Abstract
Photoacoustic microscopy (PAM) offers unprecedented sensitivity to optical absorption and opens a new window to study biological systems at multiple length- and timescales. In particular, optical-resolution PAM (OR-PAM) has pushed the technical envelope to submicron length scales and millisecond timescales. Here, we review the state of the art of OR-PAM in biophysical research. With properly chosen optical wavelengths, OR-PAM can spectrally differentiate a variety of endogenous and exogenous chromophores, unveiling the anatomical, functional, metabolic, and molecular information of biological systems. Newly uncovered contrast mechanisms of linear dichroism and Förster resonance energy transfer further distinguish OR-PAM. Integrating multiple contrasts and advanced scanning mechanisms has capacitated OR-PAM to comprehensively interrogate biological systems at the cellular level in real time. Two future directions are discussed, where OR-PAM holds the potential to translate basic biophysical research into clinical healthcare.
Introduction
Optical microscopy, capable of identifying cellular and subcellular structures based on their unique spectral signatures, has long been a driving force in modern biophysics research. Linear (1) and nonlinear (2) fluorescence microscopy have served as technical mainstays for decades. Elastic (3,4) and inelastic (5) scattering microscopy are also well established. In contrast, the development of in vivo optical absorption microscopy lags behind.
The invention of optical-resolution photoacoustic microscopy (OR-PAM) has narrowed the gap, by enabling acoustic detection of thermoelastically induced pressure waves from biomolecules’ absorption of short-pulsed or intensity-modulated light (6). Within only a few years, OR-PAM has successfully demonstrated a wide variety of absorption-based anatomical (7), functional (8,9), metabolic (10,11), molecular (12), and genetic (13) contrasts, and has found broad applications in neurology (14), vascular biology (15,16), dermatology (17), ophthalmology (18,19), and tissue engineering (20).
Photoacoustic tomography, with unique spatial capability, maintains high spatial resolution across four major length scales in biology: organelle, cell, tissue, and organ (Fig. 1 A) (21). At the cellular level, optical focusing offers OR-PAM with 2.6-μm lateral resolution, whereas optical diffusion limits its imaging depth to 1.2 mm in biological tissues (22). The optically defined lateral resolution can be scaled down to submicron (500 nm) (23) or even subwavelength (200 nm) (24) to image organelle structures, at the expense of imaging depth. In contrast, the imaging depth of photoacoustic microscopy (PAM) can be scaled up to a few millimeters with an acoustically defined lateral resolution. Such an implementation is well known as acoustic-resolution PAM, which breaks through the optical diffusion limit (25). Although the lateral resolution can be scaled between the optical and acoustic regimes, the axial resolution (typically 15 μm) is codetermined acoustically by the bandwidths of both the received photoacoustic signal and the ultrasonic detector.
Figure 1.
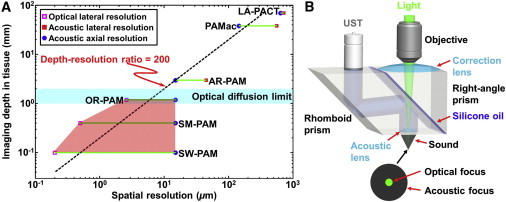
(A) Multiscale photoacoustic tomography. SW, subwavelength; SM, submicron; AR, acoustic resolution; PAMac, photoacoustic macroscopy; LA, linear array; PACT, photoacoustic computed tomography. Thepentagon zone indicates the OR-PAM regime. (B) Schematic of OR-PAM. UST, ultrasonic transducer. Reproduced from Wang and Hu (21).
Instrumentation
In a typical OR-PAM design (Fig. 1 B), the diffraction-limited optical focus is achieved by a microscope objective. To spatially separate the coaxially aligned optical excitation and acoustic detection, an optical-acoustic beam combiner with a glass-oil interface is positioned beneath the objective. A decent match in optical refractive index at the interface ensures optical transparency, whereas a significant mismatch in acoustic impedance provides acoustic reflection. To improve the sensitivity of acoustic detection, a concave acoustic lens is ground into the rhomboid prism of the beam combiner. Although confocally aligned with the optical focus, the acoustic focus (∼50 μm in diameter) is 20 times coarser than its optical counterpart due to the much longer acoustic wavelength. A portion of the generated spherical acoustic wave is converted into a plane wave by the acoustic lens and received by an unfocused ultrasonic transducer after being reflected twice at the inclined surfaces of the rhomboid prism. The second acoustic reflection is crucial to achieve high detection sensitivity, because it regains the longitudinal wave that was converted into the shear wave at the solid-oil interface (22,26,27). A correction lens is attached to the right-angle prism of the beam combiner to compensate for the optical aberrations along the light path.
Major Endogenous Contrasts
Capitalizing on the narrow nonionizing optical band of the electromagnetic spectrum, OR-PAM can identify a variety of endogenous chromophores through their unique absorption spectra (Fig. 2 A). In the ultraviolet region, DNA and RNA show strong absorption and have been used for imaging of cell nuclei (Fig. 2 B), which enables noninvasive in vivo label-free histology (7). In the visible region, hemoglobin is a predominant optical absorber (Fig. 2 C) and has been utilized to study angiogenesis and oxygen metabolism (15,22,29). The absorption spectrum of melanin spans the entire ultraviolet-visible-near-infrared region. As a major pigment in melanosomes (Fig. 2 D) and most melanoma cells (24), melanin is an ideal biomarker for both skin cancer diagnosis and treatment planning. Lipid, absorbing light in the near-infrared region, is an important component of cell membranes that plays a key role in energy storage and biological signaling. Recent technical advancement in PAM has enabled label-free visualization of lipid-rich intramuscular fat (Fig. 2 E) (30), which holds great potential in lipid biology and atherosclerosis diagnosis. Photoacoustic imaging of several other endogenous chromophores, including water (31), myoglobin (32), and bilirubin (33), has also been documented recently.
Figure 2.
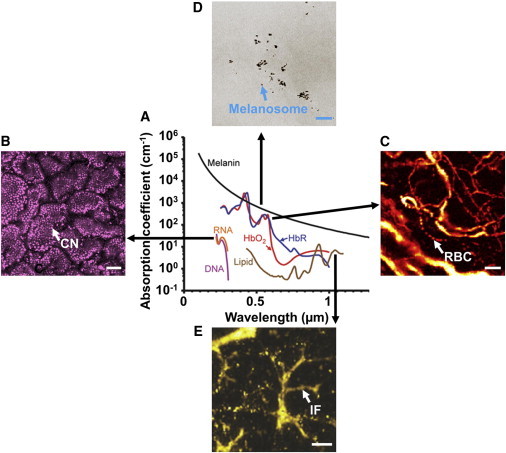
(A) Optical absorption spectra of major endogenous chromophores in biological tissues. (Magenta line) DNA, 1 g/L in cell nuclei; (orange line) RNA, 1 g/L in cell nuclei; (red line) HbO2, i.e., oxy-hemoglobin, 150 g/L in blood; (blue line) HbR, i.e., deoxy-hemoglobin, 150 g/L in blood; (black line) melanin, 14.3 g/L in human skin medium; (brown line) lipid, 20% × vol in tissue. Reproduced from Yao and Wang (50). (B) Ex vivo OR-PAM of epithelial cell nuclei (CN) in a mouse intestinal villi based on the DNA and RNA contrasts. Reproduced from Yao et al. (7). (C) In vivo OR-PAM of red blood cells (RBCs) in mouse ear vasculature based on the hemoglobin contrast. Reproduced from Hu et al. (22). (D) In vivo SW-PAM of melanosomes in a black mouse ear based on the melanin contrast. Reproduced from Zhang et al. (24). (E) Ex vivo acoustic-resolution PAM of a separate intramuscular fat (IF) sample based on the lipid contrast. Reproduced from Li et al. (30). Scale bars: 50 μm in panel B; 50 μm in panel C; 5 μm in panel D; and 1 mm in panel E.
If the cellular structure of interest consists of two or more types of chromophores, spectroscopic OR-PAM is often required to differentiate them. For example, fibroblasts contain two major chromophores: cytoplasms and nuclei. Taking advantage of their distinct absorption spectra, OR-PAM is able to distinguish them using two optical wavelengths: 422 nm for cytoplasms (Fig. 3 A) and 250 nm for nuclei (Fig. 3 B) (34). The superimposed label-free OR-PAM image of fibroblast (Fig. 3 C) matches perfectly the fluorescence image acquired after staining (Fig. 3 D).
Figure 3.
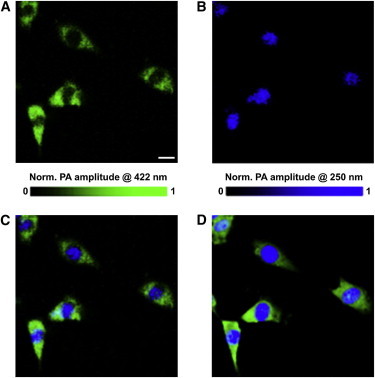
Label-free OR-PAM of fixed but unstained fibroblasts at (A) 422 nm and (B) 250 nm. (C) A superimposed image of panels A and B. (D) Fluorescence microscopy of the same sample with mitochondria and nuclei stained in different colors. Scale bar in panel A is 20 μm and applies to all panels. PA, photoacoustic. Reproduced from Zhang et al. (34).
If the absorption spectra of the chromophores of interest overlap significantly, spectral decomposition is required. A common example is to differentiate oxy-hemoglobin (HbO2) and deoxy-hemoglobin (HbR) for the quantification of hemoglobin oxygen saturation (sO2).
To decompose the experimentally measured blood absorption spectrum, we relate the photoacoustic amplitude of blood (Φ) to the concentrations of HbO2 and HbR ([HbO2] and [HbR]) as
| (1) |
where λi is the optical wavelength; μa and F are the blood absorption coefficient and optical fluence, respectively; and and are the molar extinction coefficients of HbO2 and HbR, respectively (35). By assuming that F is wavelength-independent, which is a valid approximation within the optical diffusion limit in the absence of strong intervening absorption, we can compute [HbO2] and [HbR] in relative values based on two independent measurements acquired at two wavelengths (λ1 and λ2):
| (2) |
and
| (3) |
where K is a constant prefactor.
Consequently, the sO2 can be computed as
| (4) |
New Contrasts
Dichroism
Dichroism, or polarization-dependent optical absorption, has been reported recently as a new contrast mechanism for molecular OR-PAM (36). There are three strong motivations to target dichroism:
First, dichroism is a unique molecular signature that provides an ideal specificity.
Second, the polarization-dependent optical absorption enables differential detection, which can eliminate nondichroic background and enhance sensitivity.
Third, and a more specific motivation, is that amyloid plaque, a hallmark of amyloid-associated neurodegenerative diseases, shows linear dichroism when labeled with Congo Red (37).
As a demonstration, OR-PAM examined a brain section from an APP/PS1 Alzheimer’s mouse with weak Congo-Red staining (targeting amyloid plaques) and strong Neutral-Red staining (targeting the Golgi apparatus in cells and Nissl granules in neurons, both of which are nondichroic) (Fig. 4, A and B). In both images acquired with two orthogonally polarized optical excitations, the plaque signal was obscured by the overwhelming Neutral Red-stained background (Fig. 4, C and D). Strikingly, whereas summation of the two images (Fig. 4 E) does not reveal new contrast, subtraction completely removed the nondichroic background and highlighted the quadruple-shaped dichroism feature of the amyloid plaque (Fig. 4 F).
Figure 4.
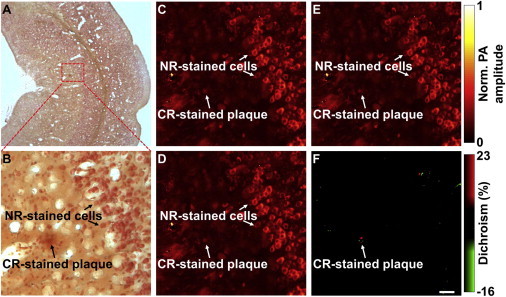
Dichroism OR-PAM of amyloid plaques in a brain section from an APP/PS1 mouse. (A) Optical microscopic photo of the brain section double-stained with Congo-Red (CR) and Neutral-Red (NR). (B) Close-up of the boxed area in panel A, showing the CR-stained amyloid plaque and the NR-stained background cells. (C and D) OR-PAM images acquired with each of the two orthogonally polarized optical irradiations. (E and F) Summation and subtraction of panels C and D, respectively. The differential detection in panel F eliminates the NR-stained nondichroic background and highlights the dichroic contrast of the CR-stained amyloid plaque. Scale bar in panel F is 100 μm and applies to all panels. PA, photoacoustic.
Previously, conventional OR-PAM demonstrated intravital amyloid plaque imaging through a cranial window (12). However, such a window may adversely influence the behavior of underlying brain tissues and bias research outcomes (38). Reflection-mode dichroism OR-PAM, with enhanced sensitivity and specificity, may be capable of visualizing individual amyloid plaques through intact skulls and create a powerful in vivo screen for anti-amyloid drugs.
Förster resonance energy transfer
The Förster resonance energy transfer (FRET) effect, encoding the distance information between molecules (typically 1–10 nm), has been a valuable tool in biophysical studies of protein interactions and conformational changes (39). OR-PAM has been adopted recently to extend the penetration of high-resolution FRET imaging (40). Unlike conventional FRET microscopy, which relies on the transition of fluorescence energy from donor to acceptor, FRET OR-PAM measures the heat production during the nonradiative dipole-dipole coupling. Therefore, if a nonfluorescent quencher is selected as the acceptor, the FRET energy will be converted into acoustic waves through heat generation and subsequent thermoelastic expansion.
To demonstrate the superior imaging depth of FRET OR-PAM, seven tubes with different concentrations of fluorescent donor Rhodamine 6G (R6G) and nonfluorescent acceptor 1,3′diethyl-4,2′quinolyoxacarbocyanide iodide (DQOCI) were imaged through freshly harvested mouse skin (Fig. 5 A). The strong optical scattering of the skin severely degrades the resolution of the fluorescence FRET image and overwhelms the quenching effect of the donor (Fig. 5 B). In contrast, OR-PAM clearly resolves all seven tubes (Fig. 5 C) and provides sufficient signal/noise for the quantification of FRET efficiency (40). Further, the cross-sectional (B-scan) FRET OR-PAM image validates an extended imaging depth of ∼1 mm (Fig. 5 D).
Figure 5.
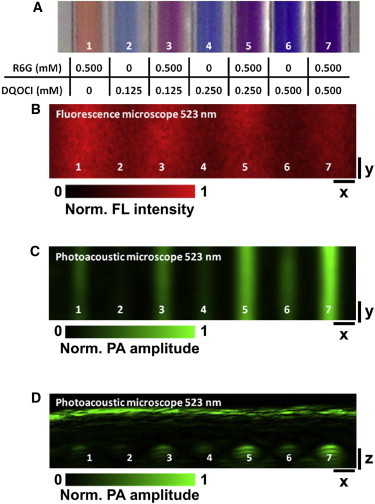
(A) Photograph of the tube phantom and tabulation of the donor (R6G) and acceptor (DQOCI) concentrations. (B) Fluorescence microscopy and (C) OR-PAM of the same tube phantom with overlaid mouse skin tissue at 523 nm. (D) B-scan OR-PAM image of the tubes acquired at 523 nm. Scale bars: 500 μm. FL, fluorescence. PA, photoacoustic. Reproduced from Wang and Wang (40).
Photoacoustic Flowoxigraphy: A New Frontier of OR-PAM
Integrating the fine length- and timescales, OR-PAM opens the door to understanding the fundamental mechanism of oxygen metabolism in biological systems. Photoacoustic flowoxigraphy, a new OR-PAM implementation, has demonstrated real-time multiparameter quantification of oxygen release from single red blood cells (RBCs) in vivo (29).
Near video-rate (20 Hz) dual-wavelength B-scan monitoring of a mouse-brain capillary in vivo clearly shows that the hemoglobin in a representative RBC is deoxygenated by 3% over a 32-μm travel distance (Fig. 6 A). Taking advantage of the ultra-short-wavelength switching time (20 μs), fast scanning speed, and high spatial resolution, multiple hemodynamic parameters—including the concentration of total hemoglobin (HbT), sO2, sO2 reduction per unit length (∇sO2), and flow speed (vf)—can be simultaneously quantified at the single-RBC level (Fig. 6 B). Further, the rate of single-RBC oxygen release (rO2) can be defined and computed in relative values as
| (5) |
where sO2in and sO2out are the sO2 values of the RBC when entering and exiting the region of interest, respectively.
Figure 6.
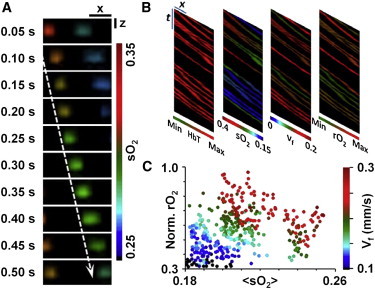
(A) Sequential snapshots of single RBCs releasing oxygen in a living mouse brain. Scale bars: 10 μm along the x direction and 30 μm along the z direction. Blood flows from left to right. (Dashed arrow) Trajectory of a single flowing RBC. (B) Simultaneous multiparameter OR-PAM of single RBCs in vivo, showing total hemoglobin concentration (HbT), sO2, flow speed (vf), and relative oxygen release rate (rO2). At each time point, the one-dimensional profile along the x axis shows the maximum amplitude projection along the depth direction. Each oblique line in the x-t images tracks one RBC. Scale bars are 10 μm and 1 s along the x axis and t axis, respectively. (C) Normalized rO2 versus ∇sO2 at varied vf. Reproduced from Wang et al. (29).
Photoacoustic flowoxigraphy experimentally shows that rO2 is proportional to the total amount of oxygen released by the RBC (∇sO2 multiplied by the pathlength), but inversely proportional to its dwell time in the field of view (pathlength divided by vf) (Fig. 6 C). The result suggests two possible underlying mechanisms for the upregulation of local oxygen metabolism at the cellular level:
-
1.
Increase in vf allows more RBCs to flow through the tissue region within a given time period; and
-
2.
More oxygen is extracted from individual RBCs, if vf is restricted.
Outlook: Translational Potential of OR-PAM to Clinic
Early detection of microvascular complications in diabetes
Diabetic microvascular complications are often asymptomatic during their early stages, and may become irreversible once symptoms develop (41). Label-free multiparameter OR-PAM of the microcirculation in vivo at high spatiotemporal resolution provides a comprehensive means for early detection of microvascular morbidity in diabetes (42).
To translate this technology to the clinic, exciting progress has been made very recently. The microvascular anatomy of a label-free human finger cuticle (Fig. 7 A) was visualized in vivo using the second-generation OR-PAM (22), which enables fiber-based instrument scanning with enhanced detection sensitivity compared with the first-generation system (6). Individual capillary loops were clearly resolved. With a dual-wavelength measurement and spectral decomposition, the blood oxygenation level within individual cuticle microvessels was further revealed (Fig. 7 B). The high spatial resolution of OR-PAM allows us to observe the sharp transition in sO2 at the tip of a representative capillary loop, which indicates a marked oxygen release from blood hemoglobin (Fig. 7 C).
Figure 7.
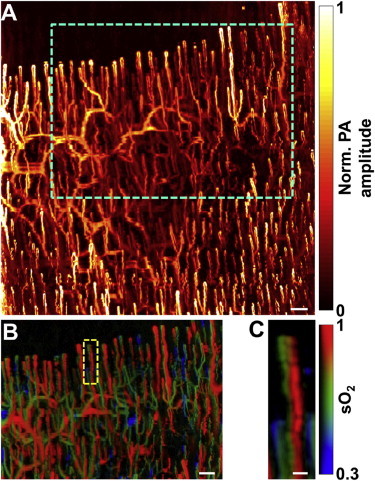
Label-free OR-PAM of (A) microvascular anatomy and (B) oxygen saturation of hemoglobin in human finger cuticle in vivo. (C) Close-up of the capillary loop indicated in B. PA, photoacoustic Scale bars: 200 μm in (A) and (B), and 50 μm in (C).
Integrating other hemodynamic parameters—including blood flow, oxygen metabolism, and pulse-wave velocity (43)—into second-generation OR-PAM would lead to a promising tool for the clinical diagnosis of microvascular complications in early-stage diabetes.
Tumor metastasis
Melanoma is malignant skin cancer with a high propensity for metastasis (44). Circulating melanoma cells (CMCs) have been regarded as a potential predictor for metastasis (45). However, blood-test-based ex vivo detection methods require a minimum number of 5000–25,000 CMCs in the blood stream, which likely corresponds to a late stage of metastasis (46).
Taking advantage of the strong optical absorption of melanin, OR-PAM holds great potential in detecting CMCs in vivo. As a proof-of-principle experiment, OR-PAM was used to monitor melanoma cells circulating in a glass microtube along with bovine blood at a concentration of 4 × 106/mL (47). The near-infrared wavelength of 1064 nm was chosen to minimize the influence of blood absorption. Similar to photoacoustic flow cytometry (48), the laser beam was focused and kept stationary at the center of the tube. CMCs passing through the optical focal zone were excited, and the generated photoacoustic signals were detected with an ultrasonic transducer. Individual melanoma cells were clearly observed with a mean signal/background of 4:1 (Fig. 8 A), and the CMC flow speed was estimated to be 1.6–3.1 mm/s (Fig. 8 B) using motion mode (M-mode) photoacoustic flow imaging (49).
Figure 8.
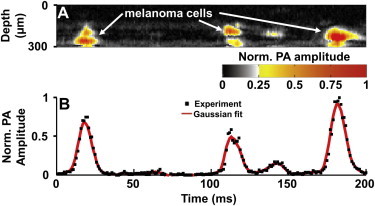
Label-free OR-PAM of melanoma cells flowing with bovine blood through a microtube. (A) M-mode PAM image tracks three melanoma cells. (B) Time course of the photoacoustic amplitude shows the transverse profile of the cells. PA, photoacoustic. Reproduced from Wang et al. (47).
Photoacoustic flowoxigraphy possesses adequate spatial and temporal resolutions for in vivo real-time CMC imaging and would enable in-depth study of the size distribution, clustering, self-seeding, and extravasation of CMCs. Operating at three optical wavelengths (two visible wavelengths for the quantification of oxygen metabolism and one near-infrared wavelength for CMC detection), photoacoustic flowoxigraphy holds the potential to unveil the relationship between CMCs and tumor progression in patients.
Acknowledgments
This work was sponsored in part by National Institutes of Health grant Nos. R01 EB008085, R01 CA134539, U54 CA136398, R01 CA157277, R01 CA159959, and DP1 EB016986 (National Institutes of Health Director’s Pioneer Award). Lihong V. Wang has a financial interest in Microphotoacoustics Inc., and Endra Inc., which, however, did not support this work.
References
- 1.White J.G., Amos W.B., Fordham M. An evaluation of confocal versus conventional imaging of biological structures by fluorescence light microscopy. J. Cell Biol. 1987;105:41–48. doi: 10.1083/jcb.105.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denk W., Strickler J.H., Webb W.W. Two-photon laser scanning fluorescence microscopy. Science. 1990;248:73–76. doi: 10.1126/science.2321027. [DOI] [PubMed] [Google Scholar]
- 3.Itzkan I., Qiu L., Perelman L.T. Confocal light absorption and scattering spectroscopic microscopy monitors organelles in live cells with no exogenous labels. Proc. Natl. Acad. Sci. USA. 2007;104:17255–17260. doi: 10.1073/pnas.0708669104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang D., Swanson E.A., Fujimoto J.G. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freudiger C.W., Min W., Xie X.S. Label-free biomedical imaging with high sensitivity by stimulated Raman scattering microscopy. Science. 2008;322:1857–1861. doi: 10.1126/science.1165758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maslov K., Zhang H.F., Wang L.V. Optical-resolution photoacoustic microscopy for in vivo imaging of single capillaries. Opt. Lett. 2008;33:929–931. doi: 10.1364/ol.33.000929. [DOI] [PubMed] [Google Scholar]
- 7.Yao D.K., Maslov K., Wang L.V. In vivo label-free photoacoustic microscopy of cell nuclei by excitation of DNA and RNA. Opt. Lett. 2010;35:4139–4141. doi: 10.1364/OL.35.004139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu S., Maslov K., Wang L.V. Functional transcranial brain imaging by optical-resolution photoacoustic microscopy. J. Biomed. Opt. 2009;14:040503. doi: 10.1117/1.3194136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi W., Shao P., Zemp R.J. In vivo dynamic process imaging using real-time optical-resolution photoacoustic microscopy. J. Biomed. Opt. 2013;18:26001. doi: 10.1117/1.JBO.18.2.026001. [DOI] [PubMed] [Google Scholar]
- 10.Yao J., Maslov K.I., Wang L.V. Label-free oxygen-metabolic photoacoustic microscopy in vivo. J. Biomed. Opt. 2011;16:076003. doi: 10.1117/1.3594786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu T., Wei Q., Zhang H.F. Combined photoacoustic microscopy and optical coherence tomography can measure metabolic rate of oxygen. Biomed. Opt. Express. 2011;2:1359–1365. doi: 10.1364/BOE.2.001359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu S., Yan P., Wang L.V. Intravital imaging of amyloid plaques in a transgenic mouse model using optical-resolution photoacoustic microscopy. Opt. Lett. 2009;34:3899–3901. doi: 10.1364/OL.34.003899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai X., Li L., Wang L.V. Multi-scale molecular photoacoustic tomography of gene expression. PLoS ONE. 2012;7:e43999. doi: 10.1371/journal.pone.0043999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsytsarev V., Hu S., Wang L.V. Photoacoustic microscopy of microvascular responses to cortical electrical stimulation. J. Biomed. Opt. 2011;16:076002. doi: 10.1117/1.3594785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oladipupo S., Hu S., Arbeit J.M. VEGF is essential for hypoxia-inducible factor-mediated neovascularization but dispensable for endothelial sprouting. Proc. Natl. Acad. Sci. USA. 2011;108:13264–13269. doi: 10.1073/pnas.1101321108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oladipupo S., Hu S., Arbeit J.M. Conditional HIF-1 induction produces multistage neovascularization with stage-specific sensitivity to VEGFR inhibitors and myeloid cell independence. Blood. 2011;117:4142–4153. doi: 10.1182/blood-2010-09-307538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Favazza C.P., Hu S., Wang L.V. In vivo multiscale photoacoustic microscopy of human skin. Proc. SPIE. 2011;7899:789946. [Google Scholar]
- 18.Hu S., Rao B., Wang L.V. Label-free photoacoustic ophthalmic angiography. Opt. Lett. 2010;35:1–3. doi: 10.1364/OL.35.000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiao S., Jiang M., Zhang H.F. Photoacoustic ophthalmoscopy for in vivo retinal imaging. Opt. Express. 2010;18:3967–3972. doi: 10.1364/OE.18.003967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai X., Paratala B.S., Wang L.V. Multiscale photoacoustic microscopy of single-walled carbon nanotube-incorporated tissue engineering scaffolds. Tissue Eng. Part C Methods. 2012;18:310–317. doi: 10.1089/ten.tec.2011.0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L.V., Hu S. Photoacoustic tomography: in vivo imaging from organelles to organs. Science. 2012;335:1458–1462. doi: 10.1126/science.1216210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu S., Maslov K., Wang L.V. Second-generation optical-resolution photoacoustic microscopy with improved sensitivity and speed. Opt. Lett. 2011;36:1134–1136. doi: 10.1364/OL.36.001134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang C., Maslov K., Wang L.V. Reflection-mode submicron-resolution in vivo photoacoustic microscopy. J. Biomed. Opt. 2012;17:020501. doi: 10.1117/1.JBO.17.2.020501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang C., Maslov K., Wang L.V. Subwavelength-resolution label-free photoacoustic microscopy of optical absorption in vivo. Opt. Lett. 2010;35:3195–3197. doi: 10.1364/OL.35.003195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H.F., Maslov K., Wang L.V. Functional photoacoustic microscopy for high-resolution and noninvasive in vivo imaging. Nat. Biotechnol. 2006;24:848–851. doi: 10.1038/nbt1220. [DOI] [PubMed] [Google Scholar]
- 26.Kinsler L.E. Wiley; New York: 2000. Fundamentals of Acoustics. [Google Scholar]
- 27.Brekhovskikh L.M. Academic Press; New York: 1980. Waves in Layered Media. [Google Scholar]
- 28.Reference deleted in proof.
- 29.Wang L., Maslov K., Wang L.V. Single-cell label-free photoacoustic flowoxigraphy in vivo. Proc. Natl. Acad. Sci. USA. 2013;110:5759–5764. doi: 10.1073/pnas.1215578110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li R., Slipchenko M.N., Cheng J.-X. Compact high power barium nitrite crystal-based Raman laser at 1197 nm for photoacoustic imaging of fat. J. Biomed. Opt. 2013;18:040502. doi: 10.1117/1.JBO.18.4.040502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu Z., Zhu Q., Wang L.V. In vivo photoacoustic tomography of mouse cerebral edema induced by cold injury. J. Biomed. Opt. 2011;16:066020. doi: 10.1117/1.3584847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang C., Cheng Y.J., Wang L.V. Label-free photoacoustic microscopy of myocardial sheet architecture. J. Biomed. Opt. 2012;17:060506. doi: 10.1117/1.JBO.17.6.060506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou Y., Zhang C., Wang L.V. Photoacoustic microscopy of bilirubin in tissue phantoms. J. Biomed. Opt. 2012;17:126019. doi: 10.1117/1.JBO.17.12.126019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang C., Zhang Y.S., Wang L.V. Label-free photoacoustic microscopy of cytochromes. J. Biomed. Opt. 2013;18:20504. doi: 10.1117/1.JBO.18.2.020504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang H.F., Maslov K., Wang L.V. Imaging of hemoglobin oxygen saturation variations in single vessels in vivo using photoacoustic microscopy. Appl. Phys. Lett. 2007;90:3. [Google Scholar]
- 36.Hu S.K.M., Yan P., Wang L.V. Biomedical Optics and 3-D Imaging. Optical Society of America; Miami, FL: 2012. Dichroism optical-resolution photoacoustic microscopy. BM2B.8. [Google Scholar]
- 37.Jin L.W., Claborn K.A., Kahr B. Imaging linear birefringence and dichroism in cerebral amyloid pathologies. Proc. Natl. Acad. Sci. USA. 2003;100:15294–15298. doi: 10.1073/pnas.2534647100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan P., Bero A.W., Lee J.M. Characterizing the appearance and growth of amyloid plaques in APP/PS1 mice. J. Neurosci. 2009;29:10706–10714. doi: 10.1523/JNEUROSCI.2637-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Periasamy A., Vogel S.S., Clegg R.M. FRET 65: a celebration of Förster. J. Biomed. Opt. 2012;17:011001. doi: 10.1117/1.JBO.17.1.011001. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y., Wang L.V. Förster resonance energy transfer photoacoustic microscopy. J. Biomed. Opt. 2012;17:086007. doi: 10.1117/1.JBO.17.8.086007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marcovecchio M.L., Tossavainen P.H., Dunger D.B. Prevention and treatment of microvascular disease in childhood type 1 diabetes. Br. Med. Bull. 2010;94:145–164. doi: 10.1093/bmb/ldp053. [DOI] [PubMed] [Google Scholar]
- 42.Krumholz A., Wang L., Wang L.V. Functional photoacoustic microscopy of diabetic vasculature. J. Biomed. Opt. 2012;17:060502. doi: 10.1117/1.JBO.17.6.060502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yeh C., Hu S., Wang L.V. Photoacoustic microscopy of blood pulse wave. J. Biomed. Opt. 2012;17:070504. doi: 10.1117/1.JBO.17.7.070504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cummins D.L., Cummins J.M., Chanmugam A. Cutaneous malignant melanoma. Mayo Clin. Proc. 2006;81:500–507. doi: 10.4065/81.4.500. [DOI] [PubMed] [Google Scholar]
- 45.Hu X., Wei C.W., Gao X. Trapping and photoacoustic detection of CTCs at the single cell per milliliter level with magneto-optical coupled nanoparticles. Small. 2013;9:2046–2052. doi: 10.1002/smll.201202085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nedosekin D.A., Sarimollaoglu M., Zharov V.P. In vivo ultra-fast photoacoustic flow cytometry of circulating human melanoma cells using near-infrared high-pulse rate lasers. Cytometry A. 2011;79:825–833. doi: 10.1002/cyto.a.21102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y., Maslov K., Wang L.V. Fiber-laser-based photoacoustic microscopy and melanoma cell detection. J. Biomed. Opt. 2011;16:011014. doi: 10.1117/1.3525643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zharov V.P., Galanzha E.I., Tuchin V.V. In vivo photoacoustic flow cytometry for monitoring of circulating single cancer cells and contrast agents. Opt. Lett. 2006;31:3623–3625. doi: 10.1364/ol.31.003623. [DOI] [PubMed] [Google Scholar]
- 49.Fang H., Wang L.V. M-mode photoacoustic particle flow imaging. Opt. Lett. 2009;34:671–673. doi: 10.1364/ol.34.000671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yao J., Wang L.V. Photoacoustic microscopy. Laser Photon Rev. 2013 doi: 10.1002/lpor.201200060. 31 Jan. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]


