Abstract
Light-harvesting pigment-protein complexes of photosystem II of plants have a dual function: they efficiently use absorbed energy for photosynthesis at limiting sunlight intensity and dissipate the excess energy at saturating intensity for photoprotection. Recent single-molecule spectroscopy studies on the trimeric LHCII complex showed that environmental control of the intrinsic protein disorder could in principle explain the switch between their light-harvesting and photoprotective conformations in vivo. However, the validity of this proposal depends strongly on the specificity of the protein dynamics. Here, a similar study has been performed on the minor monomeric antenna complexes of photosystem II (CP29, CP26, and CP24). Despite their high structural homology, similar pigment content and organization compared to LHCII trimers, the environmental response of these proteins was found to be rather distinct. A much larger proportion of the minor antenna complexes were present in permanently weakly fluorescent states under most conditions used; however, unlike LHCII trimers the distribution of the single-molecule population between the strongly and weakly fluorescent states showed no significant sensitivity to low pH, zeaxanthin, or low detergent conditions. The results support a unique role for LHCII trimers in the regulation of light harvesting by controlled fluorescence blinking and suggest that any contribution of the minor antenna complexes to photoprotection would probably involve a distinct mechanism.
Introduction
The structural flexibility of proteins is an important property that enables their regulatory switching between active and inactive functional states. Some proteins possess the ability to switch between different functional states, thereby exhibiting multifunctionality. The peripheral light-harvesting complexes of plant photosystem (PS) II are such examples: under normal conditions they ensure highly efficient transfer of absorbed solar energy to the reaction center, although under stress conditions, most of the absorbed energy is dissipated as heat (1,2). This rapidly reversible, ΔpH-dependent process is commonly referred to as qE. The properties of this regulatory function are strongly contested, in particular the site and molecular mechanism, as recently reviewed in (3).
It is commonly accepted that one or more of the peripheral antenna complexes are involved in qE. These macromolecules are composed of six homologous pigment-protein complexes from the Lhcb multigenic family (4). The first three form heterotrimers of different compositions (5,6) and are known as the major antennae, often referred to as LHCII, whereas the other three complexes exist naturally as monomers (7) and are known as the minor antennae CP29, CP26, and CP24. The proteins of these complexes bind the same pigments, but in different ratios. The crystal structures of only LHCII and CP29 have been resolved (8–10). The first indicated that each monomeric subunit of LHCII binds 14 chlorophylls (Chls) and 4 carotenoids (Cars) at fixed binding sites (8). Although one less Chl has been resolved for CP29 (9), only 8 Chls are normally identified by in vitro studies (11–14), suggesting that conventional isolation techniques of CP29 lead to a loss of Chl pigments, or that a specific subset of CP29 complexes was crystallized. The number of Chls in CP26 (9 Chls) and CP24 (10 Chls) may similarly be underestimated by in vitro studies. The minor antenna complexes bind the same xanthophyll Cars as LHCII—lutein (Lut), neoxanthin (Neo), and violaxanthin (Vio) or zeaxanthin (Zea)—but with a different occupancy (15–17).
Under high incident photon fluxes the energy delivered to the PSII reaction center can no longer be used efficiently, and the luminal pH drops from typically ∼8.0 to ∼5.5. The resulting transmembrane pH gradient triggers qE, induces the enzymatic deepoxidation of Vio into Zea within the PSII antennae (18), and protonates the antenna proteins and the PsbS protein (19,20). In addition, a rearrangement and aggregation of the antennae occurs within the membrane (21). All these processes enhance qE (1,22).
Several molecular mechanisms have been proposed for qE, the following three being the most recurrent: i), energy transfer from the terminal emitter Chls of the LHCII trimer to the S1 state of the neighboring Lut 1 molecule, followed by rapid internal conversion (23–26); ii), formation of a Chl-Chl charge-transfer (CT) state in LHCII and subsequent charge recombination (27); iii), formation of a Zea-Chl or Lut-Chl CT state in one or more of the minor antenna complexes, followed by rapid charge recombination (28–30). In each case, protein dynamics were suggested to underlie the switch between light-harvesting and photoprotective states.
Single, isolated LHCII trimers have been observed to possess the intrinsic capability to quench their fluorescence rapidly, reversibly, and for times that scale from milliseconds to minutes (31,32). Although such intermittent behavior—commonly referred to as fluorescence blinking—is shared by diverse fluorescing systems, ranging from other photosynthetic multichromophoric light-harvesting complexes (33–36) to semiconductor nanocrystals (quantum dots) (37) and single dye molecules (38), for LHCII it manifests some unique properties (39).
A direct relationship between qE and fluorescence blinking was recently proposed, based on single-molecule studies of LHCII complexes (40). It was demonstrated that each of the investigated qE-associated environmental conditions affected the fluorescence blinking such that the time- and population-averaged intensities decreased and the average dwell time in quenched states increased. The connection between qE and fluorescence blinking suggests that the intrinsic dynamic disorder of LHCII trimers, which is reflected by the observed intensity fluctuations, can be controlled by the local environment of the complexes. On a molecular scale this control features as a subtle conformational change, which shifts the population equilibrium between quenched and unquenched states. In this view, new dissipative states do not need to be invoked; instead, as more qE-related conditions are incorporated the probability for accessing one or more dissipative states increases, in agreement with the population-shift model of protein functionality (41).
The experimental blinking behavior of LHCII trimers was reproduced quantitatively by a conformational diffusion model (42). Here, the protein structural motion in the terminal emitter domain was described to contain a specific, relatively slow diffusive component that is modulated by the local environment. In the context of the Chl-to-Lut energy transfer model (23), the probability of energy transfer from the terminal emitter Chls to the electronically coupled Lut 1 molecule fluctuates extensively and on a similar timescale as fluorescence blinking, thus supporting the notion that fluorescence blinking is an intrinsic property of LHCII and associated with one of the qE mechanisms.
The protein dynamics described by this model could be extended on the same principles to other proteins of the Lhcb family, implying that the relationship between blinking and the qE-associated conditions proposed for LHCII might be a general property of all these proteins. Indeed, in vitro ensemble measurements have indicated that isolated minor antenna complexes respond qualitatively in a similar manner to qE-related conditions as isolated LHCII trimers and exhibit an even more pronounced quenching (43,44). On the other hand, only small or negligible quenching effects were observed for isolated minor antennae in some other studies (29,45–47).
In this study, the intrinsic quenching capacity of the minor antenna complexes is investigated by comparing the environmental sensitivity of their fluorescence blinking behavior to that of LHCII trimers. An important part of the study involves the contribution of the dim fraction, i.e., complexes in moderately quenched states during the entire duration of the measurement. The results support a unique role for LHCII in qE and provide an explanation for the varying quenching behavior reported in different in vitro bulk studies on isolated minor antennae. In addition, the findings suggest that artifactual or single-molecule-specific quenching effects have a negligible contribution to the observed environmental changes, thus supporting the significance of single-molecule studies to the investigation of physiological energy-dissipating processes.
Materials and Methods
Sample preparation
The complexes enriched in Vio were isolated by iso-electric focusing from PSII particles obtained from dark-adapted spinach leaves (13). To prepare the Zea-containing complexes, thylakoids were treated with 40 mM ascorbate at pH 5.5 before isolation and purification of the PSII particles (13). The pigment composition of the complexes is shown in Table 1, indicating that the Vio and Zea contents were elevated as compared to standard purification protocols. Complexes were solubilized in 20 mM HEPES at pH 8.0, 1 mM MgCl2, and 0.03% (w/v) n-dodecyl-β,D-maltoside (β-DM), which collectively mimicked the light-harvesting environment. After dilution to a few pM, a small volume was deposited onto a layer of poly-L-lysine (Sigma, Schnelldorf, Germany) and flushed with the experimental environment. The environmental variables consisted in the amount of deepoxidation (either Vio- or Zea-enriched complexes), the pH (either 8 or 5.5), and the detergent concentration (either 0.03% (w/v) or significantly lowered). Sodium citrate (15 mM) was added when a pH of 5.5 was used, and the detergent concentration was lowered by replacing the environment in which the immobilized, micelle-embedded complexes were present with a detergent-free buffer. Before flushing, oxygen was scavenged in the flushing solution by copious flushing of nitrogen gas while an enzymatic system of 200 μg/mL glucose oxidase, 7.5 mg/mL glucose, and 35 μg/mL catalase was allowed the time to react with oxygen. Measurements were performed in a hermetically sealed sample cell at 5°C and repeated on at least one other day to reduce the heterogeneity caused by slight day-to-day variations. A new sample preparation was performed for every experimental environment. Only freshly prepared samples, subject to at most one freeze-thaw cycle, were used. The freeze-thaw cycle did not notably affect the spectroscopic behavior.
Table 1.
Pigment composition of isolated photosystem II antenna proteins
| Complex | Neo | Vio | Ant | Lut | Zea | DES | Chl a/b | |
|---|---|---|---|---|---|---|---|---|
| Vio-enriched | CP24 | 28.3(18) | 25.0(12) | 0.0 | 46.7(28) | 0.0 | 0% | 1.6(1) |
| CP26 | 31.3(15) | 23.7(15) | 0.0 | 46.0(23) | 0.0 | 0% | 2.5(2) | |
| CP29 | 23.0(14) | 36.0(24) | 0.0 | 41.0(16) | 0.0 | 0% | 3.4(1) | |
| LHCII | 27.0(11) | 18.0(13) | 0.0 | 55.0(16) | 0.0 | 0% | 1.3(1) | |
| Zea-enriched | CP24 | 27.3(17) | 10.3(22) | 2.0(4) | 46.3(19) | 14.0(19) | 57% | 1.6(1) |
| CP26 | 30.0(20) | 8.0(13) | 2.7(3) | 47.3(20) | 12.0(13) | 56% | 2.5(1) | |
| CP29 | 24.3(12) | 17.0(18) | 3.7(7) | 42.3(12) | 12.7(9) | 40% | 3.4(1) | |
| LHCII | 27.0(12) | 2.0(2) | 2.0(2) | 54.0(22) | 15.0(21) | 84% | 1.3(1) |
PSII light-harvesting complexes purified by iso-electric focusing from spinach plants, obtained from the thylakoids of plants that were dark adapted for 12 h (Vio-enriched) and by prior deepoxidation of the thylakoids at pH 5.5 in the presence of 40 mM ascorbate (Zea-enriched). Neo, Vio, Ant, Lut, Zea, DES, and Chl a/b: neoxanthin, violaxanthin, antheraxanthin, lutein, zeaxanthin, deepoxidation state (Z + 0.5A)/(V + A + Z) and chlorophyll a/b ratio. Data are presented as (xanthophyll/total xanthophyll) (in %) and are means from four replicates; DES is presented as (Zea + 0.5 Ant)/(Vio + Zea + Ant); Chl a/b is presented as a molar ratio. Numbers in parenthesis denote standard deviations, as applied on the last significant digit(s), taking into account variations between different purifications.
Single-molecule spectroscopy
The experimental setup used to perform single-molecule fluorescence spectroscopy was described earlier (32,48). All complexes were excited with an intensity of ca. 250 W/cm2 at 632.8 nm, originating from a continuous wave He-Ne laser (JDS Uniphase, Eindhoven, The Netherlands). The linear polarization of the light was changed into a near-circular state by a Berek polarization compensator (5540M; New Focus, Santa Clara, CA) to further reduce Chl-specific excitation. Fluorescence counts were registered in 10-ms consecutive bins with an avalanche photodiode (SPCM-AQR-16; Perkin-Elmer Optoelectronics, Waltham, MA).
Data analysis
For each type of complex in each of the experimental environments, data sets consisting of intensity time traces of a few hundred individually measured complexes were collected and subjected to similar data screening as previously employed on LHCII trimers (31,40). In short, the single-molecule identity of the complexes was established by single-step transitions into fully quenched or photobleached states. Three additional filtering criteria involved excluding complexes exhibiting spectral bluing and intensity traces characterized by photon bursts or excessive fluctuations. Data sets containing at least 10% of such filtered out traces were disregarded altogether. In addition, only complexes surviving longer than typically one-third of the average survival time of ∼30 s were considered. Intensity traces were trimmed to the last intensity level exceeding a predefined intensity threshold, the latter of which was set relatively high for Figs. 1, 2, and 3, A–C, thereby excluding the so-called dim fraction of complexes for these figures. Screened data sets contained information from at least 100 complexes. Intensity bins of 20 ms were used, giving signal/noise ratios of typically 6–10 for the fully emitting states. Intensity levels were resolved for data in Fig. 1, employing the algorithm described in (31). Intensities were calibrated based on the best alignment of the optical setup. Presented data constitute averages from at least three different data sets and at least two batches from different protein purifications. Comparisons with in vitro ensemble results were done by calculating the time- and population-averaged intensity Ī = ΣiIiτi/Σiτi for data sets consisting of N intensities Ii and associated dwell times τi. All calculations were implemented in MATLAB (The MathWorks, Natick, MA).
Figure 1.
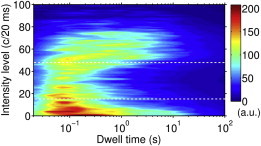
Fluorescence intensity distribution of individually measured CP29 complexes in the light-harvesting-mimicking environment, excluding the dim fraction. Density map shows the resolved intensity levels and corresponding time of residence for a set of ∼100 complexes. Dashed lines separate three categories, referred to as bright, dim, and dark states.
Figure 2.
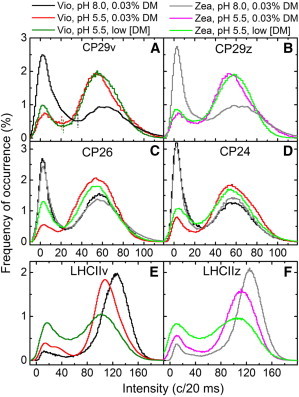
Environmental effect on fluorescence intensity distributions of individually probed PSII peripheral antennae. Histograms were constructed from consecutive 20-ms bins without resolving the intensity change points and excluding the dim fraction of complexes. Negative intensities stem from shot noise after background subtraction. Quenched and unquenched states are separated by Ithr, defined as the local minima between the histogram peaks (vertical lines in Fig. 2A). Zea, z: Zea-enriched; Vio, v: Vio-enriched; Low [DM]: low detergent concentration.
Figure 3.
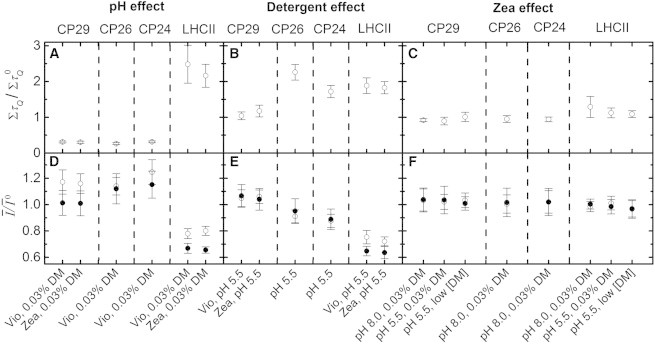
Effect of the three qE-related factors on the fluorescence intensity dynamics of individually investigated PSII peripheral antennae. (A–C) Fraction of increase in induced by the environment, where and denote the total dwell time in quenched states for complexes in the light-harvesting and quenching environment, respectively. X axes denote environmental conditions that remained constant, assuming for the detergent effect of CP26 and CP24 that Zea and Vio exhibited a similar behavior. Calculations are based on data for bright complexes in Table S1. Error bars reflect standard errors. Zea: Zea-enriched; Vio: Vio-enriched. (D–F) Similar to Fig. 3, A–C, but for the time- and population-averaged intensities (Ī) when the dim fraction was neglected (open circles) and included (solid circles). Ī0 denotes the value of Ī before the quenching environment was introduced. Error bars denote standard deviations, including calibration uncertainties of absolute intensities. See text for details.
Results
Environmental sensitivity of intensity distributions
Under continuous illumination, single complexes of CP29, CP26, and CP24 were found to display the familiar phenomenon of fluorescence blinking. Fig. 1 displays a typical intensity dwell-time distribution, shown here only for CP29 complexes under conditions that mimic the harvesting of light in vivo, i.e., Vio-enriched complexes at pH 8.0 at a detergent concentration of 0.03% β-DM. The accessed intensity levels can be broadly separated into three groups: unquenched, intermediate, and quenched, or alternatively, bright, dim, and dark. This distinction is based on the observation that the intermediate intensity states were accessed for significantly shorter average times than the quenched and unquenched states. Note that this figure includes only the results from complexes that during the measurement exhibited emission in the unquenched region (above ≈48 c/20 ms), i.e., excluding those complexes that were only weakly emitting (dim) during the whole measurement period. The latter property will be considered later.
Considering from Fig. 1 that the complexes spent most time in either unquenched (bright) or quenched (dark) states, a two-state intensity model provides a reasonable approximation to describe the primary intensity dynamics of these complexes. In addition, if the intensity threshold used to separate the two states, Ithr, is chosen well above zero (the background), the influence of shot noise on the intensity distributions is reduced significantly (31,40,49). This suggests that for such a two-state model, the accessed intensity levels do not have to be resolved when considering the environmental sensitivity of the intensity distributions.
For each of the three types of minor antennae, the changes in the intensity distributions incurred by the three environmental conditions that were associated with qE in a former study on LHCII trimers (40) were investigated, viz. Zea-containing complexes at pH 5.5 and a considerably lower detergent concentration. Distributions of 20-ms binned fluorescence intensities are shown in Fig. 2, A–D, for minor antenna complexes that were exposed to different combinations of these qE-associated conditions, and compared to the major antennae in Fig. 2, E and F. Although the distributions of the minor antennae follow mostly similar trends with respect to one another, two distinct differences are evident when compared to LHCII: under light-harvesting conditions (Vio, pH 8.0, 0.03% DM), the minor antennae spent on average considerably more time in quenched states than LHCII, and the acidic environment caused a large equilibrium shift into the opposite direction than for LHCII, enhancing the unquenched states considerably. The effect of the other two environmental factors was less dramatic. First, Zea caused a small population shift to the quenched states for LHCII, although for the minor antennae the effect was marginal (this being the reason for investigating its effect in less detail for CP26 and CP24). Second, by reducing the detergent concentration, quenched states were favored for all complexes, though the extent of the equilibrium shift was smaller for CP29 than for the other complexes.
In Fig. 3, A–C, the population shifts that resulted from the different environmental changes are quantified by the changes in ΣτQ, denoting the total time spent in quenched states. The strong divergence in the sensitivity to pH between the minor and major antennae is evident, and also the varying sensitivity of the complexes to detergent concentration. In addition, although LHCII trimers exhibited a small, positive (i.e., decreasing) Zea effect, the minor antennae showed the opposite indication, i.e., slightly favoring unquenched states for Zea-containing complexes (Fig. 3 C). The two-state intensity equilibrium is quantified further by the values of ΣτQ displayed in Table S1 in the Supporting Material.
Ensemble intensities
From Fig. 2 it is evident that the environmental changes not only affected the population equilibrium between quenched and unquenched states but also the intrinsic brightness of the complexes, indicated by the peak intensity of the broad histogram that designates the unquenched states. Evidently, both these factors influence the time- and population-averaged intensity Ī, referred to below as the ensemble intensity. Changes in this parameter can be compared directly to the extent of qE, considering that upon the simultaneous excitation of billions of complexes in an in vivo environment, their combined fluorescence signal is averaged into a single intensity level.
In Fig. 3, D–F, the effect of each of the three environmental factors on Ī is quantified. For all three minor antennae, an acidic environment increased the ensemble intensity considerably when neglecting the dim fraction (open circles), in stark contrast to LHCII. This indicates that the changes in the intrinsic brightness were not large enough to counterbalance the strong population shift to unquenched states. However, the intrinsic brightness was such that the lack of detergent had a marginal effect on Ī for CP29 and a positive (i.e., decreasing) effect on Ī for CP26 and CP24, although the extent was significantly smaller than for LHCII. Furthermore, Zea had a minor or negligible effect on all the complexes.
Effect of the dim fraction
The increase in Ī when the pH was lowered does not concur with the findings of previous in vitro bulk studies (43–45,47). An important property that has thus far been neglected in the current study is the dim fraction of complexes. To include their contribution, the intensity thresholds designating the bright population (see Fig. 1, top line) were halved. The consequent fluorescence intensity distributions are displayed in Fig. 4 for Zea-containing CP29 and LHCII complexes, the former of which represent the general trend of all the minor antennae.
Figure 4.
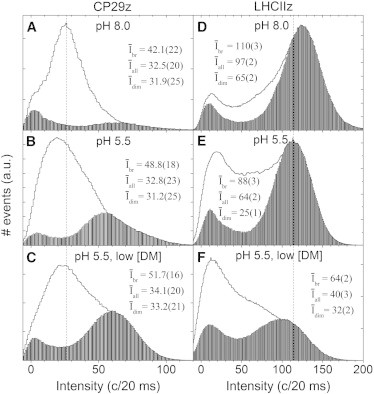
Effect of the dim fraction on the intensity distributions of PSII peripheral antennae. Histograms of consecutive 20-ms bins of fluorescence counts are shown for Zea-containing CP29 complexes (A–C) and Zea-containing LHCII trimers (D–F) under three different environmental conditions when the dim fraction was excluded (bars) and included (histogram contours). Vertical dashed lines serve as guides to monitor peak shifts. Ensemble intensities are displayed for analyses excluding the dim fraction (Ībr), including the dim fraction (Īall), and for the dim fraction only (Īdim). Numbers in parentheses denote standard deviations, as applied on the last significant digit(s).
Strikingly, in the presence of a high pH and 0.03% detergent (i.e., light-harvesting conditions), the minor antennae consisted of a very large dim fraction (Fig. 4 A), in stark contrast to LHCII (Fig. 4 D). Moreover, the dim fraction decreased strongly as more environmental changes were made, whereas for LHCII it increased considerably. For the minor antennae the ensemble intensity of the dim fraction, Īdim, was constant within the experimental error margins, and so was the average intensity of the whole ensemble, Īall, due to the dominating dim fraction. However, for LHCII, Īdim displays a more complicated behavior, whereas Īall decreased strongly with a changing environment. The latter property was enhanced by the increasing dim fraction, shifting the intensity population more strongly to quenched states (see Fig. 3, D and E, solid circles). In contrast, for the minor antennae the dim fraction reduced the shift in the intensity population (Fig. 3, D and E, solid circles).
Fig. 2 shows that when neglecting the dim fraction, under light-harvesting conditions the minor antennae spent, on average, considerably more time in quenched states than LHCII (see also Table S1). When the dim fraction is included, this difference increases substantially (see also Fig. 4, A and D). Also of interest is that the average intensity of LHCII under light-harvesting conditions is about three times that of the minor antennae, in agreement with their relative effective absorption cross sections.
Evidently, energy states associated with intermediate intensity levels play an important role for the minor antenna complexes. Hence, although the two-state intensity model provides a reasonable approximation for LHCII trimers in a light-harvesting environment, it is not valid for the minor antennae.
Discussion
Sources of quenching
The dynamic fluorescence intensity fluctuations of the PSII peripheral antenna complexes point to an intrinsic capability to dissipate absorbed energy to different extents and also indicate considerable underlying disorder. The differences in the blinking behavior between LHCII trimers and the minor antennae, in particular their differing responses to local environmental changes, strongly indicate different distributions of energy traps. In this section the relationship between the possible sources of quenching and the primary sources associated with qE will be examined by comparing this environmental sensitivity with the results from previous in vitro bulk studies, considering that the in vivo behavior of the complexes should be reflected to a reasonable extent in a qE-mimicking environment. Moreover, taking into account that all these complexes are structurally and compositionally related, valuable information on some fundamental properties of protein functionality can be obtained.
It should first be noted that triplet states as an explanation for blinking can be excluded, based on their much shorter lifetimes with respect to the experimental time resolution (39). However, accessing a dark state, such as a radical cation, via the triplet state could play a role, as demonstrated for the molecular dye R6G (50). It is unlikely that this is a dominant mechanism for the complexes investigated in this study, as this would require excitation of a Car triplet state, which has a negligible probability under the utilized conditions.
Radical-state formation in photosynthetic complexes is more frequently associated with electron transfer. This photochemical pathway was assumed to explain fluorescence blinking of allophycocyanin complexes (51,52). Moreover, each of CP24, CP26, and CP29 was observed to exhibit the formation of Car radicals after exciting their Chl b's with a high excitation repetition rate (29). It was claimed that these radicals were formed by electron transfer to nearby Chls (28), after rapid relaxation of a CT state that was delocalized over the Car and Chls (29,30). The cations were attributed primarily to Zea (29) but also to Lut (53). A couple of findings challenge the notion to relate such radical formation to the primary source of fluorescence blinking: i), only a very small fraction (<0.5%) of the complexes showed evidence for Car radicals. Their probability should increase by at least two orders of magnitude under the utilized single-molecule conditions if they were to be associated with the primary source of blinking. ii), The short lifetimes (∼150 ps) of these radical cations (30) make them unfit to account for the observed long dwell times in quenched states. Although the preceding CT states relax even faster, evidence for long-lived CT states was found for LHCII trimers under similar single-molecule conditions (32). However, these states were mostly unquenched and attributed to only ∼5% of the complexes. iii), The negligible or negative Zea effect in this study opposes its association with quenching. In addition, due to the relatively strong coordination of Zea and Vio inside the minor antenna complexes (54,55), their detachment from the complexes is deemed an unlikely explanation for this lack of Zea dependence.
A more likely way in which the xanthophylls in these complexes are involved in quenching is via direct energy transfer from the Qy energy levels of nearby Chl a’s. It is known that the S1 energies of most of these xanthophylls are lower than the Chl a Qy energies in these protein environments (56,57). As a consequence, close proximity and proper orientation between some Chl a’s and xanthophylls will lead to fast energy transfer from the Chls to the Cars and a subsequent rapid relaxation of the S1 states, thereby dissipating the energy as heat. Such a situation is likely to be achieved by the abundant structural disorder of the local protein microenvironments. Indeed, the quantitative description of LHCII’s fluorescence blinking by a model that is based on such a mechanism (42) supports the notion that this is an important source of quenching in LHCII trimers and likely the primary mechanism that underlies fluorescence blinking of all PSII peripheral antenna complexes. This mechanism would be highly sensitive to the specific protein microenvironment, type of xanthophyll, and the positions and relative orientations of the xanthophyll and neighboring Chls. The minor differences between the structurally homologous Lhcb complexes are expected to be sufficient to explain the differences in their blinking behaviors and hence their quenching capabilities.
Comparison with previous in vitro bulk studies
It should first be noted that the major and minor antenna complexes are structurally and compositionally very similar but showed a very different environmental sensitivity of their blinking dynamics, in particular with regards to the pH and the dim fraction. This signifies that the population equilibrium shifts are unlikely to be coincidental, artifactual effects caused by the single-molecule environment.
At first sight our results on the lack of sensitivity of the emission from single minor antenna complexes to a low detergent concentration, low pH, and Zea would seem to contradict previous ensemble studies (43,44). However, these studies all examined the effect of Zea and/or low pH in driving acceleration of quenching against a background of low detergent-induced aggregation of antenna complexes in solution. The conditions used in our study are quite distinct in that they evaluate the effect of each environmental condition in the absence of aggregation of the antenna complexes. One explanation is that the very large population of (permanently) dim complexes efficiently quenches the smaller population of bright complexes upon aggregation of the sample in the ensemble studies and that Zea and low pH may simply facilitate this effect by promoting aggregation. Indeed, the small effect of Zea on the population distribution of PSII antenna complexes between quenched and unquenched states in single-molecule studies supports the view that these molecules affect qE in vivo by controlling protein-protein interactions (58,59). In those ensemble studies that have examined the effect of Zea and low pH in the absence of protein aggregation it is noteworthy that these conditions had only very minor quenching effects (KD of <0.5) (29,45–47). The large dim fractions in the minor antenna samples also provide an explanation for the considerable yield of short fluorescence lifetime components compared to LHCII trimers in detergent solution (47,60).
qE based on specifically controlled disorder
Proteins are inherently dynamic and sample a vast ensemble of conformations, a concept well illustrated by the protein free-energy landscape (61), which consists of different conformational substates in dynamic equilibrium. Proteins respond to environmental changes by redistributing the relative populations of these preexisting substates, a model commonly known as conformational selection (41). An elegant example that this is also a fundamental property of photosynthetic light-harvesting proteins is our finding that isolated Lhcb complexes, which normally emit at around 680 nm, were found to occasionally fluoresce in the region around 700–730 nm, typical for Lhca complexes, whereas the latter complexes performed the exact opposite behavior (62).
The conformational selection model implies that all physiological states of the PSII peripheral antenna complexes can be accessed under any environmental conditions. Only the equilibrium between these states, and thus the access probability, may differ from the in vivo situation. This context strongly supports the proposition that qE is based on some environmental control of the protein’s structural disorder (40) and suggests that the observed intensity dynamics should at least partially account for the primary mechanism underlying qE.
Inclusion of the dim fraction of LHCII trimers resulted in a stronger correlation between quenching and qE-related conditions than observed before (40), supporting the notion that qE and fluorescence blinking share the same primary mechanism(s) for this complex. The lack of such a correlation for the minor antennae indicates that these complexes do not use their intrinsic capability of quenching in the same way as LHCII trimers. In fact, these complexes appear remarkably insensitive to the conditions we have used to simulate qE. We therefore conclude that LHCII is more sensitive to environmental changes than the minor antennae and that the structural disorder underlying blinking in all these complexes is controlled in a specific way for LHCII to exhibit a biologically relevant functionality related to qE. Of course, in vivo, when all the antenna complexes are organized in the grana membrane associated in the PSII supercomplexes, it is likely that the intrinsic dynamicity is constrained (22). Indeed, the structural changes that have been observed upon qE formation (21) suggest that these changes are necessary to release the LHCII trimers and shift the equilibrium toward increased quenching. CP29 and CP26 are tightly bound to the supercomplex and therefore probably do not have such freedom, further constraining their dynamic range. In this case, any qE-related quenching probably occurs by a different mechanism, in agreement with previous in vitro bulk studies (63–65).
Conclusion
It was shown that the minor antenna complexes of plant PSII exhibit a distinct fluorescence blinking behavior and dim fraction with respect to the structurally and compositionally related LHCII trimeric complexes. Hence, the observed environmental responses of fluorescence blinking cannot be attributed to a general property of photosynthetic light-harvesting complexes. The same conditions that, on average, induced a strong quenching of single LHCII complexes had no or only a small quenching effect on the minor antenna complexes when the dim fraction was taken into account. This indicates that the minor antennae possess an intrinsically lower degree of dynamicity than LHCII trimers and additional environmental conditions must change to appropriately shift their population equilibrium to quenched states. In addition, the results strongly support that for LHCII trimers the principal molecular mechanism underlying qE and fluorescence blinking is shared. In addition, considering that only subtle conformational changes are possible in these dense complexes, these findings strongly indicate that the environmentally controlled disorder underlying qE is a sensitive, finely tuned, and highly specific mechanism. Hence, the findings provide insights into some fundamental properties of protein dynamics in relation to protein multifunctionality.
We have also challenged the involvement of radical cations as a primary source of fluorescence blinking in these complexes, and related the phenomenon of blinking instead to excitonic interactions between Chls and Cars that are controlled by their disordered protein microenvironments.
Acknowledgments
This work was supported by the EU FP7 Marie Curie Reintegration Grant (ERG 224796) (C.I.); the CEA-Eurotalents program (PCOFUND-GA-2008-228664) (C.I.); research and equipment grants from UK Biotechnology and Biological Sciences Research Council (BBSRC) and Engineering and Physical Sciences Research Council (EPSRC) (M.P.J. and A.V.R.); Project Sunshine, University of Sheffield (P.H.); Grants from the Netherlands Organization for Scientific Research (700.58.305 and 700.56.014 from the Foundation of Chemical Sciences) (T.P.J.K., C.I., and R.v.G.), and the Advanced Investigator Grant (267333, PHOTPROT) from the European Research Council (ERC) (C.I., T.P.J.K., and R.v.G.).
Footnotes
Tjaart P. J. Krüger’s present address is Department of Physics, Faculty of Natural and Agricultural Sciences, University of Pretoria, Private bag X20, Hatfield, 0028, South Africa.
Contributor Information
Tjaart P.J. Krüger, Email: tjaart.kruger@up.ac.za.
Rienk van Grondelle, Email: r.van.grondelle@vu.nl.
Supporting Material
References
- 1.Horton P., Ruban A.V., Walters R.G. Regulation of light harvesting in green plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996;47:655–684. doi: 10.1146/annurev.arplant.47.1.655. [DOI] [PubMed] [Google Scholar]
- 2.Niyogi K.K. Photoprotection revisited: genetic and molecular approaches. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999;50:333–359. doi: 10.1146/annurev.arplant.50.1.333. [DOI] [PubMed] [Google Scholar]
- 3.Ruban A.V., Johnson M.P., Duffy C.D.P. The photoprotective molecular switch in the photosystem II antenna. Biochim. Biophys. Acta. 2012;1817:167–181. doi: 10.1016/j.bbabio.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Green B.R., Durnford D.G. The chlorophyll-carotenoid proteins of oxygenic photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996;47:685–714. doi: 10.1146/annurev.arplant.47.1.685. [DOI] [PubMed] [Google Scholar]
- 5.Caffarri S., Croce R., Bassi R. A look within LHCII: differential analysis of the Lhcbl-3 complexes building the major trimeric antenna complex of higher-plant photosynthesis. Biochemistry. 2004;43:9467–9476. doi: 10.1021/bi036265i. [DOI] [PubMed] [Google Scholar]
- 6.Jansson S. A guide to the Lhc genes and their relatives in Arabidopsis. Trends Plant Sci. 1999;4:236–240. doi: 10.1016/s1360-1385(99)01419-3. [DOI] [PubMed] [Google Scholar]
- 7.Dainese P., Bassi R. Subunit stoichiometry of the chloroplast photosystem II antenna system and aggregation state of the component chlorophyll a/b binding proteins. J. Biol. Chem. 1991;266:8136–8142. [PubMed] [Google Scholar]
- 8.Liu Z., Yan H., Chang W. Crystal structure of spinach major light-harvesting complex at 2.72 A resolution. Nature. 2004;428:287–292. doi: 10.1038/nature02373. [DOI] [PubMed] [Google Scholar]
- 9.Pan X., Li M., Chang W. Structural insights into energy regulation of light-harvesting complex CP29 from spinach. Nat. Struct. Mol. Biol. 2011;18:309–315. doi: 10.1038/nsmb.2008. [DOI] [PubMed] [Google Scholar]
- 10.Standfuss J., Terwisscha van Scheltinga A.C., Kühlbrandt W. Mechanisms of photoprotection and nonphotochemical quenching in pea light-harvesting complex at 2.5 A resolution. EMBO J. 2005;24:919–928. doi: 10.1038/sj.emboj.7600585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bassi R., Dainese P. A supramolecular light-harvesting complex from chloroplast photosystem-II membranes. Eur. J. Biochem. 1992;204:317–326. doi: 10.1111/j.1432-1033.1992.tb16640.x. [DOI] [PubMed] [Google Scholar]
- 12.Peter G.F., Thornber J.P. Biochemical composition and organization of higher plant photosystem II light-harvesting pigment-proteins. J. Biol. Chem. 1991;266:16745–16754. [PubMed] [Google Scholar]
- 13.Ruban A.V., Lee P.J., Horton P. Determination of the stoichiometry and strength of binding of xanthophylls to the photosystem II light harvesting complexes. J. Biol. Chem. 1999;274:10458–10465. doi: 10.1074/jbc.274.15.10458. [DOI] [PubMed] [Google Scholar]
- 14.Sandonà D., Croce R., Bassi R. Higher plants light harvesting proteins. Structure and function as revealed by mutation analysis of either protein or chromophore moieties. Biochim. Biophys. Acta. 1998;1365:207–214. doi: 10.1016/s0005-2728(98)00068-1. [DOI] [PubMed] [Google Scholar]
- 15.Bassi R., Croce R., Sandonà D. Mutational analysis of a higher plant antenna protein provides identification of chromophores bound into multiple sites. Proc. Natl. Acad. Sci. USA. 1999;96:10056–10061. doi: 10.1073/pnas.96.18.10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Croce R., Canino G., Bassi R. Chromophore organization in the higher-plant photosystem II antenna protein CP26. Biochemistry. 2002;41:7334–7343. doi: 10.1021/bi0257437. [DOI] [PubMed] [Google Scholar]
- 17.Passarini F., Wientjes E., Croce R. Molecular basis of light harvesting and photoprotection in CP24: unique features of the most recent antenna complex. J. Biol. Chem. 2009;284:29536–29546. doi: 10.1074/jbc.M109.036376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demmig-Adams B., Adams W.W., Björkman O. Inhibition of zeaxanthin formation and of rapid changes in radiationless energy dissipation by dithiothreitol in spinach leaves and chloroplasts. Plant Physiol. 1990;92:293–301. doi: 10.1104/pp.92.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X.P., Gilmore A.M., Niyogi K.K. Regulation of photosynthetic light harvesting involves intrathylakoid lumen pH sensing by the PsbS protein. J. Biol. Chem. 2004;279:22866–22874. doi: 10.1074/jbc.M402461200. [DOI] [PubMed] [Google Scholar]
- 20.Walters R.G., Ruban A.V., Horton P. Higher plant light-harvesting complexes LHCIIa and LHCIIc are bound by dicyclohexylcarbodiimide during inhibition of energy dissipation. Eur. J. Biochem. 1994;226:1063–1069. doi: 10.1111/j.1432-1033.1994.01063.x. [DOI] [PubMed] [Google Scholar]
- 21.Johnson M.P., Goral T.K., Ruban A.V. Photoprotective energy dissipation involves the reorganization of photosystem II light-harvesting complexes in the grana membranes of spinach chloroplasts. Plant Cell. 2011;23:1468–1479. doi: 10.1105/tpc.110.081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horton P., Johnson M.P., Ruban A.V. Photosynthetic acclimation: does the dynamic structure and macro-organisation of photosystem II in higher plant grana membranes regulate light harvesting states? FEBS J. 2008;275:1069–1079. doi: 10.1111/j.1742-4658.2008.06263.x. [DOI] [PubMed] [Google Scholar]
- 23.Ruban A.V., Berera R., van Grondelle R. Identification of a mechanism of photoprotective energy dissipation in higher plants. Nature. 2007;450:575–578. doi: 10.1038/nature06262. [DOI] [PubMed] [Google Scholar]
- 24.Bode S., Quentmeier C.C., Walla P.J. On the regulation of photosynthesis by excitonic interactions between carotenoids and chlorophylls. Proc. Natl. Acad. Sci. USA. 2009;106:12311–12316. doi: 10.1073/pnas.0903536106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao P.N., Holleboom C.P., Walla P.J. Correlation of Car S(1) → Chl with Chl → Car S(1) energy transfer supports the excitonic model in quenched light harvesting complex II. J. Phys. Chem. B. 2010;114:15650–15655. doi: 10.1021/jp1034163. [DOI] [PubMed] [Google Scholar]
- 26.Liao P.-N., Pillai S., Walla P.J. On the role of excitonic interactions in carotenoid-phthalocyanine dyads and implications for photosynthetic regulation. Photosynth. Res. 2012;111:237–243. doi: 10.1007/s11120-011-9690-9. [DOI] [PubMed] [Google Scholar]
- 27.Miloslavina Y., Wehner A., Holzwarth A.R. Far-red fluorescence: a direct spectroscopic marker for LHCII oligomer formation in non-photochemical quenching. FEBS Lett. 2008;582:3625–3631. doi: 10.1016/j.febslet.2008.09.044. [DOI] [PubMed] [Google Scholar]
- 28.Ahn T.K., Avenson T.J., Fleming G.R. Architecture of a charge-transfer state regulating light harvesting in a plant antenna protein. Science. 2008;320:794–797. doi: 10.1126/science.1154800. [DOI] [PubMed] [Google Scholar]
- 29.Avenson T.J., Ahn T.K., Fleming G.R. Zeaxanthin radical cation formation in minor light-harvesting complexes of higher plant antenna. J. Biol. Chem. 2008;283:3550–3558. doi: 10.1074/jbc.M705645200. [DOI] [PubMed] [Google Scholar]
- 30.Holt N.E., Zigmantas D., Fleming G.R. Carotenoid cation formation and the regulation of photosynthetic light harvesting. Science. 2005;307:433–436. doi: 10.1126/science.1105833. [DOI] [PubMed] [Google Scholar]
- 31.Krüger T.P.J., Ilioaia C., van Grondelle R. Fluorescence intermittency from the main plant light-harvesting complex: resolving shifts between intensity levels. J. Phys. Chem. B. 2011;115:5071–5082. doi: 10.1021/jp201609c. [DOI] [PubMed] [Google Scholar]
- 32.Krüger T.P.J., Novoderezhkin V.I., van Grondelle R. Fluorescence spectral dynamics of single LHCII trimers. Biophys. J. 2010;98:3093–3101. doi: 10.1016/j.bpj.2010.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bopp M.A., Jia Y.W., Hochstrasser R.M. Fluorescence and photobleaching dynamics of single light-harvesting complexes. Proc. Natl. Acad. Sci. USA. 1997;94:10630–10635. doi: 10.1073/pnas.94.20.10630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hofkens J., Schroeyers W., De Schryver F.C. Triplet states as non-radiative traps in multichromophoric entities: single molecule spectroscopy of an artificial and natural antenna system. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2001;57:2093–2107. doi: 10.1016/s1386-1425(01)00499-1. [DOI] [PubMed] [Google Scholar]
- 35.Unterkofler S., Pflock T., Köhler J. Fluorescence blinking of the RC-LH1 complex from Rhodopseudomonas palustris. ChemPhysChem. 2011;12:711–716. doi: 10.1002/cphc.201000588. [DOI] [PubMed] [Google Scholar]
- 36.Ying L.M., Xie X.S. Fluorescence spectroscopy, exciton dynamics, and photochemistry of single allophycocyanin trimers. J. Phys. Chem. B. 1998;102:10399–10409. [Google Scholar]
- 37.Nirmal M., Dabbousi B.O., Brus L.E. Fluorescence intermittency in single cadmium selenide nanocrystals. Nature. 1996;383:802–804. [Google Scholar]
- 38.Ambrose W.P., Goodwin P.M., Keller R.A. Single molecule detection and photochemistry on a surface using near-field optical excitation. Phys. Rev. Lett. 1994;72:160–163. doi: 10.1103/PhysRevLett.72.160. [DOI] [PubMed] [Google Scholar]
- 39.Krüger T.P.J., Ilioaia C., van Grondelle R. Fluorescence intermittency from the main plant light-harvesting complex: sensitivity to the local environment. J. Phys. Chem. B. 2011;115:5083–5095. doi: 10.1021/jp109833x. [DOI] [PubMed] [Google Scholar]
- 40.Krüger T.P.J., Ilioaia C., van Grondelle R. Controlled disorder in plant light-harvesting complex II explains its photoprotective role. Biophys. J. 2012;102:2669–2676. doi: 10.1016/j.bpj.2012.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsai C.J., Kumar S., Nussinov R. Folding funnels, binding funnels, and protein function. Protein Sci. 1999;8:1181–1190. doi: 10.1110/ps.8.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valkunas L., Chmeliov J., van Grondelle R. How photosynthetic proteins switch. J. Phys. Chem. Lett. 2012;3:2779–2784. [Google Scholar]
- 43.Ruban A.V., Young A.J., Horton P. Dynamic properties of the minor chlorophyll a/b binding proteins of photosystem II, an in vitro model for photoprotective energy dissipation in the photosynthetic membrane of green plants. Biochemistry. 1996;35:674–678. doi: 10.1021/bi9524878. [DOI] [PubMed] [Google Scholar]
- 44.Wentworth M., Ruban A.V., Horton P. Chlorophyll fluorescence quenching in isolated light harvesting complexes induced by zeaxanthin. FEBS Lett. 2000;471:71–74. doi: 10.1016/s0014-5793(00)01369-7. [DOI] [PubMed] [Google Scholar]
- 45.Amarie S., Wilk L., Wachtveitl J. Properties of zeaxanthin and its radical cation bound to the minor light-harvesting complexes CP24, CP26 and CP29. Biochim. Biophys. Acta. 2009;1787:747–752. doi: 10.1016/j.bbabio.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 46.Ballottari M., Girardon J., Bassi R. Identification of the chromophores involved in aggregation-dependent energy quenching of the monomeric photosystem II antenna protein Lhcb5. J. Biol. Chem. 2010;285:28309–28321. doi: 10.1074/jbc.M110.124115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crimi M., Dorra D., Bassi R. Time-resolved fluorescence analysis of the recombinant photosystem II antenna complex CP29. Effects of zeaxanthin, pH and phosphorylation. Eur. J. Biochem. 2001;268:260–267. doi: 10.1046/j.1432-1033.2001.01874.x. [DOI] [PubMed] [Google Scholar]
- 48.Rutkauskas D., Novoderezkhin V., van Grondelle R. Fluorescence spectral fluctuations of single LH2 complexes from Rhodopseudomonas acidophila strain 10050. Biochemistry. 2004;43:4431–4438. doi: 10.1021/bi0497648. [DOI] [PubMed] [Google Scholar]
- 49.Crouch C.H., Sauter O., Pelton M. Facts and artifacts in the blinking statistics of semiconductor nanocrystals. Nano Lett. 2010;10:1692–1698. doi: 10.1021/nl100030e. [DOI] [PubMed] [Google Scholar]
- 50.Zondervan R., Kulzer F., Orrit M. Photoblinking of rhodamine 6G in poly(vinyl alcohol): radical dark state formed through the triplet. J. Phys. Chem. A. 2003;107:6770–6776. [Google Scholar]
- 51.Goldsmith R.H., Moerner W.E. Watching conformational- and photo-dynamics of single fluorescent proteins in solution. Nat. Chem. 2010;2:179–186. doi: 10.1038/nchem.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Loos D., Cotlet M., Hofkens J. Single-molecule spectroscopy selectively probes donor and acceptor chromophores in the phycobiliprotein allophycocyanin. Biophys. J. 2004;87:2598–2608. doi: 10.1529/biophysj.104.046219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Avenson T.J., Ahn T.K., Fleming G.R. Lutein can act as a switchable charge transfer quencher in the CP26 light-harvesting complex. J. Biol. Chem. 2009;284:2830–2835. doi: 10.1074/jbc.M807192200. [DOI] [PubMed] [Google Scholar]
- 54.Bassi R., Pineau B., Marquardt J. Carotenoid-binding proteins of photosystem II. Eur. J. Biochem. 1993;212:297–303. doi: 10.1111/j.1432-1033.1993.tb17662.x. [DOI] [PubMed] [Google Scholar]
- 55.Ruban A.V., Young A.J., Horton P. The effects of illumination on the xanthophyll composition of the photosystem-II light-harvesting complexes of spinach thylakoid membranes. Plant Physiol. 1994;104:227–234. doi: 10.1104/pp.104.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Polívka T., Sundström V. Ultrafast dynamics of carotenoid excited States-from solution to natural and artificial systems. Chem. Rev. 2004;104:2021–2071. doi: 10.1021/cr020674n. [DOI] [PubMed] [Google Scholar]
- 57.Polívka T., Zigmantas D., Bassi R. Carotenoid S(1) state in a recombinant light-harvesting complex of Photosystem II. Biochemistry. 2002;41:439–450. doi: 10.1021/bi011589x. [DOI] [PubMed] [Google Scholar]
- 58.Johnson M.P., Ruban A.V. Arabidopsis plants lacking PsbS protein possess photoprotective energy dissipation. Plant J. 2010;61:283–289. doi: 10.1111/j.1365-313X.2009.04051.x. [DOI] [PubMed] [Google Scholar]
- 59.Ruban A.V., Phillip D., Horton P. Carotenoid-dependent oligomerization of the major chlorophyll a/b light harvesting complex of photosystem II of plants. Biochemistry. 1997;36:7855–7859. doi: 10.1021/bi9630725. [DOI] [PubMed] [Google Scholar]
- 60.Moya I., Silvestri M., Bassi R. Time-resolved fluorescence analysis of the photosystem II antenna proteins in detergent micelles and liposomes. Biochemistry. 2001;40:12552–12561. doi: 10.1021/bi010342x. [DOI] [PubMed] [Google Scholar]
- 61.Frauenfelder H., Sligar S.G., Wolynes P.G. The energy landscapes and motions of proteins. Science. 1991;254:1598–1603. doi: 10.1126/science.1749933. [DOI] [PubMed] [Google Scholar]
- 62.Krüger T.P.J., Wientjes E., van Grondelle R. Conformational switching explains the intrinsic multifunctionality of plant light-harvesting complexes. Proc. Natl. Acad. Sci. USA. 2011;108:13516–13521. doi: 10.1073/pnas.1105411108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Holzwarth A.R., Miloslavina Y., Jahns P. Identification of two quenching sites active in the regulation of photosynthetic light-harvesting studied by time-resolved fluorescence. Chem. Phys. Lett. 2009;483:262–267. [Google Scholar]
- 64.Miloslavina Y., de Bianchi S., Holzwarth A.R. Quenching in Arabidopsis thaliana mutants lacking monomeric antenna proteins of photosystem II. J. Biol. Chem. 2011;286:36830–36840. doi: 10.1074/jbc.M111.273227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Bianchi S., Betterle N., Dall’Osto L. Arabidopsis mutants deleted in the light-harvesting protein Lhcb4 have a disrupted photosystem II macrostructure and are defective in photoprotection. Plant Cell. 2011;23:2659–2679. doi: 10.1105/tpc.111.087320. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


