Abstract
The olfactory system encodes information about molecules by spatiotemporal patterns of activity across distributed populations of neurons and extracts information from these patterns to control specific behaviors. Recent studies used in vivo recordings, optogenetics, and other methods to analyze the mechanisms by which odor information is encoded and processed in the olfactory system, the functional connectivity within and between olfactory brain areas, and the impact of spatiotemporal patterning of neuronal activity on higher-order neurons and behavioral outputs. The results give rise to a faceted picture of olfactory processing and provide insights into fundamental mechanisms underlying neuronal computations. This review focuses on some of this work presented in a Mini-Symposium at the Annual Meeting of the Society for Neuroscience in 2012.
Introduction
Olfactory systems have evolved to perform sophisticated, survival-relevant analyses of the molecular environment. Odor information is sampled by a large number of odorant receptors, transformed into electrical activity by olfactory sensory neurons (OSNs), and presented to the brain as combinatorial patterns of activity across discrete input channels, the olfactory glomeruli. In vertebrates, these input patterns are processed in the olfactory bulb (OB) by neuronal circuits consisting of principal neurons, the mitral/tufted (MT) cells, and a variety of local interneurons. Output of the OB is conveyed to multiple cortical and subcortical target areas including piriform cortex, anterior olfactory nucleus, olfactory tubercle, cortical amygdala, and entorhinal cortex. Many of these brain areas are interconnected and project back to the OB. Consistent with these centrifugal projections, the activity of neurons in the OB and higher brain areas is not only modulated by odors but also by behavioral variables and context-dependent cues (Kay and Laurent, 1999; Doucette and Restrepo, 2008).
The array of glomeruli exhibits a coarse chemotopic organization that is, however, not as distinct as topographic maps in other sensory systems and does not predict the arrangement of glomeruli at fine spatial scales (Friedrich and Korsching, 1997; Uchida et al., 2000; Mori et al., 2006; Soucy et al., 2009), raising the question whether interactions between neurons associated with different glomeruli are organized by topography (Yokoi et al., 1995) or by other principles. At least some interglomerular interactions are sparse and extend over long distances (Fantana et al., 2008; Kim et al., 2011, 2012), but the underlying rules, if any, remain obscure. In piriform cortex, no obvious topography was detected in afferent projections, intracortical connectivity, and odor-evoked activity patterns (Johnson et al., 2000; Stettler and Axel, 2009; Yaksi et al., 2009; Franks et al., 2011; Ghosh et al., 2011; Miyamichi et al., 2011; Sosulski et al., 2011). The principles governing functional connectivity within and between olfactory brain areas are therefore an important subject of research in olfaction.
Patterns of odor-evoked activity exhibit temporal structure that may arise from at least three sources. First, temporal structure may be imposed upon a stimulus by odor-sampling mechanisms such as sniffing in mammals or antennal flicking in arthropods (Wachowiak, 2011). Second, slow modulations of firing rates and fast oscillatory synchronization can be generated by synaptic interactions in the OB and elsewhere (Laurent, 2002). Third, the concentration of an odor can fluctuate in time due to movements of the carrier medium (air or water). Such fluctuations may convey important information about an odor source, as demonstrated for insects (Murlis et al., 1992). This raises the question how spatiotemporal odor representations are organized and decoded, and how features of such representations may be reflected in olfactory behaviors.
These and other questions were addressed in recent studies using a wide range of techniques including optogenetics (Yizhar et al., 2011). Using light rather than odors to stimulate neurons in the olfactory system allowed, for example, for systematic and precise manipulation of activity patterns in space and time, even in awake, behaving animals. The results have challenged prevailing hypotheses such as the notion that precise timing of sensory responses is not exploited for olfactory coding. Together, recent results provided new insights into fundamental mechanisms of neuronal processing and substantially refined the current picture of olfactory processing in vertebrates. The focus of this review is on some of this work presented in a Mini-Symposium at the 42nd Annual Meeting of the Society for Neuroscience. Other recent findings, including results from invertebrates, can, unfortunately, not be reviewed in depth.
Odor representations by activity patterns across glomeruli
In the main olfactory system, axons of OSNs expressing the same odorant receptor converge onto a few defined glomeruli within the OB (Buck, 2000). Because an odorant activates multiple odorant receptors, odor information is encoded combinatorially by dynamic patterns of multiple responsive glomeruli (Leveteau and MacLeod, 1966; Friedrich and Korsching, 1997; Spors and Grinvald, 2002; Spors et al., 2006; Koulakov et al., 2007). Positions of individual glomeruli are conserved between individuals with a precision of one or a few glomerular diameters (Strotmann et al., 2000; Soucy et al., 2009). This stereotyped and precise layout of the glomerular array does not, however, imply a “chemotopic” spatial organization similar to topographic maps in other sensory systems. Rather, chemotopy is defined functionally, referring to a systematic mapping of features in molecular stimulus space onto spatial coordinates in the brain. In a chemotopic map, it may thus be expected that responses to odorants with different functional groups are mapped to distinct spatial domains of the OB, and that glomeruli responding to similar odorants are located in close proximity to each other.
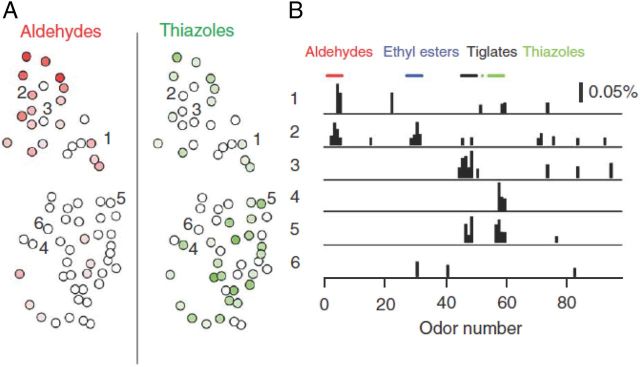
In rodents, a chemotopic organization of the glomerular array was proposed based on the finding that odors with different functional groups and carbon chain length activated glomeruli or MT cells within different domains of the OB (Johnson and Leon, 2000; Uchida et al., 2000; Meister and Bonhoeffer, 2001; Mori et al., 2006). However, other wide-field imaging studies, some of which used >100 odorants, have produced a more complex picture (Friedrich and Korsching, 1997; Bozza et al., 2004; Soucy et al., 2009; Murthy, 2011; Ma et al., 2012). Although spatial biases of glomerular responses to particular functional groups were observed at a coarse scale (∼1 mm in rodents), apparent functional domains were fractured and spatially overlapping (Fig. 1A). Moreover, no obvious chemotopic organization was observed at finer spatial scales; rather, responses of neighboring glomeruli were as diverse as those of more distant ones (Soucy et al., 2009; but see also Ma et al., 2012) (Fig. 1B). An estimate for the spatial scale of polysynaptic interactions among MT cells associated with different glomeruli may, in first approximation, be derived from the length of their basal dendrites and by the distance of short axon cell projections. Both these lengths are in the range of 1 mm or less (Shepherd et al., 2004). Responses of MT cells may thus be influenced by functionally heterogeneous glomeruli scattered throughout a large region (Fantana et al., 2008), essentially unconstrained by chemotopic rules (D. F. Albeanu and V. Murthy, unpublished observations).
Figure 1.
Distributed glomerular responses in the OB. A, Glomeruli responding to odorants with different functional groups (aldehydes, thiazoles) are distributed and interspersed on the dorsal surface of the OB. B, Odor response spectra of glomeruli labeled in A showing that individual glomeruli can respond to multiple classes of odorants, and that neighboring glomeruli often have non-overlapping response profiles (modified from Soucy et al., 2009).
Spatiotemporal activity patterns in the OB
In the OB, glomerular activity patterns are processed by a network of MT cells and various classes of local interneurons including GABAergic periglomerular cells in superficial layers, GABAergic granule cells in deeper layers, glutamatergic external tufted cells, short-axon cells releasing both GABA and dopamine, and others (Wachowiak and Shipley, 2006) (Fig. 2A). This network has been proposed to perform various tasks including a normalization of response intensity, a decorrelation of overlapping activity patterns, and feature extraction (Yokoi et al., 1995; Friedrich and Laurent, 2001; Arevian et al., 2008; Niessing and Friedrich, 2010; Olsen et al., 2010; Padmanabhan and Urban, 2010; Wiechert et al., 2010; Koulakov and Rinberg, 2011; Cleland and Linster, 2012).
Figure 2.
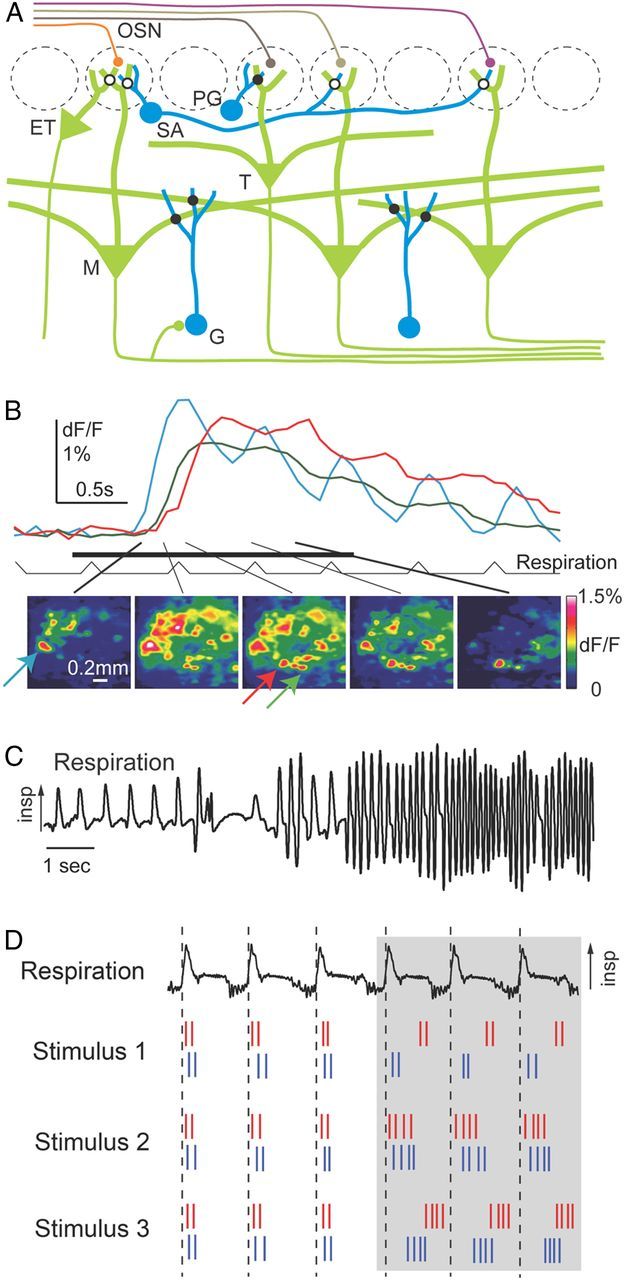
A, Schematic architecture of the olfactory bulb. Filled circles on the intersection of processes denote bidirectional synaptic connections; filled circles that abut neurons indicate polarized axo-dendritic synapses. Open circles indicate connections that are suspected but not directly confirmed or are mediated by diffuse extrasynaptic signaling. ET, External tufted; G, granule; M, mitral; PG, periglomerular; SA, short-axon; T, tufted. B, Top, Response time course of three glomeruli (see arrows with corresponding colors below) to ethyl butyrate (1% saturated vapor). Bottom, Patterns of fluorescence signals in 154 ms time windows centered on the time points indicated by lines (modified from Spors et al., 2006). C, Respiration (measured as intranasal pressure) in an awake, freely moving rat, showing changes in frequency, amplitude and duration of individual “sniffs”—as well as pauses in breathing—within a ∼15 s span. Arrow indicates direction of inspiration (modified from Wachowiak, 2011). D, Spike trains from two sister MT cells (red and blue) and respiration (top). Sister cell responses to different odors have similar firing rates, but can have different phases (modified from Dhawale et al., 2010).
A variety of approaches has recently contributed to a more advanced understanding of olfactory processing in the OB and higher brain areas. Transgenic and viral methods have been used to target fluorescent proteins, genetically encoded calcium indicators, and optogenetic probes to defined glomeruli and neuron types. Together with advanced imaging technology, these approaches allow for electrophysiological and optical recordings from identified neurons and glomeruli in vitro and in vivo. Moreover, the combination of optogenetic stimulation and electrophysiology permits the identification of neuron types in extracellular recordings (Lima et al., 2009; Dhawale et al., 2010; Cohen et al., 2012). Importantly, optogenetic activation of OSNs or MT cells enables precise spatial and temporal control over the evoked activity patterns. This is desired for systematic approaches in sensory physiology but has been a notorious problem in olfaction. Moreover, recent in vivo studies in rodents achieved detailed analyses of spatial and temporal activity patterns in awake animals rather than under anesthesia (Cury and Uchida, 2010; Shusterman et al., 2011; Smear et al., 2011; Gschwend et al., 2012; Miura et al., 2012). This is an important step because activity patterns in the OB can differ significantly under these conditions (Adrian, 1950; Rinberg et al., 2006).
Many concepts of olfactory processing in vertebrates are based largely on olfactory coding by spatial patterns or neuronal identity (Yokoi et al., 1995; Cleland and Linster, 2012). However, odor-evoked activity patterns in the OB are also highly dynamic, sometimes evolving over hundreds of milliseconds or even seconds (Laurent, 2002). Moreover, odors can evoke oscillatory population activity in the theta, beta, and gamma frequency ranges that reflects the rhythmic synchronization of stimulus-specific neuronal ensembles. Pioneering work in insects, but also in vertebrates, indicates that this dynamics may convey additional stimulus information and may play important roles in the processing of odor representations (Laurent, 2002). Recent studies have now examined temporal patterning of activity in the rodent OB in detail.
Optical imaging using voltage- and calcium-sensitive dyes revealed odor-specific latencies and dynamics of glomerular responses (Spors and Grinvald, 2002; Spors et al., 2006) (Fig. 2B) and extracellular recordings uncovered rich temporal patterning of MT cell responses (Cury and Uchida, 2010; Dhawale et al., 2010; Shusterman et al., 2011). Importantly, olfactory processing in rodents is temporally constrained by sniffing, which changes in frequency from 2–3 Hz during slow breathing to 7–10 Hz during active exploration (Fig. 2C). Information processing is thus likely to occur within successive 100–200 ms epochs of transient activity (Cury and Uchida, 2010; Shusterman et al., 2011).
The prominence of respiration-driven dynamics at all levels of the mammalian olfactory CNS suggests that olfactory processing networks actively shape the temporal structure of odor-evoked activity. Indeed, external tufted cells can be entrained to phasic inputs in vitro, which enhance their drive onto MT cell activity at frequencies corresponding to natural sniffing (Hayar et al., 2004; Wachowiak and Shipley, 2006). Likewise, inhalation-locked temporal patterning of MT cell activity in vivo actually becomes sharper at higher sniff frequencies, even though it becomes weaker in sensory inputs (Carey and Wachowiak, 2011; Shusterman et al., 2011). Even in awake mice, where spontaneous activity is high, odor responses of MT cells are phase locked to the sniff cycle with a precision of ∼10 ms, as revealed by temporally precise odor stimulation in head-fixed animals (Shusterman et al., 2011).
In awake mice performing odor-guided tasks, odor-evoked activity patterns across MT cells exhibit complex, phase-locked temporal dynamics within the timeframe of a sniff cycle (Chaput, 1986; Cury and Uchida, 2010; Shusterman et al., 2011; for anesthetized animals, see Dhawale et al., 2010; Carey and Wachowiak, 2011) (Fig. 2D). These temporal ensemble patterns contained substantial odor information, particularly during the first ∼100 ms after inhalation onset. More pronounced firing rate modulations during short time windows within a sniff (∼30 ms), but not firing rates averaged over the entire sniff (∼160 ms), were correlated with shorter behavioral reaction times on a trial-by-trial basis, consistent with the hypothesis that the dynamics of odor responses is used for odor identification (Cury and Uchida, 2010).
When sniffing frequency was decreased by changing the task design, the duration of inhalation and the activity patterns during this phase were largely preserved, indicating that the dynamics of MT activity during the initial phase of the sniff cycle is remarkably stable (Cury and Uchida, 2010). In another study using head-fixed animals, trial-to-trial variations in sniff duration were associated with temporal stretching or compression of the MT cell activity pattern. As a consequence, the phase of MT cell responses relative to the sniff cycle was invariant to variability in sniff duration (Shusterman et al., 2011). This temporal scaling may be explained by the assumption that faster inhalation produces a steeper rise in odor concentration in the nose and, thus, faster responses of the bulbar network. The somewhat different observations of these studies may now be further explored.
To examine whether mice are able to detect subtle differences in the timing of sensory input, OSNs expressing channelrhodopsin 2 (Chr2) were stimulated by light in mice implanted with an optical fiber in the nasal cavity (Smear et al., 2011). This approach made it possible to vary the timing of the stimulus relative to the sniffing cycle without changing the spatial pattern of stimulation. Mice could be trained to discriminate with >90% accuracy between two brief (1 ms) light pulses presented during inhalation and 100 ms later. Even when the temporal differences between two stimuli were as small as 10 ms, mice were able to perform the task above chance level (Fig. 3). Hence, mice can solve an olfactory discrimination task purely based on temporal cues in the stimulus. MT cells, however, responded to optical stimulation at different times with different firing rates, indicating that temporal information in the stimulus is transformed into different spatial patterns of neuronal activity in the brain, which may be the basis for behavioral decisions (Smear et al., 2011). The striking temporal acuity of the system and the ease with which mice learn to discriminate between temporal cues suggests that information contained in the timing of responses is also used during natural odor stimulation.
Figure 3.
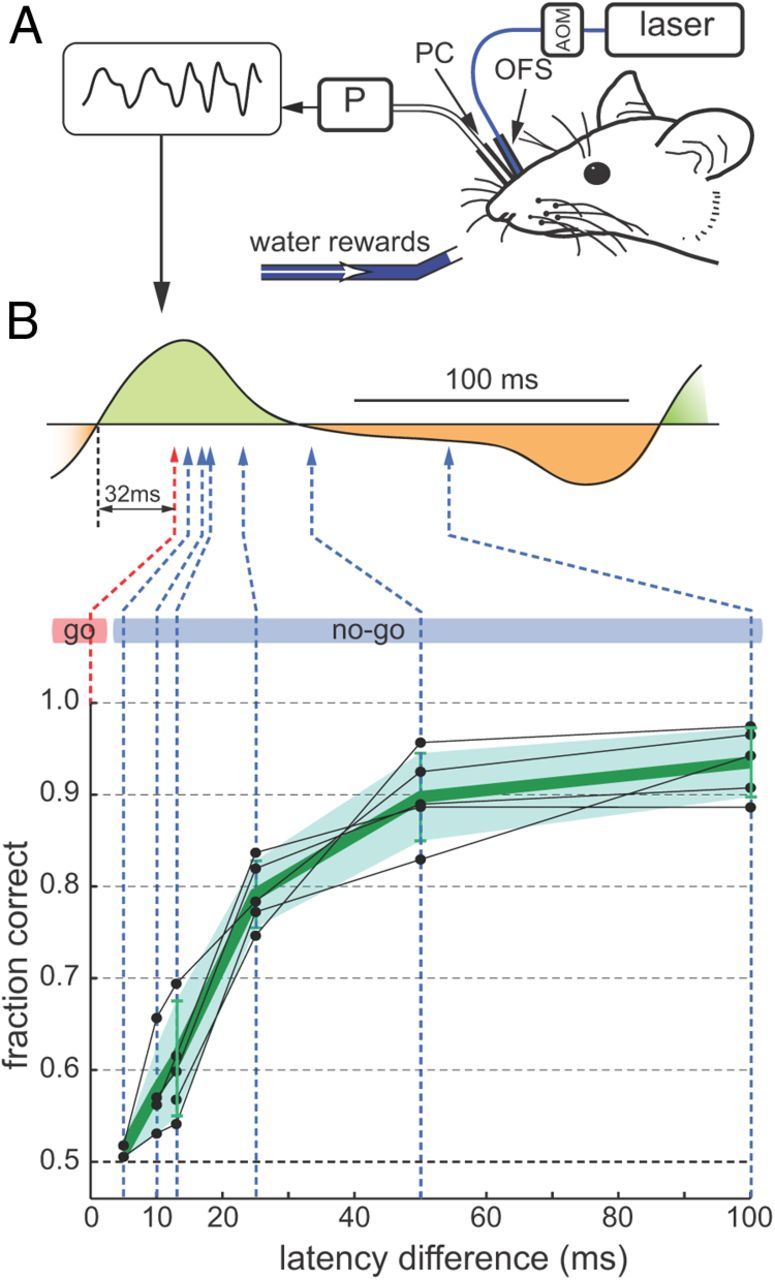
Perception of stimulus time differences relatively to the sniffing cycle. A, Mice were implanted with a nasal optical fiber stub (OFS) to deliver light, gated by an acousto-optic modulator (AOM). A nasal pressure cannula (PC) coupled to a pressure sensor (P) measured sniffing. Inverted intranasal pressure signal is shown at top left. B, Top, Schematic of the sniff time discrimination problem, shown relative to a typical sniff waveform, with inhalation shaded green and exhalation shaded orange. Light was delivered 32 ms after inhalation onset (red arrow) in “go” trials and sometimes later (blue arrow) in “no-go” trials. Bottom, Performance of individual OMP-Chr2 mice (n = 5) (black dots). Green line and shaded region show average performance and SD (modified from Smear et al., 2011).
Output from each glomerulus is transmitted to higher brain areas by multiple (20–50) MT cells (sister cells). Since these cells receive common sensory input, the question arises whether they carry redundant information to downstream circuits (Chen et al., 2009; Kazama and Wilson, 2009; Padmanabhan and Urban, 2010), or whether they are functionally diverse. To address this question, a digital micromirror device was used to optically stimulate individual glomeruli in mice expressing Chr2 in OSNs, and sister cells connected to the stimulated glomerulus were identified by their time-locked spike responses. Subsequent odor stimulation showed that sister cells responded to odorants with similar firing rate changes but differed in their spike timing (Dhawale et al., 2010). At rest, sister cells spiked in a synchronized manner. During odor presentation, however, temporal firing patterns of sister cells became decorrelated in a stimulus-specific manner, to the same degree as among non-sister cells. While the common excitatory sensory input can readily explain the similar changes in average firing of sister cells, mechanisms underlying temporal decorrelation remain elusive. One possibility is that sister cells receive distinct lateral inhibitory inputs from juxtaglomerular or granule cells. Together, these recent results argue for the coexistence of information in the rate and in the timing of action potential output from MT cells (Cury and Uchida, 2010; Dhawale et al., 2010; Shusterman et al., 2011). Moreover, the number of functionally distinct output channels of the OB is higher than the number of input channels (glomeruli).
Transformations of spatiotemporal activity patterns in the OB and the underlying mechanisms are now further analyzed by optogenetic approaches. For example, in vitro studies predict that GABAergic (GAD65+) periglomerular interneurons play a role in shaping both the overall excitability and the temporal response pattern of MT cells (Gire and Schoppa, 2009; Shao et al., 2012). This hypothesis is now being tested by expressing the inhibitory opsin Arch in these neurons. Similar approaches are also used to test the functions of other interneurons in the OB of mice and zebrafish. Moreover, recent studies indicate that biophysical variation among neurons of the same type can contribute to the computational function of the OB network (Padmanabhan and Urban, 2010; Angelo et al., 2012).
Transformations of odor representations in olfactory cortex
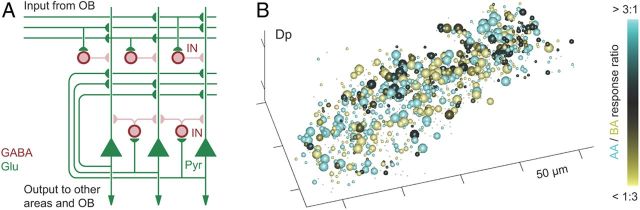
The largest target area of the OB is the piriform cortex, a three-layered paleocortical brain area that has been proposed to establish synthetic representations of olfactory objects and to function as an auto-associative memory network (Haberly, 2001; Wilson and Sullivan, 2011; Chapuis and Wilson, 2012). Pyramidal neurons in piriform cortex receive nontopographic excitatory input from diverse sets of MT cells (Nagayama et al., 2010; Ghosh et al., 2011; Miyamichi et al., 2011; Sosulski et al., 2011; Igarashi et al., 2012) and form sparse connections with other pyramidal neurons independent of distance (Johnson et al., 2000; Franks et al., 2011). Moreover, pyramidal neurons receive powerful inhibitory input from different classes of local GABAergic interneurons (Poo and Isaacson, 2009; Stokes and Isaacson, 2010; Suzuki and Bekkers, 2010) (Fig. 4A). Consistent with the distributed character of afferent and intrinsic connections, large-scale multiphoton calcium imaging showed that odors evoke scattered, moderately sparse patterns of activity in piriform cortex and in the homologous brain area in zebrafish, the posterior zone of the dorsal telencephalon (Dp) (Stettler and Axel, 2009; Yaksi et al., 2009) (Fig. 4B). These studies also demonstrated that activity patterns evoked by binary mixtures deviated substantially from responses to the individual components. Optical stimulation of glomeruli using Chr2 or glutamate uncaging showed that piriform neurons integrate spatially distributed inputs from the OB, often exhibiting superlinear responses to multiple inputs (Arenkiel et al., 2007; Davison and Ehlers, 2011). These results are consistent with the notion that odor representations are synthetic in nature (Haberly, 2001; Wilson and Sullivan, 2011).
Figure 4.
Responses in higher olfactory areas. A, Simplified scheme of piriform cortex. B, Distributed, overlapping activity patterns evoked by two dissimilar odors (AA, amino acid; BA, bile acid) in Dp of adult zebrafish, measured by two-photon calcium imaging. Plot symbols indicate neurons, size of spheres indicates response magnitude to stronger stimulus, color code indicates response ratio (modified from Yaksi et al., 2009). IN, Interneuron; Pyr, pyramidal neuron.
Extracellular recordings in rats performing an odor discrimination task showed that neurons in piriform cortex responded to odor stimulation with simple, burst-like activities time locked to inhalation onset (Miura et al., 2012). Spike counts of these bursts conveyed more reliable and rapid information than temporal patterns, suggesting that the significance of temporal patterns is greatly reduced in the anterior piriform cortex compared with the olfactory bulb. Cortical circuits may therefore detect timing differences between MT cells and transform them into a firing rate response. In theory, this could be accomplished by various mechanisms including delay lines, feedforward inhibition, or recurrent inhibition (Perez-Orive et al., 2002; Luna and Schoppa, 2008; Stokes and Isaacson, 2010). These issues can now be further explored by temporally precise optical stimulation of MT cells in the OB.
The impact of temporally patterned MT cell activity on higher-order neurons was examined in area Dp of adult zebrafish, the homolog of piriform cortex in other vertebrates. Since zebrafish inhabit slow or still waters and do not sniff, natural stimuli are unlikely to exhibit pronounced temporal structure. However, temporal patterning of MT cell activity results from interactions among neurons in the OB. Odor stimuli with a rise time of ∼600 ms produced MT cell activity patterns that were dynamically reorganized before they approached a steady state after a few hundred milliseconds (Friedrich and Laurent, 2001; Niessing and Friedrich, 2010). Concomitantly, subsets of MT cells rhythmically synchronized their action potentials at a frequency near 20 Hz. Activity patterns across synchronized MT cells conveyed information about the molecular category of an odorant, but information about its precise identity was represented mostly by ensembles of nonsynchronized MT cells during the steady state (Friedrich et al., 2004). The information that is retrieved from MT activity patterns therefore depends on the impact of synchronization on higher-order neurons, and on the time window during the response.
To examine how activity patterns are temporally filtered in Dp, axon terminals of OSNs expressing Chr2 in the OB were optically stimulated using a digital micromirror array. Using this approach, it was possible to vary oscillatory synchrony among distributed ensembles of MT cells without major changes in firing rates (Blumhagen et al., 2011; Zhu et al., 2012). Surprisingly, firing rates of Dp neurons did not increase with synchrony in their inputs, and odor stimulation evoked only small oscillatory membrane potential fluctuations. Although these fluctuations influenced action potential timing, firing rates of Dp neurons depended primarily on the magnitude of a large and slow depolarization, which in turn depended on the balance between excitatory and inhibitory synaptic input (Yaksi et al., 2009; Blumhagen et al., 2011). Neuronal circuits in Dp therefore attenuate the impact of synchrony, at least in part because passive neuronal properties act as strong low-pass filters. Moreover, most responses occurred during the decorrelated steady state of MT cell input (Blumhagen et al., 2011). Temporal filtering therefore tunes Dp neurons to those features of input patterns that are particularly informative about precise odor identity.
The olfactory brain, like other sensory areas, does not simply integrate sensory information in a passive manner to create a complete and accurate representation of the sensory scene. Instead, sensory responses are modulated by the behavioral state and prior experience even at the earliest processing stages. Such top-down modulation is thought to involve projections from central back to more peripheral brain areas (Knudsen, 2007; Restrepo et al., 2009), which are prominent in the olfactory system. The OB itself receives dense projections from olfactory cortex as well as neuromodulatory inputs from other areas. Moreover, different cortical regions are interconnected with each other (Shepherd et al., 2004; Matsutani and Yamamoto, 2008). Recent in vitro studies have begun to uncover the synaptic properties of centrifugal axons projecting back to the OB (Gao and Strowbridge, 2009), but the cellular targets of feedback projections, their functional properties, and their effects on olfactory processing in vivo remain largely unknown.
Optogenetic methods have a particular advantage over other techniques when examining connectivity across different areas because long-distance projection axons can be selectively activated near the target regions (Petreanu et al., 2007). This capability has recently been exploited in rodents to study functional connectivity patterns among the anterior olfactory nucleus, the anterior piriform cortex, and posterior piriform cortex (Hagiwara et al., 2012). Moreover, optogenetic stimulation of pyramidal neurons demonstrated that excitatory connectivity within piriform cortex is extremely widespread and sparse, whereas inhibitory connections are more local (Franks et al., 2011).
Optogenetic manipulations are also used to examine the functional role of feedback connections from cortical areas to the OB. Initial studies have focused on the dense bilateral projections from the anterior olfactory nucleus to the OB (F. Markopoulos, D. Rokni, D. Gire, and V. Murthy, unpublished data). By expressing Chr2 in the anterior olfactory nucleus and optical stimulation of the projecting axons in the OB, feedback was found to provide strong synaptic input to interneurons in the glomerular layer and to granule cells. Consistent with this connectivity pattern, activation of feedback projection triggered disynaptic inhibition in mitral/tufted cells. Intriguingly, optical stimulation of feedback projections from the anterior olfactory nucleus could also excite mitral cells with sufficient strength to trigger precisely timed spikes under some conditions. These methods can now be adapted to study the role of cortical feedback on odor coding and perception, for example by activating or inhibiting specific sources of feedback during odor-guided behaviors.
Conclusions and outlook
Careful quantitative measurements and manipulations of spatiotemporal activity patterns have refined current views of information processing at successive stages in the olfactory system. These studies also highlighted the power of optogenetic approaches to control neuronal activity patterns in space and time, and to explore systematic relationships between activity patterns and behavioral outputs. These approaches may now be used to address further important questions such as the roles of defined cell types in network function, the functions of different higher brain areas, and the role of top-down projections in the olfactory system.
Footnotes
Work in the authors' laboratories is funded by the Max Planck Society, Novartis Research Foundation, Swiss Nationalfonds, Human Frontiers Science Program, Deutsche Forschungsgemeinschaft (Grant SPP 1392), the European Union, Howard Hughes Medical Institute, National Institute on Deafness and Other Communication Disorders, Cold Spring Harbor Laboratory Startup, Whitehall Foundation, and the German Israeli Foundation.
References
- Adrian ED. The electrical activity of the mammalian olfactory bulb. Electroencephalogr Clin Neurophysiol. 1950;2:377–388. doi: 10.1016/0013-4694(50)90075-7. [DOI] [PubMed] [Google Scholar]
- Angelo K, Rancz EA, Pimentel D, Hundahl C, Hannibal J, Fleischmann A, Pichler B, Margrie TW. A biophysical signature of network affiliation and sensory processing in mitral cells. Nature. 2012;488:375–378. doi: 10.1038/nature11291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenkiel BR, Peca J, Davison IG, Feliciano C, Deisseroth K, Augustine GJ, Ehlers MD, Feng G. In vivo light-induced activation of neural circuitry in transgenic mice expressing channelrhodopsin-2. Neuron. 2007;54:205–218. doi: 10.1016/j.neuron.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arevian AC, Kapoor V, Urban NN. Activity-dependent gating of lateral inhibition in the mouse olfactory bulb. Nat Neurosci. 2008;11:80–87. doi: 10.1038/nn2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumhagen F, Zhu P, Shum J, Schärer YP, Yaksi E, Deisseroth K, Friedrich RW. Neuronal filtering of multiplexed odour representations. Nature. 2011;479:493–498. doi: 10.1038/nature10633. [DOI] [PubMed] [Google Scholar]
- Bozza T, McGann JP, Mombaerts P, Wachowiak M. In vivo imaging of neuronal activity by targeted expression of a genetically encoded probe in the mouse. Neuron. 2004;42:9–21. doi: 10.1016/s0896-6273(04)00144-8. [DOI] [PubMed] [Google Scholar]
- Buck LB. The molecular architecture of odor and pheromone sensing in mammals. Cell. 2000;100:611–618. doi: 10.1016/s0092-8674(00)80698-4. [DOI] [PubMed] [Google Scholar]
- Carey RM, Wachowiak M. Effect of sniffing on the temporal structure of mitral/tufted cell output from the olfactory bulb. J Neurosci. 2011;31:10615–10626. doi: 10.1523/JNEUROSCI.1805-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapuis J, Wilson DA. Bidirectional plasticity of cortical pattern recognition and behavioral sensory acuity. Nat Neurosci. 2012;15:155–161. doi: 10.1038/nn.2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaput MA. Respiratory-phase-related coding of olfactory information in the olfactory bulb of awake freely-breathing rabbits. Physiol Behav. 1986;36:319–324. doi: 10.1016/0031-9384(86)90023-5. [DOI] [PubMed] [Google Scholar]
- Chen TW, Lin BJ, Schild D. Odor coding by modules of coherent mitral/tufted cells in the vertebrate olfactory bulb. Proc Natl Acad Sci U S A. 2009;106:2401–2406. doi: 10.1073/pnas.0810151106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland TA, Linster C. On-center/inhibitory-surround decorrelation via intraglomerular inhibition in the olfactory bulb glomerular layer. Front Integr Neurosci. 2012;6:5. doi: 10.3389/fnint.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature. 2012;482:85–88. doi: 10.1038/nature10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cury KM, Uchida N. Robust odor coding via inhalation-coupled transient activity in the mammalian olfactory bulb. Neuron. 2010;68:570–585. doi: 10.1016/j.neuron.2010.09.040. [DOI] [PubMed] [Google Scholar]
- Davison IG, Ehlers MD. Neural circuit mechanisms for pattern detection and feature combination in olfactory cortex. Neuron. 2011;70:82–94. doi: 10.1016/j.neuron.2011.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawale AK, Hagiwara A, Bhalla US, Murthy VN, Albeanu DF. Non-redundant odor coding by sister mitral cells revealed by light addressable glomeruli in the mouse. Nat Neurosci. 2010;13:1404–1412. doi: 10.1038/nn.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucette W, Restrepo D. Profound context-dependent plasticity of mitral cell responses in olfactory bulb. PLoS Biol. 2008;6:e258. doi: 10.1371/journal.pbio.0060258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantana AL, Soucy ER, Meister M. Rat olfactory bulb mitral cells receive sparse glomerular inputs. Neuron. 2008;59:802–814. doi: 10.1016/j.neuron.2008.07.039. [DOI] [PubMed] [Google Scholar]
- Franks KM, Russo MJ, Sosulski DL, Mulligan AA, Siegelbaum SA, Axel R. Recurrent circuitry dynamically shapes the activation of piriform cortex. Neuron. 2011;72:49–56. doi: 10.1016/j.neuron.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich RW, Korsching SI. Combinatorial and chemotopic odorant coding in the zebrafish olfactory bulb visualized by optical imaging. Neuron. 1997;18:737–752. doi: 10.1016/s0896-6273(00)80314-1. [DOI] [PubMed] [Google Scholar]
- Friedrich RW, Laurent G. Dynamic optimization of odor representations in the olfactory bulb by slow temporal patterning of mitral cell activity. Science. 2001;291:889–894. doi: 10.1126/science.291.5505.889. [DOI] [PubMed] [Google Scholar]
- Friedrich RW, Habermann CJ, Laurent G. Multiplexing using synchrony in the zebrafish olfactory bulb. Nat Neurosci. 2004;7:862–871. doi: 10.1038/nn1292. [DOI] [PubMed] [Google Scholar]
- Gao Y, Strowbridge BW. Long-term plasticity of excitatory inputs to granule cells in the rat olfactory bulb. Nat Neurosci. 2009;12:731–733. doi: 10.1038/nn.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Larson SD, Hefzi H, Marnoy Z, Cutforth T, Dokka K, Baldwin KK. Sensory maps in the olfactory cortex defined by long-range viral tracing of single neurons. Nature. 2011;472:217–220. doi: 10.1038/nature09945. [DOI] [PubMed] [Google Scholar]
- Gire DH, Schoppa NE. Control of on/off glomerular signaling by a local GABAergic microcircuit in the olfactory bulb. J Neurosci. 2009;29:13454–13464. doi: 10.1523/JNEUROSCI.2368-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gschwend O, Beroud J, Carleton A. Encoding odorant identity by spiking packets of rate-invariant neurons in awake mice. PLoS One. 2012;7:e30155. doi: 10.1371/journal.pone.0030155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberly LB. Parallel-distributed processing in olfactory cortex: new insights from morphological and physiological analysis of neuronal circuitry. Chem Senses. 2001;26:551–576. doi: 10.1093/chemse/26.5.551. [DOI] [PubMed] [Google Scholar]
- Hagiwara A, Pal SK, Sato TF, Wienisch M, Murthy VN. Optophysiological analysis of associational circuits in the olfactory cortex. Front Neural Circuits. 2012;6:18. doi: 10.3389/fncir.2012.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayar A, Karnup S, Shipley MT, Ennis M. Olfactory bulb glomeruli: external tufted cells intrinsically burst at theta frequency and are entrained by patterned olfactory input. J Neurosci. 2004;24:1190–1199. doi: 10.1523/JNEUROSCI.4714-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi KM, Ieki N, An M, Yamaguchi Y, Nagayama S, Kobayakawa K, Kobayakawa R, Tanifuji M, Sakano H, Chen WR, Mori K. Parallel mitral and tufted cell pathways route distinct odor information to different targets in the olfactory cortex. J Neurosci. 2012;32:7970–7985. doi: 10.1523/JNEUROSCI.0154-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Leon M. Modular representations of odorants in the glomerular layer of the rat olfactory bulb and the effects of stimulus concentration. J Comp Neurol. 2000;422:496–509. doi: 10.1002/1096-9861(20000710)422:4<496::aid-cne2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Johnson DM, Illig KR, Behan M, Haberly LB. New features of connectivity in piriform cortex visualized by intracellular injection of pyramidal cells suggest that “primary” olfactory cortex functions like “association” cortex in other sensory systems. J Neurosci. 2000;20:6974–6982. doi: 10.1523/JNEUROSCI.20-18-06974.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay LM, Laurent G. Odor- and context-dependent modulation of mitral cell activity in behaving rats. Nat Neurosci. 1999;2:1003–1009. doi: 10.1038/14801. [DOI] [PubMed] [Google Scholar]
- Kazama H, Wilson RI. Origins of correlated activity in an olfactory circuit. Nat Neurosci. 2009;12:1136–1144. doi: 10.1038/nn.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Phillips ME, Chang AY, Patel HK, Nguyen KT, Willhite DC. Lateral connectivity in the olfactory bulb is sparse and segregated. Front Neural Circuits. 2011;5:5. doi: 10.3389/fncir.2011.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Chang AY, McTavish TS, Patel HK, Willhite DC. Center-surround vs. distance-independent lateral connectivity in the olfactory bulb. Front Neural Circuits. 2012;6:34. doi: 10.3389/fncir.2012.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen EI. Fundamental components of attention. Annu Rev Neurosci. 2007;30:57–78. doi: 10.1146/annurev.neuro.30.051606.094256. [DOI] [PubMed] [Google Scholar]
- Koulakov AA, Rinberg D. Sparse incomplete representations: a potential role of olfactory granule cells. Neuron. 2011;72:124–136. doi: 10.1016/j.neuron.2011.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulakov A, Gelperin A, Rinberg D. Olfactory coding with all-or-nothing glomeruli. J Neurophysiol. 2007;98:3134–3142. doi: 10.1152/jn.00560.2007. [DOI] [PubMed] [Google Scholar]
- Laurent G. Olfactory network dynamics and the coding of multidimensional signals. Nat Rev Neurosci. 2002;3:884–895. doi: 10.1038/nrn964. [DOI] [PubMed] [Google Scholar]
- Leveteau J, MacLeod P. Olfactory discrimination in the rabbit olfactory glomerulus. Science. 1966;153:175–176. doi: 10.1126/science.153.3732.175. [DOI] [PubMed] [Google Scholar]
- Lima SQ, Hromádka T, Znamenskiy P, Zador AM. PINP: a new method of tagging neuronal populations for identification during in vivo electrophysiological recording. PLoS One. 2009;4:e6099. doi: 10.1371/journal.pone.0006099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna VM, Schoppa NE. GABAergic circuits control input-spike coupling in the piriform cortex. J Neurosci. 2008;28:8851–8859. doi: 10.1523/JNEUROSCI.2385-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Qiu Q, Gradwohl S, Scott A, Yu EQ, Alexander R, Wiegraebe W, Yu CR. Distributed representation of chemical features and tunotopic organization of glomeruli in the mouse olfactory bulb. Proc Natl Acad Sci U S A. 2012;109:5481–5486. doi: 10.1073/pnas.1117491109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsutani S, Yamamoto N. Centrifugal innervation of the mammalian olfactory bulb. Anat Sci Int. 2008;83:218–227. doi: 10.1111/j.1447-073X.2007.00223.x. [DOI] [PubMed] [Google Scholar]
- Meister M, Bonhoeffer T. Tuning and topography in an odor map on the rat olfactory bulb. J Neurosci. 2001;21:1351–1360. doi: 10.1523/JNEUROSCI.21-04-01351.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Mainen ZF, Uchida N. Odor representations in olfactory cortex: distributed rate coding and decorrelated population activity. Neuron. 2012;74:1087–1098. doi: 10.1016/j.neuron.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamichi K, Amat F, Moussavi F, Wang C, Wickersham I, Wall NR, Taniguchi H, Tasic B, Huang ZJ, He Z, Callaway EM, Horowitz MA, Luo L. Cortical representations of olfactory input by trans-synaptic tracing. Nature. 2011;472:191–196. doi: 10.1038/nature09714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Takahashi YK, Igarashi KM, Yamaguchi M. Maps of odorant molecular features in the mammalian olfactory bulb. Physiol Rev. 2006;86:409–433. doi: 10.1152/physrev.00021.2005. [DOI] [PubMed] [Google Scholar]
- Murlis J, Elkinton JS, Carde RT. Odor plumes and how insects use them. Annu Rev Entomol. 1992;37:505–532. [Google Scholar]
- Murthy VN. Olfactory maps in the brain. Annu Rev Neurosci. 2011;34:233–258. doi: 10.1146/annurev-neuro-061010-113738. [DOI] [PubMed] [Google Scholar]
- Nagayama S, Enerva A, Fletcher ML, Masurkar AV, Igarashi KM, Mori K, Chen WR. Differential axonal projection of mitral and tufted cells in the mouse main olfactory system. Front Neural Circuits. 2010;4:120. doi: 10.3389/fncir.2010.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessing J, Friedrich RW. Olfactory pattern classification by discrete neuronal network states. Nature. 2010;465:47–52. doi: 10.1038/nature08961. [DOI] [PubMed] [Google Scholar]
- Olsen SR, Bhandawat V, Wilson RI. Divisive normalization in olfactory population codes. Neuron. 2010;66:287–299. doi: 10.1016/j.neuron.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan K, Urban NN. Intrinsic biophysical diversity decorrelates neuronal firing while increasing information content. Nat Neurosci. 2010;13:1276–1282. doi: 10.1038/nn.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Orive J, Mazor O, Turner GC, Cassenaer S, Wilson RI, Laurent G. Oscillations and sparsening of odor representations in the mushroom body. Science. 2002;297:359–365. doi: 10.1126/science.1070502. [DOI] [PubMed] [Google Scholar]
- Petreanu L, Huber D, Sobczyk A, Svoboda K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat Neurosci. 2007;10:663–668. doi: 10.1038/nn1891. [DOI] [PubMed] [Google Scholar]
- Poo C, Isaacson JS. Odor representations in olfactory cortex: “sparse” coding, global inhibition, and oscillations. Neuron. 2009;62:850–861. doi: 10.1016/j.neuron.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restrepo D, Doucette W, Whitesell JD, McTavish TS, Salcedo E. From the top down: flexible reading of a fragmented odor map. Trends Neurosci. 2009;32:525–531. doi: 10.1016/j.tins.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinberg D, Koulakov A, Gelperin A. Sparse odor coding in awake behaving mice. J Neurosci. 2006;26:8857–8865. doi: 10.1523/JNEUROSCI.0884-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z, Puche AC, Liu S, Shipley MT. Intraglomerular inhibition shapes the strength and temporal structure of glomerular output. J Neurophysiol. 2012;108:782–793. doi: 10.1152/jn.00119.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM, Chen WR, Greer CA. Olfactory bulb. In: Shepherd GM, editor. The synaptic organization of the brain. Oxford: Oxford UP; 2004. pp. 165–216. [Google Scholar]
- Shusterman R, Smear MC, Koulakov AA, Rinberg D. Precise olfactory responses tile the sniff cycle. Nat Neurosci. 2011;14:1039–1044. doi: 10.1038/nn.2877. [DOI] [PubMed] [Google Scholar]
- Smear M, Shusterman R, O'Connor R, Bozza T, Rinberg D. Perception of sniff phase in mouse olfaction. Nature. 2011;479:397–400. doi: 10.1038/nature10521. [DOI] [PubMed] [Google Scholar]
- Sosulski DL, Bloom ML, Cutforth T, Axel R, Datta SR. Distinct representations of olfactory information in different cortical centres. Nature. 2011;472:213–216. doi: 10.1038/nature09868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucy ER, Albeanu DF, Fantana AL, Murthy VN, Meister M. Precision and diversity in an odor map on the olfactory bulb. Nat Neurosci. 2009;12:210–220. doi: 10.1038/nn.2262. [DOI] [PubMed] [Google Scholar]
- Spors H, Grinvald A. Spatio-temporal dynamics of odor representations in the Mammalian olfactory bulb. Neuron. 2002;34:301–315. doi: 10.1016/s0896-6273(02)00644-x. [DOI] [PubMed] [Google Scholar]
- Spors H, Wachowiak M, Cohen LB, Friedrich RW. Temporal dynamics and latency patterns of receptor neuron input to the olfactory bulb. J Neurosci. 2006;26:1247–1259. doi: 10.1523/JNEUROSCI.3100-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettler DD, Axel R. Representations of odor in the piriform cortex. Neuron. 2009;63:854–864. doi: 10.1016/j.neuron.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Stokes CC, Isaacson JS. From dendrite to soma: dynamic routing of inhibition by complementary interneuron microcircuits in olfactory cortex. Neuron. 2010;67:452–465. doi: 10.1016/j.neuron.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strotmann J, Conzelmann S, Beck A, Feinstein P, Breer H, Mombaerts P. Local permutations in the glomerular array of the mouse olfactory bulb. J Neurosci. 2000;20:6927–6938. doi: 10.1523/JNEUROSCI.20-18-06927.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Bekkers JM. Inhibitory neurons in the anterior piriform cortex of the mouse: classification using molecular markers. J Comp Neurol. 2010;518:1670–1687. doi: 10.1002/cne.22295. [DOI] [PubMed] [Google Scholar]
- Uchida N, Takahashi YK, Tanifuji M, Mori K. Odor maps in the mammalian olfactory bulb: domain organization and odorant structural features. Nat Neurosci. 2000;3:1035–1043. doi: 10.1038/79857. [DOI] [PubMed] [Google Scholar]
- Wachowiak M. All in a sniff: olfaction as a model for active sensing. Neuron. 2011;71:962–973. doi: 10.1016/j.neuron.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachowiak M, Shipley MT. Coding and synaptic processing of sensory information in the glomerular layer of the olfactory bulb. Seminars in cell and developmental biology. 2006;17:411–423. doi: 10.1016/j.semcdb.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Wiechert MT, Judkewitz B, Riecke H, Friedrich RW. Mechanisms of pattern decorrelation by recurrent neuronal circuits. Nat Neurosci. 2010;13:1003–1010. doi: 10.1038/nn.2591. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM. Cortical processing of odor objects. Neuron. 2011;72:506–519. doi: 10.1016/j.neuron.2011.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksi E, von Saint Paul F, Niessing J, Bundschuh ST, Friedrich RW. Transformation of odor representations in target areas of the olfactory bulb. Nat Neurosci. 2009;12:474–482. doi: 10.1038/nn.2288. [DOI] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011;71:9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Yokoi M, Mori K, Nakanishi S. Refinement of odor molecule tuning by dendrodendritic synaptic inhibition in the olfactory bulb. Proc Natl Acad Sci U S A. 1995;92:3371–3375. doi: 10.1073/pnas.92.8.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P, Fajardo O, Shum J, Zhang Schärer YP, Friedrich RW. High-resolution optical control of neuronal activity patterns in space and time using a digital micromirror device in zebrafish. Nat Protoc. 2012;7:1410–1425. doi: 10.1038/nprot.2012.072. [DOI] [PubMed] [Google Scholar]