytokine expression in avian cells in response to invasion by Salmonella typhimurium, Salmonella enteritidis and Salmonella gallinarum. Microbiology 12:3217-3226. [DOI] [PubMed] [Google Scholar]
- 10.Kaiser, P., G. Underwood, and F. Davison. 2003. Differential cytokine responses following Marek's disease virus infection of chickens differing in resistance to Marek's disease. J. Virol. 77:762-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kogut, M. H., E. D. McGruder, B. M. Hargis, D. E. Corrier, and J. R. DeLoach. 1994. Dynamics of avian inflammatory response to Salmonella-immune lymphokines: changes in avian blood leukocyte populations. Inflammation 18:373-388. [DOI] [PubMed] [Google Scholar]
- 12.Kogut, M. H., E. D. McGruder, B. M. Hargis, D. E. Corrier, and J. R. DeLoach. 1994. Characterization of the pattern of inflammatory cell influx in chicks following the intraperitoneal administration of live Salmonella enteritidis and Salmonella enteritidis-immune lymphokines. Poult. Sci. 74:8-17. [DOI] [PubMed] [Google Scholar]
- 13.Kogut, M. H., G. I. Tellez, E. D. McGruder, B. M. Hargis, J. D. Williams, D. E. Corrier, and J. D. DeLoach. 1994. Heterophils are decisive components in early responses of chickens to Salmonella enteritidis infections. Microb. Pathog. 16:141-151. [DOI] [PubMed] [Google Scholar]
- 14.Kogut, M. H., E. D. McGruder, B. M. Hargis, D. E. Corrier, and J. R. DeLoach. 1995. In vivo activation of heterophil function in chickens following injection with Salmonella enteritidis-immune lymphokines. J. Leukoc. Biol. 57:56-62. [DOI] [PubMed] [Google Scholar]
- 15.Laurent, F., R. Mancassola, S. Lacroix, R. Menezes, and M. Naciri. 2001. Analysis of chicken mucosal immune response to Eimeria tenella and Eimeria maxima infection by quantitative reverse transcription-PCR. Infect. Immun. 69:2527-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li, Q. J., S. Lu, R. D. Ye, and M. Martins-Green. 2000. Isolation and characterization of a new chemokine receptor gene, the putative chicken CXCR1. Gene 257:307-317. [DOI] [PubMed] [Google Scholar]
- 17.Liang, T. S., J. K. Hartt, S. Lu, M. Martins-Green, J. L. Gao, and P. M. Murphy. 2001. Cloning, mRNA distribution, and functional expression of an avian counterpart of the chemokine receptor/HIV coreceptor CXCR4. J. Leukoc. Biol. 69:297-305. [PubMed] [Google Scholar]
- 18.Luster, A. D. 1998. Chemokines—chemotactic cytokines that mediate inflammation. N. Engl. J. Med. 338:436-445. [DOI] [PubMed] [Google Scholar]
- 19.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moody, A., S. Sellers, and N. Bumstead. 2000. Measuring infectious bursal disease virus RNA in blood by multiplex real-time quantitative RT-PCR. J. Virol. Methods 85:55-64. [DOI] [PubMed] [Google Scholar]
- 21.Rajashekara, G., E. Haverly, D. A. Halvorson, K. E. Ferris, D. C. Lauer, and K. V Nagaraja. 2000. Multidrug-resistant Salmonella Typhimurium DT104 in poultry. J. Food Prot. 63:155-161. [DOI] [PubMed] [Google Scholar]
- 22.Schneider, K., R. Klaas, B. Kaspers, and P. Staeheli. 2001. Chicken interleukin-6 cDNA structure and biological properties. Eur. J. Biochem. 268:4200-4206. [DOI] [PubMed] [Google Scholar]
- 23.Sick, C., K. Schneider, P. Staeheli, and K. C. Weining. 2000. Novel chicken CXC and CC chemokines. Cytokine 12:181-186. [DOI] [PubMed] [Google Scholar]
- 24.Smith, H. W., and J. F. Tucker. 1975. The effect of antibiotic therapy on the faecal excretion of Salmonella typhimurium by experimentally infected chickens. J. Hyg. (London) 75:275-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thorns, C. J. 2000. Bacterial food-borne zoonoses. Rev. Sci. Tech. 9:226-239. [DOI] [PubMed] [Google Scholar]
- 26.Wall, P. G., and L. R. Ward. 1999. Epidemiology of Salmonella enterica serotype Enteritidis phage type 4 in England and Wales, p. 19-26. In A. M. Saeed (ed.), Salmonella enterica serotype Enteritidis in humans and animals. Iowa State University Press, Ames, Iowa.
- 27.Wallis, T. S., and E. E. Galyov. 2000. Molecular basis of Salmonella-induced enteritis. Mol. Microbiol. 36:997-1005. [DOI] [PubMed] [Google Scholar]
- 28.Weining, K. C., C. Sick, B. Kaspers, and P. Staeheli. 1998. A chicken homologue of mammalian interleukin-1: cDNA cloning and purification of active recombinant protein. Eur. J. Biochem. 258:994-1000. [DOI] [PubMed] [Google Scholar]
- 29.Zhang, S., R. A. Kingsley, R. L. Santos, H. Andrews-Polymenis, M. Raffatellu, J. Figueiredo, J. Nunes, R. M. Tsolis, L. G. Adams, and A. J. Baumler. 2003. Molecular pathogenesis of Salmonella enterica serotype Typhimurium-induced diarrhoea. Infect. Immun. 71:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zlotnik, A., and O. Yoshie. 2000. Chemokines: a new classification system and their role in immunity. Immunity. 12:121-127. [DOI] [PubMed] [Google Scholar]