Abstract
Elucidating the biological basis for sex differences in diseases can reveal their pathophysiology and guide the development of individualized treatments. Here we review evidence for the novel concept that receptor signaling can be sex biased such that the specific pathways engaged by ligand binding are determined by sex. As an example, this review focuses on the receptor for corticotropin-releasing factor (CRF), a stress-related peptide implicated in diverse psychiatric and medical disorders that are more prevalent in females. There is evidence for sex biases in CRF receptor coupling to G-proteins and β-arrestin that render females more sensitive to acute stress and less able to adapt to chronic stress. Taken with evidence for sex biased signaling in other receptor systems, the studies demonstrate the broad potential impact of this characteristic in determining sex differences in disease and therapeutic efficacy and underscore the importance of studying females in medical and pharmacological research.
Biased Signaling at seven-transmembrane receptors
Understanding of how seven-transmembrane receptors (7-TMR) function has undergone an evolution over the last decade and transformed approaches to drug development. Rather than the simple model in which ligand binding initiates a cascade of reactions that is determined by receptor coupling to specific guanine nucleotide binding proteins (G proteins), it is now recognized that 7-TMRs can associate with multiple G proteins. Moreover, evidence for the association of 7-TMRs with β-arrestin adaptor proteins that scaffold the receptor to other signaling pathways allows for the engagement of diverse patterns of G-protein-independent signaling [1]. Complementary to these discoveries was the demonstration of ligand bias, whereby the binding of specific ligands can direct a selective subset of reactions within the broad network of possible receptor-mediated reactions [2]. This engenders diversity in the consequences of ligand–receptor interactions that can be utilized to design more targeted therapeutics lacking adverse effects.
The focus of studies upon which this revolution has been based is primarily pharmacologic in nature, in that most studies of biased agonists involve synthetic compounds. However, there are a few examples of endogenous ligands that direct receptor activity in a biased manner including endogenous ligands for chemokine receptors, metabotropic glutamate 1a receptors, μ-opiate receptors (MOR) and the β2 adrenergic receptors [3–6]. A related idea that has broad physiological and therapeutic implications is that receptor signaling initiated by the same endogenous ligand may be biased towards a set of pathways depending on the physiological state, condition or stage of development. For example, β-adrenergic receptor (βAR) signaling differs in the fetus and neonate compared to the adult, as a result of differences in the types of adenylyl cyclase isoforms and ratio of stimulatory to inhibitory G proteins [7]. Recently, evidence has emerged demonstrating that sex can determine the state of coupling of G proteins and β–arrestin to the receptor for the stress-related peptide, corticotropin-releasing factor (CRF) [8]. Notably, these sex differences at the molecular level translate to functional differences in neuronal responses to CRF that could account for the increased vulnerability of females to stress-related disorders [8, 9]. Given the diverse stress-related disorders that are more prevalent in females, sex biased CRF receptor signaling has broad clinical implications. Here we introduce CRF as a neuropeptide that orchestrates the stress response and review current knowledge of CRF receptor signaling. Convergent evidence for sex differences in CRF receptor association with Gs and β-arrestin is reviewed. How sex differences in CRF receptor signaling could contribute to stress-related pathology is discussed in light of studies using genetic models of the excessive CRF that is thought to occur in stress-related diseases. Finally, evidence for sex biased signaling by other 7-TMRs that underscores the broader influence of this phenomenon is described with therapeutic implications.
CRF, stress and disease
Sex differences in disease prevalence are reported for many diseases but are particularly apparent for stress-related psychiatric and medical diseases, including anxiety, depression, post-traumatic stress disorder (PTSD), irritable bowel syndrome (IBS), inflammatory disorders and metabolic syndrome, many of which are nearly two times more prevalent in females (see http://www.hcp.med.harvard.edu/ncs/ftpdir/NCS-R_Lifetime_Prevalence_Estimates.pdf) [10, 11]. Certain disorders that have been associated with stress, such as drug abuse are reported to be more prevalent in males (see http://www.hcp.med.harvard.edu/ncs/ftpdir/NCS-R_Lifetime_Prevalence_Estimates.pdf). However, this may be confounded by the decreased exposure of women to drugs because when women are exposed to drugs they develop addiction faster than men [12]. Although there are caveats of population-based studies of disease prevalence, sex differences in the expression of diverse stress-related pathology emphasize the importance of studying stress response circuitry and mediators in females.
The 41-amino acid neuropeptide, CRF, stands out as an orchestrator of the stress response. Stressors trigger CRF release from neurons in the paraventricular hypothalamic nucleus that project to the median eminence where CRF enters the portal circulation and can contact the anterior pituitary corticotrophs and intitiate adrenocorticotropic hormone (ACTH) secretion [13]. This neurohormone action of CRF begins the endocrine cascade that elicits adrenal corticosteroid release and is considered to be a hallmark of stress. Although this is a general response to all stressors, the circuitry underlying activation of CRF neurons of the paraventricular hypothalamic nucleus is stressor specific [14]. In addition to engaging this neuroendocrine circuit, stressors activate CRF cell bodies in limbic and autonomic-related brain regions that project to monoamine nuclei and other brain regions. Within these circuits CRF serves as a brain neurotransmitter that is poised to regulate autonomic, behavioral and cognitive limbs of the stress response [15]. The complementary neurohormone and neurotransmitter actions of CRF in brain can act in concert to coordinate a multicomponent response to stressors [16]. This compelling concept of CRF as an orchestrator of many limbs of the stress response is supported by evidence for stress-mediated CRF release, electrophysiological and behavioral effects using pharmacological tools as well as genetic models [16].
By directing processes that are integral to surviving life-threatening challenges, CRF is of obvious high biological significance. However, because maladaptive stress responses have been linked to disease, this function also implicates CRF in diseases ranging from metabolic disorders to depression many of which have overlapping co-morbid features indicative of common underlying pathophysiology [17]. To this end, inappropriate CRF release or excessive CRF that is not counterbalanced is thought to be a pathophysiological factor in many of the stress-related diseases that are more prevalent in females including PTSD, depression, IBS and metabolic syndrome [17–20]. One potential mechanism linking sex differences in these diseases to excessive CRF is through direct regulation of the CRF gene by estradiol. The CRF promotor contains half-palindromic estrogen response elements and cyclic AMP response elements that are thought to mediate estradiol increases in CRF expression [21]. The effect of estrogen on the CRF promotor is an example of a sex difference at a presynaptic level that can result in excessive CRF in females with ensuing pathological consequences. More recently, sex differences on the postsynaptic side of the CRF synapse have been described that can account for sex differences in the cellular, behavioral and pathological consequences of stress [8].
CRF1 signaling
CRF exerts its effects through two receptor subtypes, CRF1 and CRF2. Genes for CRF1 and CRF2 have been cloned [22, 23] and their distinct distribution, pharmacological specificity, signaling and trafficking have been described (see for review [16]. This review focuses on CRF1, the receptor subtype that is the most prominent in brain and that is thought to mediate most aspects of the stress response including ACTH release, arousal and anxiogenic effects. Notably, the evidence for sex biases in CRF receptor signaling are based on studies of CRF1 described below. Reviews of CRF2 structure, signaling and function can be found in [24].
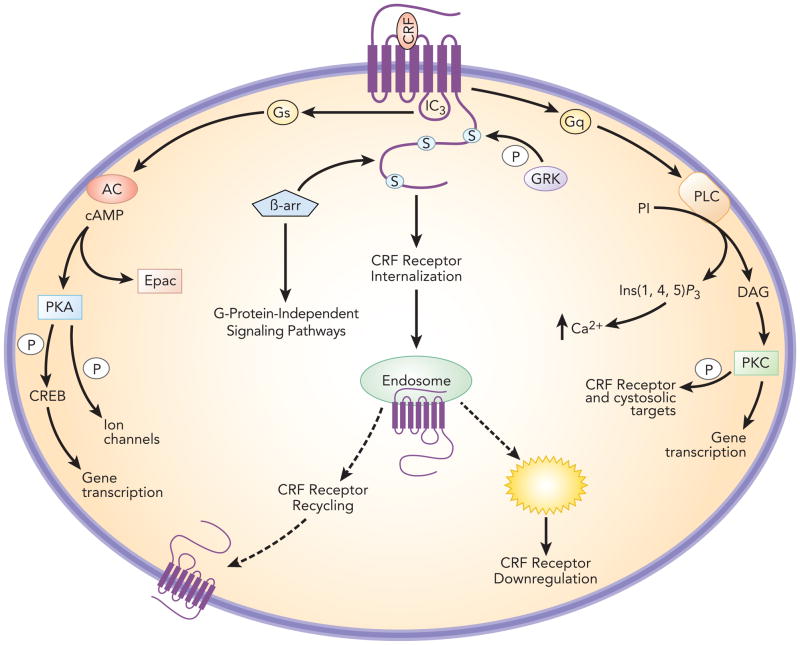
CRF1 is a Class B G-protein coupled receptor (GPCR). In the brain, the primary mode of CRF1 signal transduction is through Gsα, which binds to the third intracellular loop [24] (Figure 1). This activates adenylyl cyclase with consequent formation of cyclic AMP and activation of protein kinase A (PKA). Cellular activities associated with CRF-related PKA activation include regulation of neuronal activity, calcium mobilization [25], gene transcription, and dendritic growth [26]. For example, CRF activation of PKA phosphorylates ion channels that determine neuronal excitability and calcium release [27, 28]. Phosphorylation of cyclic AMP response element binding protein (CREB) by PKA regulates gene transcription [29]. Independent of PKA, cyclic AMP activates Epac, a guanine nucleotide exchange factor that engages extracellular signal-regulated kinase-mitogen activated protein kinase (ERK-MAPK) pathways and promotes intracellular calcium mobilization [30]. CRF1 can also couple to Gq and activate phospholipase C to promote calcium mobilization via inositol triphosphate and protein kinase C (PKC) activation through diacylglycerol [24]. Although CRF1-Gq signaling has been mostly described in peripheral cells or cell lines, recent examples of PKC-dependent CRF1-mediated neuronal effects have been reported, including activation of ventral tegmental dopamine neurons [31] and induction of climbing fiber long-term depression [32].
Figure 1.
Schematic depicting signal transduction pathways associated with CRF1. The primary mode of signaling in brain is through CRF1 coupling to Gsα, activation of adenylyl cyclase and activation of protein kinase A (PKA). CRF1 coupling to Gq and engagement of pathways linked to activation of phospholipase C has also been reported. CRF1 is phosphorylated on the carboxy tail by G-protein receptor kinases (GRK), which promote recruitment of β-arrestin 2. The association of CRF1 with β-arrestin 2 promotes internalization into endosomes where it can be recycled back to the plasma membrane or degraded by lysosomes resulting in receptor downregulation. β-arrestin 2 can also serve to link CRF1 to G-protein-independent signaling.
CRF1 internalization has been demonstrated and the underlying molecular mechanisms characterized in cells [33, 34]. Following agonist binding, CRF1 is sequentially phosphorylated on its carboxyl tail and third intracellular loop by G protein receptor kinases (GRKs). This promotes the recruitment and binding of β–arrestin 2, which uncouples and sterically hinders Gs binding and promotes internalization by a dynamin-dependent process. Agonist- and stress-induced CRF1 internalization in vivo has been demonstrated in male rat locus coeruleus (LC) neurons [35, 36]. CRF1 internalization into early endosomes in LC dendrites is apparent 5 min after CRF microinfusion into the LC and this becomes more pronounced by 30 min after injection [35]. Notably, the neuronal response to CRF single infusion outlasts this time period. However, a subsequent CRF infusion even administered 24 h later is ineffective, providing evidence for downregulation [37]. Acute stress also initiates CRF1 internalization into male rat LC dendrites that is apparent 1 h and 24 h after the stress, and this is completely prevented by pretreatment with a selective CRF1 antagonist [36]. CRF1 is associated with early endosomes at early and later timepoints. However, by 24 h after swim stress substantially more CRF1 is associated with multivesicular bodies, indicative of receptor degradation and downregulation. CRF1 downregulation at this time is consistent with a decreased maximum response of the CRF dose-response curve for LC neuronal activation seen in male rats 24 h following swim stress (see below) [9]. In addition to promoting receptor internalization β-arrestin 2 acts as a scaffold to promote receptor association with G-protein-independent signaling pathways including, Src, Akt, ERK and Rho, thereby allowing CRF to regulate a wider range of cellular processes (see below).
Sex differences in CRF1 neuronal responses
A target of CRF neurotransmission in the brain is the pontine nucleus LC [38], which is the major source of norepinephrine in brain. CRF containing axon terminals synapse with LC dendrites, and CRF microinfused directly onto LC neurons in vivo or in vitro increases discharge activity of the cells by inhibiting potassium currents [39–41]. CRF-induced activation of the LC-norepinephrine system during stress is thought to be important for initiating arousal and promoting cognitive flexibility [38, 39, 42]. However, inappropriate or persistent activation of this system would be expressed as hyperarousal, sleep disturbances and inability to concentrate, symptoms that characterize many stress-related disorders. Although LC spontaneous discharge rates and responses to sensory stimuli are comparable between male and female rats, sensitivity to CRF is markedly different [9]. LC neurons of female rats are more sensitive to CRF as indicated by a shift to the left of the CRF dose-response curve for LC activation in female rats compared to males. Notably, these sex differences are unrelated to adult hormonal status and are observed whether males and females are gonadaly intact or gonadectomized. The increased sensitivity of female LC neurons to exogenously administered CRF translates to an enhanced response of LC neurons to stressors [9].
In addition to the sex difference in neuronal sensitivity of unstressed rats to CRF, sex determines how stress history regulates the subsequent effect of CRF on LC neuronal responses [9]. For example, in male rats that have been exposed to shock or swim stress the CRF dose-response curve for LC activation shifts to the left and the maximal magnitude of activation decreases with the net effect that the neurons are more sensitive to low doses of CRF and less sensitive to higher doses [9, 43]. By contrast, in female rats, the CRF dose-response curve for LC activation is unaffected by stress history. These sex differences in LC sensitivity to CRF and in the regulation of LC responses to CRF by a history of stress suggest differences in CRF receptor signaling [9].
Sex differences in CRF1-Gs-dependent signaling
CRF activation of LC neurons is differentially attenuated by the PKA antagonist, Rp-cAMP-S, which almost completely blocks the effect in females, while producing only a partial attenuation in male rats, consistent with differential CRF1 signaling [8]. Confirmatory evidence for sex differences in CRF1 signaling was derived from immunoprecipitation of CRF1 from rat cortex, a tissue of high CRF1 expression and lacking CRF2 [44]. Immunoprecipitated CRF1 from female rat cortex co-immunoprecipitated three times more Gs compared to male rat cortex, and similar to the electrophysiological differences, these effects were unrelated to adult female hormonal status [8]. In rats with a history of swim stress, CRF1-Gs association increased in males to a magnitude that matched that of unstressed females, but the same stress history had no effect in females. Thus, sex differences in LC sensitivity to CRF were mirrored by sex differences in CRF1-Gs coupling, implicating this as a molecular mechanism underlying sex differences in physiological responses.
Sex differences in CRF1 receptor trafficking
The initial descriptions of stress-induced CRF1 internalization in vivo were based on studies of LC neurons from male rats and are consistent with the observation of a decreased maximum response in the CRF dose-response curve for LC activation observed at the same time following stress [36]. In contrast to males, swim stress does not promote CRF1 internalization in LC neurons of female rats or decrease the CRF maximal response [8]. The cellular localization of CRF1 is remarkably opposite in male and female LC neurons. In unstressed male rats, CRF1 is roughly evenly distributed between the plasma membrane and cytoplasm. Swim stress shifts this distribution so that approximately 70% of CRF1 is cytoplasmic, indicative of internalization. In females, the pattern of CRF1 localization is opposite, with a predominantly cytoplasmic localization in the unstressed state and a shift towards a more even distribution between cytoplasmic and plasma membrane compartments.
Sex differences in stress-induced CRF1 trafficking can be attributed to differences in stress-induced CRF1-β-arrestin 2 association because this is a critical molecular step in the process of CRF1 internalization. Following swim stress, CRF1 association with β-arrestin 2 greatly increased in males, consistent with stress-induced CRF1 internalization [8]. However, in females stress CRF1-β-arrestin 2 association remained low. As for other measures, the differences in β-arrestin 2 association were unrelated to adult hormonal status. The deficit in β-arrestin 2 association with CRF1 in females relative to males can account for the compromised ability to internalize CRF1 and to decrease the maximal effect of CRF following stress in females.
Given that the acute response to a stressor is adaptive and critical for survival, the enhanced response in females may provide an evolutionary advantage. For example, by increasing activity in LC projections to the medial prefrontal cortex, cognitive flexibility is enhanced and this would improve chances for survival in a dynamic, life-threatening environment [42]. It is when the stress response becomes dysfunctional, when it is elicited and/or persists in the absence of a stressor, that sex differences in CRF1 signaling render females more vulnerable to stress-related pathology. A dysfunctional stress response has been attributed to increased expression and/or activity of the CRF system.
Consequences of sex differences in CRF1 signaling in conditions of CRF overexpression
Increased CRF1-Gs coupling together with decreased CRF1 internalization would render female neurons more sensitive to CRF and less able to adapt to excessive CRF (Figure 2). This is clinically relevant because excessive CRF has been implicated in many stress-related disorders that are more prevalent in females [18–20]. The pathological condition of excessive CRF has been modeled using CRF overexpressing mice (CRF-OE) [45, 46]. A well-characterized CRF-OE model is a transgenic line in which CRF expression is under control of the metallothionein promoter [45]. Unlike certain conditional CRF-OE models, these mice have elevated CRF expression in brain neurons in most regions that typically express CRF [47]. These mice exhibit evidence of hypothalamic-pituitary-adrenal axis overactivity, including adrenal hypertrophy and elevated plasma adrenocorticotropin and corticosterone [45]. Additionally, these mice also show anxiogenic effects in many animal tests of anxiety-related behavior [48].
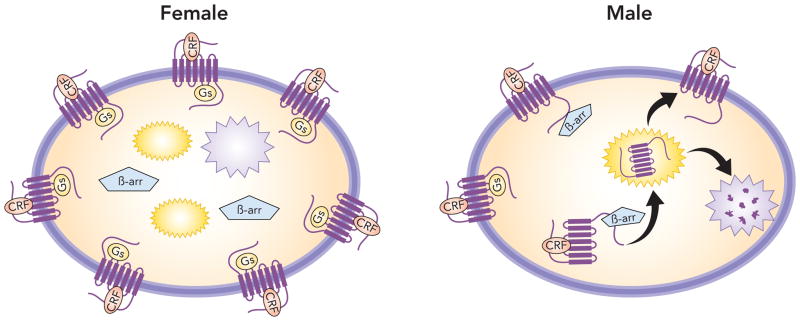
Figure 2.
Sex differences in CRF trafficking. The schematic depicts differential CRF1 trafficking in conditions of excess CRF. In females because β-arrestin 2 binding to CRF1 is compromised, more CRF1 is on the plasma membrane and is free to couple to Gs. In males because β-arrestin 2 associates with CRF1 the receptor internalizes into early endosomes (yellow structures) and can be recycled back to the plasma membrane or be degraded by lysosomes (purple structures) so that less receptor is on the plasma membrane (downregulation). β-arrestin 2 hinders the association of Gs to CRF1.
The LC of both male and female CRF-OE mice shows a comparable increase in CRF innervation compared to wildtype mice [47]. Supporting the idea that conditions of excessive CRF like those seen in stress-related diseases would selectively impact neurons of females, LC neuronal disc6harge rates were three times higher in female CRF-OE mice compared to male CRF-OE mice or male or female wild-type mice [47]. In contrast, the CRF-OE condition does not affect LC neuronal activity of male mice. The differential impact of CRF overexpression could be attributed to differential cellular localization of CRF1 (Figure 2). For male CRF-OE mice, CRF1 had a predominant cytoplasmic localization compared to wild-type male mice, which exhibited a roughly equivalent cellular distribution of CRF1 on the plasma membrane and within the cytoplasm. This internalization could serve to protect neurons of male CRF-OE mice from excess CRF. By contrast, CRF1 was predominately localized to the plasma membrane of female CRF-OE mice where it would be available to be activated by excessive levels of CRF (Figure 2). The inability of neurons in female CRF-OE mice to internalize CRF1, an effect that may be in part attributed to a defect in β-arrestin 2 association, results in an overactivated LC-norepinephrine system. Because overactivation of the LC-NE system translates to the hyperarousal symptoms that define many stress-related psychiatric disorders including PTSD, depression, anxiety and IBS [49], this can account for the increased prevalence in females.
Sex Biased CRF Signaling
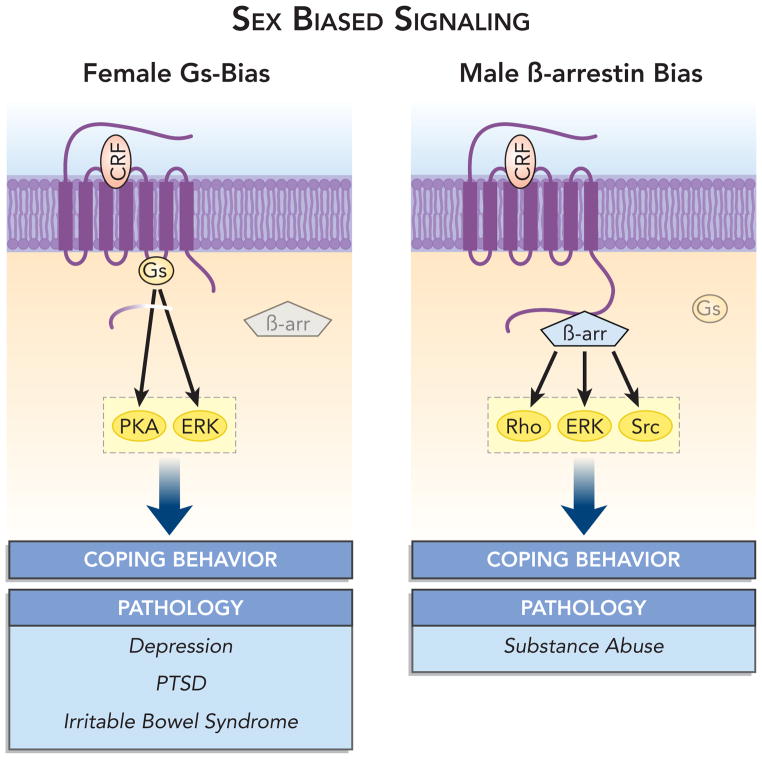
In addition to enabling receptor internalization, it is now well recognized that β-arrestin 2 can engage G-protein-independent signaling cascades by scaffolding receptors to signaling molecules [1]. Given this function, the implications of sex differences in CRF1-β-arrestin association are much broader than can be attributed to differences in CRF1 internalization alone. β-arrestin 2 signaling includes mitogen-activated protein kinase (e.g., ERK2, JNK3, and p38), tyrosine kinases (e.g., c-SRC), AKT, PI3 kinase and RhoA (for review see,[2, 50]). A compromised ability of female CRF1 receptors to associate with β-arrestin 2 would bias CRF1 signaling towards Gs-related pathways and PKA-dependent processes. In contrast, in male neurons β-arrestin 2 G-protein independent signaling would be favored. By engaging sex specific signaling pathways, CRF released during stress would have sex-specific cellular consequences that could translate to distinct physiological and/or behavioral responses (Figure 3). This could account for sexually distinct stress-coping styles. However, differences would be most pronounced when CRF is in excess as has been proposed to occur in stress-related psychiatric disorders and is modeled by CRF-OE mice. Under these conditions, sex differences in CRF signaling could be expressed as differences in the expression of stress-related pathology.
Figure 3.
Sex biased CRF1 signaling. In females, the decreased ability of CRF1 to associate with β-arrestin2 biases signaling through Gs-related pathways. In contrast, in males CRF1 receptor can associate with β-arrestin2, which results in a relative bias towards β-arrestin 2-related pathways. Because of the sex difference in association of CRF1 with these molecules, the interaction of CRF with its receptor can produce sex specific cellular responses that can translate to different physiological and behavioral coping responses and different pathology. The specific pathologies shown have not been directly linked to the cellular pathways but are listed only as stress-related pathologies that have been reported to be more prevalent in one sex than the other.
To date it is not known which, if any, β-arrestin 2-related pathways are engaged by CRF1. Some CRF1-mediated effects require Rho signaling that has been associated with β-arrestin 2 [26]. Notably, in HEK 293 cells, CRF1 activation of ERK/MAPK signaling requires CRF1 internalization, consistent with a β-arrestin 2 mechanism [51]. Because Gs-protein and β-arrestin 2 signaling regulate phosphorylation dynamics in cells, excessive CRF would be predicted to give rise to sexually differentiated phosphoprotein profiles and sex differences in these profiles may explain differences in the expression of stress-related pathology. Preliminary results of a deep phosphoproteomic analysis of cortex of male and female CRF overexpressing (CRF-OE) mice using stable isotope labeling of whole mouse and high resolution mass spectrometry are currently identifying sex differences between in phosphoprotein patterns in cortex elicited by conditions of excessive CRF [52]. These studies are confirming the model of sex biased signaling and providing a discovery platform for new targets for the treatment of stress-related diseases.
An important consideration in predicting the consequences of sex biased CRF1 signaling is the ability to generalize to all CRF1 expressing cells. As for other 7-TMRs, cell type specificity of CRF1 signaling has been described and interpretations must consider that sex biases may be present in one region and not another, resulting in both distinct and shared consequences of stress in males and females. Nonetheless, the finding that receptor immunoprecipitation studies in cortex were consistent with trafficking and physiological responses in the LC argues for some degree of regional generalization. Evidence that female rats are more vulnerable to CRF-induced reinstatement of cocaine seeking behavior, an effect thought to be mediated by CRF1 receptors in the bed nucleus of the stria terminalis also supports a generalization of sex biased CRF1 signaling to other regions [53].
Sex biased signaling of other receptors
Given the shared characteristics of different GPCRs, sex biased signaling would be predicted to be a property of other GPCRs. Although this has not yet been systematically studied for receptors other than CRF1, evidence for differential signaling in males and females exists for several GPCRs and in some cases there is evidence for differential coupling of GPCRs to G-proteins, as has been demonstrated for CRF1. For example, sex differences in βAR-Gs coupling have been demonstrated in rat hepatocytes, with increased coupling in hepatocytes isolated from females. This sex difference was functionally relevant because it resulted in a 3-fold greater glycogenolysis response to βAR agonists in females compared to males. This was attributed to increased βAR-Gs coupling because there was no difference in βAR affinity or number and responses to forskolin or GTP-γ-S were comparable between male and female hepatocytes [54]. Sex differences in μ-opioid receptor (MOR) coupling to G-proteins have been suggested to play a role in differential adaptation to opiate withdrawal in the spinal cord [55]. Here the release of the potent endogeous opioid, endomorphin 2, is regulated by MOR, which inhibits release through a Gi-protein coupled mechanism. Withdrawal of MOR agonists after chronic administration shifts MOR coupling towards the stimulatory Gs protein in males and this is accompanied by enhanced endomorphin 2 release that can mitigate withdrawal signs in males. This switch in MOR coupling from Gi to Gs during withdrawal does not occur in females. The lack of this adaptive effect in females could result in hyperalgesia during withdrawal as well as an increased severity of other withdrawal signs depending on the generalization of the adaptation. Notably, the sex difference in MOR coupling suggests sex specific treatments for acute opiate withdrawal. For example, pharmacological elevation or administration of endomorphin like compounds may be effective in females.
Although to date examples of sex differences in receptor coupling are limited, there are many examples of sex differences in levels or activity of receptor signaling molecules. For example, sex differences in ERK and AKT regulation by neonatal ventral hippocampal lesion, a model of schizophrenia, suggest mechanisms whereby differential dopamine signaling may underlie sex differences in vulnerability to schizophrenia [56]. Sex differences in behavioral responses to cocaine have been attributed to differences in the PKA and the DARPP-32 cascade in rat nucleus accumbens [57, 58]. Sex differences in phosphorylation of cannabinoid receptors that could lead to differential receptor trafficking have been demonstrated and implicated in sex differences in stress sensitivity [59]. In peripheral tissue, aortic contraction mediated by serotonin 2A receptors is greater in males compared to females as a result of increased RhoA and Rho-kinase activity, in the absence of increased expression [60]. Given that the concept has been relatively unexplored, these few examples of evidence of sex differences in signaling of prominent neurotransmitter receptors underscore the potential impact that this could have in determining resilience/vulnerability to disease as well as to transform therapeutics.
Clinical and therapeutic implications of sex biased receptor signaling
Sex biased receptor coupling and signaling has important ramifications for understanding disease and developing therapeutics. Focusing on the CRF system alone, it implies that the cellular reactions initiated by stressors will differ to some extent in males and females and this could account for a different expression of stress-related pathology. Given the many and diverse diseases that have been linked to stress, elucidating how differences in CRF1 signal transduction translate to different pathological consequences will have a broad clinical impact. The phenomenon underscores the importance of including both males and females in research of stress-related diseases. Evidence that sex biased signaling extends to other receptor systems such as the βAR and MOR emphasizes the importance of studying females in general biomedical research.
The therapeutic implications of sex biased receptor signaling are perhaps more relevant because most drugs target GPCRs. The development of biased agonists that differentially recruit β-arrestin or G-protein signaling is refining therapeutics to provide drugs with increased selectivity and fewer side effects. If the sex differences in β-arrestin2 recruitment demonstrated for CRF1 generalize to other GPCRs, this strategy may be sex specific for certain drugs. Sex differences in receptor coupling imply structural differences, perhaps through post-translational modifications so that just as endogenous agonists may have different effects so will drugs designed to manipulate receptor function. This makes it imperative to design drugs taking the potential for sex differences into consideration, particularly if the drugs are being designed to treat a disease that is more prevalent in one sex.
Concluding remarks
This review integrates convergent findings supporting the novel concept of sex differences in receptor signaling and trafficking, using CRF1 as a model. Sex differences in Gs coupling would confer differences in agonist sensitivity and in the case of CRF, differences in acute responses to stressors. Differences in receptor association with β-arrestin influence receptor trafficking and the ability to adapt to the excessive CRF that is predicted to be present in diseases related to severe or chronic stress. For females this would translate to an enhanced sensitivity to acute stress and decreased ability to adapt to chronic or repeated stress. Given evidence for β-arrestin 2 signaling that is independent of Gs signaling, the broader implication of this model is that stressors may initiate different cellular reactions in males and females and this may be a basis of sex differences in coping responses and/or pathology elicited by stress. Identifying specific consequences of sex biased CRF1 signaling has the potential to reveal the molecular basis for sex disparities in stress-related disease and this knowledge can further be used to elucidate pathophysiology.
Evidence suggests that sex biased signaling generalizes to other GPCRs. Future systematic studies of sex bias in other receptor models could provide the knowledge to transform approaches to diagnosing and treating the many diseases that exhibit sex differences.
Highlights.
Receptor signaling can be sex biased such that different pathways are engaged in males and females.
The CRF receptor is an example of a receptor that exhibits sex biased signaling.
Sex biased signaling may underlie sex differences in disease vulnerability and drug sensitivity.
Sex biased signaling underscores the importance of including both sexes in biomedical research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Rita J. Valentino, The Children’s Hospital of Philadelphia, Philadelphia, PA 19104
Elisabeth Van Bockstaele, Thomas Jefferson University, Philadelphia, PA.
Debra Bangasser, Temple University, Philadelphia, PA 19122.
References
- 1.Shenoy SK, Lefkowitz RJ. Seven-transmembrane receptor signaling through beta-arrestin. Sci STKE. 2005;2005:cm10. doi: 10.1126/stke.2005/308/cm10. [DOI] [PubMed] [Google Scholar]
- 2.Violin JD, Lefkowitz RJ. Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci. 2007;28:416–422. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Reiner S, et al. Differential signaling of the endogenous agonists at the beta2-adrenergic receptor. J Biol Chem. 2010;285:36188–36198. doi: 10.1074/jbc.M110.175604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emery AC, et al. Ligand bias at metabotropic glutamate 1a receptors: molecular determinants that distinguish beta-arrestin-mediated from G protein-mediated signaling. Mol Pharmacol. 2012;82:291–301. doi: 10.1124/mol.112.078444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rivero G, et al. Endomorphin-2: a biased agonist at the mu-opioid receptor. Mol Pharmacol. 2012;82:178–188. doi: 10.1124/mol.112.078659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zidar DA. Endogenous ligand bias by chemokines: implications at the front lines of infection and leukocyte trafficking. Endocr Metab Immune Disord Drug Targets. 2011;11:120–131. doi: 10.2174/187153011795564160. [DOI] [PubMed] [Google Scholar]
- 7.Slotkin TA, et al. Ontogenesis of beta-adrenoceptor signaling: implications for perinatal physiology and for fetal effects of tocolytic drugs. J Pharmacol Exp Ther. 2003;306:1–7. doi: 10.1124/jpet.102.048421. [DOI] [PubMed] [Google Scholar]
- 8.Bangasser DA, et al. Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry. 2010;15:877, 896–904. doi: 10.1038/mp.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curtis AL, et al. Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology. 2006;31:544–554. doi: 10.1038/sj.npp.1300875. [DOI] [PubMed] [Google Scholar]
- 10.Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58:15–25. doi: 10.1002/art.23177. [DOI] [PubMed] [Google Scholar]
- 11.Lin JD, et al. Gender differences in the prevalence of metabolic syndrome and its components among adults with disabilities based on a community health check up data. Res Dev Disabil. 2013;34:516–520. doi: 10.1016/j.ridd.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Becker JB, et al. Sex differences in the neural mechanisms mediating addiction: a new synthesis and hypothesis. Biol Sex Differ. 2012;3:14. doi: 10.1186/2042-6410-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vale W, et al. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- 14.Li HY, et al. Distinct mechanisms underlie activation of hypothalamic neurosecretory neurons and their medullary catecholaminergic afferents in categorically different stress paradigms. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:2359–2364. doi: 10.1073/pnas.93.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray TS. Amygdaloid CRF pathways. Role in autonomic, neuroendocrine, and behavioral responses to stress. Ann N Y Acad Sci. 1993;697:53–60. doi: 10.1111/j.1749-6632.1993.tb49922.x. [DOI] [PubMed] [Google Scholar]
- 16.Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 17.Chrousos GP. Stress, chronic inflammation, and emotional and physical well-being: concurrent effects and chronic sequelae. J Allergy Clin Immunol. 2000;106:S275–S291. doi: 10.1067/mai.2000.110163. [DOI] [PubMed] [Google Scholar]
- 18.Bremner JD, et al. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry. 1997;154:624–629. doi: 10.1176/ajp.154.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larauche M, et al. Stress and visceral pain: from animal models to clinical therapies. Exp Neurol. 2012;233:49–67. doi: 10.1016/j.expneurol.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nemeroff C, et al. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226:1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- 21.Vamvakopoulos NC, Chrousos GP. Evidence of direct estrogenic regulation of human corticotropin-releasing hormone gene expression. Potential implications for the sexual dimorphism of the stress response and immune/inflammatory reaction. J Clin Invest. 1993;92:1896–1902. doi: 10.1172/JCI116782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lovenberg TW, et al. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci (USA) 1995;92:836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perrin MH, et al. Comparison of an agonist, urocortin, and an antagonist, astressin, as radioligands for characterization of corticotropin-releasing factor receptors. J Pharmacol Exp Ther. 1999;288:729–734. [PubMed] [Google Scholar]
- 24.Hauger RL, et al. Role of CRF receptor signaling in stress vulnerability, anxiety, and depression. Ann N Y Acad Sci. 2009;1179:120–143. doi: 10.1111/j.1749-6632.2009.05011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dermitzaki I, et al. Roles of protein kinase A (PKA) and PKC on corticotropin-releasing hormone (CRH)-induced elevation of cytosolic calcium from extra-and intra-cellular sources. Hormones (Athens) 2004;3:252–258. doi: 10.14310/horm.2002.11134. [DOI] [PubMed] [Google Scholar]
- 26.Swinny JD, Valentino RJ. Corticotropin-releasing factor promotes growth of brain norepinephrine neuronal processes through Rho GTPase regulators of the actin cytoskeleton in rat. Eur J Neurosci. 2006;24:2481–2490. doi: 10.1111/j.1460-9568.2006.05129.x. [DOI] [PubMed] [Google Scholar]
- 27.Haug T, Storm JF. Protein kinase A mediates the modulation of the slow Ca(2+)-dependent K(+) current, I(sAHP), by the neuropeptides CRF, VIP, and CGRP in hippocampal pyramidal neurons. J Neurophysiol. 2000;83:2071–2079. doi: 10.1152/jn.2000.83.4.2071. [DOI] [PubMed] [Google Scholar]
- 28.Lee AK, Tse A. Mechanism underlying corticotropin-releasing hormone (CRH) triggered cytosolic Ca2+ rise in identified rat corticotrophs. J Physiol. 1997;504 (Pt 2):367–378. doi: 10.1111/j.1469-7793.1997.367be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bayatti N, et al. Corticotropin-releasing hormone-mediated induction of intracellular signaling pathways and brain-derived neurotrophic factor expression is inhibited by the activation of the endocannabinoid system. Endocrinology. 2005;146:1205–1213. doi: 10.1210/en.2004-1154. [DOI] [PubMed] [Google Scholar]
- 30.Traver S, et al. The phenotypic differentiation of locus ceruleus noradrenergic neurons mediated by brain-derived neurotrophic factor is enhanced by corticotropin releasing factor through the activation of a cAMP-dependent signaling pathway. Mol Pharmacol. 2006;70:30–40. doi: 10.1124/mol.106.022715. [DOI] [PubMed] [Google Scholar]
- 31.Wanat MJ, et al. Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J Physiol. 2008;586:2157–2170. doi: 10.1113/jphysiol.2007.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmolesky MT, et al. The neuropeptide corticotropin-releasing factor regulates excitatory transmission and plasticity at the climbing fibre-Purkinje cell synapse. Eur J Neurosci. 2007;25:1460–1466. doi: 10.1111/j.1460-9568.2007.05409.x. [DOI] [PubMed] [Google Scholar]
- 33.Holmes KD, et al. Differential regulation of corticotropin releasing factor 1alpha receptor endocytosis and trafficking by beta-arrestins and Rab GTPases. J Neurochem. 2006;96:934–949. doi: 10.1111/j.1471-4159.2005.03603.x. [DOI] [PubMed] [Google Scholar]
- 34.Oakley RH, et al. Carboxyl-terminal and intracellular loop sites for CRF1 receptor phosphorylation and beta-arrestin-2 recruitment: a mechanism regulating stress and anxiety responses. Am J Physiol Regul Integr Comp Physiol. 2007;293:R209–222. doi: 10.1152/ajpregu.00099.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reyes BA, et al. Agonist-induced internalization of corticotropin-releasing factor receptors in noradrenergic neurons of the rat locus coeruleus. Eur J Neurosci. 2006;23:2991–2998. doi: 10.1111/j.1460-9568.2006.04820.x. [DOI] [PubMed] [Google Scholar]
- 36.Reyes BA, et al. Stress-induced intracellular trafficking of corticotropin-releasing factor receptors in rat locus coeruleus neurons. Endocrinology. 2008;149:122–130. doi: 10.1210/en.2007-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conti LH, Foote SL. Effects of pretreatment with corticotropin-releasing factor on the electrophysiological responsivity of the locus coeruleus to subsequent corticotropin-releasing factor challenge. Neuroscience. 1995;69:209–219. doi: 10.1016/0306-4522(95)00222-5. [DOI] [PubMed] [Google Scholar]
- 38.Valentino RJ, Van Bockstaele E. Convergent regulation of locus coeruleus activity as an adaptive response to stress. Eur J Pharmacol. 2008;583:194–203. doi: 10.1016/j.ejphar.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Curtis AL, et al. Activation of the locus coeruleus noradrenergic system by intracoerulear microinfusion of corticotropin-releasing factor: effects on discharge rate, cortical norepinephrine levels and cortical electroencephalographic activity. J Pharmacol Exp Ther. 1997;281:163–172. [PubMed] [Google Scholar]
- 40.Jedema HP, Grace AA. Corticotropin-releasing hormone directly activates noradrenergic neurons of the locus ceruleus recorded in vitro. J Neurosci. 2004;24:9703–9713. doi: 10.1523/JNEUROSCI.2830-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Bockstaele EJ, et al. Corticotropin-releasing factor-containing axon terminals synapse onto catecholamine dendrites and may presynaptically modulate other afferents in the rostral pole of the nucleus locus coeruleus in the rat brain. J Comp Neurol. 1996;364:523–534. doi: 10.1002/(SICI)1096-9861(19960115)364:3<523::AID-CNE10>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 42.Snyder K, et al. Corticotropin-releasing factor in the norepinephrine nucleus, locus coeruleus, facilitates behavioral flexibility. Neuropsychopharmacology. 2012;37:520–530. doi: 10.1038/npp.2011.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Curtis AL, et al. Previous stress alters corticotropin-releasing factor neurotransmission in the locus coeruleus. Neuroscience. 1995;65:541–550. doi: 10.1016/0306-4522(94)00496-r. [DOI] [PubMed] [Google Scholar]
- 44.Van Pett K, et al. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 45.Stenzel-Poore MP, et al. Overproduction of corticotropin-releasing factor in transgenic mice: a genetic model of anxiogenic behavior. J Neurosci. 1994;14:2579–2584. doi: 10.1523/JNEUROSCI.14-05-02579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu A, et al. Conditional mouse mutants highlight mechanisms of corticotropin-releasing hormone effects on stress-coping behavior. Mol Psychiatry. 2008;13:1028–1042. doi: 10.1038/mp.2008.51. [DOI] [PubMed] [Google Scholar]
- 47.Bangasser DA, et al. Increased vulnerability of the brain norepinephrine system of females to corticotropin-releasing factor overexpression. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heinrichs SC, et al. Learning impairment in transgenic mice with central overexpression of corticotropin-releasing factor. Neuroscience. 1996;74:303–311. doi: 10.1016/0306-4522(96)00140-6. [DOI] [PubMed] [Google Scholar]
- 49.Wong ML, et al. Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: relation to hypercortisolism and corticotropin-releasing hormone. Proc Natl Acad Sci U S A. 2000;97:325–330. doi: 10.1073/pnas.97.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 51.Punn A, et al. Identification of signaling molecules mediating corticotropin-releasing hormone-R1alpha-mitogen-activated protein kinase (MAPK) interactions: the critical role of phosphatidylinositol 3-kinase in regulating ERK1/2 but not p38 MAPK activation. Mol Endocrinol. 2006;20:3179–3195. doi: 10.1210/me.2006-0255. [DOI] [PubMed] [Google Scholar]
- 52.Valentino RJ. The sex biased phosphoproteome: a nove approach towards understanding the molecular basis for sex differences in neuropsychiatric diseases. Neuropsychopharmacology. 2012;38:S272–S273. [Google Scholar]
- 53.Buffalari DM, et al. Corticotrophin releasing factor (CRF) induced reinstatement of cocaine seeking in male and female rats. Physiol Behav. 2012;105:209–214. doi: 10.1016/j.physbeh.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yagami T, et al. Sex difference in adrenergic receptor-mediated glycogenolysis in rat livers. Jpn J Pharmacol. 1990;54:365–374. doi: 10.1254/jjp.54.365. [DOI] [PubMed] [Google Scholar]
- 55.Chakrabarti S, et al. Pleiotropic opioid regulation of spinal endomorphin 2 release and its adaptations to opioid withdrawal are sexually dimorphic. J Pharmacol Exp Ther. 2012;340:56–63. doi: 10.1124/jpet.111.186874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bychkov E, et al. Sex differences in the activity of signalling pathways and expression of G-protein-coupled receptor kinases in the neonatal ventral hippocampal lesion model of schizophrenia. Int J Neuropsychopharmacol. 2011;14:1–15. doi: 10.1017/S1461145710000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nazarian A, et al. Sex differences in basal and cocaine-induced alterations in PKA and CREB proteins in the nucleus accumbens. Psychopharmacology. 2009;203:641–650. doi: 10.1007/s00213-008-1411-5. [DOI] [PubMed] [Google Scholar]
- 58.Zhou L, et al. Basal and cocaine-induced sex differences in the DARPP-32-mediated signaling pathway. Psychopharmacology. 2009;203:175–183. doi: 10.1007/s00213-008-1388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xing G, et al. Cannabinoid receptor expression and phosphorylation are differentially regulated between male and female cerebellum and brain stem after repeated stress: implication for PTSD and drug abuse. Neurosci Lett. 2011;502:5–9. doi: 10.1016/j.neulet.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 60.Nuno DW, et al. RhoA activation contributes to sex differences in vascular contractions. Arterioscler Thromb Vasc Biol. 2007;27:1934–1940. doi: 10.1161/ATVBAHA.107.144675. [DOI] [PubMed] [Google Scholar]