Abstract
Glucagon-like peptides (GLP-1/2) are co-produced and highlighted as key modulators to improve glucose homeostasis and insulin sensitivity after bariatric surgery. However, it is unknown if CNS GLP-2 plays any physiological role in the control of glucose homeostasis and insulin sensitivity. We show that mice lacking GLP-2 receptor (GLP-2R) in POMC neurons display glucose intolerance and hepatic insulin resistance. GLP-2R activation in POMC neurons is required for GLP-2 to enhance insulin-mediated suppression of hepatic glucose production (HGP) and gluconeogenesis. GLP-2 directly modulates excitability of POMC neurons in GLP-2R- and PI3K-dependent manners. GLP-2 initiates GLP-2R-p85α interaction and facilitates PI3K-Akt-dependent FoxO1 nuclear exclusion in POMC neurons. Central GLP-2 suppresses basal HGP and enhances insulin sensitivity, which are abolished in POMC-p110α KO mice. Thus, CNS GLP-2 plays a key physiological role in the control of hepatic glucose production through activating PI3K-dependent modulation of membrane excitability and nuclear transcription of POMC neurons in the brain.
Keywords: Glucagon-like peptides and receptors, Glucose homeostasis, Hypothalamus, Insulin sensitivity
Introduction
Insulin resistance is an important factor in the development of the metabolic syndrome and type 2 diabetes, in which hepatic glucose production (HGP) is not suppressed sufficiently by insulin. Through the gut-brain axis, gut hormones play a key role in the regulation of energy balance and glucose homeostasis(Murphy and Bloom, 2006). In response to food intake, glucagon-like peptide-2 (GLP-2) is co-produced with GLP-1 from endocrine L cells in the gut and preproglucagonergic (PPG) neurons in the brain. GLP-1/2 are key signals for the brain and pancreas to control energy balance and glucose homeostasis and highlighted as key modulators to improve glucose homeostasis and insulin sensitivity after gastric bypass surgery(Field et al., 2010). GLP-1/2 receptor agonists and DPP-IV antagonists are used clinically for the prevention and treatment of diabetes, inflammatory bowel disease, and short bowel syndrome(Dong and Brubaker, 2012). Thus, elucidation of GLP-1/2 signaling and action in the control of glucose homeostasis may reveal novel targets for the treatment of malnutrition, obesity and diabetes.
Through a specific Gs protein-coupled receptor (GLP-2R), GLP-2 is essential for maintaining intestinal homeostasis and barrier function, slowing gastric emptying and gut motility, improving mucosal blood flow and nutrient absorption, and enhancing immune defense(Bahrami et al., 2010b; Guan et al., 2003). Being a key organ for digestion, absorption, and assimilation, the gastrointestinal (GI) tract plays a crucial role in controlling energy intake. Except for its enterotrophic role, however, GLP-2-mediated metabolic action (e.g., glucose homeostasis) has not been adequately explored. In the GI tract, GLP-2 regulates glucose homeostasis by promoting intestinal glucose absorption(Guan et al., 2003) and pancreatic glucagon secretion(Meier et al., 2006). In contrast, GLP-2R global deficiency increases glucagon secretion and accentuates hyperglycemia and impaired glucose tolerance in leptin-deficient (Lepob/ob) mice(Bahrami et al., 2010a). The GLP-2R is expressed not only in endocrine cells (such as enteroendocrine cells and pancreatic α-cells), but also in neurons (such as enteric neurons, vagal sensory neurons, and central neurons)(Guan et al., 2006; Nelson et al., 2007). Specifically, Glp2r mRNA is expressed in key regions of the brain, including the hypothalamus, hippocampus, and the brainstem(Guan et al., 2012; Tang-Christensen et al., 2000; Wang and Guan, 2010). As a neurotransmitter, GLP-2 may mediate PPG neuron-induced synaptic transmission linking the hypothalamus and the brainstem(Tang-Christensen et al., 2000), and acts as a key, physiological satiation signal (for short-term energy availability) in the control of feeding behavior and gastric emptying, which may contribute to the homeostatic, long-term control of energy balance and body weight(Guan et al., 2012). However, it is unknown if CNS GLP-2 plays any physiological role in the control of glucose homeostasis and insulin sensitivity.
The hypothalamus plays pivotal roles not only in the regulation of energy balance but also in the maintenance of glucose homeostasis(Schwartz and Porte, Jr., 2005). Pro-opiomelanocortin (POMC) is expressed in the arcuate nucleus of the hypothalamus (ARC) and the nucleus of the tractus solitarius (NTS) of the brainstem. POMC neurons are perfectly positioned to integrate long-term adiposity and short-term satiety signals for energy homeostasis(Cone, 2005). Direct action of leptin and insulin on POMC neurons regulates hepatic glucose production in mice and rats(Berglund et al., 2012; Hill et al., 2010; Lin et al., 2010). GLP-2R is localized to the mouse ARC POMC neurons and icv administration of GLP-2 increases ARC Pomc mRNA abundance(Guan et al., 2012). Therefore, we hypothesized that GLP-2R in POMC neurons plays an important, physiological role in the control of glucose homeostasis and insulin sensitivity.
The PI3K-Akt-FoxO1 axis is a key signaling pathway mediating hormonal and nutritional actions in the brain. PI3K-dependent Akt activation inhibits FoxO1 activity. GLP-2 activates PI3K-Akt signaling in hippocampal neurons(Shi et al., 2010). As a major catalytic subunit of PI3K, p110α is required for leptin- and insulin-induced PI3K activity in the hypothalamus(Koch et al., 2010). Thus, we hypothesized that PI3K signaling in POMC neurons is required for CNS GLP-2 to promote glucose homeostasis and enhance insulin sensitivity.
In the present study, we show that Glp2r deletion in POMC neurons impairs postprandial glucose tolerance and hepatic insulin sensitivity. GLP-2 directly modulates postsynaptic excitability of POMC neurons in GLP-2R- and PI3K-dependent manners. By inducing GLP-2R-p85α interaction, GLP-2 activates PI3K-Akt-FoxO1 signaling in POMC neurons. Glp2r and p110α in POMC neurons are required for icv GLP-2 to suppress HGP. Therefore, the CNS GLP-2R plays a key role in the control of glucose homeostasis and insulin sensitivity via GLP2R-PI3K-mediated central melanocortin system, which is independent of conventional incretin-mediated insulin action.
Results
GLP-2R deletion in POMC neurons impairs hepatic insulin sensitivity
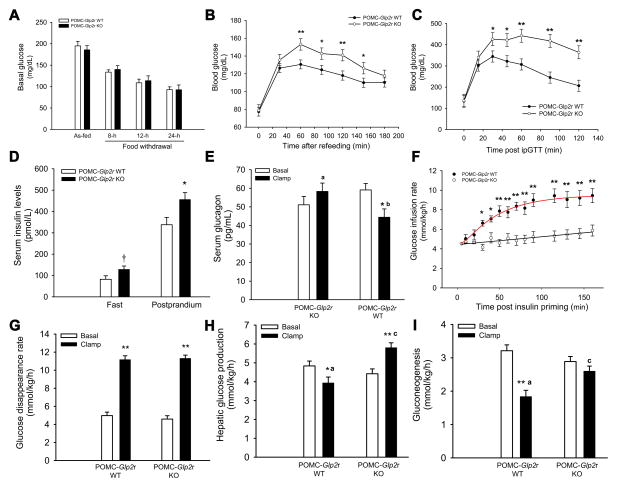
GLP-2R in POMC neurons is required for the control of feeding behavior and gastric emptying. However, it is unknown if GLP-2R in POMC neurons modulates glucose homeostasis and insulin sensitivity. To determine the functional role of GLP-2R in POMC neurons, we genetically engineered mice that lack GLP-2R specifically in POMC neurons by crossing Glp2rflox/flox mice with POMC-cre mice. Glp2r deletion in POMC neurons was validated by immuohistochemistry(Guan et al., 2012). Moreover, Glp2r mRNA expression was markedly attenuated in the neurons within the ARC of POMC-Glp2r KO (= POMC-cre+/0, Glp2rflox/flox) mice as compared to WT (POMC-cre0/0, Glp2rflox/flox) mice (Fig. S1). There were no differences in Glp2r mRNA expression in non-ARC regions such as the paraventricular nucleus of the hypothalamus (PVH) and hippocampus (Fig. S1). Glp2r protein expression was not altered in the PVH either (Fig. S1). Of note, 94.5 ± 4.9 % of POMC-GFP neurons were GLP-2R-immunoreactive, while 48.2 ± 8.5% of GLP-2R-immunoreactive cells were POMC-GFP neurons, indicating GLP-2R expression in non-POMC neurons within the ARC. POMC-Glp2r KO mice (at ~10 week-old) displayed glucose intolerances during postprandial status and ipGTT, though fasting and as-fed glucose levels were not altered (Fig. 1A–C). Moreover, serum insulin levels in the KO mice were increased at fast and 2 h postprandial statuses (Fig. 1D), probably suggesting insulin resistance per se. To dissect tissue-specific insulin effects, we determined the mouse whole-body insulin sensitivity using hyperinsulinemic euglycemic clamp coupled with stable isotopic tracers (2H2O and 6,6-2H2-glucose). The protocol for quantifying glucose kinetics is shown in Fig. S2. Note that [1] insulin was infused at a relative low rate; [2] the ratio of labeled glucose vs unlabeled glucose was kept constant in the infusate and thus was diluted exclusively by endogenous glucose; and [3] the physiological equilibration of labeled glucose was achieved within ~3 h, conditions that allow us to examine glucose homeostasis under relatively physiological conditions. It took 2~3 h for 12 h-fasted 10-wk old mice to reach a plateau of isotopic enrichment (i.e., 2H-glucose). In fact, glucose infusion rate reached a plateau within 2~3 h when blood glucose was clamped at ~110 mg/dL. Of note, estimates for glucose kinetics were influenced by insulin infusion rate. When insulin was infused from 2.5 to 5.0 mU/kg/min, glucose infusion rate (GIR) was increased by ~20% (from 7.65 ± 0.30 to 9.21 ± 0.35 mmol/kg/h) and endogenous glucose production (i.e., hepatic glucose production, HGP) decreased by ~85% (from 1.93 ± 0.14 to 0.33 ± 0.25 mmol/kg/h), though glucose disappearance rate (Rd, i.e., glucose uptake by peripheral tissues) was not altered (from 9.75 ± 0.28 to 9.35 ± 0.42 mmol/kg/h). When insulin was infused at a higher rate of 5.0 mU/kg/min, HGP was completely suppressed and undistinguished between genotypes. Therefore, insulin was primed at 150 mU/kg and infused continuously at 2.5 mU/kg/min while glucose (30%) was infused at varied rates to maintain an euglycemic level (~110 ± 5 mg/dL) throughout this study.
Figure 1. GLP-2R deletion in POMC neurons impairs glucose homeostasis and insulin sensitivity.
A. Blood glucose levels at 8~24-h fast or as-fed.
B. Blood glucose levels during refeeding.
C. POMC-Glp2r KO mice display glucose intolerance.
D. Serum insulin levels at 12-h fast and 2-h postprandial statuses.
E. GLP-2R deletion in POMC neurons impairs insulin-suppressed glucagon secretion.
F. POMC-Glp2r KO mice display whole-body insulin resistance indicated by lower glucose infusion rates during the clamp.
G. Glucose disappearance rate (Rd) was not altered in POMC-Glp2r KO mice.
H and I. POMC-Glp2r KO mice display hepatic insulin sensitivity indicated by higher endogenous glucose production (i.e., hepatic glucose production) and gluconeogenesis. 10-wk-old mice (with similar BW and fat mass) were assessed for glucose tolerance by intraperitoneal (ip) glucose tolerance test; and quantified for glucose homeostasis and insulin sensitivity using stable isotopic tracers during hyperinsulinemic euglycemic clamp. Data are expressed as means ± SEM (n = 8~12 per group); †, *, and **P < 0.10, 0.05, and 0.01, respectively, denoting significance between genotypes at the same time point or between baseline and clamp within a genotype; a,b and a,cP < 0.05 and 0.01, respectively, denoting significance between genotypes during the clamp (C–H). See also Figure S1 & S2 and Table S3.
GLP-2R deletion in POMC neurons impairs whole-body glucose metabolism (Fig. 1F–I). For the insulin clamp, there were no differences in body weight or fat mass between two groups (Table S3). In the WT mice, infusion of insulin (2.5 mU/kg/min) increased Rd by 2.5-fold, decreased HGP by 20%, and decreased gluconeogenesis (GNG) by 43% when compared to their basal values. Importantly, GIR (an indicator of whole-body insulin sensitivity) decreased by 26.6% in POMC-Glp2r KO mice compared to their WT littermates. During the clamp, Rd (an indicator of insulin-stimulated uptake of glucose by peripheral tissues) was not different between two genotypes. However, HGP during the clamp (an indicator of insulin-suppressed glucose production by liver) increased by 47.5% in POMC-Glp2r KO mice compared to their WT littermates. Proportionally, gluconeogenesis also increased by 41.4% in the KO mice. In agreement with glucose kinetics data, hepatic mRNA abundances of G6Pase and PEPCK (two key gluconeogenic genes) after the insulin clamp were higher in the POMC-Glp2r KO than those in the WT mice (Fig. S2).
In terms of blood hormonal profile, we found that, compared to the WT littermate, the POMC-Glp2r KO mice displayed higher serum insulin concentrations specifically at postprandial status (Fig. 1D). However, there was no difference in basal serum GLP-1 (7-36)amide concentrations (the KO vs WT mice: 1.83 ± 0.01 vs 1.85 ± 0.05 pM). Glucagon is a major driving force behind increased HGP while glucagon secretion is regulated by vagal input. Since GLP-2R global KO mice display enhanced secretion of glucagon, we wanted to determine if increased HGP in POMC-Glp2r KO mice is associated with increased glucagon secretion. Notably, blood glucagon concentrations during the clamp were suppressed in the WT mice, but not altered in the KO mice (Fig. 1E), although their basal levels were similar. These data indicate that Glp2r deletion in POMC neurons leads to hepatic insulin resistance associated with impairment in insulin-suppressed secretion of glucagon, suggesting that GLP-2R in POMC neurons is required for insulin-mediated suppression of HGP (i.e., gluconeogenesis).
GLP-2R activation in POMC neurons is required for GLP-2 to promote glucose homeostasis and enhance insulin sensitivity
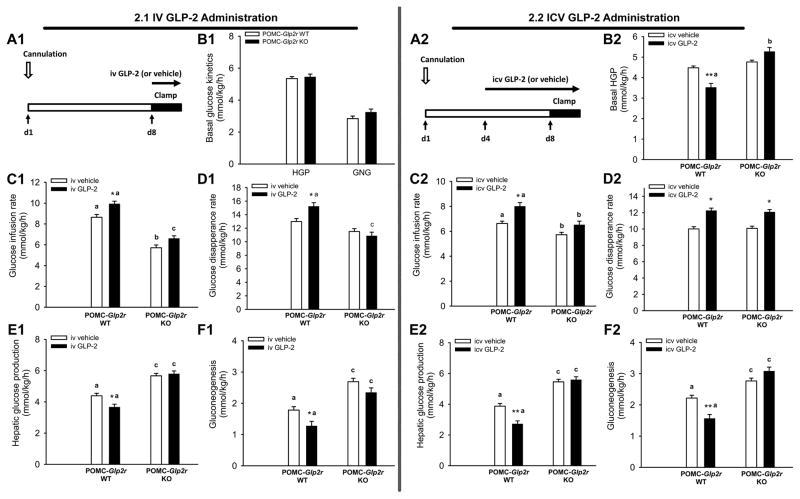
POMC neurons in the ARC can sense peripheral hormones and nutrients. We speculate if peripheral GLP-2 may directly act on POMC neurons in the ARC where blood brain barrier is semi-permeable. Alternatively, peripheral GLP-2 may act GLP-2R on the vagal (afferent) neurons and consequently activates neurons in the brainstem and hypothalamus, i.e., GLP-2 may indirectly acts on ARC POMC neurons. Thus, we hypothesized that GLP-2R on POMC neurons was required for acute iv GLP-2 to promote glucose homeostasis. To address it, we examined glucose homeostasis under acute 3-h iv infusion of GLP-2 (at 500 pmol/kg/h) or vehicle during insulin clamp (Fig. 2.1). Consistently, there were no differences in basal HGP or gluconeogenesis between the WT and KO mice prior to iv GLP-2 (or vehicle) infusion. Acute iv infusion (3~4 h) of GLP-2 enhanced the whole-body insulin sensitivity (indicated by increased GIR) in the WT mice through further increasing peripheral glucose disposal (indicated by increased Rd) and suppressing HGP and gluconeogenesis. However, iv GLP-2-induced enhancement in insulin sensitivity was negated in POMC-Glp2r KO mice, indicating that Glp2r in POMC neurons is required for iv GLP-2 acute action in the control of insulin sensitivity and glucose homeostasis.
Figure 2. Peripheral and central GLP-2 augments hepatic insulin sensitivity through GLP-2R function in POMC neurons.
2.1 iv GLP-2 administration.
2.2 icv GLP-2 administration.
A1 and A2. Protocols for quantifying glucose homeostasis and insulin sensitivity under peripheral and central infusion of GLP-2.
B1 and B2. Hepatic glucose production or gluconeogenesis at the baseline.
C1–F1 and C2–F2. Peripheral and central GLP-2 improve insulin sensitivity in the WT mice during the insulin clamp, respectively, as indicated by higher glucose infusion rate, lower hepatic glucose production, and gluconeogenesis. Notably, central GLP-2, not peripheral GLP-2, enhanced glucose disappearance rate in the WT mice. There effects were negated in POMC-Glp2r KO mice.
10-wk-old mice (with similar BW and fat mass) were infused peripherally with GLP-2 (500 pmol/kg/h) via a jugular vein catheter for 3 h or centrally with GLP-2 (250 μM at a rate of 0.5 μL/h) via a microosmotic pump for 3 d; and meanwhile quantified for insulin sensitivity using stable isotopic tracers during hyperinsulinemic euglycemic clamp. Data are expressed as means ± SEM (n = 8~12 per group); ** or *P < 0.01 or 0.05 denoting significance between genotypes during the clamp; a,b and a,cP < 0.05 and 0.01, respectively, denoting significance between genotypes during the clamp. See also Figure S2 and Table S3.
As we reported recently, icv GLP-2 suppresses food intake and gastric emptying, and this is mediated by Glp2r in POMC neurons(Guan et al., 2012). It is unknown if and how icv GLP-2 promotes glucose homeostasis and insulin sensitivity. We hypothesized that GLP-2R in POMC neurons was a key site for central GLP-2 action on peripheral glucose homeostasis. To test it, we performed the insulin clamp in mice after a 3-d icv infusion of GLP-2 (Fig. 2.2). 3-d icv infusion of GLP-2 enhanced the whole-body insulin sensitivity (indicated by increased GIR) in the WT mice through further increasing peripheral glucose disposal (indicated by increased Rd) and suppressing HGP and gluconeogenesis. However, icv GLP-2-induced suppression of HGP and gluconeogenesis was negated in POMC-Glp2r KO mice, indicating that Glp2r in POMC neurons is required for icv GLP-2 to suppress hepatic glucose production. Of note, icv GLP-2 suppressed basal HGP. Interestingly, icv GLP-2 still enhanced peripheral glucose disposal (indicated by increased Rd) in the KO mice. This is consistent with a current view that ARC POMC neurons control glucose production while VHM (or DMH) neurons modulate glucose disposal. Both iv GLP-2 and icv GLP-2 might act on POMC neurons in the ARC to suppress HGP. However, icv GLP-2 might also act on GLP-2R in the DMH and stimulate glucose disposal (Rd) where iv GLP-2 could not reach. Regardless of GLP-2 origin, GLP-2R activation is required for GLP-2 to promote glucose homeostasis.
GLP-2 differentially modulates the excitability of POMC neurons in a PI3K-dependent manner
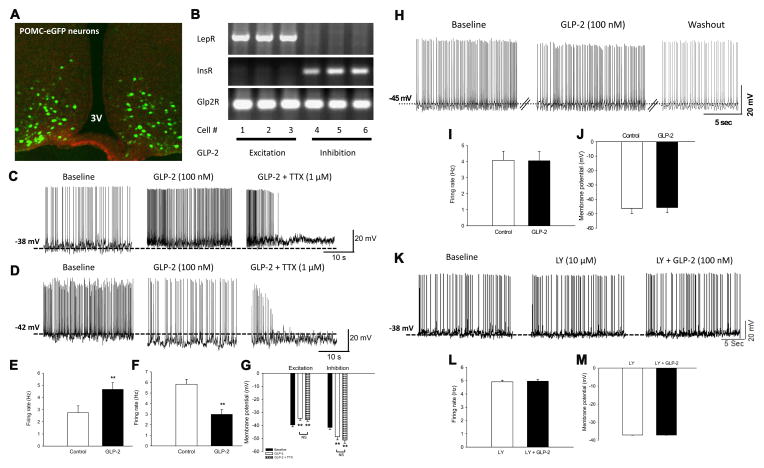
POMC neurons in the hypothalamus play a key role in the regulation of HGP. The metabolic phenotype of POMC-Glp2r KO mice suggests that GLP-2R in POMC neurons is involved in the control of glucose homeostasis. Moreover, icv infusion of GLP-2 (at 250 pmol/μL at 0.5 μl/h for 3 d) improved glucose homeostasis (Fig. 2.2) with increased ARC Pomc mRNA abundance. Though GLP-2R is expressed in POMC neurons, it is unknown if GLP-2 can directly modulate their excitability. To determine GLP-2-induced action on POMC neuronal excitability, we performed whole-cell patch-clamp recording on brain slices from POMC-Glp2r WT (=POMC-eGFP+/0, Glp2rflox/flox) and POMC-Glp2r KO (=POMC-cre+/0, POMC-eGFP+/0, Glp2rflox/flox) mice. POMC neurons were identified by POMC promoter-driven eGFP expression (Fig. 3A).
Figure 3. GLP-2 directly modulates membrane excitability of hypothalamic POMC neurons in GLP-2R- and PI3K-dependent manners.
A. POMC neurons in the arcuate are identified by POMC promoter-driven GFP expression.
B. mRNA expression of hormonal receptors in recorded POMC neurons was determined by single cell RT-PCR.
C, E, and G. GLP-2 depolarizes POMC neurons expressing leptin receptor. GLP-2 (100 nM) depolarized 22 of 44 POMC neurons as indicated by increased spontaneous firing rate and decreased resting membrane potential. Under the presence of tetrodotoxin (TTX, 1 μM), GLP-2 still depolarized 10 of 22 POMC neurons, indicating a direct action on membrane depolarization of POMC neurons independent of action potential (AP)-mediated synaptic transmission. This subgroup of POMC neurons expressed leptin receptor (LepR) mRNA, but not insulin receptor (InsR) mRNA (B).
D, F, and G. GLP-2 hyperpolarizes POMC neurons expressing insulin receptor. GLP-2 (100 nM) also hyperpolarized 18 of 44 POMC neurons as indicated by decreased firing rate and increased resting membrane potential. Under the presence of TTX (1 μM), GLP-2 still hyperpolarized 10 of 22 POMC neurons, indicating a direct action on membrane hyperpolarization of POMC neurons independent of AP-mediated synaptic transmission. This subgroup of POMC neurons expressed InsR mRNA, but not LepR mRNA (B).
H–J. GLP-2R in POMC neurons is required for GLP-2-induced modulation of membrane excitability. GLP-2 (100 nM) neither depolarized nor hyperpolarized 11 of 12 of POMC neurons with GLP-2R deletion. Neither spontaneous firing rate nor resting membrane potential were observed in response to GLP-2 in POMC neurons with Glp2r deletion, indicating GLP-2-mediated action on membrane potential through GLP-2R function in POMC neurons.
K–M. GLP-2 action on POMC neuronal excitability is blocked by PI3K inhibitor. GLP-2 (100 nM) did not excite or inhibit POMC neurons (n=12) after the brain slides were treated with LY-294002 (10 μM), indicating GLP-2 action in a PI3K-dependent manner. Panels (C, D, and K) show representative traces of the whole-cell patch clamp recordings on POMC neurons in WT (i.e., Glp2rflox/flox; POMC-GFP+/0) brain slices, while Panel H representative traces of the whole-cell patch clamp recordings on POMC neurons in KO (i.e., Glp2rflox/flox; POMC-Cre+/0; POMC-GFP+/0) brain slices. Membrane potential and firing rate were measured by the whole-cell current clamp recording, and analyzed with Mini Analysis Program. After the whole-cell patch clamp recording, single neurons were ad hoc acquired for RT-PCR. Data are expressed as means ± SEM; **P < 0.01 denoting significance between control and GLP-2 (± TTX); and NS denoting no significance (P > 0.1) between GLP-2 and GLP-2 plus TTX (G). See also Figure S3.
We found that GLP-2 (perfused at 100 nM) depolarized ~50% (22 of 44) POMC neurons as indicated by increased spontaneous firing rate and decreased resting membrane potential (Fig. 3C, E, and G). Hormonal regulation of POMC neuron activity involves both cell autonomous and presynaptic effects. Of note, GLP-2 effects occurred with an onset < 5 min in our preparation (Fig. S3), similar to leptin effects on membrane potential of POMC neurons(Hill et al., 2008). The GLP-2-induced depolarization was partially reversible within ~18 min, probably pointing to GLP-2R-mediated homologous desensitization. To define if this excitation is mediated by action potential (AP)-dependent synaptic transmission, tetrodotoxin (TTX, a fast sodium channel blocker, 1 μM) was co-perfused with GLP-2. Clearly, GLP-2 still depolarized ~45% (10 of 22) POMC neurons (as membrane potential decreased by 3.97 ± 0.8 mV; n = 10; Fig. 3G), indicating a direct action on membrane depolarization in POMC neurons independent of AP-mediated synaptic transmission. Representative traces of TTX pre-blockage procedure were shown in Fig. S3. Moreover, single-cell RNA was obtained ad hoc after the recording. Interestingly, the single-cell RT-PCR showed that leptin receptor (LepR) mRNA was expressed, but insulin receptor (InsR) mRNA was not, in this subgroup of POMC neurons (Fig. 3B).
In contrast, GLP-2 (100 nM) also hyperpolarized ~40% (18 of 44) POMC neurons as indicated by decreased spontaneous firing rate and increased resting membrane potential (Fig. 3D, F, and G). In the presence of TTX (1 μM), GLP-2 still hyperpolarized ~45% (10 of 22) POMC neurons (as membrane potential increased by −9.6 ± 1.7 mV; n = 10; Fig. 3G), indicating a direct action on membrane hyperpolarization in POMC neurons independent of AP-mediated synaptic transmission. Moreover, the single-cell RT-PCR showed that InsR was expressed, but LepR mRNA was not, in this subgroup of POMC neurons (Fig. 3B).
Importantly, GLP-2 (100 nM) neither depolarized nor hyperpolarized ~90% (11 of 12) POMC neurons in the brain slices from the POMC-Glp2r KO mice (Fig. 3H). GLP-2-induced excitation (or inhibition) was abolished in POMC neurons with Glp2r deletion since no changes were observed in spontaneous firing rate or resting membrane potential in slices from these mice (Fig. 3I–J), further indicating GLP-2-mediated direct action on membrane potential (not attributable to synaptic transmission) through GLP-2R function in POMC neurons. Interestingly, the resting membrane potential was at −45 mV, i.e., approximately 5 mV lower in POMC neurons from the POMC-Glp2r KO mice than those from the WT mice. These data not only indicate that GLP-2 differentially modulates membrane excitability of POMC neurons, but also suggest that Glp2r is required for maintaining the basal membrane potential of POMC neurons.
GLP-2 modulates firing rate and membrane potential of POMC neurons in a PI3K-dependent manner. PI3K signaling is required for leptin and insulin to activate TRPC and KATP channels, respectively, resulting in depolarization and hyperpolarization of POMC neurons (to suppress and promote glucose production). GLP-2 rapidly phosphorylates Akt in primary neurons in a PI3K-dependent manner(Shi et al., 2010), suggesting that the PI3K-Akt signaling pathway may underlie GLP-2-induced modulation of POMC neurons. Therefore, we examined whether PI3K activation is required for GLP-2 action on neuronal excitability, and found that pretreatment of brain slides with LY-294002 (a PI3K inhibitor, 10 μM) completely blocked the effects of GLP-2 (100 nM) on POMC neurons (n=12)(Fig. 3K–M), indicating that GLP-2 modulates firing rate and membrane potential of POMC neurons in a PI3K-dependent manner.
To further define which ion channels were attributed to GLP-2-modulated neuronal excitability, we assessed how POMC neurons responded to GLP-2 in the presence of tolbutamide (an ATP-dependent K+ channel blocker). While 37.5% of POMC neurons were excited (as indicated by increased firing rate and decreased membrane potential, Fig. S3), 62.5% of POMC neurons were unresponsive to GLP-2 (100 nM) in the presence of tolbutamide (200 μM), indicating either K+ATP blockage or unresponsiveness per se. Thus, K+ATP activation was responsible for GLP-2-induced inhibition of POMC neurons.
In summary, we show that GLP-2 directly modulates excitability of POMC neurons in GLP-2R- and PI3K-dependent manners. GLP-2 depolarizes one subgroup of POMC neurons expressing LepR, but hyperpolarizes another subgroup of POMC neurons expressing InsR, indicating that GLP-2 is a key modulator in the central melanocortin system that may mediate CNS GLP-2-induced suppression of HGP. However, little is known about CNS GLP-2-mediated intracellular signaling pathways in these neurons.
GLP-2R binds to PI3K (p85α) and activates PI3K-Akt-FoxO1 signaling in POMC neurons
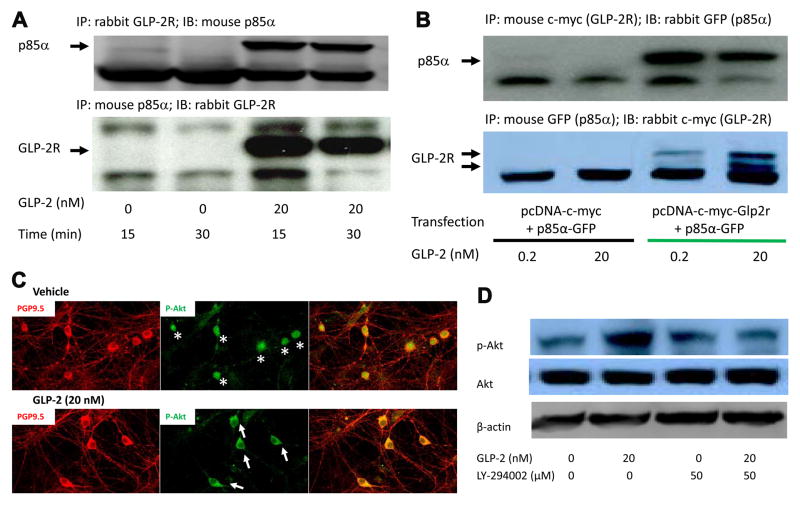
PI3K is recruited to the phosphotyrosine residues (consensus sequence YXXM) via SH2 domains in the regulatory domain (p85), leading to changes in the catalytic domain (p110) conformation and then PI3K activation. Through GLP-2R primary sequence analysis, we found a putative p85α-binding motif (YXXM) in the GLP-2R amino acid sequence (Fig. S4).
To determine if GLP-2R directly interacts with the PI3K regulatory subunit (p85α), we performed immunoprecipitation (IP) in primary neurons (DIV 7) treated with GLP-2 at 20 nM for 15~30 min. Since it is technically challenging to culture mature neurons from the hypothalamus, neurons from the hippocampus were cultured for investigating GLP-2R function as we reported previously(Wang and Guan, 2010). We found that in the co-immunoprecipited (co-IP) protein complex with rabbit GLP-2R antibody, a protein band of the p85α at the predicted size was detected by immunoblotting (IB) using mouse p85α antibody (in Fig. 4A). Conversely, in co-IP protein complex with mouse p85α antibody, a protein band of the GLP-2R at the predicted size was detected by IB using rabbit GLP-2R antibody. Of note, this interaction (between GLP-2R and p85α) was activated by GLP-2 addition and refeeding (Fig. S4.B). Moreover, this GLP-2R-p85α interaction was confirmed in HEK293 cells transfected with Glp2r-c-myc and p85α-GFP using antibodies against c-myc and GFP (Fig. 4B). Note that c-myc multiple bands are related to GLP-2R glycosylation in HEK293 cells. Moreover, the p85α binding motif (YXXM) on human Glp2r was mutated to the AXXA sequence to determine if the motif was responsible for GLP-2R-PI3K activation. In wild-type Glp2r-transfected cells, GLP-2 (at 20 nM for 30 min) enhanced Akt phosphorylation at both Ser473 and Thr308 (Fig. S4). In the mutant Glp2r-transfected cells, in contrast, GLP-2 enhanced Akt phosphorylation only at Ser473, but not at Thr308, suggesting that the p85α binding motif (of GLP-2R) was essential for GLP-2-induced phosphorylation of Akt Thr308, and thus assumedly required for the Akt full activation.
Figure 4. GLP-2 activates PI3K-dependent Akt signaling by inducing GLP-2R-p85α interaction.
A. GLP-2R interacts with p85α in cultured hippocampal neurons. Up panel: in GLP-2R-co-immunoprecipited (co-IP) protein complex, a protein band (p85α) was detected by immunoblotting (IB). Bottom panel: Conversely, in p85α-co-IP protein complex, a protein band (GLP-2R) was detected by IB.
B. GLP-2R interacts with p85α in transfected HEK 293 cells. The GLP-2R-p85α interaction was confirmed in HEK 293 cells transfected with Glp2r-c-myc plus p85α-GFP, but not in the control. Up panel: in c-myc (i.e., GLP-2R)-co-IP protein complex, a protein band (GFP, i.e., p85α) was detected by IB. Bottom panel: Conversely, in GFP (i.e., p85α)-co-IP protein complex, a protein band (c-myc, i.e., GLP-2R) was detected by IB.
C. GLP-2 induces p-AKT membrane localization in cultured neurons. p-Akt Ser473 was translocated to the plasma membrane (indicated in white arrows in C) in cultured neurons after treated with GLP-2 (at 20 nM for 30 min).
D. GLP-2 increases PI3K-dependent phosphorylated Akt abundance in cultured neurons. p-Akt Ser473 abundance increased in cultured neurons after treated with GLP-2 (at 20 nM for 30 min), but not in the pretreatment of PI3K inhibitor (LY294002, 50 μM).
Hippocampal neurons and transfected HEK 293 cells were cultured, and treated with vehicle, GLP-2, or GLP-2 plus LY294002 for immunocytochemistry or immunoblotting after co-immunoprecipitation. See also Figure S4.
To define if GLP-2 activates PI3K-Akt signaling in cultured neurons, we determined the protein abundance and cellular localization of phosphorylated Akt (Akt as a PI3K substrate). It is known that PI3K phosphorylates PIP2 to generate PIP3 at the plasma membrane where Akt is bound to PIP3 and activated by PDK1. GLP-2 (20 nM for 30 min) increased the p-Akt S473 protein abundance in cultured neurons, and this effect was abolished after pretreatment with LY-294002 (50 μM, Fig. 4D). Importantly, the p-Akt was translocated to the plasma membrane (in white arrows in the bottom middle panel of Fig. 4C) in cultured neurons after treatment with GLP-2 (20 nM) for 30 min, while p-Akt was mainly localized to the nucleus (in white stars in the top middle panel of Fig. 4C). In summary, we show in cultured primary neurons and transfected cells that GLP-2 activates the PI3K-dependent Akt signaling through GLP-2R-p85α interaction.
FoxO1 is a downstream target of the PI3K/Akt signaling pathway and regulates energy balance(Kim et al., 2006). Importantly, Akt1 phosphorylates FoxO1, leading to its retention in the cytoplasm (i.e., nuclear exclusion) and disinhibition of target gene expression (such as Pomc and Cpe) in POMC neurons(Plum et al., 2009; Kim et al., 2006). Thus, FoxO1-GFP nuclear exclusion not only indicates transcriptional function of FoxO1 per se, but also represents activation of PI3K-Akt signaling pathway in a neuron-specific manner(Fukuda et al., 2008). Therefore, we tested if GLP-2-induced activation of PI3K-Akt signaling pathway was associated with FoxO1-GFP nucleocytoplasmic shuttling in POMC neurons. To quantify if GLP-2 acutely induces PI3K-dependent nuclear exclusion of FoxO1-GFP in POMC neurons, brain slices were cultured and treated with GLP-2 (20 nM for 30 min). Of note, FoxO1-GFP was expressed in a cre-dependent manner. To determine if Glp2r in POMC neurons is required for GLP-2 to enhance Akt-FoxO1 signaling, we analyzed the cellular distribution of p-Akt and FoxO1-GFP in brain slices by immunocytochemistry. As shown in Fig. S5, FoxO1-GFP was localized exclusively to the nucleus with attenuated p-Akt in ARC POMC neurons of POMC-Glp2r KO (= POMC-cre+/0; Glp2rflox/flox; FoxO1-GFP+/0) brain slices, while FoxO1-GFP was localized to both the nucleus and cytoplasm with basal p-Akt in POMC neurons of the WT (= POMC-cre+/0; FoxO1-GFP+/0) brain slides. Moreover, GLP-2 promoted Akt phosphorylation and FoxO1-GFP nuclear exclusion in the brain slices from the WT mice. In response to GLP-2, FoxO1-GFP was maintained in the nucleus of POMC neurons, though p-Akt signaling was enhanced in adjacent neurons, in the brain slices of POMC-Glp2r KO mice. These data suggest that Glp2r in POMC neurons is required for GLP-2 to activate Akt-FoxO1 signaling.
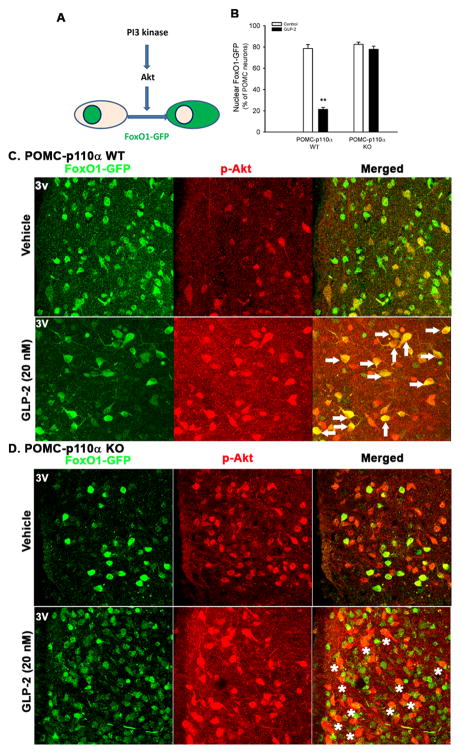
To genetically define if GLP-2 activates the PI3K-Akt-FoxO1 signaling in POMC neurons, we monitored the nuclear exclusion of FoxO1-GFP in POMC neurons expressing or lacking p110α (the PI3K catalytic subunit) in response to GLP-2 treatment (20 nM for 30 min). GLP-2 acutely induced nuclear exclusion of FoxO1-GFP in POMC neurons as decreased from ~80% to ~20% of FoxO1-GFP exclusively localized to the nucleus in POMC-p110α WT brain slices (i.e., from POMC-cre+/0; FoxO1-GFP+/0 mice), while this nuclear exclusion was abolished in POMC neurons in POMC-p110α KO brain slices (i.e., from POMC-cre+/0; p110αflox/flox; FoxO1-GFP+/0 mice)(Fig. 5B). Interestingly, FoxO1-GFP in the cytoplasm was co-localized with p-AKT (as shown in yellow, see arrows) in POMC neurons of the WT brain slides (Fig. 5C). In contrast, p-Akt signaling was enhanced, but not co-localized with FoxO1-GFP in POMC neurons of the KO brain slides (indicated in stars in Fig. 5D). Those pharmacological and genetic data indicate that GLP-2R interacts physically with the PI3K regulatory subunit (p85α), and is required for the basal activation of p-Akt in POMC neurons. Moreover, GLP-2 enhances PI3K-dependent p-Akt signaling, facilitating FoxO1 nuclear exclusion in hypothalamic POMC neurons.
Figure 5. GLP-2 activates the PI3K-Akt-FoxO1 signaling in POMC neurons.
A. FoxO1-GFP nuclear exclusion as readout of PI3K activation.
B. GLP-2 induces FoxO1-GFP nuclear exclusion in POMC neurons in a p110α-dependent manner.
C. GLP-2 induces FoxO1-GFP nuclear exclusion in POMC neurons.
In GLP-2-treated WT brain slice (i.e., from POMC-cre+/0; FoxO1-GFP+/0 mice), FoxO1-GFP is mainly localized to the cytoplasm of POMC neurons where p-AKT staining was enhanced (in arrows).
D. In GLP-2-treated KO brain slides (i.e., from POMC-cre+/0; p110αflox/flox; FoxO1-GFP+/0 mice), FoxO1-GFP is still dominantly localized to the nucleus of POMC neurons although p-Akt signaling is enhanced in adjacent cells (in stars).
Brain slices (250 μm) were cultured and treated with vehicle or GLP-2 (at 20 nM for 30 min). FoxO1-GFP and p-Akt Ser473 were immunostained and quantified. Data are expressed as means ± SEM; **P < 0.01 denoting significance between vehicle and GLP-2 within a genotype. See also Figure S5.
PI3K in POMC neurons is required for icv GLP-2-enhanced insulin sensitivity and glucose homeostasis
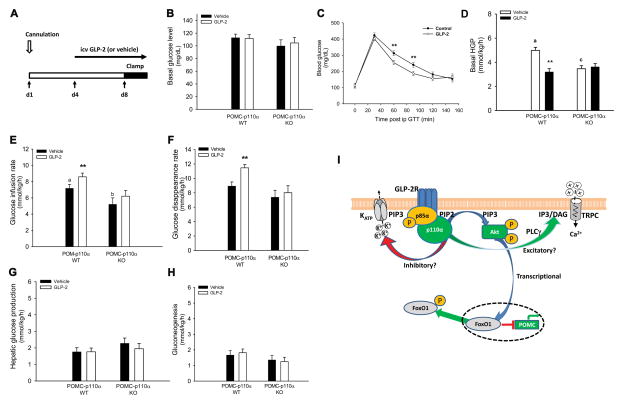
As we demonstrated above, Glp2r in POMC neurons is essential for insulin-mediated suppression of HGP. To determine icv GLP-2 affects glucose homeostasis and insulin sensitivity, we infused into icv through an osmotic pump with GLP-2 (at 250 pmol/μL at 0.5 μl/h for 3 d), which led to suppressed basal HGP in the WT mice as shown in Fig. 2.2. In another experiment, C57BL/6J mice were icv infused with GLP-2 for 8 d and had increased glucose tolerance (during ipGTT)(Fig. 6C). Fasting glucose levels were not altered by 8-d icv infusion of GLP-2. Moreover, basal HGP in POMC-p110α WT (= POMC-cre0/0, p110αflox/flox) mice was suppressed (-28%) by 3-d icv infusion of GLP-2, and this CNS GLP-2 suppression of HGP was abolished in POMC-p110α KO (= POMC-cre+/0, p110αflox/flox) mice (Fig. 6). During the insulin clamp, blood glucose concentrations were clamped at the same level (108.0 ± 6.5 mg/dL) in all four groups (Fig. S6). Of note, there was no difference in BW between treatments or genotypes when the insulin clamp was performed (Table S3). In the vehicle groups, p110α deletion in POMC neurons negatively regulated the basal HGP (5.08 ± 0.30 vs 2.92 ± 0.54 mmol/kg/h, respectively, for the WT and KO mice). Furthermore, icv infusion of GLP-2 enhanced whole-body insulin action in the WT mice (as increased GIR, i.e., 8.40 ± 0.36 and 7.43 ± 0.40 mmol/kg/h, respectively, for the GLP-2 and vehicle groups), but did not impact it in POMC-p110α KO mice. It is important to note that GIR in the vehicle control was lower in POMC-p110α KO mice compared to their WT littermates. Consistently, icv infusion of GLP-2 enhanced insulin-stimulated glucose uptake and utilization by peripheral tissues in the WT mice (as increased Rd, i.e., 8.93 ± 0.58 and 11.49 ± 0.46 mmol/kg/h, respectively, for the GLP-2 and vehicle groups), but did not improve it in the KO mice. Of note, icv infusion of GLP-2 did not further decrease insulin-suppressed HGP or gluconeogenesis in both genotypes. These data clearly demonstrate that CNS GLP-2 suppresses HGP and enhances insulin sensitivity. PI3K in POMC neurons is required for CNS GLP-2-mediated improvement in insulin sensitivity. In fact, p110α in POMC neurons is required to maintain basal HGP and insulin sensitivity.
Figure 6. p110α in POMC neurons is required for icv GLP-2-enhnaced insulin sensitivity and glucose homeostasis.
A. Protocol for quantifying glucose homeostasis and insulin sensitivity under 3-d icv infusion of GLP-2.
B. Blood glucose concentrations after an overnight fast.
C. 8-d icv infusion of GLP-2 augments glucose tolerance under ipGTT.
D. Basal hepatic glucose production is suppressed by 3-d icv infusion of GLP-2 in the WT, but not KO.
E–F. Insulin sensitivity during the clamp is enhanced by 3-d icv infusion of GLP-2 as indicated by higher glucose infusion rate and glucose disappearance rate in the WT mice, but not in the KO mice.
G–H. Hepatic glucose production/gluconeogenesis during the clamp is not further suppressed by icv GLP-2.
I. A model for CNS GLP-2R action on POMC neurons in suppressing hepatic glucose production.
Transcriptionally, CNS GLP-2 activates PI3K-Akt-FoxO1 signaling in POMC neurons as indicated by initiating GLP-2R-PI3K regulatory subunit (p85α) interaction, enhancing PI3K-dependent Akt (S473) phosphorylation, and inducing FoxO1 nuclear exclusion in POMC neurons, disinhibiting Pomc expression. In addition, through the PI3K-dependent signaling pathway, GLP-2 excites and inhibits LepR-positive and InsR-positive POMC neurons, respectively. GLP-2-mediated direct action on POMC neurons contributes at least in part to suppressing hepatic glucose production and gluconeogenesis probably through vagal efferent output to the liver.
10-wk-old mice (with similar BW and fat mass) were infused icv with vehicle (aCSF) or GLP-2 (250 μM at 0.5 μL/h) via a microosmotic pump. Glucose homeostasis and insulin sensitivity were quantified using intraperitoneal (ip) glucose tolerance test after 8-d icv infusion; and stable isotopic tracers (2H2O and 6,6-2H2-D-glucose) coupled with hyperinsulinemic euglycemic clamp after 3-d icv infusion. Data are expressed as means ± SEM (n = 8 per group); **P < 0.01 denoting significance in WT mice between vehicle and GLP-2 at the same points (C), during baseline (D), or during clamp (E–F); a,b or a,cP < 0.05 or 0.01 denoting significance between genotypes during the baseline (B) or clamp (C). See also Figure S2 & S6 and Table S3.
Discussion
The first major finding in this study is that CNS GLP-2R activation suppresses HGP under both basal and hyperinsulinemic conditions. It is unknown if central GLP-2 plays any roles in the regulation of glucose homeostasis and insulin sensitivity. We show that chronic icv infusion of GLP-2 in the WT mice improved glucose tolerance, suppressed basal HGP, and increased ARC Pomc mRNA abundance, suggesting that CNS GLP-2 promotes glucose homeostasis probably through the central melanocortin system. Leptin direct action on POMC neurons lowers glucagon levels, improves hepatic insulin sensitivity, but does not influence insulin-stimulated glucose disposal. Analogously, POMC-Glp2r KO mice display a reversed metabolic phenotype (i.e., increased glucose intolerance, displayed hepatic insulin resistance, impaired insulin-inhibited glucagon secretion, but unaltered insulin-stimulated glucose disposal), suggesting that GLP-2R in POMC neurons plays a physiological role in the control of glucose homeostasis. We speculate that GLP-2, secreted from enteroendocrine L cells in response to food intake and probably transported across the semi-permeable blood-brain barrier in the hypothalamus, activates GLP-2R in POMC neurons to initiate a negative feedback control of HGP at the postprandial condition. In addition, GLP-1/2 are released from preproglucagon (PPG)-positive neurons in the brainstem NTS. Preproglucagon-GFP neurons project widely to the mouse autonomic control areas (including the hypothalamic ARC)(Llewellyn-Smith et al., 2011). PPG neurons are directly excited by leptin and accounted in part for its anti-obesity action, yet little is known about downstream neural transmission. Thus, GLP-2 (released from PPG neurons in the brainstem NTS) might function as a key neural transmitter in the hypothalamic-brainstem neurocircuits to fine-tune glucose homeostasis (including HGP) and energy balance. Regardless of GLP-2 origin, our results indicate that GLP-2R activation in POMC neurons is required for maintaining glucose homeostasis and insulin sensitivity. This HGP-suppressing action is physiologically and pharmacologically important since excessive HGP is a hallmark of T2DM patients. In future studies, it will be important to determine if enhanced CNS GLP-2 signaling is a key contributor to improving glucose homeostasis and insulin sensitivity in diabetes after gastric bypass surgery.
The second major finding is that GLP-2 differentially modulates membrane excitability of POMC neurons in GLP-2R- and PI3K-dependent manners. GLP-2 directly depolarizes LepR-positive POMC neurons or hyperpolarizes InsR-positive POMC neurons. To sort out if GLP-2-mediated action on POMC neurons is attributed to cell autonomous and presynaptic effects, we found that under the presence of TTX, GLP-2 still has a direct action on the membrane depolarization (or hyperpolarization). Therefore, GLP-2 action on POMC neurons cannot be attributed to action potential-dependent presynaptic transmission. In fact, GLP-2-mediated effects are abolished in POMC neurons with Glp2r deletion, pointing again to a direct action of GLP-2-GLP2R on membrane potential. Moreover, in the presence of the PI3K inhibitor, GLP-2-induced depolarization and excitation were blocked in POMC neurons, indicating a PI3K-dependent action. Furthermore, activation of K+ATP channels was attributed to GLP-2-induced inhibition of POMC neurons (Fig. S3). In the context of regulating Ca2+ influx, GLP-2R interacts in vitro with calmodulin(Mahon and Shimada, 2005), a protein partner with TRPC channels(Nilius et al., 2007).
Our finding that GLP-2 differentially affects membrane excitability of POMC neurons in distinct subgroups, which is consistent with the functional heterogeneity of hypothalamic POMC neurons(Williams et al., 2010). For example, leptin-responsive POMC neurons (i.e., acutely activated by leptin) are segregated from insulin-responsive POMC neurons (i.e., acutely inhibited by insulin). PI3K signaling is essential for leptin-induced activation and insulin-induced inhibition of POMC neurons. Leptin and serotonin depolarize POMC neurons via PI3K-dependent PLCγ-generated diacylglycerol activation of TRPC channels(Sohn et al., 2011); in contrast, insulin hyperpolarizes POMC neurons via PI3K-generated PIP3 activation of KATP channels(Plum et al., 2006). Therefore, through activating the GLP-2R-PI3K signaling pathway, GLP-2 depolarizes LepR-positive POMC neurons, while hyperpolarizes InsR-positive POMC neurons, both resulting in decreases in HGP. However, it is currently unclear about distinct properties and functions of these subgroups of POMC neurons. Further studies are warranted to define GLP-2R-mediated action on ion channel activities of POMC neurons in segregated subgroups, which will ultimately determine GLP-2-mediated modulation of glucose homeostasis.
The third major finding is that GLP-2 activates GLP-2R-PI3K signaling in POMC neurons. As mentioned earlier, PI3K signaling is essential for leptin-induced depolarization and insulin-induced hyperpolarization of POMC neurons, resulting from enhanced activities of TRPC channels and KATP channels, respectively(Hill et al., 2008; Plum et al., 2006; Sohn et al., 2011). Through inducing GLP-2R-p85α interaction, GLP-2 rapidly phosphorylates Akt in primary neurons in a PI3K-dependent manner (Shi et al., 2010). Moreover, p85α binding motif on GLP-2R is required for GLP-2 to induce Akt full activation in transfected HEK cells (Fig. S4). These data suggest that the PI3K-Akt signaling pathway may underlie GLP-2-induced modulation of POMC neurons. Basal PI3K signaling in POMC neurons is severely compromised in POMC-Glp2r KO mice as indicated by decreases in Akt phosphorylation and FoxO1 nuclear exclusion. In contrast, GLP-2 activates PI3K-Akt-FoxO1 signaling in POMC neurons (as indicated by increases in Akt phosphorylation and FoxO1 nuclear exclusion), and this effect is abolished in the brain slices from mice with deletion of Glp2r or p110α in POMC neurons. As reported, the basal PI3K activity in POMC neurons is attenuated in POMC-p110a KO mice(Al-Qassab et al., 2009). Moreover, PI3K activation also induces nuclear exclusion of FoxO1, abrogating FoxO1’s inhibition of Pomc and Cpe expression(Kim et al., 2006). Thus, p110α deletion in POMC neurons inactivates PI3K signaling, probably inhibiting the expression and release of α-MSH. Combined with our data that GLP-2 enhances Akt phosphorylation via initiating GLP-2R-p85α interaction and GLP-2 activates the PI3K-Akt-FoxO1 signaling in POMC neurons, we conclude that GLP-2 has a direct action on membrane excitability of POMC neurons via activating GLP-2R-PI3K signaling. Thus, we speculate that via the same PI3K signaling cascade as leptin and insulin act, GLP-2 may activate TRPC channels, exciting LepR-expressing POMC neurons; or activate KATP channels, inhibiting InsR-expressing POMC neurons as shown in our proposed model (Fig. 6I). However, additional studies are warranted to test this speculation in the future.
The fourth major finding is that PI3K in POMC neurons is required for CNS GLP-2-mediated suppression of HGP. The PI3K signaling pathway in POMC neurons is involved not only in energy balance but also glucose homeostasis(Morton et al., 2005; Hill et al., 2009). Increased PI3K-Akt activity in POMC neurons improves insulin sensitivity, whereas decreased PI3K-Akt signaling impairs glucose homeostasis through a hypothalamus-vagal-liver neurocircuit(German et al., 2009). Direct action of insulin and leptin on POMC neurons is required to maintain glucose homeostasis(Hill et al., 2010; Obici et al., 2002). Local infusion of GLP-1 into the ARC decreases HGP(Sandoval et al., 2008). Mice with p85α (or p110α) deletion in POMC neurons display improved (or impaired) insulin sensitivity(Hill et al., 2009), while mice with PDK1 deletion in POMC neurons exhibit hyperglycemia(Belgardt et al., 2008), which links to increased FoxO1 nuclear localization (in POMC neuron) and decreased Pomc gene expression. In this study, we found that p110α deletion in POMC neurons lowers basal HGP, but reduces whole-body insulin sensitivity, pointing out that PI3K signaling in POMC neurons is required not only for basal glucose homeostasis (basal HGP), but also for insulin sensitivity. Moreover, icv infusion of GLP-2 suppresses HGP; however, this suppression is abolished in POMC-p110α KO mice.
POMC neurons are not only localized in the hypothalamic ARC, but also in the brainstem NTS. Theoretically, POMC cre-mediated recombination might occur in POMC neurons within these two nuclei. However, POMC-Cre is expressed mainly in the ARC(Balthasar et al., 2004). In this study, we cannot definitively define the physiological role of GLP-2R in POMC neurons at the nuclei-specific or the subtype-specific manner. Of note, POMC-Cre is transiently expressed in embryonic non-POMC neurons in the brain(Padilla et al., 2012). Ultimately, the POMC-Cre mediated recombination of each floxed allele is unique, depending upon endogenous recombination efficiency. Except for the hypothalamus and hippocampus, Glp2r mRNA and GLP-2R protein are not expressed in the brain regions where POMC-Cre is highly transiently expressed(Padilla et al., 2012). It is well documented that the hippocampus plays a major role in memory and learning, but does not in metabolism. Moreover, Glp2r mRNA expression in the hippocampus is not attenuated in POMC-Glp2r KO. Thus, based on our in situ hybridization and qRT-PCR data, we believe that the transient POMC-Cre activity during development is not sufficient to induce GLP-2R deletion in non-ARC regions (such as hippocampus).
In summary, we demonstrate that [1] Glp2r deletion in POMC neurons impairs glucose tolerance and hepatic insulin sensitivity. Glp2r activation in POMC neurons is required for GLP-2 to enhance insulin-mediated suppression of hepatic HGP and gluconeogenesis. [2] GLP-2 directly modulates postsynaptic membrane excitability of hypothalamic POMC neurons in GLP-2R- and PI3K-dependent manners. [3] GLP-2 activates the PI3K-Akt-FoxO1 signaling in POMC neurons by initiating GLP-2R-p85α protein interaction, anchoring p-Akt membrane localization, enhancing PI3K-dependent Akt phosphorylation, and inducing PI3K-Akt-dependent nuclear exclusion of FoxO1-GFP in POMC neurons. [4] icv GLP-2 augments glucose tolerance, suppresses basal HGP, and enhances insulin sensitivity, which requires PI3K (p110α) activation in POMC neurons. We conclude that CNS GLP-2 suppresses hepatic glucose production at least in part through directly modulating membrane excitability and nuclear transcription of POMC neurons via activating the PI3K-Akt-FoxO1 signaling pathway. Thus, GLP-2R activation in POMC neurons is essential for the maintenance of energy balance and glucose homeostasis.
Experimental Procedures
The protocols of this study were approved by the Animal Care and Use Committee of Baylor College of Medicine. Transgenic mice were generated using the Cre-LoxP system and provided ad libitum access to water and food. Experimental Procedures in detail are provided in the supplemental information.
Assessing Glucose Homeostasis and Insulin Sensitivity Using Hyperinsulinemic Euglycemic Clamp
Glp2rflox/flox (or p110αflox/flox) mice were crossed with POMC-cre+/0 to generate POMC-specific Glp2r (or p110α) WT mice and KO mice. To validate Glp2r deletion in POMC neurons, Glp2r protein expression was determined by immunohistochemistry and the Glp2r mRNA expression by in situ hybridization(Wang and Guan, 2010; Guan et al., 2012). POMC-Glp2r (or p110α) WT and KO male mice were icv infused with vehicle or GLP-2 (250 μM at a rate of 0.5 μL/h) for the insulin clamp (Figure S2). In addition, POMC-Glp2r WT and KO male mice were iv infused with vehicle or GLP-2 (at 500 pmol/kg/h). During the insulin clamp, mice were primed-continuously infused with stable isotopic tracers (2H2O and 6,6-2H2-D-glucose). Glucose kinetics was quantified in conscious mice at the steady status. Hypothalamus and liver samples were harvested for qRT-PCR; and blood samples collected for insulin, GLP-1, and glucagon concentrations by ELISA.
Defining GLP-2R Signaling in Neurons
To examine if GLP-2R directly interacts with PI3K regulatory subunit (p85α), primary neurons were dissociated from the hippocampus and cultured (Wang and Guan, 2010). On DIV 7, neurons were treated with vehicle or GLP-2 (at 20 nM for 30 min) and harvested for co-immunoprecipitation and immunoblotting. Neurons cultured on slides were used for immunohistochemistry. GLP-2R-p85α interaction was confirmed in HEK 293 cells transiently transfected with cDNAs of p85α and GLP-2R (expressing the p85α binding motif or its mutant sequence). To further monitor FoxO1-eGFP nuclear–cytoplasmic shuttling, (Rosa26)FoxO1-eGFP reporter mice were crossed with POMC-cre for WT mice; and then bred with Glp2rflox/flox (or p110αflox/flox) mice for KO mice. Brain slices (250 μm) were treated with vehicle or GLP-2 (at 20 nM for 30 min); and immunostained for GFP and p-Akt.
Electrophysiological Recordings
To determine if GLP-2 directly modulates excitability of POMC neurons, membrane potential and firing rate were measured by the whole-cell current clamp recordings(Sohn et al., 2011) from POMC neurons in brain slices. For electrophysiological recordings, POMC-GFP+/0 mice were crossed with Glp2rflox/flox mice to obtain the WT mice; and then bred with POMC-cre+/0 mice to generate POMC-specific Glp2r KO mice. GLP-2 or other reagents were applied to bath solution. After the patch-clamp recording, the cytoplasm of the recorded neuron was ad hoc acquired via the recording-pipette for single-cell RT-PCR.
Statistical Analysis
Using the mixed procedure (SAS 9.2), we analyzed data with the fixed effects (including genotype, treatment, and time); the random effect (including individual mouse); and repeated measures (at different time points in the same mouse). Thus, a full model for analysis of covariance (ANCOVA) includes BW (and/or lean mass), genotype (WT vs KO), treatment (vehicle vs GLP-2 or inhibitor), and their interactions. Least squares mean of each variable was reported and difference considered significant at p < 0.05. Error bars were indicated by SEM.
Supplementary Material
GLP-2R in POMC neurons is required for GLP-2 to promote glucose homeostasis
GLP-2 modulates excitability of POMC neurons in GLP-2R- and PI3K-dependent manners
GLP-2 activates GLP-2R-PI3K-FoxO1 signaling pathway in POMC neurons
Central GLP-2 enhances hepatic insulin sensitivity in PI3K-dependent manner
Acknowledgments
The authors thank Zhuo Yang, Maria Truong, Shixiang Wen, An Hong Nguyen, Jian Qi, Wei Wang, Guangchen Zhang, Shaji Chacko, Daniel Donaldson, Jerome Stubblefield, Fang Zou, Liangru Zhu, Qiang Tong, Darryl Hadsell, Marta Fiorotto, Juan Marini, and Dennis Bier at Baylor College of Medicine for scientific and technical support. We also thank Yuanzhong Xu at the University of Texas Health Science Center at Houston for technical support. This work is supported by the USDA CRIS 6250-51000-055, NIH Grants DK075489 and DK084125, and the National Natural Science Foundation of China Grant 30728016 (X. G.); and NIH P30DK079638 (L. C.).
Abbreviation
- ARC
arcuate nucleus of the hypothalamus
- GLP-2
glucagon-like peptide-2
- GLP-2R
GLP-2 receptor
- HGP
hepatic glucose production
- NTS
nucleus of the tractus solitaries
- PI3K
phosphoinositide 3-kinases
- PPG
preproglucagon
- POMC
pro-opiomelanocortin
Footnotes
Author Contributions: X. Shi and X. Guan contributed to the study concept and design, acquisition of data, statistical analysis, interpretation of data, and drafting the manuscript. In addition, X. Guan obtained funding, supervised the study, and finalized the manuscript. X. Li and B. Chang contributed to the design and generation of the Glp2r floxed mice. F. Zhou, Y. Wang, Q. Tong, and D. Li assisted with cell culture, in situ hybridization, and electrophysiological recording. Y. Xu, M. Fukuda, J. J. Zhao, D. F. Li, D. G. Burrin and L. Chan provided technical support and critically reviewed the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Al-Qassab H, Smith MA, Irvine EE, Guillermet-Guibert J, Claret M, Choudhury AI, Selman C, Piipari K, Clements M, Lingard S, Chandarana K, Bell JD, Barsh GS, Smith AJ, Batterham RL, Ashford ML, Vanhaesebroeck B, Withers DJ. Dominant role of the p110beta isoform of PI3K over p110alpha in energy homeostasis regulation by POMC and AgRP neurons. Cell Metab. 2009;10:343–354. doi: 10.1016/j.cmet.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrami J, Longuet C, Baggio LL, Li K, Drucker DJ. Glucagon-like peptide-2 receptor modulates islet adaptation to metabolic stress in the ob/ob mouse. Gastroenterology. 2010a;139:857–868. doi: 10.1053/j.gastro.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Bahrami J, Yusta B, Drucker DJ. ErbB activity links the glucagon-like peptide-2 receptor to refeeding-induced adaptation in the murine small bowel. Gastroenterology. 2010b;138:2447–2456. doi: 10.1053/j.gastro.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr, Elmquist JK, Lowell BB. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Belgardt BF, Husch A, Rother E, Ernst MB, Wunderlich FT, Hampel B, Klockener T, Alessi D, Kloppenburg P, Bruning JC. PDK1 deficiency in POMC-expressing cells reveals FOXO1-dependent and -independent pathways in control of energy homeostasis and stress response. Cell Metab. 2008;7:291–301. doi: 10.1016/j.cmet.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Berglund ED, Vianna CR, Donato J, Jr, Kim MH, Chuang JC, Lee CE, Lauzon DA, Lin P, Brule LJ, Scott MM, Coppari R, Elmquist JK. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. J Clin Invest. 2012;122:1000–1009. doi: 10.1172/JCI59816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- Dong CX, Brubaker PL. Ghrelin, the proglucagon-derived peptides and peptide YY in nutrient homeostasis. Nat Rev Gastroenterol Hepatol. 2012;9:705–715. doi: 10.1038/nrgastro.2012.185. [DOI] [PubMed] [Google Scholar]
- Field BC, Chaudhri OB, Bloom SR. Bowels control brain: gut hormones and obesity. Nat Rev Endocrinol. 2010;6:444–453. doi: 10.1038/nrendo.2010.93. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Jones JE, Olson D, Hill J, Lee CE, Gautron L, Choi M, Zigman JM, Lowell BB, Elmquist JK. Monitoring FoxO1 localization in chemically identified neurons. J Neurosci. 2008;28:13640–13648. doi: 10.1523/JNEUROSCI.4023-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German J, Kim F, Schwartz GJ, Havel PJ, Rhodes CJ, Schwartz MW, Morton GJ. Hypothalamic leptin signaling regulates hepatic insulin sensitivity via a neurocircuit involving the vagus nerve. Endocrinology. 2009;150:4502–4511. doi: 10.1210/en.2009-0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X, Karpen HE, Stephens J, Bukowski JT, Niu S, Zhang G, Stoll B, Finegold MJ, Holst JJ, Hadsell D, Nichols BL, Burrin DG. GLP-2 receptor localizes to enteric neurons and endocrine cells expressing vasoactive peptides and mediates increased blood flow. Gastroenterology. 2006;130:150–164. doi: 10.1053/j.gastro.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Guan X, Shi X, Li X, Chang B, Wang Y, Li DP, Chan L. GLP-2 receptor in POMC neurons suppresses feeding behavior and gastric motility. Am J Physiol Endocrinol Metab. 2012;303:E853–E864. doi: 10.1152/ajpendo.00245.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X, Stoll B, Lu X, Tappenden KA, Holst JJ, Hartmann B, Burrin DG. GLP-2-mediated up-regulation of intestinal blood flow and glucose uptake is nitric oxide-dependent in TPN-fed piglets 1. Gastroenterology. 2003;125:136–147. doi: 10.1016/s0016-5085(03)00667-x. [DOI] [PubMed] [Google Scholar]
- Hill JW, Elias CF, Fukuda M, Williams KW, Berglund ED, Holland WL, Cho YR, Chuang JC, Xu Y, Choi M, Lauzon D, Lee CE, Coppari R, Richardson JA, Zigman JM, Chua S, Scherer PE, Lowell BB, Bruning JC, Elmquist JK. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab. 2010;11:286–297. doi: 10.1016/j.cmet.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK. Acute effects of leptin require PI3K signaling in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest. 2008;118:1796–1805. doi: 10.1172/JCI32964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JW, Xu Y, Preitner F, Fukuda M, Cho YR, Luo J, Balthasar N, Coppari R, Cantley LC, Kahn BB, Zhao JJ, Elmquist JK. Phosphatidyl inositol 3-kinase signaling in hypothalamic proopiomelanocortin neurons contributes to the regulation of glucose homeostasis. Endocrinology. 2009;150:4874–4882. doi: 10.1210/en.2009-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Pak YK, Jang PG, Namkoong C, Choi YS, Won JC, Kim KS, Kim SW, Kim HS, Park JY, Kim YB, Lee KU. Role of hypothalamic Foxo1 in the regulation of food intake and energy homeostasis. Nat Neurosci. 2006;9:901–906. doi: 10.1038/nn1731. [DOI] [PubMed] [Google Scholar]
- Koch C, Augustine RA, Steger J, Ganjam GK, Benzler J, Pracht C, Lowe C, Schwartz MW, Shepherd PR, Anderson GM, Grattan DR, Tups A. Leptin rapidly improves glucose homeostasis in obese mice by increasing hypothalamic insulin sensitivity. J Neurosci. 2010;30:16180–16187. doi: 10.1523/JNEUROSCI.3202-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HV, Plum L, Ono H, Gutierrez-Juarez R, Shanabrough M, Borok E, Horvath TL, Rossetti L, Accili D. Divergent regulation of energy expenditure and hepatic glucose production by insulin receptor in agouti-related protein and POMC neurons. Diabetes. 2010;59:337–346. doi: 10.2337/db09-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon MJ, Shimada M. Calmodulin interacts with the cytoplasmic tails of the parathyroid hormone 1 receptor and a sub-set of class b G-protein coupled receptors. FEBS Lett. 2005;579:803–807. doi: 10.1016/j.febslet.2004.12.056. [DOI] [PubMed] [Google Scholar]
- Meier JJ, Nauck MA, Pott A, Heinze K, Goetze O, Bulut K, Schmidt WE, Gallwitz B, Holst JJ. Glucagon-like peptide 2 stimulates glucagon secretion, enhances lipid absorption, and inhibits gastric acid secretion in humans. Gastroenterology. 2006;130:44–54. doi: 10.1053/j.gastro.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Gelling RW, Niswender KD, Morrison CD, Rhodes CJ, Schwartz MW. Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell Metab. 2005;2:411–420. doi: 10.1016/j.cmet.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Murphy KG, Bloom SR. Gut hormones and the regulation of energy homeostasis. Nature. 2006;444:854–859. doi: 10.1038/nature05484. [DOI] [PubMed] [Google Scholar]
- Nelson DW, Sharp JW, Brownfield MS, Raybould HE, Ney DM. Localization and activation of glucagon-like peptide-2 receptors on vagal afferents in the rat. Endocrinology. 2007;148:1954–1962. doi: 10.1210/en.2006-1232. [DOI] [PubMed] [Google Scholar]
- Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med. 2002;8:1376–1382. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]
- Padilla SL, Reef D, Zeltser LM. Defining POMC neurons using transgenic reagents: impact of transient Pomc expression in diverse immature neuronal populations. Endocrinology. 2012;153:1219–1231. doi: 10.1210/en.2011-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum L, Lin HV, Dutia R, Tanaka J, Aizawa KS, Matsumoto M, Kim AJ, Cawley NX, Paik JH, Loh YP, DePinho RA, Wardlaw SL, Accili D. The obesity susceptibility gene Cpe links FoxO1 signaling in hypothalamic pro-opiomelanocortin neurons with regulation of food intake. Nat Med. 2009;15:1195–1201. doi: 10.1038/nm.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum L, Ma X, Hampel B, Balthasar N, Coppari R, Munzberg H, Shanabrough M, Burdakov D, Rother E, Janoschek R, Alber J, Belgardt BF, Koch L, Seibler J, Schwenk F, Fekete C, Suzuki A, Mak TW, Krone W, Horvath TL, Ashcroft FM, Bruning JC. Enhanced PIP3 signaling in POMC neurons causes KATP channel activation and leads to diet-sensitive obesity. J Clin Invest. 2006;116:1886–1901. doi: 10.1172/JCI27123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval DA, Bagnol D, Woods SC, D’Alessio DA, Seeley RJ. Arcuate glucagon-like peptide 1 receptors regulate glucose homeostasis but not food intake. Diabetes. 2008;57:2046–2054. doi: 10.2337/db07-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Porte D., Jr Diabetes, obesity, and the brain. Science. 2005;307:375–379. doi: 10.1126/science.1104344. [DOI] [PubMed] [Google Scholar]
- Shi X, Li X, Wang Y, Zhang K, Zhou F, Chan L, Li D, Guan X. Glucagon-like peptide-2 (GLP-2) stimulated protein synthesis through the PI3-kinase-dependent Akt-mTOR signaling pathway. Am J Physiol Endocrinol Metab. 2010 doi: 10.1152/ajpendo.00620.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn JW, Xu Y, Jones JE, Wickman K, Williams KW, Elmquist JK. Serotonin 2C Receptor Activates a Distinct Population of Arcuate Pro-opiomelanocortin Neurons via TRPC Channels. Neuron. 2011;71:488–497. doi: 10.1016/j.neuron.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang-Christensen M, Larsen PJ, Thulesen J, Romer J, Vrang N. The proglucagon-derived peptide, glucagon-like peptide-2, is a neurotransmitter involved in the regulation of food intake. Nat Med. 2000;6:802–807. doi: 10.1038/77535. [DOI] [PubMed] [Google Scholar]
- Wang Y, Guan X. GLP-2 potentiates L-type Ca2+ channel activity associated with stimulated glucose uptake in hippocampal neurons. Am J Physiol Endocrinol Metab. 2010;298:E156–E166. doi: 10.1152/ajpendo.00585.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KW, Margatho LO, Lee CE, Choi M, Lee S, Scott MM, Elias CF, Elmquist JK. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J Neurosci. 2010;30:2472–2479. doi: 10.1523/JNEUROSCI.3118-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.