Abstract
The control of Listeria monocytogenes infection depends on the rapid activation of the innate immune system, likely through Toll-like receptors (TLR), since mice deficient for the common adapter protein of TLR signaling, myeloid differentiation factor 88 (MyD88), succumb to Listeria infection. In order to test whether TLR2 is involved in the control of infections, we compared the host response in TLR2-deficient mice with that in wild-type mice. Here we show that TLR2-deficient mice are more susceptible to systemic infection by Listeria than are wild-type mice, with a reduced survival rate, increased bacterial burden in the liver, and abundant and larger hepatic microabscesses containing increased numbers of neutrophils. The production of tumor necrosis factor, interleukin-12, and nitric oxide and the expression of the costimulatory molecules CD40 and CD86, which are necessary for the control of infection, were reduced in TLR2-deficient macrophages and dendritic cells stimulated by Listeria and were almost abolished in the absence of MyD88, coincident with the high susceptibility of MyD88-deficient mice to in vivo infection. Therefore, the present data demonstrate a role for TLR2 in the control of Listeria infection, but other MyD88-dependent signals may contribute to host resistance.
Toll-like receptors (TLRs) participate in innate immunity by detecting different invading pathogens (2, 3) and stimulating cell activation through adapter molecules, such as myeloid differentiation factor 88 (MyD88), Toll-interleukin-1 receptor domain-containing adapter protein (TIRAP), and Toll-interleukin-1 receptor domain-containing adapter inducing beta interferon (TRIF), and transcription factors, such as nuclear factor κB (NF-κB), which allows the transcription of a set of proinflammatory cytokine genes (27). TLRs are expressed on a wide variety of cells, such as monocytes/macrophages, dendritic cells (DC), B cells, T cells, mast cells, and epithelial cells, but the pattern of expression of individual TLRs differs for specific cell types. TLRs recognize conserved molecular structures on pathogens (3), discriminating gram-positive and gram-negative bacteria from fungi and other pathogens (30). Bacterial lipoteichoic acid (LTA), peptidoglycans, and lipoarabinomannan (2) and zymosan from yeast (3) induce cell activation in a TLR2-dependent fashion. This broad and unusual spectrum of pathogen recognition may be explained by the fact that TLR2 forms heterodimers in association with TLR6 or TLR1 (22).
Listeria monocytogenes is a gram-positive facultative intracellular bacterium, and its virulence is due to its capacity to penetrate into mammalian cells, to use their cellular machinery, and to evolve highly sophisticated strategies to evade the host immune response. After internalization of the bacteria, by phagocytosis or by penetration in the case of nonphagocytic cells, macrophages, natural killer cells, and neutrophils are recruited to inhibit the growth of the pathogen. Infected macrophages secrete inflammatory cytokines, such as tumor necrosis factor (TNF), interleukin-12 (IL-12), and several chemokines, allowing the recruitment and activation of immune cells. IL-12 participates in the development of T lymphocytes expressing Th1-type cytokines such as interferon gamma (IFN-γ), TNF, and IL-2. The synergy between TNF and IL-12 augments the expression of major histocompatibility complex class II and costimulatory molecules, allowing optimal bacterial antigen presentation to T cells (10) and the activation of T and natural killer cells. This results in the secretion of IFN-γ, which in combination with TNF leads to full macrophage activation and bacterial killing through the production of nitric oxide (NO) (4, 10). Furthermore, activated macrophages secrete IL-6, IL-1, and chemokines, which also control lymphocyte and neutrophil recruitment and activation (7). Microabscesses comprised of different cell types, such as activated macrophages, neutrophils, and others, are formed in the parenchyma of infected organs.
Recent reports showed that TLR2 may play a protective role during infections by gram-positive bacteria. Indeed, TLR2-deficient mice are more susceptible to Staphylococcus aureus (28) and Streptococcus pneumoniae subsp. meningitis infections (9). In vitro studies with human monocytes revealed that TLR2 is required for macrophage activation in response to Listeria (12). Furthermore, Seki et al. showed that TLR2-deficient mice present a deficit in circulating TNF and IL-12 p40 production in response to Listeria infection in vivo (26). However, the innate immune response to and control of infection by a 5 × 105 Listeria intraperitoneal inoculum were reportedly normal in TLR2-deficient mice, whereas the presence of MyD88 was critical for the control of infection (11).
In order to clarify the role of TLR2 in the control of Listeria infection, we used TLR2-deficient mice and examined their responses to Listeria infection in vitro and in vivo. Our study provides the first evidence that TLR2 plays a role in the control of Listeria infection.
MATERIALS AND METHODS
Mice and reagents.
Six- to 12-week-old TLR2-deficient (−/−) and TLR2 control (+/+) mice (obtained from Carsten Kirschning, Munich, Germany [33]) and MyD88−/− mice (1) were used in this study. All mice were backcrossed for seven generations onto a C57BL/6 background. The results of the infection were verified at backcross 10. TLR2−/− and TLR2+/+ littermates obtained by intercrossing of heterozygous (+/−) parents were used for direct comparisons. All mice were bred under specific-pathogen-free conditions at the Transgenose Institute (CNRS, Orleans, France).
Culturing of bacteria.
L. monocytogenes (L028 strain from P. Cossart, Pasteur Institute, Paris, France) was cultured in Trypticase soy broth (soybean casein digest medium; Biovalley), divided into aliquots, and stored in 30% glycerol at −80°C at a concentration of 5 × 109 CFU/ml.
Heat-killed L. monocytogenes (HKLM) was prepared by incubation at 60°C for 1 h followed by two washes with sterile phosphate-buffered saline (PBS).
Primary macrophage and DC cultures.
Murine bone marrow cells were isolated from femurs and were differentiated into macrophages after culturing at 106 cells/ml for 7 days in Dulbecco's modified Eagle's medium (Sigma) supplemented with 20% horse serum and 30% L929 cell-conditioned medium (as a source of macrophage colony-stimulating factor [20]). Three days after being washed and recultured in fresh medium, the cell preparation contained a homogenous population of macrophages (>97% CD11b+ cells). Alternatively, murine bone marrow cells were differentiated into myeloid DCs (>98% CD11c+ cells) after culturing at 2 × 106 cells/ml for 10 days in RPMI medium supplemented with 10% fetal calf serum, glutamine, antibiotics, and 4% J558L cell-conditioned medium as a source of granulocyte-macrophage colony-stimulating factor, as described previously (19).
Bone-marrow-derived macrophages (BMDM) and DCs (BMDC) were plated in 96-well microculture plates (at 105cells/well) and stimulated with lipopolysaccharide (LPS) (from Escherichia coli serotype O111:B4, at 100 ng/ml; Sigma), bacterial lipoprotein (BLP) [Pam3Cys-Ser-(Lys)4, at 0.5 μg/ml; EMC Microcollections], HKLM (at a bacteria-to-cell ratio of 200), and Listeria (at a bacteria-to-cell ratio of 2). After 24 h of stimulation, the supernatants were harvested for cytokine determination.
Cytokine determination.
TNF, IL-12 p40, and IFN-γ levels were quantified by using a commercial enzyme-linked immunosorbent assay (ELISA) (Duoset; R&D Systems, Abingdon, United Kingdom) according to the recommendations of the manufacturer.
Flow cytometry.
After stimulation, macrophages and DCs were harvested, washed once in PBS, and incubated on ice at 105 cells/50 μl with 2% serum for 20 min. After centrifugation (10 min at 200 × g and 4°C), macrophages were incubated in PBS-0.5% bovine serum albumin with primary antibodies (anti-CD40-PE clone 2G9, anti-CD86-PE clone GL1, anti-CD11b-PerCP Cy5.5 clone M1/70, and anti-CD11c-APC clone HL3) for 20 min in the dark. All antibodies were from BD Pharmingen (San Diego, Calif.). After being washed with PBS-0.5% bovine serum albumin, cells were analyzed on a Becton Dickinson LSR analyzer.
Infection of mice.
Littermate TLR2−/− and TLR2+/+ mice were injected intravenously in the caudal vein at 1 × 105, 2 × 105, or 3 × 105 CFU/mouse, and MyD88−/− mice were injected at 2 × 105 CFU/mouse. Circulating IFN-γ levels were measured in the sera 5, 8, 24, 48, and 72 h after infection. On day 2, livers and spleens were harvested. The numbers of viable bacteria in organ homogenates were determined by plating serial dilutions on Trypticase soy broth plates (Biovalley). Plates were incubated at 37°C, and numbers of CFU were enumerated after 24 h.
Histology and immunohistochemistry.
Samples of livers and spleens were fixed in 10% buffered formalin (Shandon, Pittsburgh, Pa.). Tissues were dehydrated in ethanol and embedded in paraffin. Sections (4 μm thick) were cut and stained with hematoxylin and eosin for evaluation of pathological changes. Microabscesses were quantified by counting of 20 microscopic fields at a ×100 magnification. For immunohistochemical analysis, livers and spleens were embedded with Tissue-Tek (Sakura, Zoeterwoude, The Netherlands) in cryomolds, immediately frozen on dry ice, and stored at −80°C. The frozen tissues were cut into 5-μm thick sections on a cryostat (Leica, Nussloch, Germany), air dried, and stored at −80°C before being fixed in acetone (10 min at 4°C). Endogenous peroxidase activity was blocked by methanol in 1% H2O2 (30 min). Endogenous biotin in the liver was blocked with a PBS-0.1% avidin solution (20 min) and PBS-0.01% biotin (20 min). The tissue sections were incubated with an appropriate normal serum (30 min) before incubation for 2 h at 37°C with the primary antibody. Antibodies to GR1, F4/80, and inducible nitric oxide synthase (iNOS) were from BD Pharmingen. The sections were then incubated for 30 min at 37°C with the appropriate biotinylated secondary antibody. Avidin-biotin peroxidase complexes were added to the sections for 30 min (ABC vector kit; Vector, Burlingame, Calif.), washed, and revealed with diaminobenzidine substrate (Dako, Glostrup, Denmark). After a rinse in PBS, the sections were mounted in Eukitt reagent (Kindler & Co., Freiburg, Germany).
Nitrite measurements.
Nitrite concentrations in supernatants from macrophages were determined by use of Griess reagent (1% sulfanilamide in 2.5% phosphoric acid-0.1% n-1-napthylethylenediamide dichlorique [14]). After a 30-min incubation at room temperature with agitation, the absorbance at 540 nm was measured. NO2− was quantified by using NaNO2 as a standard.
Statistical analysis.
The statistical evaluation of differences between the experimental groups was done with the log rank test for survival curves and with Student's t test.
RESULTS
Impaired TNF and IL-12 p40 production and CD40 and CD86 expression in TLR2- and MyD88-deficient macrophages and DCs stimulated by Listeria.
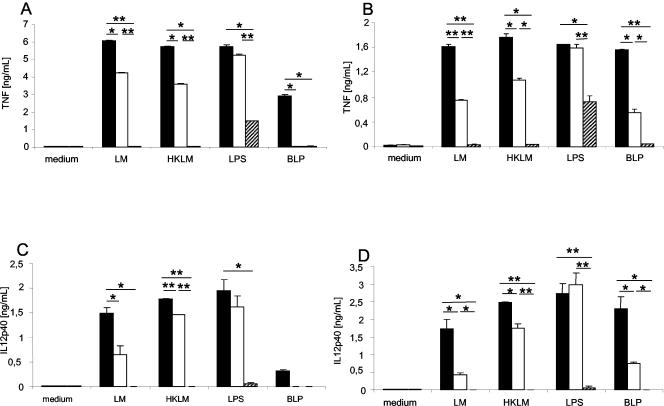
For characterization of the role of TLR2 in the production of proinflammatory cytokines in response to Listeria, primary BMDM and BMDC from TLR2+/+, TLR2−/−, and MyD88−/− mice were stimulated with Listeria and HKLM. A 30 to 40% decrease in TNF production was found after Listeria or HKLM activation of TLR2−/− macrophages (Fig. 1A) and DCs (Fig. 1B) compared to that for wild-type cells. A similar reduction in TNF production was also observed for BLP, a TLR2 agonist. As expected, no difference was visible after stimulation of TLR2−/− macrophages with LPS, a typical TLR4 agonist. For comparison, TNF secretion was completely abolished in MyD88−/− macrophages stimulated with Listeria or HKLM, indicating that this adapter protein of the TLR signaling pathway is critical (Fig. 1A and B). Similar results were obtained for the secretion of IL-12 p40 by macrophages and DCs in response to Listeria, HKLM, LPS, or BLP stimulation (Fig. 1C and D).
FIG. 1.
Impaired TNF and IL-12 p40 production in TLR2- and MyD88-deficient macrophages and DCs infected with Listeria (LM) or stimulated with HKLM in vitro. Shown are in vitro responses of BMDM and BMDC to HKLM, Listeria, LTA, and LPS by TLR2+/+ (black bars), TLR2−/− (white bars), and MyD88−/− (hatched bars) mice. Macrophages (A and C) and DCs (B and D) were stimulated for 24 h with Listeria (2×), HKLM (200×), BLP (0.5 μg/ml), and LPS (100 ng/ml). The concentrations of TNF (A and B) and IL-12 p40 (C and D) in supernatants were determined by ELISA. Results are from one representative experiment of three independent experiments and are expressed as means ± standard deviations (SD). *, P < 0.05; **, P < 0.01.
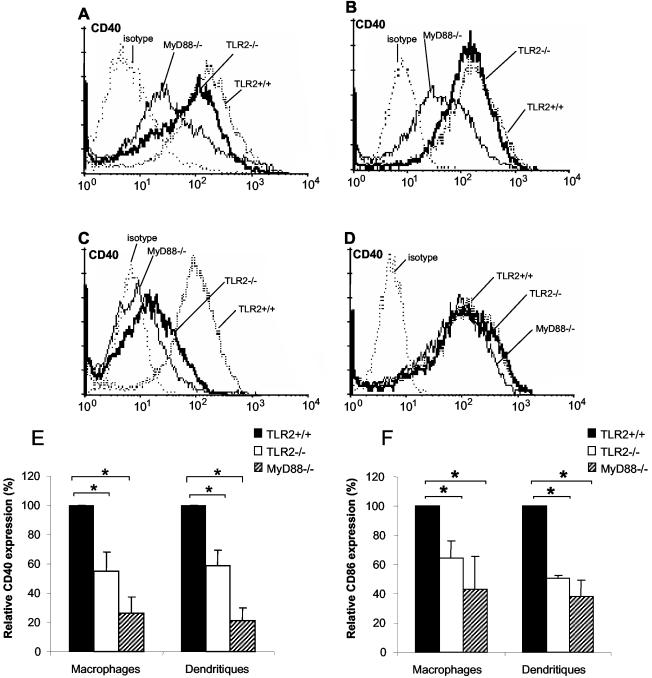
Activated macrophages and DCs express costimulatory molecules such as CD40 and CD86 that are necessary for the development of adaptive immunity. We wondered whether TLR2 is required for the optimal expression of these costimulatory molecules upon activation by Listeria. Listeria induced the upregulation of CD40 expression by CD11b+ cells (Fig. 2). This expression was reduced in Listeria-infected TLR2−/− macrophages compared to wild-type macrophages, while CD40 expression was almost completely abolished in MyD88−/− macrophages (Fig. 2A). The profile observed after stimulation with HKLM was similar in the case of MyD88−/− mice, but no reduction in CD40 expression was observed for TLR2−/− macrophages (Fig. 2B). Finally, CD40 expression induced by the TLR2 agonist BLP was largely TLR2 and MyD88 dependent (Fig. 2C), while that induced by the TLR4 agonist LPS was TLR2 and MyD88 independent (Fig. 2D). These data demonstrate that Listeria activates TLR2, as does the TLR2 agonist BLP, but they also suggest the existence of additional TLR-dependent signaling pathways. The levels of CD40 (Fig. 2E) and CD86 (Fig. 2F) expression upon Listeria stimulation were compared for TLR2+/+, TLR2−/−, and MyD88−/− macrophages and DCs. An analysis of the geometric mean fluorescence intensity relative to that for wild-type controls showed a parallel decrease in CD40 and CD86 costimulatory molecule expression levels in both TLR2−/− macrophages and DCs (∼40%). A similar but more pronounced reduction was observed for MyD88−/− cells for CD40 (∼70%) and CD86 (∼60%) expression (Fig. 2E and F).
FIG. 2.
Reduced CD40 and CD86 expression in TLR2- and MyD88-deficient macrophages and DCs infected with L. monocytogenes in vitro. BMDM from TLR2+/+, TLR2−/−, and MyD88−/− mice were stimulated for 24 h with Listeria (2×) (A), HKLM (200×) (B), BLP (0.5 μg/ml) (C), and LPS (100 ng/ml) (D). BMDM were labeled with an anti-CD11b antibody (>97% CD11b+) and analyzed by fluorescence-activated cell sorting for CD40 expression. Unstimulated controls showed essentially no CD40 expression, similar to the isotype controls (dotted lines). Results are from one representative experiment (a pool of two mice) of three independent experiments. The levels of CD40 (E) and CD86 (F) expression by CD11b+ BMDM and CD11c+ BMDC from TLR2+/+, TLR2−/−, and MyD88−/− mice stimulated for 24 h by Listeria (2×) were also compared. The results, expressed as geometric mean fluorescence intensities relative to those of wild-type controls, were calculated from three independent experiments and are expressed as means ± SD. *, P < 0.05.
Therefore, live Listeria activates macrophages and DCs through TLR2 engagement and MyD88 signaling, resulting in the expression of costimulatory molecules and the synthesis of TNF and IL-12 p40.
Impaired NO production in TLR2- and MyD88-deficient macrophages and DCs stimulated by Listeria.
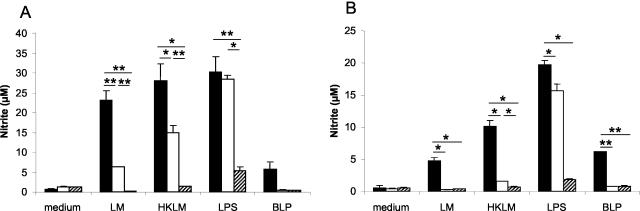
NO is a critical mediator in the killing of various intracellular parasitic microbes and plays a distinct role in host protection (5). We determined whether NO synthesis depends on TLR2 signaling. Listeria-stimulated TLR2−/− macrophages secreted four times less NO than their wild-type counterparts (Fig. 3A), whereas 50 and 80% reductions were observed for macrophages stimulated with HKLM and BLP, respectively. As expected, no difference in NO production was found for TLR2−/− macrophages stimulated by LPS (Fig. 3A). NO synthesis was completely dependent on the MyD88 signaling pathway, as it was undetectable in supernatants from MyD88−/− macrophages stimulated with Listeria, HKLM, or BLP (Fig. 3A). Upon LPS stimulation, MyD88−/− macrophages produced residual NO, likely mediated by a MyD88-independent signaling pathway (Fig. 3A). Upon stimulation, DCs behaved similarly to macrophages, although they produced slightly lower NO levels (Fig. 3B).
FIG. 3.
Impaired NO production in TLR2- and MyD88-deficient macrophages and DCs infected with Listeria (LM) or stimulated with HKLM in vitro. BMDM (A) and BMDC (B) from TLR2+/+ (black bars), TLR2−/− (white bars), and MyD88−/− (hatched bars) mice were stimulated for 24 h with Listeria (2×), HKLM (200×), BLP (0.5 μg/ml), and LPS (100 ng/ml). Supernatants were recovered, and nitrite was measured by use of the Griess reagent. Results are from one representative experiment (n = 2) of two independent experiments. Results are expressed as means ± SD. *, P < 0.05; **, P < 0.01.
Thus, macrophage and DC production of the antibacterial mediator NO, necessary for the killing of Listeria, is partially TLR2 dependent, and this response is mediated via MyD88.
Increased susceptibility of TLR2-deficient mice to Listeria infection.
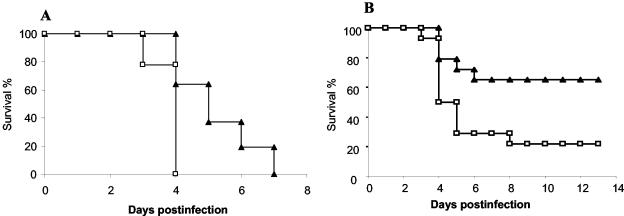
Since Listeria activates macrophages and DCs in a TLR2-dependent manner, we next asked whether TLR2 signaling contributes to resistance to in vivo infections. We compared the resistance of TLR2+/+ and TLR2−/− mice after a systemic infection with Listeria. Listeria injected intravenously (i.v.) at a dose of 3 × 105 CFU/mouse was lethal within 4 days for TLR2-deficient mice, while control mice survived for 4 to 7 days (Fig. 4A). At a lower infectious dose of 105 CFU/mouse, only 3 of 14 TLR2-deficient mice survived the infection, whereas 9 of 14 control mice had long-term survival (Fig. 4B). Therefore, these data clearly show that TLR2 has a protective effect for the control of Listeria infection in vivo.
FIG. 4.
Increased susceptibility of TLR2−/− mice to L. monocytogenes. TLR2+/+ (triangles) and TLR2−/− (squares) mice were inoculated i.v. with 3 × 105 CFU/mouse (A) and 1 × 105 CFU/mouse (B) and were monitored for survival. For panel A, P = 0.03, and for panel B, P = 0.022 by the log rank test. (A) Results are from 11 TLR2+/+ mice and 9 TLR2−/− mice. (B) Results are from 14 mice per group. The results shown are the combination of two identical experiments.
Increased hepatic bacterial load in TLR2-deficient mice.
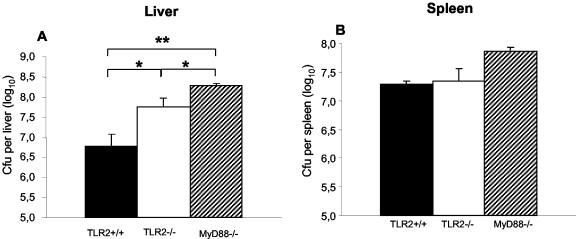
In view of the increased sensitivity of TLR2−/− mice to Listeria infection, we asked whether the bacterial clearance could be reduced. The bacterial loads in the spleens and livers of TLR2+/+, TLR2−/−, and MyD88−/− mice infected with 2 × 105 CFU of Listeria per mouse were analyzed. TLR2−/− mice displayed 1 log higher CFU counts in the liver than did control mice, while MyD88−/− mice had slightly higher CFU counts than did TLR2−/− mice (Fig. 5A). No difference in CFU was apparent in the spleen for TLR2−/− and TLR2+/+ mice, whereas a slight, but not significant, difference was observed between TLR2−/− and MyD88−/− mice (Fig. 5B). Later time points (day 5 after the i.v. injection of a lower dose of 2 × 104 CFU/mouse) revealed the same trend, with increased CFU counts in the livers of TLR2−/− mice (data not shown).
FIG. 5.
Increased bacterial loads after infection of TLR2−/− mice with L. monocytogenes. TLR2+/+ (black bars), TLR2−/− (white bars), and MyD88−/− (hatched bars) mice were infected with 2 × 105 CFU of Listeria i.v. and sacrificed at day 2 postinfection, and the total numbers of CFU per liver (A) and spleen (B) were determined. Results are from one representative experiment of two independent experiments. Results are expressed as means ± SD. *, P < 0.05, **, P < 0.01.
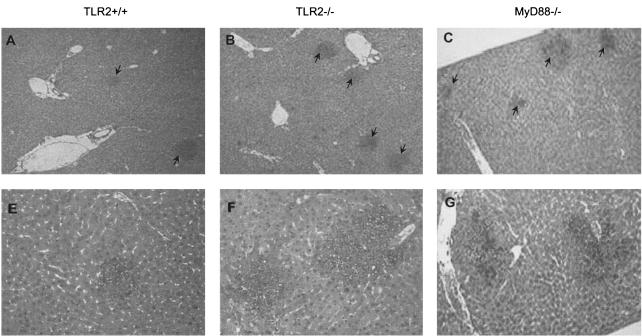
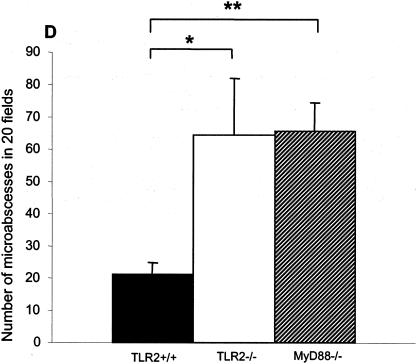
Microscopic examination at a low magnification revealed distinct hepatic microabscesses in TLR2- and MyD88-deficient mice (Fig. 6A to C) at 2 days postinfection. The microabscesses from MyD88-deficient mice were generally larger and in part confluent. Their numbers in TLR2- and MyD88-deficient mice were significantly increased and different from that in control mice (Fig. 6D). Furthermore, the microabscesses were larger and had less defined boundaries in TLR2−/− and MyD88−/− mice (Fig. 6E to G) than in control mice (Fig. 6D). No significant difference was apparent between TLR2−/− and MyD88−/− mice in the number of microabscesses in the liver (Fig. 6D) and in the morphology of microabscesses (Fig. 6F and G). Therefore, the absence of TLR2 signaling is associated with an enhanced formation of more loosely organized microabscesses in the livers of infected mice.
FIG. 6.
Increased hepatic microabscesses in TLR2-deficient infected mice. Two days after i.v. infection with 2 × 105 CFU of Listeria, the livers of infected TLR2+/+ (A), TLR2−/− (B), and MyD88−/− (C) mice contained multiple microabscesses. (D) Numbers of microabscesses per 20 fields quantified and expressed as means ± SD (*, P < 0.05). Histologic samples were stained with hematoxylin and eosin (magnification, ×200). (E, F, and G) Liver microabscesses from wild-type, TLR2−/−, and MyD88−/− mice, respectively, at a higher magnification (×400). Results are from one representative experiment of two independent experiments.
Recruitment of immune cells to microabscesses.
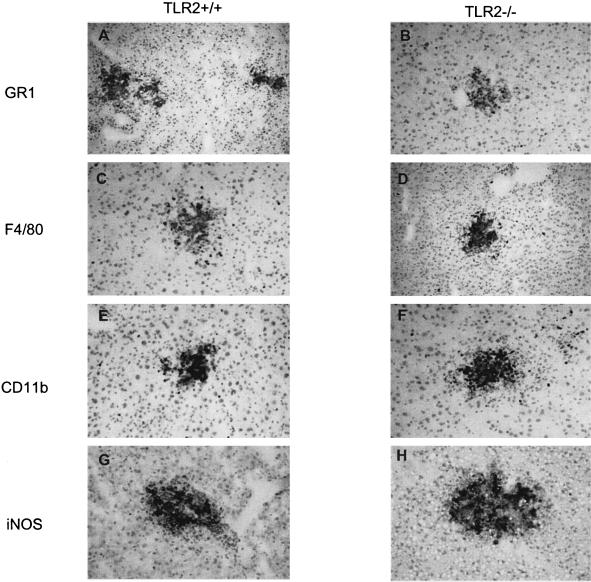
In view of the enhanced inflammatory response, we asked whether the composition of the microabscesses was different in the absence of TLR2. Therefore, we characterized immune inflammatory cells in the hepatic microabscesses from Listeria-infected TLR2+/+ and TLR2−/− mice. The number of neutrophils detected with an anti-GR1 monoclonal antibody seemed to be increased for TLR2-deficient mice, concomitant with the increase in size and number of microabscesses (Fig. 7A and B). In contrast, the expression of F4/80- and CD11b-positive macrophages was not different for the two experimental groups (Fig. 7C to F). Interestingly, CD11c-positive DCs were absent from the hepatic microabscesses 2 days after infection. Very few T and B cells were detected at the periphery of microabscesses (data not shown). The expression of iNOS, as judged by immunohistochemistry, was not reduced in TLR2-deficient mice after 2 days of infection (Fig. 7G and H). These results indicate that the recruitment of mononuclear cells is not impaired in the absence of TLR2 and that neutrophils are slightly more abundant in microabscesses.
FIG. 7.
Immune cell recruitment to microabscesses after L. monocytogenes infection. Images of the immunohistochemistry of TLR2+/+ (A, C, E, and G) and TLR2−/− (B, D, F, and H) livers 2 days after i.v. infection with 2 × 105 CFU/mouse are shown. Immunolabeling with GR1 (A and B), F4/80 (C and D), CD11b (E and F), and iNOS (G and H) is shown in brown (magnification, ×200).
Reduced IFN-γ production in TLR2-deficient mice during early infection of Listeria.
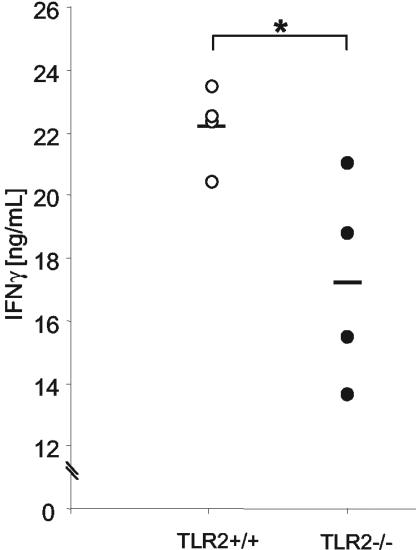
Since IFN-γ plays a critical role in the control of Listeria infections and since IFN-γ is detectable in the sera of mice upon infection, we determined whether IFN-γ was induced in TLR2-deficient mice (7, 8, 10). Circulating IFN-γ levels were measured in the sera of TLR2−/− and TLR2+/+ mice 5, 8, 24, 48, and 72 h after Listeria infection. We detected IFN-γ expression 24 h after Listeria infection for both strains, while at earlier and later time points no IFN-γ was detected in the sera (data not shown). Figure 8 shows that IFN-γ levels at 24 h were significantly lower in the absence of TLR2 (P < 0.05). Therefore, the data suggest that TLR2 receptor expression is necessary for the optimal induction of IFN-γ upon Listeria infection.
FIG. 8.
Requirement of TLR2 for IFN-γ induction in early L. monocytogenes infection. TLR2+/+ (white circles) and TLR2−/− (black circles) mice were inoculated i.v. with 2 × 105 CFU of Listeria. Blood was taken at 24 h postinfection, and IFN-γ levels in serum were measured by ELISA. Results are from one representative experiment of two independent experiments and are expressed as means ± SD. *, P < 0.05.
DISCUSSION
Here we report that TLR2 plays a role in the protective immune response to the intracellular gram-positive pathogen L. monocytogenes. Indeed, our data show a reduced activation of macrophages and DCs, with an increased bacterial burden in the liver and an augmented mortality, for TLR2−/− mice infected by Listeria. Furthermore, by comparing TLR2−/− and MyD88−/− mice, we confirmed that MyD88 is essential for the control of Listeria infection, suggesting that other TLRs and/or IL-1 and IL-18 signaling play additional roles in infection control (11, 26).
TLR2 was previously shown to serve as a receptor for gram-positive bacteria and to play a critical role in resistance to infection (2, 3, 17, 18, 28, 31). We showed that TLR2−/− mice succumb to Listeria infection with reduced bacterial clearance, although MyD88−/− mice are even more susceptible. Our finding contrasts with a recent report showing no difference in CFU in livers of TLR2−/− and control mice 3 days after intraperitoneal infection with 5 × 105 Listeria, i.e., half the 50% lethal dose (LD50) (11). We found an increased susceptibility of the TLR2-deficient mice for two different i.v. doses, corresponding roughly to the LD30 and LD100. The discrepancy may be related to the difference in the route of Listeria injection (i.v. rather than intraperitoneal), which may favor more rapid bacterial colonization of the liver. It could also be due to the fact that we used L. monocytogenes strain L028, whereas the previous work was based on the EGD strain (11). Furthermore, we demonstrated the TLR2 dependence of the inflammatory response to Listeria infection with distinct increased microabscesses containing neutrophils and macrophages together with augmented bacterial loads in the livers of TLR2−/− mice. These effects were even more pronounced in MyD88−/− mice. Neutrophils play a key role in the early stage of Listeria infection (10, 23), and increased neutrophil recruitment and microabscess formation in TLR2−/− mice may represent a compensatory mechanism due to insufficient macrophage activation.
We showed here the reduced TNF and IL-12 p40 production by TLR2−/− macrophages and DCs stimulated in vitro by live Listeria or HKLM. It was previously shown that TLR2-deficient Kupffer cells secrete less TNF in response to Listeria than those from control mice (11, 26). In addition, upon Listeria infection, TLR2-deficient macrophages and DCs produced reduced amounts of the mediator NO and expressed lower levels of costimulatory molecules such as CD40 and CD86. These effects were even more pronounced for cells from MyD88−/− mice.
NO synthesis is known to be critical for the control of infection, as the production of this mediator endows macrophages with cytostatic and cytotoxic activities against bacteria (29). The lower CD40 and CD86 expression levels by macrophages and DCs observed after Listeria stimulation in the absence of TLR2 should not affect the establishment of an efficient adaptive immune response against Listeria. Indeed, MyD88-deficient mice were shown to generate effective protective immunity to Listeria (32), and no alternative signaling pathway to MyD88 has been described for TLR2. Reduced antigen-presenting cell (APC) activation and pro-inflammatory cytokine secretion in the absence of TLR2 signaling further contribute to the lower IFN-γ production observed with the sera of TLR2−/− mice. Indeed, APC-derived IL-12 triggers NK cells to produce IFN-γ, which itself as a feedback loop activates macrophages and recruits neutrophils, which are both important for the clearance of Listeria (29). Therefore, a low IFN-γ level in turn prevents effective macrophage activation, thereby reducing the killing of Listeria by macrophages (8) and the generation of protective immunity (34).
Cooperation between TLR2 and TLR6 or TLR1 for different TLR2 agonists has been shown (6, 22). Further studies will be necessary to clarify whether TLR2 heterodimerization with either TLR1 or TLR6 is required for Listeria-induced activation of APCs and for the control of Listeria infection in vivo. Furthermore, other TLRs may be implicated in the in vivo response to Listeria infection. Indeed, Hayashi et al. demonstrated that Listeria is recognized by TLR5 through its flagellin (15). Further, TLR9, which is implicated in inflammatory responses induced by bacterial, nonmethylated DNA (16), could be another candidate. Interestingly, the implication of IL-1 in the control of Listeria infection has been controversial. No role was shown when IL-1 receptor 1-deficient mice were used (13), whereas IL-1 was shown to participate in the development of anti-Listeria responses (24, 25). A critical role for IL-18 in the control of Listeria infection has also been documented (21), although only a limited higher susceptibility of IL-18-deficient mice was reported (26). Edelson and Unanue (11) documented that mice that are deficient in caspase-1 have minimally increased spleen Listeria titers, but an ∼2-log increase in liver titers, supporting a role for IL-1 and IL-18 in the innate response to Listeria. Since the signaling of IL-1 and IL-18 is MyD88 dependent (1), the difference between MyD88- and TLR2-deficient mice in host resistance could indeed be due to defective signaling of IL-1 or IL-18. However, in view of the controversial results obtained by different groups, experiments are required to directly compare infections in caspase-1-, IL-1 receptor 1-, and IL-18-deficient mice.
In summary, we show here that the innate immune receptor TLR2 contributes to the effective control of an early Listeria infection in vivo, although compensatory mechanisms may be activated for the subsequent control of infection and bacterial clearance.
Editor: F. C. Fang
REFERENCES
- 1.Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143-150. [DOI] [PubMed] [Google Scholar]
- 2.Aderem, A., and R. J. Ulevitch. 2000. Toll-like receptors in the induction of the innate immune response. Nature 406:782-787. [DOI] [PubMed] [Google Scholar]
- 3.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. [DOI] [PubMed] [Google Scholar]
- 4.Beckerman, K. P., H. W. Rogers, J. A. Corbett, R. D. Schreiber, M. L. McDaniel, and E. R. Unanue. 1993. Release of nitric oxide during the T cell-independent pathway of macrophage activation. Its role in resistance to Listeria monocytogenes. J. Immunol. 150:888-895. [PubMed] [Google Scholar]
- 5.Boockvar, K. S., D. L. Granger, R. M. Poston, M. Maybodi, M. K. Washington, J. B. Hibbs, Jr., and R. L. Kurlander. 1994. Nitric oxide produced during murine listeriosis is protective. Infect. Immun. 62:1089-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bulut, Y., E. Faure, L. Thomas, O. Equils, and M. Arditi. 2001. Cooperation of Toll-like receptor 2 and 6 for cellular activation by soluble tuberculosis factor and Borrelia burgdorferi outer surface protein A lipoprotein: role of Toll-interacting protein and IL-1 receptor signaling molecules in Toll-like receptor 2 signaling. J. Immunol. 167:987-994. [DOI] [PubMed] [Google Scholar]
- 7.Cousens, L. P., and E. J. Wing. 2000. Innate defenses in the liver during Listeria infection. Immunol. Rev. 174:150-159. [DOI] [PubMed] [Google Scholar]
- 8.Dai, W. J., W. Bartens, G. Kohler, M. Hufnagel, M. Kopf, and F. Brombacher. 1997. Impaired macrophage listericidal and cytokine activities are responsible for the rapid death of Listeria monocytogenes-infected IFN-gamma receptor-deficient mice. J. Immunol. 158:5297-5304. [PubMed] [Google Scholar]
- 9.Echchannaoui, H., K. Frei, C. Schnell, S. L. Leib, W. Zimmerli, and R. Landmann. 2002. Toll-like receptor 2-deficient mice are highly susceptible to Streptococcus pneumoniae meningitis because of reduced bacterial clearing and enhanced inflammation. J. Infect. Dis. 186:798-806. [DOI] [PubMed] [Google Scholar]
- 10.Edelson, B. T., and E. R. Unanue. 2000. Immunity to Listeria infection. Curr. Opin. Immunol. 12:425-431. [DOI] [PubMed] [Google Scholar]
- 11.Edelson, B. T., and E. R. Unanue. 2002. MyD88-dependent but Toll-like receptor 2-independent innate immunity to Listeria: no role for either in macrophage listericidal activity. J. Immunol. 169:3869-3875. [DOI] [PubMed] [Google Scholar]
- 12.Flo, T. H., O. Halaas, E. Lien, L. Ryan, G. Teti, D. T. Golenbock, A. Sundan, and T. Espevik. 2000. Human Toll-like receptor 2 mediates monocyte activation by Listeria monocytogenes, but not by group B streptococci or lipopolysaccharide. J. Immunol. 164:2064-2069. [DOI] [PubMed] [Google Scholar]
- 13.Glaccum, M. B., K. L. Stocking, K. Charrier, J. L. Smith, C. R. Willis, C. Maliszewski, D. J. Livingston, J. J. Peschon, and P. J. Morrissey. 1997. Phenotypic and functional characterization of mice that lack the type I receptor for IL-1. J. Immunol. 159:3364-3371. [PubMed] [Google Scholar]
- 14.Green, S. J., C. A. Nacy, and M. S. Meltzer. 1991. Cytokine-induced synthesis of nitrogen oxides in macrophages: a protective host response to Leishmania and other intracellular pathogens. J. Leukoc. Biol. 50:93-103. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410:1099-1103. [DOI] [PubMed] [Google Scholar]
- 16.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 17.Kim, J., M. T. Ochoa, S. R. Krutzik, O. Takeuchi, S. Uematsu, A. J. Legaspi, H. D. Brightbill, D. Holland, W. J. Cunliffe, S. Akira, P. A. Sieling, P. J. Godowski, and R. L. Modlin. 2002. Activation of Toll-like receptor 2 in acne triggers inflammatory cytokine responses. J. Immunol. 169:1535-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koedel, U., B. Angele, T. Rupprecht, H. Wagner, A. Roggenkamp, H. W. Pfister, and C. J. Kirschning. 2003. Toll-like receptor 2 participates in mediation of immune response in experimental pneumococcal meningitis. J. Immunol. 170:438-444. [DOI] [PubMed] [Google Scholar]
- 19.Lutz, M. B., N. Kukutsch, A. L. Ogilvie, S. Rossner, F. Koch, N. Romani, and G. Schuler. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223:77-92. [DOI] [PubMed] [Google Scholar]
- 20.Muller, M., H. P. Eugster, M. Le Hir, A. Shakhov, F. Di Padova, C. Maurer, V. F. Quesniaux, and B. Ryffel. 1996. Correction or transfer of immunodeficiency due to TNF-LT alpha deletion by bone marrow transplantation. Mol. Med. 2:247-255. [PMC free article] [PubMed] [Google Scholar]
- 21.Neighbors, M., X. Xu, F. J. Barrat, S. R. Ruuls, T. Churakova, R. Debets, J. F. Bazan, R. A. Kastelein, J. S. Abrams, and A. O'Garra. 2001. A critical role for interleukin 18 in primary and memory effector responses to Listeria monocytogenes that extends beyond its effects on interferon gamma production. J. Exp. Med. 194:343-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ozinsky, A., D. M. Underhill, J. D. Fontenot, A. M. Hajjar, K. D. Smith, C. B. Wilson, L. Schroeder, and A. Aderem. 2000. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between Toll-like receptors. Proc. Natl. Acad. Sci. USA 97:13766-13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogers, H. W., M. P. Callery, B. Deck, and E. R. Unanue. 1996. Listeria monocytogenes induces apoptosis of infected hepatocytes. J. Immunol. 156:679-684. [PubMed] [Google Scholar]
- 24.Rogers, H. W., K. C. Sheehan, L. M. Brunt, S. K. Dower, E. R. Unanue, and R. D. Schreiber. 1992. Interleukin 1 participates in the development of anti-Listeria responses in normal and SCID mice. Proc. Natl. Acad. Sci. USA 89:1011-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogers, H. W., C. S. Tripp, R. D. Schreiber, and E. R. Unanue. 1994. Endogenous IL-1 is required for neutrophil recruitment and macrophage activation during murine listeriosis. J. Immunol. 153:2093-2101. [PubMed] [Google Scholar]
- 26.Seki, E., H. Tsutsui, N. M. Tsuji, N. Hayashi, K. Adachi, H. Nakano, S. Futatsugi-Yumikura, O. Takeuchi, K. Hoshino, S. Akira, J. Fujimoto, and K. Nakanishi. 2002. Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes in mice. J. Immunol. 169:3863-3868. [DOI] [PubMed] [Google Scholar]
- 27.Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335-376. [DOI] [PubMed] [Google Scholar]
- 28.Takeuchi, O., K. Hoshino, and S. Akira. 2000. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J. Immunol. 165:5392-5396. [DOI] [PubMed] [Google Scholar]
- 29.Tripp, C. S., S. F. Wolf, and E. R. Unanue. 1993. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc. Natl. Acad. Sci. USA 90:3725-3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Underhill, D. M., A. Ozinsky, A. M. Hajjar, A. Stevens, C. B. Wilson, M. Bassetti, and A. Aderem. 1999. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401:811-815. [DOI] [PubMed] [Google Scholar]
- 31.Vasselon, T., and P. A. Detmers. 2002. Toll receptors: a central element in innate immune responses. Infect. Immun. 70:1033-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Way, S. S., T. R. Kollmann, A. M. Hajjar, and C. B. Wilson. 2003. Cutting edge: protective cell-mediated immunity to Listeria monocytogenes in the absence of myeloid differentiation factor 88. J. Immunol. 171:533-537. [DOI] [PubMed] [Google Scholar]
- 33.Werts, C., R. I. Tapping, J. C. Mathison, T. H. Chuang, V. Kravchenko, I. Saint Girons, D. A. Haake, P. J. Godowski, F. Hayashi, A. Ozinsky, D. M. Underhill, C. J. Kirschning, H. Wagner, A. Aderem, P. S. Tobias, and R. J. Ulevitch. 2001. Leptospiral lipopolysaccharide activates cells through a TLR2-dependent mechanism. Nat. Immunol. 2:346-352. [DOI] [PubMed] [Google Scholar]
- 34.Xiong, H., S. Ohya, Y. Tanabe, and M. Mitsuyama. 1997. Persistent production of interferon-gamma (IFN-gamma) and IL-12 is essential for the generation of protective immunity against Listeria monocytogenes. Clin. Exp. Immunol. 108:456-462. [DOI] [PMC free article] [PubMed] [Google Scholar]