Abstract
BACKGROUND
Our previous work showed a survival advantage with L-arginine (L-Arg) pretreatment in a swine model of severe hemorrhagic shock. This study was designed to evaluating whether the benefit is sustained when L-Arg is given during resuscitation and whether the mechanism is mediated by enzymatic activation of nitric oxide (NO) synthesis.
METHODS
Adult rats (n =30) underwent 40% blood volume loss and were resuscitated with saline (3×shed blood volume). Animals were divided into five treatment groups of six animals each: (1) Sham, (2) Control (resuscitation alone), (3) L-Arg (300 mg/kg) with resuscitation, (4) L-Arg +L-nitroarginine methyl ester pretreatment, and (5) D-arginine (300 mg/kg) with resuscitation. Animals were observed for 240 minutes postresuscitation or until death. Hemodynamic, metabolic, histologic, and survival outcomes were measured.
RESULTS
Administration of L-Arg after hemorrhage and before resuscitation significantly improved outcomes, relative to the control group. The L-Arg infusion improved terminal arterial pressures, lowered lactate, improved small bowel histologic signs of reperfusion injury, and increased survival (p <0.05). Endpoints of the L-Arg group were similar to the Sham group. The benefits of L-Arg infusion were abolished or attenuated when animals were pretreated with L-nitroarginine methyl ester and potentiated with D-arginine, suggesting a NO-specific mechanism of L-Arg. Finally, severe shock and resuscitation injury significantly elevated circulating asymmetric dimethylarginine levels, which are potent competitive inhibitors of NO synthetase.
CONCLUSION
L-Arg infusion during resuscitation offers a significant functional, metabolic, and survival benefit after severe hemorrhagic shock. The mechanism seems to be by activation of NO synthesis with its attendant benefits to local perfusion and inflammation after global reperfusion.
Keywords: L-Arginine, hemorrhage, shock, nitric oxide, nitric oxide synthetase, L-NAME
Trauma is the leading cause of death in the first four decades of life, and the fifth leading cause overall.1 In the early phase of hospital treatment, most of these patients die, succumb to neurologic injury, or hemorrhage.2 The insult of blood loss results in an inability for the oxygen supply to meet the demands of the body. Trauma and hypovolemia trigger multiple compensatory mechanisms, which include activation of the sympathetic nervous system, cardiac adjustments, hormonal changes, renal volume, and electrolyte alterations that act to preserve oxygenation and tissue blood flow. Some microcirculatory beds are rerouted to sustain flow to essential organs. In the hospital setting, hemorrhage is controlled and trauma-hemorrhage victims are resuscitated with intravenous crystalloid fluids to restore oxygen delivery. Despite these salvage mechanisms, victims may develop irretrievable loss of capillary bed perfusion (the no-reflow phenomenon), immune suppression, and systemic inflammation.2 After resuscitation, there may be ischemia-reperfusion injury that may precipitate further tissue damage, immunosuppression, sepsis, multiple organ failure, and death.3
Injury and blood loss manifest clinically with tachycardia, tachypnea, hypoxia, and hypotension. Persistent hypovolemia causes hypoperfused tissues and cellular hypoxia resulting in increased anaerobic cellular activity and increased levels of lactate.3,4 The endpoints of resuscitation are determined by a combination of clinical, laboratory, and invasive monitoring. Blood pressure, heart rate, and urine output, base deficit, and lactate are all used to monitor the extent of hemorrhagic shock.5
The role of the L-Arginine (L-Arg)-nitric oxide (NO) pathway in the regulation of tissue perfusion and modulation of the inflammatory response continues to evolve. L-Arg is an amino acid that may have antioxidant and immunomodulatory activity.6 NO is a downstream product of L-Arg oxidation formed by NO synthetase (NOS). The enzyme exists in an inducible form, which is activated by immunologic and inflammatory responses and a constitutive form that continuously produces low levels of NO, mainly in the vascular endothelium.7,8 Some studies have suggested that the augmentation of the L-Arg-NO pathway by the infusion of L-Arg can restore the depressed cardiac output and improve tissue hypoperfusion seen after trauma and hemorrhage.9–11 Other studies suggest that augmenting this pathway by providing L-Arg or NO donors may attenuate systemic and regional inflammation and improve outcomes after shock with or without the preceding trauma.12–14 Our previous study demonstrated increased survival of swine receiving L-Arg before lethal hemorrhagic shock, trauma, and resuscitation (unpublished data). The mechanism of these salutary effects of L-Arg have yet to be systematically demonstrated. The major aim of this study was to demonstrate that the beneficial effects seen after L-Arg administration in the setting of hemorrhagic shock are due to metabolism through the NO pathway.
METHODS
Experimental Design
Adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing 250 to 350 g were used in these studies. All animals were allowed food and water ad libitum before the experiment. Animals were randomized into five groups (6 animals per group).
-
Group 1
Animals were sham operated and maintained under anesthesia for the same time period as others.
-
Group 2
Animals were subjected to trauma-hemorrhage (40% blood volume) and resuscitated with normal saline (3×shed blood volume).
-
Group 3
Animals were treated with L-Arg (300 mg/kg, intravenously [i.v.]) in 1.0 mL normal saline immediately before resuscitation. This dose was based on a previous study that demonstrated improved perfusion after trauma-hemorrhage with L-Arg administration.9 The total volume of normal saline for resuscitation included the 1.0 mL vehicle for the L-Arg.
-
Group 4
Identical to group 3, animals were pretreated with the NOS inhibitor L-nitroarginine methyl ester (L-NAME) at 10 mg/kg, i.v. in 0.2 mL saline 10 minutes before L-Arg administration and resuscitation.15
-
Group 5
Animals were treated with D-arginine (D-Arg, 300 mg/kg, i.v.) in normal saline (pH adjusted to 7.4) immediately before resuscitation. This is the biologically inactive enantiomer of L-Arg, regarding NOS metabolism.
All procedures were performed in accordance with and approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University.
Model of Hemorrhage and Resuscitation
Animals were administered isofluorane inhalant (4%) and 96% supplemental oxygen for 2 to 4 minutes to induce general anesthesia. The isofluorane was maintained at a lower level (1%–2%) for the duration of the experiment. A small abdominal incision was made to insert a temperature probe (ADInstuments, Denver, CO). The abdominal incision was closed and secured around the probe. A rectal temperature probe was also inserted and connected by a feedback temperature controller to a heat lamp above the animal. A heating board was also placed beneath the animal to provide supplemental heat. Bilateral femoral cut downs and arterial cannulations were performed with polyethylene-90 catheters. The femoral vein was cannulated on one side with a PE-50 catheter. All catheters were prepared with the anticoagulate triodecylmethylammonium chloride heparin complex to reduce clotting. Occasionally, catheters were flushed with a 0.005% heparinized saline solution. The arterial catheter and the intraabdominal temperature probe were connected to a Powerlab data acquisition system (ADInstruments, Denver, CO) controlled by a notebook computer running Chart v5 (ADInstruments). This allowed for continuous recording of heart rate, blood pressure, and core temperature throughout the experiment. A baseline recording was taken for a period of 10 to 20 minutes. Rats were then bled through one of the arterial catheters, initially at a rate of 1 mL/min. The first 2.2 mL removed were used for a baseline arterial blood gas (0.2 mL), and the remainder (2 mL) was centrifuged at 13,000g for 10 minutes to separate the plasma, which was frozen at −20°C for later analysis. Additional arterial blood gasses were obtained after hemorrhage and every hour after resuscitation for 4 hours. The bleed was controlled to not allow the mean arterial pressure (MAP) to drop below 35 mm Hg and was generally held between 30 to 35 mm Hg during the hemorrhage period. Rats were bled to reduce their blood volume by 40%, which was estimated by the relationship 40% blood volume =0.4 ×65 mL/kg × animal weight (kg). After the total 40% blood volume was removed, hemorrhaging was stopped and an additional 15 minutes elapsed before resuscitation. Sham animals were treated identically to the other animals except they were not bled, resuscitated, or treated. Animals were excluded if they did not survive before resuscitation or if their baseline arterial oxygen saturations were below 400 mm Hg while breathing 95% oxygen. Shed blood was not used for resuscitation, and the animals were not heparinized at any point of the experiment. The total time of resuscitation varied by weight of the animal but was approximately 25 to 30minutes. Animals were observed for 4 hours postresuscitation or until they died. The animal was deemed terminal when the MAP dropped below 30 mm Hg. Animals were killed with anesthetic overdose of isoflurane.
Preparation and Analysis of Histologic Sections
At 4 hours postresuscitation or on the animal’s death, small bowel samples from the distal ileum were obtained and placed in a 10% formalin solution. Tissue was embedded in paraffin, sectioned at 4 μm, and stained with hematoxylin and eosin. The sections were qualitatively examined and scored based predominately on the degree of epithelial denudation, microvilli injury, and crypt inflammation as previously described.16 One investigator, with previous experience in assessing bowel ischemia analyzed the samples under blinded conditions.
Asymmetric Dimethylarginine
Asymmetric dimethylarginine (ADMA) concentrations were measured in plasma of sham and control rats by a commercially available ELISA kit (Euroimmune US, LLC, Boonton, NJ) using standard laboratory practices and techniques.
Statistical Analysis
The data were analyzed with SAS 9.13 and expressed as the means ± standard deviation. Differences in most mean values were determined by a univariate repeated-measures analysis of variance model. Rat survival was determined by the Fisher’s Exact Test and the Wilcoxon Test. Histology scores were analyzed by a multivariant repeated measures analysis of variance model. Statistical significance was set at a p value of 0.05.
RESULTS
Rat Demographics
Rats undergoing hemorrhage and resuscitation had an average weight of about 311 g (range 270 –370 g). The average duration of surgery for most animals was ~1 hour. The average hemorrhage volume was 8.1 mL during an average period of 24 minutes.
Hemodynamics and Lactate
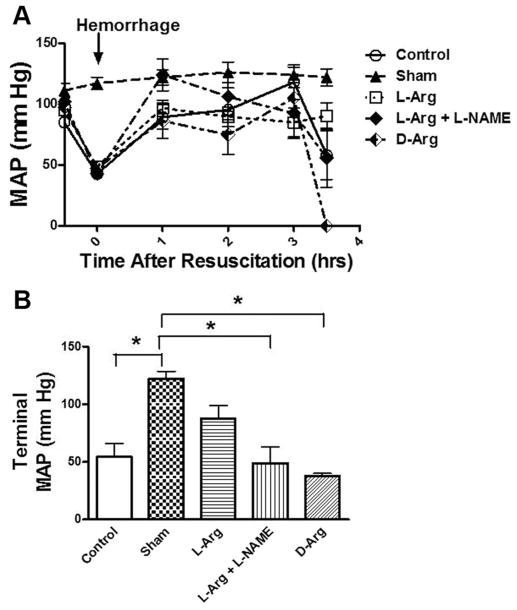
MAP was continuously monitored throughout the study (Fig. 1). The MAP of sham animals showed very little variation throughout the study. The other animal groups showed a decrease MAP in response to hemorrhage and improvement in MAP with resuscitation. The animals that received L-Arg were able to sustain this higher MAP for a longer duration in the post resuscitation period. The terminal values are shown in Figure 1B and highlight the beneficial effects of L-Arg administration before resuscitation. Those animals that received L-Arg had a higher terminal MAP that was not significantly different from the Shams. The MAP of Controls was significantly lower, when compared with Shams.
Figure 1.
Mean arterial pressure (MAP) in rats before hemorrhage (baseline) after hemorrhage (arrow) and after 1–3.5 h of reperfusion after resuscitation with saline (A). Five groups of rats were studied and include the values that were observed at the end of the study (B). *p <0.05, values are mean ± standard error of the mean, n =6 per group.
Serum lactates were measured at the same time points (Fig. 2). Sham lactates were <2 mmol/L/L throughout the study. In all other groups, lactates rose with hemorrhage and decreased with resuscitation. During the reperfusion period, the lactate levels continued to rise in the L-NAME, D-Arg, Control, and L-Arg groups. However, the rise was markedly higher in the L-NAME, D-Arg, and Control animals, relative to the L-Arg and Sham groups. The values of mean serum lactate on termination of the experiment and/or death of the animal are also shown in Figure 2B. The control animals had a serum lactate that was significantly higher, when compared with sham animals. In contrast, L-Arg animals were not significantly different, when compared with shams. L-NAME treated animals were significantly higher, when compared with all animal groups.
Figure 2.
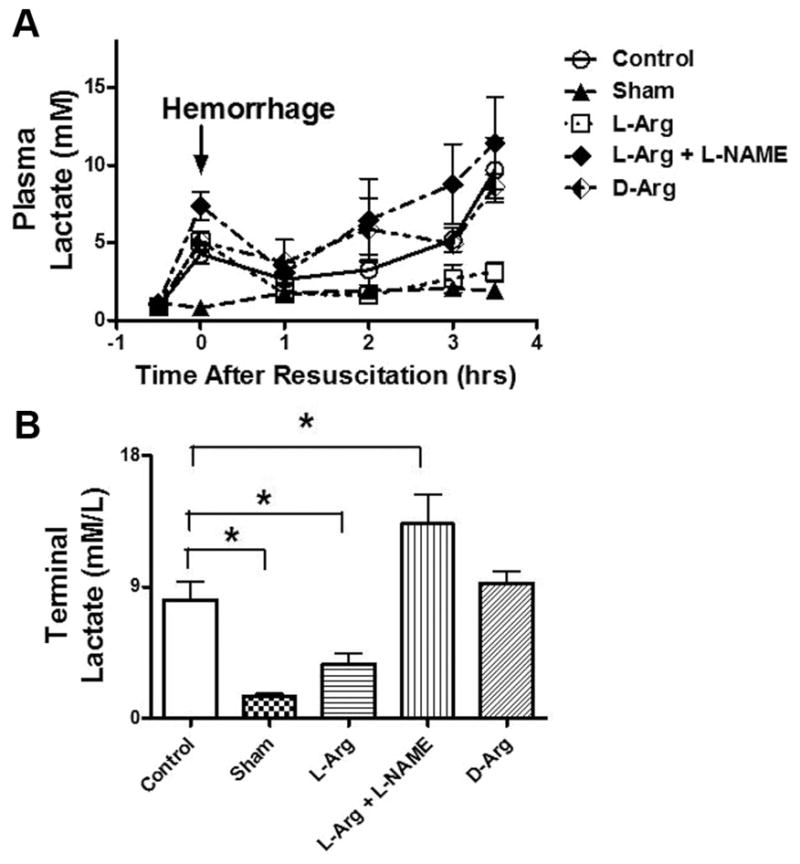
Plasma lactate concentrations in rats before hemorrhage (baseline) after hemorrhage (arrow) and after 1–3.5 h of reperfusion after resuscitation with saline (A). Five groups of rats were studied and include the values that were observed at the end of the study (B). *p <0.05, values are mean ± standard error of the mean, n =6 per group.
Oxygenation
The response of oxygenation to severe shock in this model and the effects of L-Arg and related agents are shown in Figure 3. The PaO2 was significantly reduced after hemorrhage and resuscitation in the untreated control group, relative to the sham and L-Arg group. Terminal L-Arg PaO2 values were also significantly higher compared with L-NAME and D-Arg animals (Fig. 3).
Figure 3.
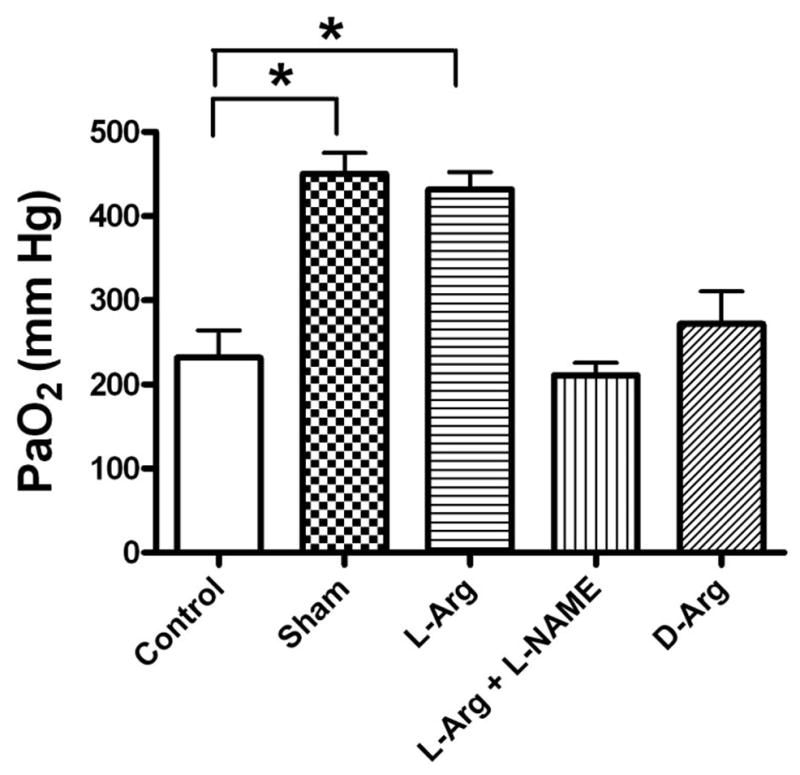
End of study PaO2 values in rats after hemorrhage and resuscitation in five groups of rats. *p, 0.05, values are mean ± standard error of the mean, n =6 per group.
Survival
There was no difference between the overall survival of the Sham and L-Arg groups, and both groups were significantly higher than Control animals (Fig. 4). The animals treated with L-NAME had a survival rate that was significantly lower than L-Arg and Sham animals. These results are shown in Figure 4.
Figure 4.
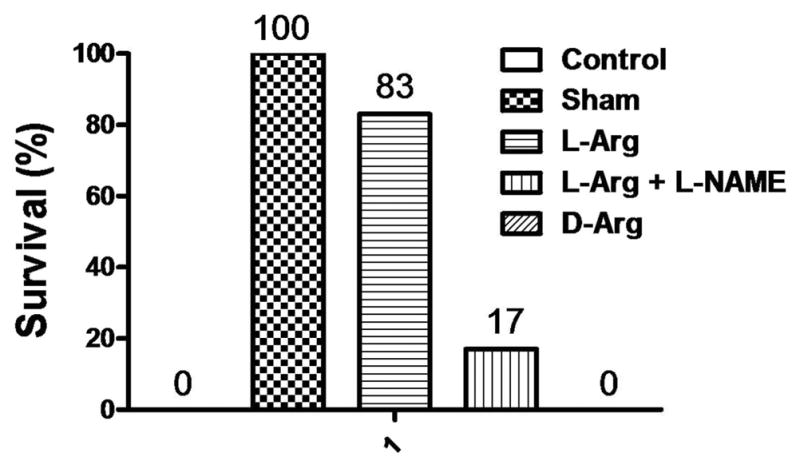
Rat survival rates after hemorrhage and 3.5 h of resuscitation in the five groups. Values are expressed as percent survival, n =6 per group.
Intestinal Reperfusion injury
Small bowel histologic scores after severe hemorrhagic shock and 4 hours after resuscitation is shown in Figure 5, whereas representative sample photomicrographs from each of the five groups is shown in Figure 6. Higher scores are associated with increased mucosal epithelial cell denudation, shortened villus heights, leukocyte infiltration, and crypt erosion. Scores were significantly reduced in L-Arg treated animals, relative to the bowel samples obtained from D-Arg treated rats. Furthermore, the shock-induced increase in histologic injury scores (Fig. 5) was dramatically reduced with L-Arg treatment after hemorrhage and before resuscitation.
Figure 5.
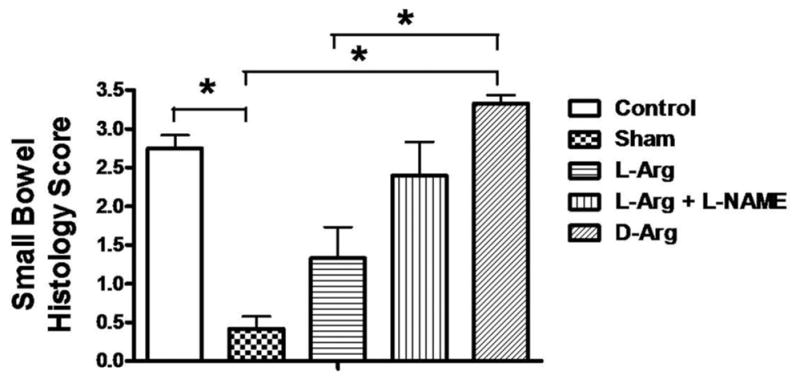
Small bowel histology scores from samples of ileum retrieved at the end of the study in the five treatment groups of rats. The grading scale is described in the Methods section. *p <0.05, values are mean ± standard error of the mean, n =6 per group.
Figure 6.
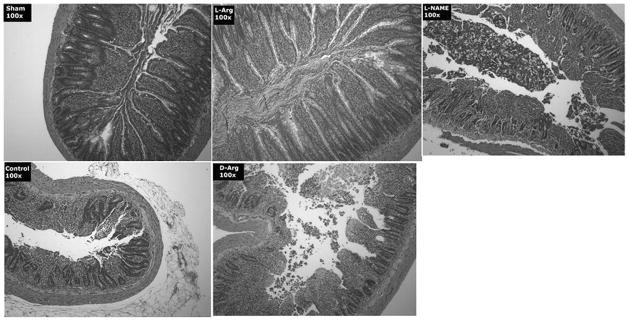
Representative photomicrographs of sections of rat ileum examined under light microscopy after staining with hematoxylin and eosin. Samples were harvested from the five treatment groups at the end of the study. The control samples show severe reperfusion injury characterized by profound loss of villus height and denudation of the villus epithelia. Samples from rats treated with L-Arg are much less affected with less loss of epithelial cells and more defined crypt architecture. The rats treated with L-NAME before L-Arg or the ones treated with D-Arg are similar to the control group. The Sham-negative control group shows essentially normal small bowel.
Asymmetric Dimethylarginine
Levels of ADMA were measured in Sham and Control Animals. The hemorrhage-resuscitated animals had significantly higher concentrations of ADMA compared with the controls. The results are shown in Figure 7.
Figure 7.
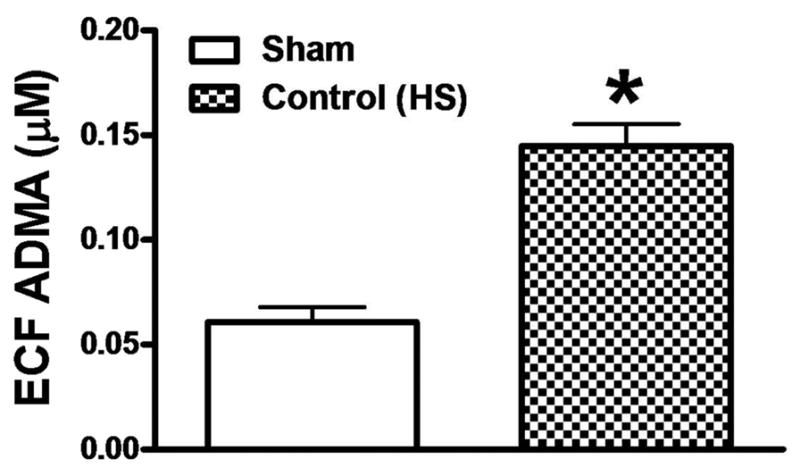
Asymmetric dimethylarginine (ADMA) concentrations in rat plasma at the end of the study in the control (untreated) hemorrhaged group and the Sham group. Values in the plasma were normalized to the estimated extracellular fluid (ECF) volume to control for the volume expansion in that compartment in the hemorrhaged group after the saline resuscitation. p <0.05, values are mean ± standard error of the mean, n =6 per group.
DISCUSSION
Hemorrhage leading to irreversible shock is the major cause of early death after trauma injury. Early in hemorrhage, the body shuts down nonessential capillary beds to preserve perfusion to essential organs such as the heart and brain.17,18 After a period of time, the capillary beds that have been shut down are irretrievably lost, and flow through these beds cannot be reestablished despite hemorrhage control and resuscitation. This has been referred to as the no reflow phenomenon.19 The cause of “no reflow” is capillary narrowing from endothelial cell and parenchymal cell swelling due to the ATP-dependant loss of cell volume control mechanisms during ischemia20,21 and clogging due to activated neutrophils from early inflammation.22
L-Arg is a precursor for NO catalyzed by the enzyme NOS. Studies show that increasing NO availability can improve perfusion and attenuate inflammation in a wide variety of clinical scenarios.9–11,23 Our previous studies have shown that pretreatment with L-Arg improves survival in a swine model of severe trauma and hemorrhagic shock and resuscitation injury (unpublished data).
The likely mechanism of action of L-Arg in shock is by increased NO synthesis by mass action shift in enzyme activity. However, nobody has systematically or conclusively demonstrated this, which is our major objective in this study. Enhanced synthesis of NO during shock then may modulate the inflammatory response at reperfusion by preventing the upregulation and expression of cellular adhesion molecules on the surface of the capillary endothelial cells, fixed tissue macrophages, granulocytes, platelets, and mononuclear cells. This prevents the adhesion of activated immune competent cells to the capillary wall, which attenuates target organ injury.14,24,25 This is part of our central hypothesis.
Others suggest that arginine may play a non-NO role because the circulating arginine levels are 10-fold higher than the Km of the NOS reaction.26 This would not shift NO synthesis with further substrate loading. However, this “arginine paradox” may be reconciled by the presence of endogenous NO synthesis inhibitors in the system, which would pull the reactions to the left and increase the sensitivity of the system for endogenous substrate. In fact, ADMA derived by enzymatic hydrolysis of proteins containing methylated arginine act as competitive inhibitors of endogenous NOS.27,28 Competition of exogenous arginine with these endogenous inhibitors may explain apparent NO effects with exogenous arginine infusions. Other arginine effects not attributable to NO may be nonselective effects on pituitary growth hormone release12,14 or amino acid protective effects on membranes during ischemia.6,29,30
In this study, we demonstrated that the same benefit is observed in another mammalian species and that the benefit is maintained even when the L-Arg infusion is given with resuscitation after the insult, thus making the observation clinically appealing. Furthermore, this study has demonstrated an improvement in MAP, serum lactate, and PaO2, when compared with control hemorrhage-resuscitated animals. These benefits were not seen after D-Arg administration, suggesting that these advantages were not merely amino-acid-mediated salutary effects on reperfusion injury.29,31 Finally, the benefits of L-Arg in hemorrhagic shock are reversed or attenuated in the presence of L-NAME, strongly suggesting that these benefits are NO mediated.
We also identified that L-Arg attenuates reperfusion-induced small bowel reperfusion injury as assessed by tissue histology. Histologic analysis revealed that L-Arg-treated animals had greater preservation of mucosal architecture and was similar to the sham animals. This effect was clearly NO mediated because the salutary effects of L-Arg on the intestine were blocked by the NOS inhibitor L-NAME, and the NOS inactive enantiomer D-Arg was ineffective. The response may be mediated by NO counterbalancing the intense intestinal vasoconstriction induced by hemorrhagic shock or by reduced expression of adhesion molecules and reduced postreperfusion inflammation associated with intestinal ischemia and reperfusion.32 Whatever the precise molecular mechanism, the intestinal sparing effect of L-Arg in hemorrhagic shock likely contributes to the observed benefits in animal survival because global intestinal ischemia contributes to severe hemorrhagic shock and resuscitation injury.33,34
It is difficult to imagine how exogenous L-Arg stimulates NO synthesis when the estimated amount of free L-Arg far exceeds the Km of NOS enzyme. It has been proposed that the presence of circulating endogenous competitive inhibitors of NOS may reconcile the arginine paradox. Multiple studies from the cardiovascular and nutrition literature suggest that ADMA is an endogenous NOS inhibitor and is synthesized in normal humans.35 This could make the local environment for NOS substrate limited and restore sensitivity to added substrate. Furthermore, increased levels of ADMA have been associated with increased inflammation and higher mortality in the ICU.27,36 In fact, plasma ADMA levels were found to be one of the most important independent risk factor for trauma-induced mortality in the ICU.36 In our study, ADMA levels were significantly higher in the Control group, when compared with the Sham. This suggests that shock results in increased ADMA levels that in turn, reduce NO production by acting as competitive inhibitor of NOS. Increasing L-Arg levels by exogenous infusion under these conditions then would overcome the competitive inhibition leading to increased NO production that is responsible for restoring perfusion, attenuating inflammation, and improving survival after hemorrhagic shock. The data presented in this study are consistent with this mechanism.
CONCLUSIONS
L-Arg infusion during resuscitation improves cardiovascular and metabolic changes in severe hemorrhagic shock and improves survival. The mechanism of the benefit seems to be increased NO generation by NOS. The benefit occurs at resuscitation, thus making this effect clinically attractive.
Footnotes
Presented, in part, at the Committee on Trauma, Virginia Chapter, State and Region III Resident competition, AAST Annual Meeting in HI (2008). Formal presentation of this article will occur at Podium Presentation for EAST Meeting, FL, 2009.
DISCLOSURE
Supported by Institutional NRSA T-32 grant GM008695–07A1, AAST education foundation grant, and a grant from the State of Virginia (Trauma Fund).
References
- 1.Jones AS, Kaufman LS, Slakever DS. Cost of Injury in the United States, A Report to Congress. MD: The Johns Hopkins University; 1989. [Google Scholar]
- 2.Rotstein OD. Modeling the two-hit hypothesis for evaluating strategies to prevent organ injury after shock/resuscitation. J Trauma. 2003;54:S203–S206. doi: 10.1097/01.TA.0000064512.62949.92. [DOI] [PubMed] [Google Scholar]
- 3.Holecroft J, Anderson J, Sena M. Shock, ACS Surgery: Principles of Practice. WebMD. 2006 Available at http://www.acssurgery.com.
- 4.Manikis P, Jankowski S, Zhang H, Kahn RJ, Vincent JL. Correlation of serial blood lactate levels to organ failure and mortality after trauma. Am J Emerg Med. 1995;13:619– 622. doi: 10.1016/0735-6757(95)90043-8. [DOI] [PubMed] [Google Scholar]
- 5.Porter JM, Ivatury RR. In search of the optimal end points of resuscitation in trauma patients: a review. J Trauma. 1998;44:908–914. doi: 10.1097/00005373-199805000-00028. [DOI] [PubMed] [Google Scholar]
- 6.Zhong Z, Wheeler MD, Li X, et al. L-Glycine: a novel antiinflammatory, immunomodulatory, and cytoprotective agent. Curr Opin Clin Nutr Metab Care. 2003;6:229–240. doi: 10.1097/00075197-200303000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Albina JE, Reichner JS. Nitric oxide in inflammation and immunity. New Horiz. 1995;3:46– 64. [PubMed] [Google Scholar]
- 8.Fukuto JM, Mayer B. The enzymology of nitric oxide synthetase. In: Freelisch M, Stamler JS, editors. Methods in Nitric Oxide Research. New York: Wiley; 1998. pp. 147–161. [Google Scholar]
- 9.Angele MK, Fitzal F, Smail N, et al. L-Arginine attenuates trauma-hemorrhage-induced liver injury. Crit Care Med. 2000;28:3242–3248. doi: 10.1097/00003246-200009000-00020. [DOI] [PubMed] [Google Scholar]
- 10.Angele MK, Smail N, Wang P, Cioffi WG, Bland KI, Chaudry IH. L-Arginine restores the depressed cardiac output and regional perfusion after trauma-hemorrhage. Surgery. 1998;124:394– 401. [PubMed] [Google Scholar]
- 11.Angele MK, Smail N, Knoferl MW, Ayala A, Cioffi WG, Chaudry IH. L-Arginine restores splenocyte functions after trauma and hemorrhage potentially by improving splenic blood flow. Am J Physiol. 1999;276:C145–C151. doi: 10.1152/ajpcell.1999.276.1.C145. [DOI] [PubMed] [Google Scholar]
- 12.Isidori A, Lo MA, Cappa M. A study of growth hormone release in man after oral administration of amino acids. Curr Med Res Opin. 1981;7:475–481. doi: 10.1185/03007998109114287. [DOI] [PubMed] [Google Scholar]
- 13.Miyabe M, Yanagi K, Ohshima N, Sato S, Fukuda T, Toyooka H. Sodium nitroprusside decreases leukocyte adhesion and emigration after hemorrhagic shock. Anesth Analg. 2002;94:296–301. doi: 10.1097/00000539-200202000-00012. table. [DOI] [PubMed] [Google Scholar]
- 14.Sato S, Suzuki A, Nakajima Y, Iwamoto T, Bito H, Miyabe M. S-Nitroso-N-acetylpenicillamine (SNAP) during hemorrhagic shock improves mortality as a result of recovery from vascular hyporeactivity. Anesth Analg. 2000;90:362–368. doi: 10.1097/00000539-200002000-00023. [DOI] [PubMed] [Google Scholar]
- 15.Youn TJ, Kim HS, Kang HJ, et al. Inhibition of nitric oxide synthesis increases apoptotic cardiomyocyte death and myocardial angiotensin-converting enzyme gene expression in ischemia/reperfusion-injured myocardium of rats. Heart Vessels. 2001;16:12–19. doi: 10.1007/pl00007274. [DOI] [PubMed] [Google Scholar]
- 16.Mangino MJ, Anderson CB, Murphy MK, Brunt E, Turk J. Mucosal arachidonate metabolism and intestinal ischemia-reperfusion injury. Am J Physiol. 1989;257:G299–G307. doi: 10.1152/ajpgi.1989.257.2.G299. [DOI] [PubMed] [Google Scholar]
- 17.Vatner SF. Effects of hemorrhage on regional blood flow distribution in dogs and primates. J Clin Invest. 1974;54:225–235. doi: 10.1172/JCI107757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Race D, Cooper E, Rosenbaum M. Hemorrhagic shock: the effect of prolonged low flow on the regional distribution of blood and its modification by hypothermia. Ann Surg. 1968;167:454– 466. doi: 10.1097/00000658-196804000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nanobashvili J, Neumayer C, Fuegl A, et al. Development of ‘no-reflow’ phenomenon in ischemia/reperfusion injury: failure of active vasomotility and not simply passive vasoconstriction. Eur Surg Res. 2003;35:417– 424. doi: 10.1159/000072226. [DOI] [PubMed] [Google Scholar]
- 20.Behmanesh S, Kempski O. Mechanisms of endothelial cell swelling from lactacidosis studied in vitro. Am J Physiol Heart Circ Physiol. 2000;279:H1512–H1517. doi: 10.1152/ajpheart.2000.279.4.H1512. [DOI] [PubMed] [Google Scholar]
- 21.Kretschmar K, Engelhardt T. Swelling of capillary endothelial cells contributes to traumatic hemorrhagic shock-induced microvascular injury: a morphologic and morphometric analysis. Int J Microcirc Clin Exp. 1994;14:45– 49. doi: 10.1159/000178205. [DOI] [PubMed] [Google Scholar]
- 22.Kaminski KA, Bonda TA, Korecki J, Musial WJ. Oxidative stress and neutrophil activation—the two keystones of ischemia/reperfusion injury. Int J Cardiol. 2002;86:41–59. doi: 10.1016/s0167-5273(02)00189-4. [DOI] [PubMed] [Google Scholar]
- 23.Daughters K, Waxman K, Nguyen H. Increasing nitric oxide production improves survival in experimental hemorrhagic shock. Resuscitation. 1996;31:141–144. doi: 10.1016/0300-9572(95)00922-1. [DOI] [PubMed] [Google Scholar]
- 24.Biffl WL, Moore EE, Moore FA, Barnett C. Nitric oxide reduces endothelial expression of intercellular adhesion molecule (ICAM)-1. J Surg Res. 1996;63:328–332. doi: 10.1006/jsre.1996.0270. [DOI] [PubMed] [Google Scholar]
- 25.Symington PA, Ma XL, Lefer AM. Protective actions of S-nitroso-N-acetylpenicillamine (SNAP) in a rat model of hemorrhagic shock. Methods Find Exp Clin Pharmacol. 1992;14:789–797. [PubMed] [Google Scholar]
- 26.Vukosavljevic N, Jaron D, Barbee KA, Buerk DG. Quantifying the L-arginine paradox in vivo. Microvasc Res. 2006;71:48–54. doi: 10.1016/j.mvr.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Boger RH. Asymmetric dimethylarginine (ADMA): a novel risk marker in cardiovascular medicine and beyond. Ann Med. 2006;38:126–136. doi: 10.1080/07853890500472151. [DOI] [PubMed] [Google Scholar]
- 28.Millatt LJ, Whitley GS, Li D, et al. Evidence for dysregulation of dimethylarginine dimethylaminohydrolase I in chronic hypoxia-induced pulmonary hypertension. Circulation. 2003;108:1493–1498. doi: 10.1161/01.CIR.0000089087.25930.FF. [DOI] [PubMed] [Google Scholar]
- 29.Mangino MJ, Murphy MK, Grabau GG, Anderson CB. Protective effects of glycine during hypothermic renal ischemia-reperfusion injury. Am J Physiol. 1991;261:F841–F848. doi: 10.1152/ajprenal.1991.261.5.F841. [DOI] [PubMed] [Google Scholar]
- 30.Nishimura Y, Lemasters JJ. Glycine blocks opening of a death channel in cultured hepatic sinusoidal endothelial cells during chemical hypoxia. Cell Death Differ. 2001;8:850– 858. doi: 10.1038/sj.cdd.4400877. [DOI] [PubMed] [Google Scholar]
- 31.Mangino JE, Kotadia B, Mangino MJ. Characterization of hypothermic intestinal ischemia-reperfusion injury in dogs. Effects of glycine. Transplantation. 1996;62:173–178. doi: 10.1097/00007890-199607270-00005. [DOI] [PubMed] [Google Scholar]
- 32.Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci USA. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lefer AM. Interaction between myocardial depressant factor and vasoactive mediators with ischemia and shock. Am J Physiol. 1987;252:R193–R205. doi: 10.1152/ajpregu.1987.252.2.R193. [DOI] [PubMed] [Google Scholar]
- 34.Lillehei RC. The intestinal factor in irreversible hemorrhagic shock. Surgery. 1957;42:1043–1054. [PubMed] [Google Scholar]
- 35.Vallance P, Leone A, Calver A, Collier J, Moncada S. Endogenous dimethylarginine as an inhibitor of nitric oxide synthesis. J Cardiovasc Pharmacol. 1992;20(suppl 12):S60–S62. doi: 10.1097/00005344-199204002-00018. [DOI] [PubMed] [Google Scholar]
- 36.Nijveldt RJ, Teerlink T, Van Der HB, et al. Asymmetrical dimethylarginine (ADMA) in critically ill patients: high plasma ADMA concentration is an independent risk factor of ICU mortality. Clin Nutr. 2003;22:23–30. doi: 10.1054/clnu.2002.0613. [DOI] [PubMed] [Google Scholar]