Abstract
The blood brain barrier protects the brain from circulating compounds and drugs. The ATP-binding cassette (ABC) transporter P-glycoprotein (Pgp) is involved with the barrier, both preventing the influx of agent from the blood into the brain and facilitating the efflux of compounds from the brain into the blood, raising the possibility of a similar role for other transporters. Multidrug resistance associated protein (MRP), a 190 kDa protein similar to Pgp is also ABC transport that has been implicated in the blood brain barrier. The current study explores its role in opioid action. Immunohistochemically, it is localized in the choroid plexus in ratsand can be selectively downregulated by antisense treatment at both the level of mRNA, as shown by RT-PCR, and protein, as demonstrated immunohistochemically. Behaviorally, downregulation of MRP significantly enhances the analgesic potency of systemic morphine in MRP knockout mice and in antisense-treated rats by lowering the blood brain barrier. Following intracerebroventricular administration, a number of compounds, including some opioids, are rapidly secreted from the brain into the blood where they contribute to the overall analgesic effects by activating peripheral systems. MRP plays a role in this efflux. Downregulating MRP expression leads to a corresponding decrease in the transport and a diminished analgesic response from opioids administered intracerebroventricularly. Thus, the transporter protein MRP plays a role in maintaining the blood-brain barrier and modulates the activity of opioids.
Keywords: opioid, transporter, peripheral, analgesia, MRP, tolerance, transport, efflux
Introduction
The blood-brain barrier impedes the entry of compounds from the blood to the brain, thereby providing protection from circulating agents that might influence brain function. However, evidence suggests that this barrier, which contains a number of transporters, may have a second role in the secretion of compounds from the brain to the peripheral circulation (Banks et al., 1993;Banks and Kastin, 1987;Banks and Kastin, 1996;King et al., 2001). For example, tumor necrosis factor (TNF-α) given intracerebroventricularly rapidly appears intact in the circulation despite the fact that intravenous TNF-α does not gain appreciable entry into the brain (Bodnar et al., 1989). Others have explored the secretions of other cytokines from the brain into the systemic circulation (Goodman et al., 1990;McClain et al., 1991;Romero et al., 1996). In a series of elegant studies, Reichlin and colleagues demonstrated that i.c.v. injections of interleukin-1β stimulated the secretion of interleukin-6 in the brain which then appeared in the peripheral circulation (Romero et al., 1996).
P-glycoprotein (Pgp) is a well established transporter that has been associated with the blood brain barrier, both structurally (Cordon-Cardo et al., 1989;Hegmann et al., 1992;Tatsuta et al., 1992) and functionally (Regina et al., 1998;Schinkel et al., 1994;Schinkel et al., 1995a;Schinkel et al., 1995b;Schinkel et al., 1996). Like other members of the ATP binding cassette (ABC) transporters, Pgp effluxes a wide range of molecules. Initial studies focused upon chemotherapeutic agents and the role of increased Pgp expression with chemoresistance. Among the large number of substrates are the opioids. Pgp has been directly implicated in opioid actions (Aquilante et al., 1999;Callaghan and Riordan, 1993;Chen and Pollack, 1998;King et al., 2001;Thompson et al., 2000;Zong and Pollack, 2000). Elimination of Pgp greatly enhances the actions of systemic morphine by permitting the increased entry of the drug into the brain. However, opioids administered directly into the brain can be secreted into the circulation through a Pgp-medatiated transport system, as shown through antisense and knockout paradigms (King et al., 2001). These findings suggest an expanded role for Pgp beyond the blood-brain barrier to include the efflux of neuro-active agents from the brain to the periphery. Many neurotransmitters and neuromodulators have important targets on peripheral tissues, raising the possibility that this system may provide an important communication link between the brain and peripheral organs.
Multidrug resistance associate protein (MRP), a 190 kDa protein and a member of the ATP-binding cassette (ABC) superfamily of transport proteins has long been associated with drug resistance (Cole et al., 1994;Cole and Deeley, 1996;Cole and Deeley, 1998;Grant et al., 1994;Loe et al., 1996). Yet, MRP and Pgp are quite dissimilar structurally, sharing only approximately 15% amino acid homology. In vitro studies have shown that MRP and Pgp efflux the same anti-tumor chemotherapeutic agents from tumor cells, although with different affinities. Like Pgp, MRP has a wide spectrum of substrates, of which opioids are only a small number. Analysis of human and murine tissues reveals a wide distribution of MRP mRNA among a variety of tissues, with moderate expression in the brain (Loe et al., 1996). In the current study, we have examined the potential role of MRP in the blood brain barrier and its modulation of opioid action.
Materials and Methods
DPDPE, β-endorphin, [D-Ala2,MePhe4,Gly(ol)5]enkephalin (DAMGO), morphine, oxymorphone and naltrexone were gifts from Research Technology Branch of the National Institute on Drug Abuse. Chloramine T, and sodium metabisulfate were purchased from the Sigma Chemical Co. (St. Louis, MO), Ketamine HCl was purchased from Fort Dodge Laboratories, Inc. (Fort Dodge, IA) and Na[125I] was purchased from New England Nuclear (Boston, MA). Antisense oligodeoxynucleotides were synthesized by Midland Certified Reagent (Midland, TX).
β-Endorphin, DPDPE, morphine, oxymorphone and DAMGO were iodinated with Na125I (DuPont, Wilmington, DE) and chloramine T with a peptide/NaI molar ratio of 10:1, as previously described (Goldberg et al., 1998). The iodinated compounds were purified by HPLC over a Rainin Microsorb-MV C18 reverse-phase column (Woburn, MA) with an acetonitrile gradient (10%-60%).
Male Sprague-Dawley rats (250-275 g; Charles River Laboratories, Wilmington, MA), male Crl:CD-1® (ICR) BR mice (25-30g; Charles River Laboratories, Wilmington, MA) and male MRP−/− mice and wild-type controls (Taconic Farms) were maintained on a 12 h light/dark cycle with food and water available ad libitum. Wild-type (FVB/N) and MRP−/− (Mdr1a−/−; FVB/NMRPtm1N7) mice were purchased from Taconic Farms. Intracerebroventricular (i.c.v.) cannulae were implanted in rats as previously described (Rossi et al., 1997b) and animals were allowed one week to recover from the surgery. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee (IA CUC) of Memorial Sloan-Kettering Cancer Center (#90-05-010). The animal care systems of the MSKCC are fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and are in compliance with the Guide for the Care and use of Laboratory Animals. We are also in compliance with the Animal Welfare Act and agree to adhere to the Public Health Service “Principles for the Use of Animals” (NIH Manual Chapter 4,206).
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Male Sprague-Dawley rats were sacrificed and the choroid plexus isolated from the lateral ventricles. Total cytoplasmic RNA of the choroid plexus was isolated and purified by the Ultraspec RNA isolation system (Biotecx Laboratories, Houston, TX). Methods were based upon prior approaches (Pan et al., 1999). Complementary DNA (cDNA) was synthesized from 1 μg total RNA at 37° C for 1 hour using 200 ng random hexamer oligonucleotide, 200 U of Superscript II reverse transcriptase (GibcoBRL, Grand Island, NY), 10 mM dNTP, and 0.1M DTT in a reaction volume of 20 μL.
Two oligodeoxynucleotides (Clonetech, Palo Alto,CA) targeting the rat glyceraldehyde 3-phosphate dehydrogenase were designed at positions 35-60 (sense primer, 5′-TGAAGGTCGGTGTCAACGGATTTGGC-3′) and 994-1017 (antisense primer, 5′-CATGTAGGCCATGAGGTCCACCAC-3′) and utilized in PCRs to quantify the total RNA concentration and used as internal standards. Two oligodeoxynucleotides were designed on the basis of the nucleotide sequence of rat MRP at positions 3049-3073 (sense primer, 5′-CAGTGACTCTGACAACTTGAATGGG-3′) and 3537-3559 (antisense primer, 5′-ACGGATAATGGGCAAACCTGTG-3′) and used in PCRs to amplify cDNA fragment encoding the rat MRP in the choroid plexus. After the reverse transcription, PCR reactions were carried out for 35 cycles using T. aquaticus polymerase (Sigma, St. Louis, MO) to obtain high amplification in the presence of α 32P-dCTP (New England Nuclear). Each cycle consisted of melting step (45 s at 90°C), an annealing step (45 s at 60°C) and an extension step (1 min at 72°C). A 2% agrose gel was used to run the PCR products. The intensity of the bands were visualized on XO-MAT AR film (Kodak, Rochester, NY).
Immunohistochemical Studies
Rats were perfused transcardially with 0.1M phosphate buffered saline (pH 7.4) and fixed with 4% formaldehyde in phosphate buffer. Upon the removal of the brain, it was placed in 4% formaldehyde for 2 hr at room temperature and transferred to 30% sucrose and stored at 4°C overnight. Sections (40 μ) were pre-incubated at room temperature for 30 min in 3% NGST (normal goat serum triton) and then incubated overnight at room temperature with primary antibody, MRPr1 (1:5000) (Kamiya Biomedical Co.; Seattle, WA). The sections were washed with 1% NGST, incubated with the secondary anti-mouse IgG antibody (Vector Laboratories; Burlingame, CA) at room temperature for 1 hr, washed with phosphate buffered saline (PBS), incubated with Elite ABC (Vector Laboratories; Burlingame, CA) at room temperature for 1 hr and washed with PBS. These sections were reacted at room temperature with nickel-DAB until the background increases and washed with PBS. Brain sections were photographed and digitized with Photoshop without modification.
Antisense Studies
The rat MRP antisense oligodeoxynucleotides were based upon rat sequences. These antisense oligodeoxynucleotides were synthesized by Midland Certified Reagent Co., purified, and dissolved in 0.9% saline. The antisense targeting rat MRP (GenBank accession No.X6393, 5′-CGCCGCATAAGACCGAGAGGA-3′) corresponds to nucleotide 456-476. Four base pairs in the MRP antisense were switched to generate the mismatch control (5′-CCGCGACTAAGCACGAGGAGA-3′). Rats received the antisense oligodeoxynucleotides (10 μg in 2 μL, i.c.v.) daily for five days and were tested on the sixth day for either efflux of radiolabeled compounds from the brain or for analgesia.
Efflux studies
Groups of rats received the [125I]compound (i.c.v.; 5-10×106 c.p.m.) at time zero and 200 μl of blood samples were drawn from an indwelling jugular catheter at the indicated times into tubes containing EDTA (70 μL, 0.2 mM). Samples were centrifuged and aliquots of plasma (100 μl) were counted. Prior studies have shown that the radioactivity in the plasma is primarily unchanged compound (King et al., 2001).
Analgesia Studies
Analgesia was assessed quantally in the radiant heat tail-flick assay, as previously described (Rossi et al., 1993;Rossi et al., 1994;Rossi et al., 1996;Rossi et al., 1997a). Baseline latencies ranged from 2-3 s and a cutoff of 10 s was implemented to minimize tissue damage in analgesic animals. Rat studies were assessed using graded responses while mice studies assessed analgesia quantally as an increase equal to or greater than twice the baseline value for that animal (Le Bars et al., 2001). To assess the involvement of possible peripheral mechanisms, the distal portion of the tail was immersed in a DMSO solution containing the antagonist naltrexone (3 mM), which provides a pharmacological effect localized only to the region of the tail exposed to the solution (King et al., 2001; Kolesnikov and Pasternak, 1999) (King et al., 2001;Kolesnikov and Pasternak, 1999). As a control, a more proximal region of the tail that was not exposed to the naltrexone was tested.
In mice analgesia studies, antisense targeting mouse MRP (5 μg in 2 μL, ic.v.; GenBank acession No.NM_008576, GCTATGCTGCTGTGTTGCTGG; corresponds to nucleotide 2766-2786) was administered on days 1, 3 and 5. On day six, analgesia was assessed (as described above). Two base pairs in the MRP antisense were switched to generate the mismatch control (GCTTAGCTGCTGTTGTGCTGG).
Results
MRP expression in the brain
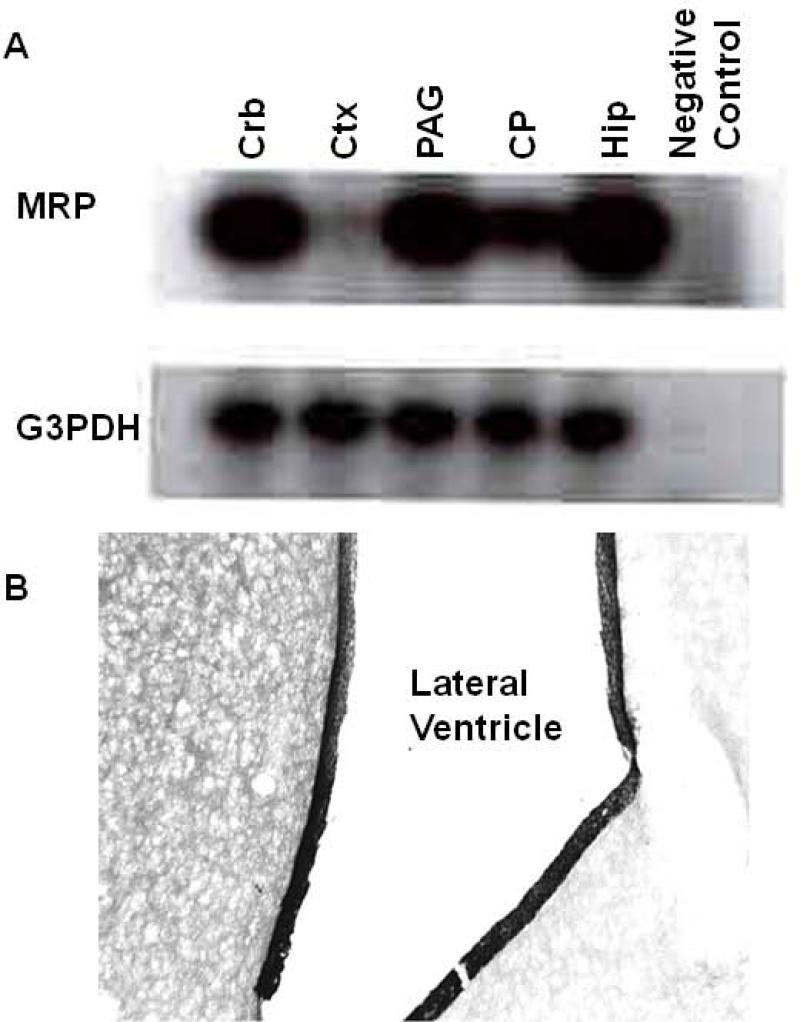
The importance of Pgp in the blood brain barrier raised the question of whether or not other transporters, like MRP, may have similar functions. First, we examined the expression of MRP within the brain. RT-PCR revealed the presence of MRP mRNA in a number of brain structures, including the choroid plexus, the cerebellum (CRB), cortex (CTX), periaqueductal gray (PAG), and hippocampus (HIP) (Fig 1a). However, its expression was not homogeneous. Expression was greatest in the cerebellum, periaqueductal gray and hippocampus, with moderate levels in the choroid plexus and the lowest levels in the cortex. Immunohistochemistry confirmed the expression of MRP within the choroid plexus (Fig 1b), with dense labeling around the border of the ventricle showing a distribution similar to that previously observed with P-glycoprotein (King et al., 2001).
Fig 1. Identification of MRP in rat brain.
a) RT-PCR was performed to determine the level of MRP mRNA expression in the rat cerebellum (CRB), frontal cortex (CTX), periaqueductal gray (PAG), choroid plexus (CP), and hippocampus (HIP). Total cytoplasmic RNA from the indicated regions were isolated and purified by the Ultraspec RNA isolation system (Biotecx Laboratories; Houston, TX) as described in methods. Loading was assessed using the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (G3PDH).
b) Sections of rat brain through the lateral ventricle to view the choroid plexus were prepared as described and then reacted with the MRP1 monoclonal antibody, as described in methods. Labeling was most restricted to the cells lining the ventricle.
The role of MRP in opioid efflux from the brain
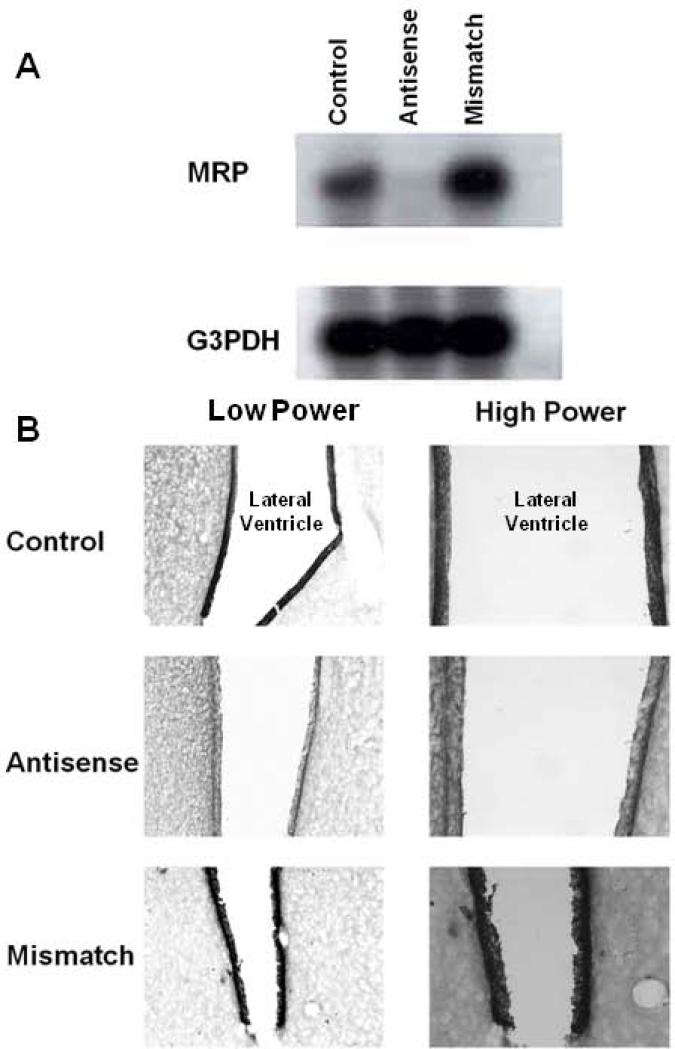
The presence of MRP in the choroid plexus suggested that it might be involved with the blood brain barrier. Prior work from our group has shown that in addition to reducing the entry of compounds from the circulation into the brain, Pgp also actively secretes compounds in the opposite direction, secreting them from the brain into the circulation (King et al., 2001). In these studies administration of a series of opioids and opioid peptides into the lateral ventricle led to their transport and detection into the peripheral circulation (King et al., 2001). To assess whether MRP also is involved in the transport of compounds from the brain to the periphery, we selectively downregulated MRP using antisense approaches previously established in our laboratory to selectively downregulate opioid receptors, neuronal nitric oxide synthase and P-glycoprotein (King et al., 2001;King et al., 1997;Kolesnikov et al., 1997;Rossi et al., 1994;Rossi et al., 1995;Standifer et al., 1994;Standifer et al., 1996). Unmodified antisense oligodeoxynucleotides were administered intracerebroventricularly over a period of five days and animals tested on the sixth day. Downregulation of MRP mRNA was confirmed with RT-PCR (Fig. 2a), with the specificity of the response confirmed by the inactivity of a mismatch control in which the order of only four bases was switched. Immunohistochemical studies confirmed the actions of the antisense at the protein level (Fig. 2). Control rats showed intense labeling in the walls of the ventricle (Fig. 2b), which was not appreciably altered in mismatch control animals (Fig. 2d). However, little immunohistochemical staining could be detected following antisense treatment (Fig. 2c). Thus, the antisense paradigm downregulated MRP expression levels for both mRNA and protein.
Fig 2. Effects of MRP antisense treatment on MRP expression.
The effects of antisense treatment on MRP expression were assessed at the mRNA and protein levels. a) MRP mRNA expression was analyzed using RT-PCR in control, MRP antisense treated and mismatch control rats. All the samples contained the same predicted MRP PCR fragment (530 bp), but the levels in the antisense treated animals were dramatically reduced. Loading was assessed by PCR of G3PDH, which gave the predicted 982 bp fragment in all lanes with equal intensity. b) MRP immunohistochemical staining was used to assess expression at the protein level. Sections of rat brain containing choroid plexus were obtained and reacted with MRPr1 monoclonal antibody, as describe in methods. Staining was carried out on control, antisense and mismatch control animals. The figures show a low power overview along with a higher power of the same section.
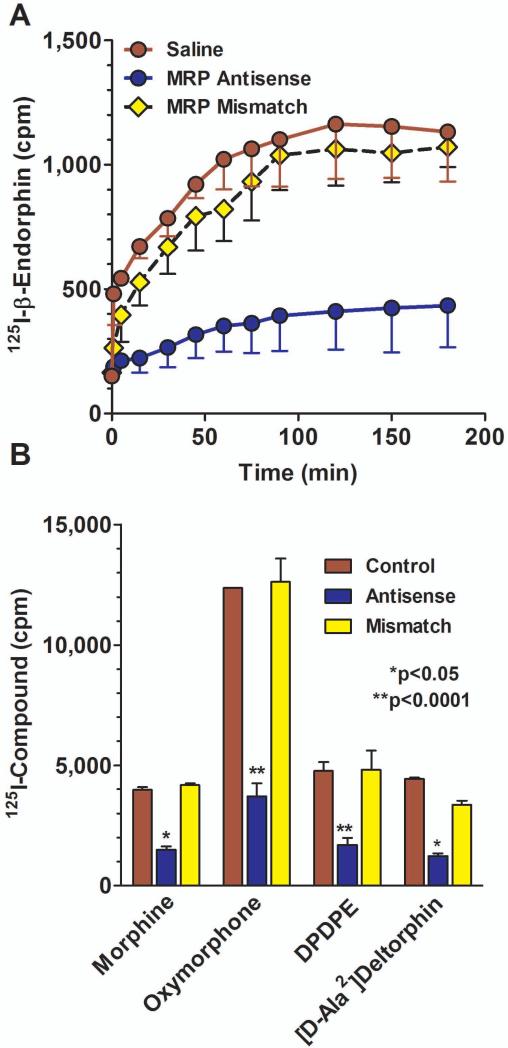
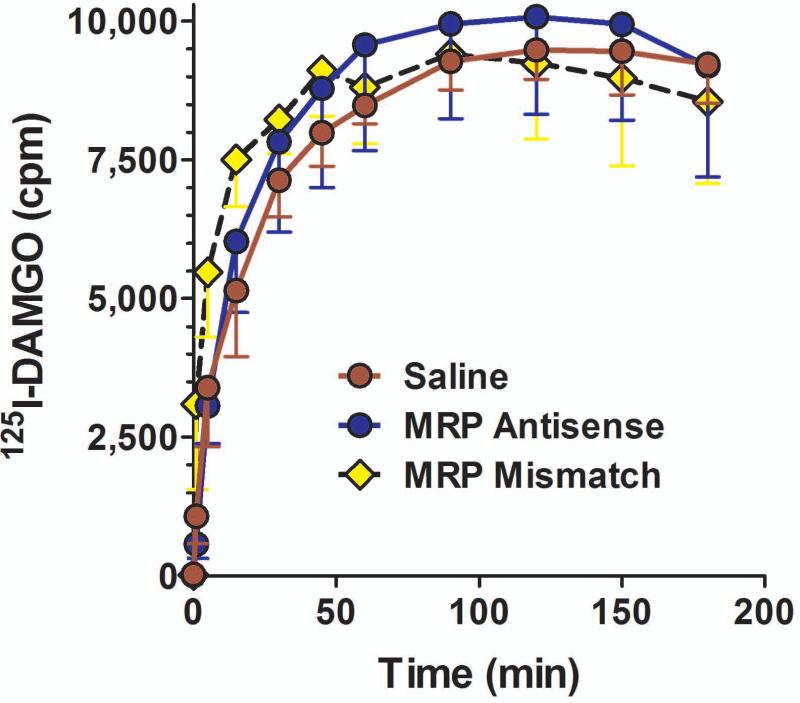
We next examined the role of MPR in the brain-to-blood transport of a series of structurally dissimilar [125]opioids. [125I]β-endorphin administered intracerebroventricularly rapidly appeared in the peripheral circulation (Fig. 3a), as previously shown (King et al., 2001). Treatment of the rats with the mismatch oligodeoxynucleotide was without effect, but the antisense treatment significantly lowered the efflux of the drug (F22,72 = 3.373; p<0.0001). Posthoc analysis (Dunnets) revealed that the efflux in the MRP antisense group, but not the mismatch group, was significantly different from control (p<0.01). Antisense treatment produced a similar blockade of [125I]morphine (F20,88 = 1.73; p<0.05), [125I]oxymorphone (F20,33 = 4.63; p<0.0001), [125I]deltorphin II (F20,121 = 1.77; p<0.05), and [125I]DPDPE (F12,63 = 4.65; p<0.0001) efflux from the brain into the periphery. However, the MRP antisense did not block the transport of all compounds tested. The transport into the circulation of [125I]DAMGO and [125I]dynorphin A was not significantly affected by the antisense treatment. The inactivity of the MRP antisense against [125I]DAMGO transport (Fig. 3) illustrated the relatively stringent specificity of the transporter since the efflux of another pentapeptide, DPDPE, was blocked by the MRP antisense. It also distinguished Pgp from MRP since [125I]DAMGO transport was lowered by Pgp antisense treatment (King et al., 2001).
Fig 3. Effects of peptide secretion from the brain to periphery by MRP antisense treatment.
a) Groups of rats (n = 3) were either treated with saline, MRP antisense, or mismatch control daily for 5 consecutive days. On day 6, [125I]β-endorphin was administered i.c.v. and aliquots of plasma assessed for radioactivity at the indicated time points. ANOVA revealed significant differences among the groups (p<0.0001), with a post hoc Dunnett analysis showing that the antisense group was significantly different from the others (p<0.05).
b) Groups of rats were either treated with saline, MRP antisense, or mismatch control daily for 5 consecutive days. On day 6, [125I]morphine, [125I]oxymorphone, [125I]DPDPE or [125I]deltorphin II (8X106 cpm, i.c.v.) was administered and aliquots of plasma withdrawn from the jugular catheter and counted for radioactivity in the gamma counter. Results are the peak effect time point for control radiodrug. MRP antisense significantly decreased the efflux of these compounds from the brain into the blood.
The role of MRP in systemic opioid analgesia
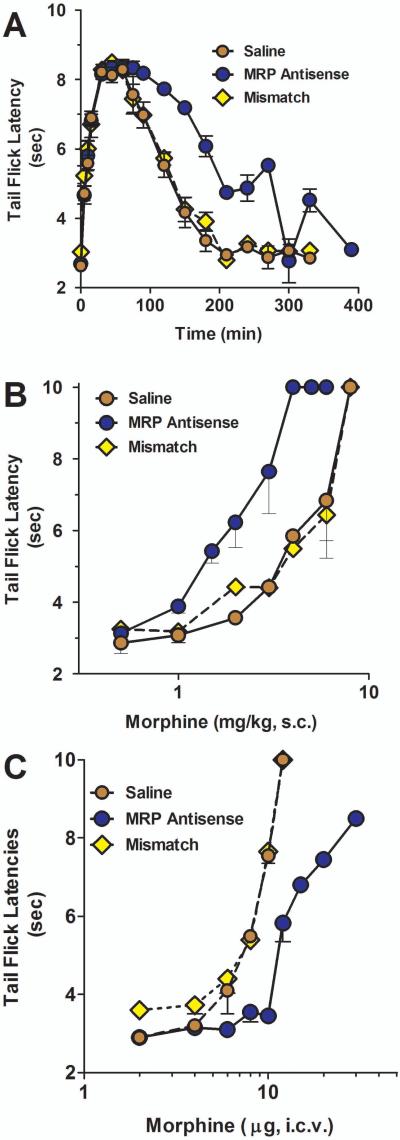
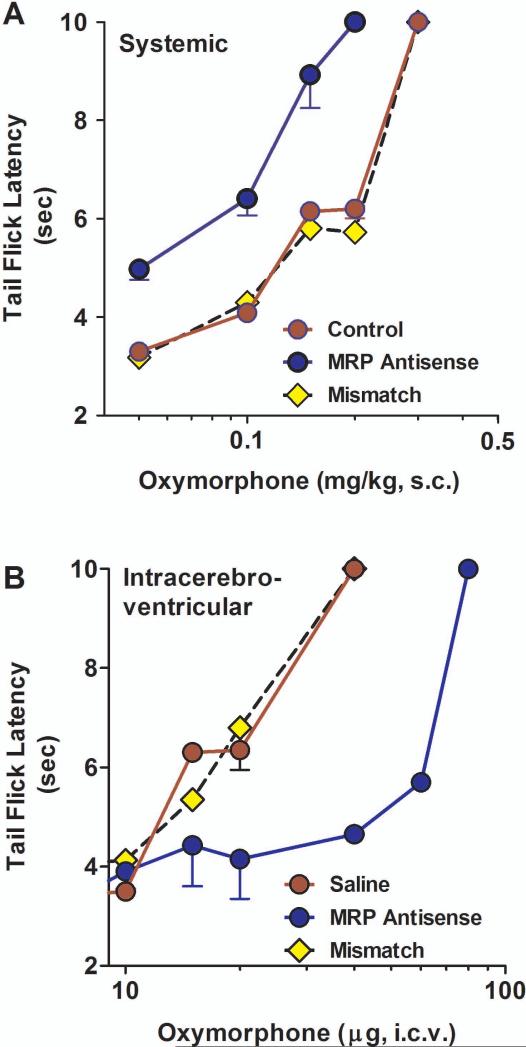
P-glycoprotein modulates morphine analgesia (Drewe et al., 2000;King et al., 2001;Letrent et al., 1998;Letrent et al., 1999;Thompson et al., 2000;Zong and Pollack, 2000). As a component of the blood brain barrier, Pgp impedes the entry of morphine into the brain. Downregulation of Pgp leads to higher morphine levels in the brain and increased analgesic responses. To assess whether MRP had a similar role in the blood-brain barrier, we explored the role of MRP in the production of morphine and oxymorphone mediated analgesia. Downregulation of MRP using antisense enhanced systemic morphine analgesia. At a fixed dose, morphine analgesia displayed a greater peak response and longer duration of action than control animals (data not shown). This prolonged duration of morphine analgesia was still evident when the dose of morphine in the antisense-treated animals was decreased to give similar peak effects as those in the control and mismatch groups (p<0.01) (Fig. 5a). Dose-response studies revealed a significant 2-fold enhanced potency of systemic morphine following downregulation of MRP (p<0.05; Fig 5b, Table 1). Oxymorphone analgesia also was influenced by MRP. Transport studies demonstrated the MDR-dependent transport of oxymorphone from the brain to the peripheral circulation, indicating that oxymorphone was a substrate for the transporter. Downregulation of MRP by antisense treatment shifted the analgesic dose-response curve of systemic oxymorphone approximately 3-fold to the left (Fig. 6a; Table 1).
Fig 5. Effect of MRP on morphine analgesia in rats.
a) Groups of rats (n ≥ 4) received either saline, MRP1 antisense (10 μg; i.c.v.) or mismatch (10 μg; i.c.v.) for five consecutive days. On day six, the animals received equianalgesic doses of morphine determined to yield similar peak effects (saline and mismatch, morphine, 4.5 mg/kg, s.c.; MRP antisense, morphine, 2 mg/kg, s.c.) and analgesia was assessed at the indicated times.
b) Groups of rats (n ≥ 4) received either saline, MRP1 antisense (10 μg; i.c.v.) or mismatch (10 μg; i.c.v.) for five consecutive days. On day six, morphine cumulative dose response curves were generated. Analgesia was assessed as tailflick latency.
c) Groups of rats (n ≥ 4) received either saline, MRP1 antisense (10 μg; i.c.v.) or mismatch (10 μg; i.c.v.) for five consecutive days. On day six, the animals received the indicated morphine dose supraspinally and morphine analgesia was assessed.
Table 1.
Effect of antisense treatment on opioid analgesia
| Saline | Antisense | Mismatch | Antisense Shift | |
|---|---|---|---|---|
| Systemic (mg/kg, s.c.) | ||||
| Morphine | 3.5 (2.2, 5.5) | 1.3 (1.1, 1.6) | 3.9 (2.0, 4.1) | 2.7 |
| Oxymorphone | 0.20 (0.16, 0.25) | 0.08 (0.07, 0.09) | 0.24 (0.21, 0.28) | 2.5 |
| Supraspinal (μg, i.c.v) | ||||
| Morphine | 2.2 (1.5, 3.0) | 10.6 (8.4, 13.3) | 2.2 (1.9, 2.4) | 4.8 |
| Oxymorphone | 16.7 (12.5, 21) | 104 (91, 107) | 16.8 (13.5, 20.8) | 6.2 |
The ED50 values with 95% confidence limits were calculated from the dose-response curves in the figures. The shift by antisense was determined by the ratio of the ED50 of the saline and antisense groups.
Fig 6. Effect of MRP1 antisense on oxymorphone analgesia in rats.
a) Groups of rats (n ≥ 4) received either saline, MRP1 antisense (10 μg; i.c.v.) or mismatch (10 μg; i.c.v.) for five consecutive days. On day six, systemic oxymorphine cumulative dose response curves were generated.
b) Groups of rats (n ≥ 4) received either saline, MRP1 antisense (10 μg; i.c.v.) or mismatch (10 μg; i.c.v.) for five consecutive days. On day six, the animals received the inducated oxymorphone dose supraspinally and oxymorphone analgesia was assessed.
The role of MRP in morphine tolerance
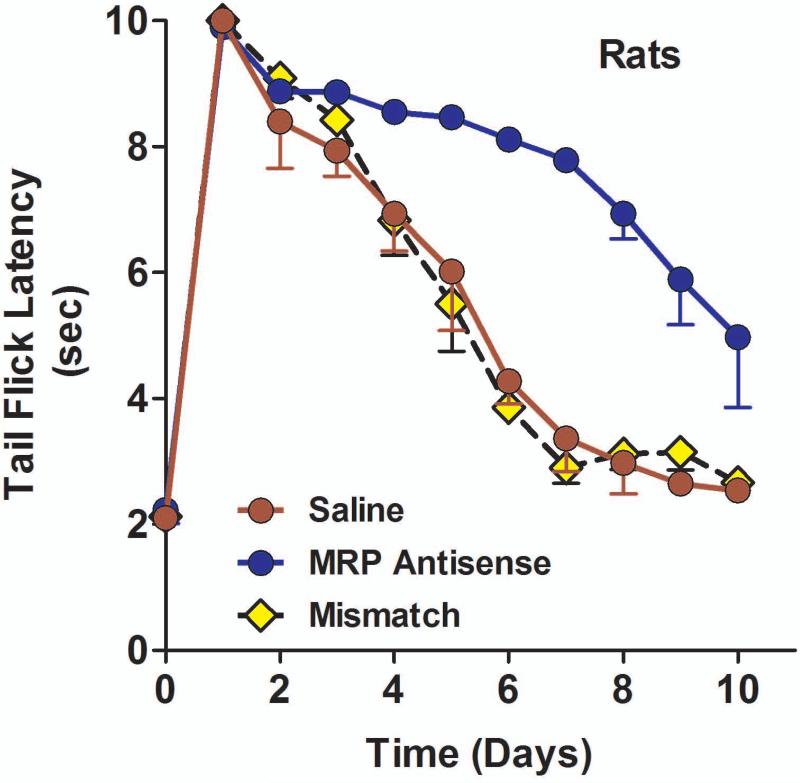
A number of studies have implicated Pgp in morphine tolerance (Aquilante et al., 1999;Hassan et al., 2009;King et al., 2001;Letrent et al., 1999;Mercer and Coop, 2011), leading us to explore MRP in this model. Morphine responses in control and mismatch-treated animals were highest on the first day and rapidly declined over a week. Downregulating MRP expression slowed the development of morphine tolerance (F2,11 = 22.5; p<0.001) (Fig 7). Post hoc analysis revealed that the antisense treated animals were significantly different (p<0.05) from either the saline or mismatch controls. In the MRP antisense-treated group, morphine retained a response near to those in naïve animals for almost a week. Similar results were seen in mice (data not shown), where an antisense treated group showed no decline in analgesic response over the 10 day test (80% to 70%) whereas no analgesic response was seen in the saline group at 7 days compared with 80% on the first day (p<0.004 Fisher Exact Test).
Fig 7. Effect of MRP1 antisense on morphine tolerance.
Groups of rats (n ≥ 4) received either saline, MRP1 antisense (10 μg; i.c.v.) or mismatch (10 μg; i.c.v.) for five consecutive days. On day six, the animals started receiving peak equi-analgesic doses of morphine (saline and mismatch, morphine, 4.5 mg/kg, s.c.; MRP antisense, morphine, 2 mg/kg, s.c.) daily along with either saline, MRP1 antisense or mismatch (i.c.v.). Analgesia was assessed on the indicated days. b) Groups of mice (n ≥ 10) received saline MRP1.
The role of MRP in intracerebroventricular morphine analgesia
Prior studies with Pgp revealed an important role for transport in mechanisms alone can mediate opioid analgesia, their importance became clearer with the demonstration of synergy between peripheral and central sites (Kolesnikov et al., 1996b). Peripheral doses that had little effect alone still markedly potentiated the actions of intracerebroventricular or intrathecal morphine. Although the level of morphine in the circulation following intracerebroventricular administration is low, it still contributed to the overall analgesia (King et al., 2001). In the earlier studies, the downregulation of Pgp lowered the analgesic response to intracerebroventricular morphine due to the diminished secretion of the drug into the systemic circulation. This analgesic effect was opposite to that seen with systemic morphine, where the loss of Pgp enabled higher brain concentrations and a greater analgesic response.
We therefore examined MRP to see if it, too, elicited a similar effect. Like Pgp, downregulating MRP significantly lowered the analgesic response of supraspinal morphine, shifting the dose-response curve approximately 5-fold to the right (p<0.05; Fig 5c; Table 1). Similarly, supraspinal oxymorphone analgesia was decreased approximately 6-fold following antisense treatment, as demonstrated by the shift in the dose-response curves (Fig 6b).
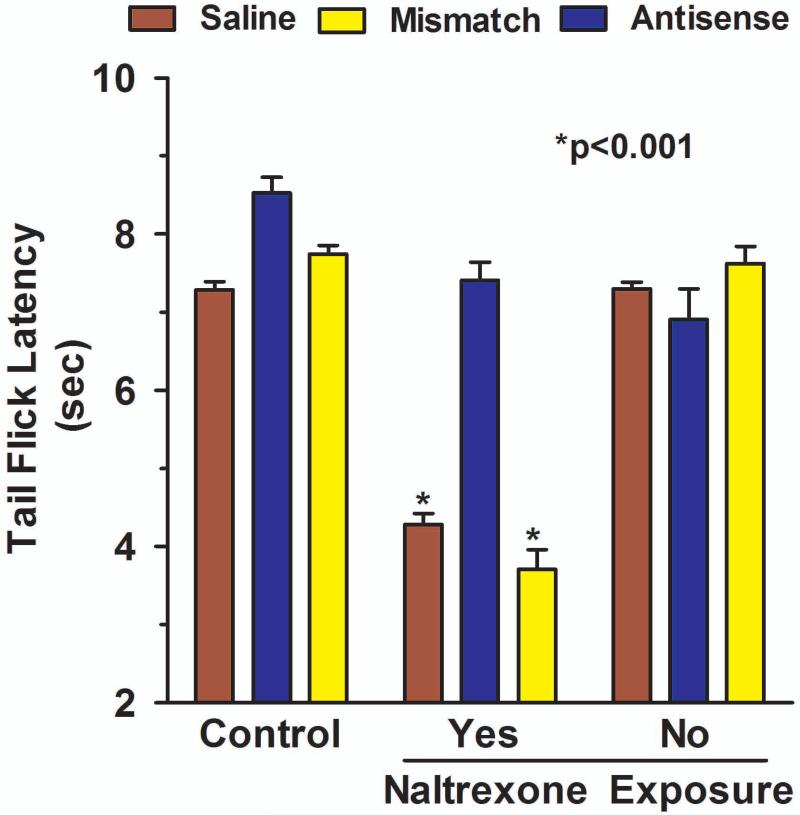
To assess whether this shift was due to the loss of a peripheral site of action, we utilized a paradigm in which peripheral sites were selectively blocked by the opioid antagonist naltrexone administered topically (Kolesnikov et al., 1996a;Kolesnikov and Pasternak, 1999;Kolesnikov et al., 1996b). In this approach, the distal portion of the tail is immersed into a solution of naltrexone in DMSO. Administered in this way, the actions of the antagonist are limited to only the exposed region of the tail, with no appreciable systemic absorption (Kolesnikov and Pasternak, 1999). We next examined the potential role of MRP.
In these studies, we administered doses of morphine that had equianalgesic peak effects in control, mismatch and antisense groups. Since the antisense treatment lowered the response (Fig. 5c), this group received a higher morphine dose (30 μg) than the dose in the other two groups (10 μg) so that their peak responses would be similar. Following intracerebroventricular morphine administration, the analgesic response in control and mismatch animals was lowered on regions of the tail exposed to topical naltrexone (Fig. 8), implying the importance of a peripheral mechanism in the analgesic response. Testing on the proximal region of the tail not exposed to the antagonist offered an important control. If the naltrexone were acting systemically, it should also have blocked the response on the proximal region to the same extent as the distal region which was exposed to the solution containing the drug. Thus, the differences between the proximal and distal responses in the control and mismatch groups confirmed the topical nature of the antagonism (Fig. 8). In the antisense group, the higher morphine dose (30 μg) produced a similar response as in the control and mismatch groups that received a lower morphine dose (10 μg), as expected (Fig. 8) Unlike the other two groups, topical naltrexone had no effect in the MRP antisense treated group (Fig. 8). Thus, downregulation of MRP eliminated the peripheral component of centrally administered morphine analgesic.
Fig 8. Effect of MRP1 antisense on peripheral opioid alkaloids analgesia.
Groups of rats (n ≥ 4) received either saline, MRP1 antisense (10 μg, i.c.v.) or mismatch (10 μg, i.c.v.) for five consecutive days. On day six, they received the indicated morphine dose (saline and mismatch, morphine, 10 μg, i.c.v.; MRP1 antisense, morphine, 30 μg, i.c.v.) supraspinally. Fifteen minutes later, analgesia was assessed and the distal portion of their tails were immersed in a 3 mM naltrexone solution. Tail flick latencies were determined on the region exposed to naltrexone and a more proximal control region that had not been exposed to naltrexone.
MRP knockout mice
Knockout mice offer another model for assessing the role of MRP. Unlike the antisense paradigm, there is a complete loss of MRP in these mice. The effects of the loss of MRP in the knockout model were quite similar to those seen using antisense in the rats. Systemic morphine was over twice as potent in the MRP−/− mice (Fig 9a; Table 2). However, when given supraspinally, morphine was significantly less active in the MRP−/− mice compared to the wild-type mice (p<0.05) (Fig 9b). The decrease in supraspinal morphine analgesia activity in the MRP knockout mice reflected the loss of a peripheral mechanism of action. In the wild-type mice, topical naltrexone lowered the analgesic activity of centrally administered morphine (0.8 μg, i.c.v.). In contrast, topical naltrexone was without effect against an equi-analgesic dose (2.1 μg, i.c.v.) of centrally administered morphine in the MRP−/− mice (Fig 9c).
Fig 9. Morphine analgesia in MRP knockout mice.
a) Cumulative dose response curves were generated in groups (n ≥10) of for both wild-type and MRP knockout mice, with ED50 values of 4.3 mg/kg, s.c. (3.0, 6.4) and 1.7 mg/kg, s.c. (1.1, 2.5), respectively.
b) Groups of wild-type (n ≥ 10) and MRP knockout mice (n ≥ 10) mice received a dose of morphine (700 ng, i.c.v.) supraspinally and analgesia was assessed fifteen minutes later. There was a significant decrease in the morphine analgesic response in the MRP knockout mice compared to the wild-type (p<0.05).
c) Groups of wild-type (n ≥10) and MRP knockout mice (n ≥10) mice received peak equianalgesic doses of morphine (wild-type, morphine 700 ng, i.c.v.; MRP−/−, morphine, 2.1 μg, i.c.v.). Fifteen minutes later, the distal portion of their tails were immersed in a 3 mM naltrexone solution and analgesia assessed both proximally on a region not exposed to naltrexone and distally on a region exposed to the naltrexone.
Table 2.
Systemic morphine analgesia in MRP knockout mice
| ED50 Value (mg/kg, s.c.) | |
|---|---|
| Wildtype mice | 4.3 (3.0, 6.4) |
| MRP Knockout mice | 1.7 (1.1, 2.6) |
ED50 values are from the dose-response curves in Figure 9.
Discussion
The blood-brain barrier, a complex structure which is designed to isolate the brain from circulating drugs, toxins, and xenobiotics. In addition to the well established structural components, it also includes transporters, such as P-glycoprotein (Mercer and Coop, 2011;Rao et al., 1999;Schinkel et al., 1994;Schinkel et al., 1996). Within the blood-brain barrier, the P-glycoprotein transporter pumps drugs that diffuse into the brain from the blood back into the blood. Evidence now suggests that these transporters also pump agents from the brain directly into the circulation. Earlier studies have demonstrated the active transport of a wide range of compounds from the brain into the circulation, including TNF-α (Bodnar et al., 1989), cytokines such as IL-6 (Romero et al., 1996) and others (Abe et al., 1998;Banks et al., 1993;Banks and Kastin, 1987;Banks and Kastin, 1994;Banks and Kastin, 1996;Gao et al., 2000). More recently we documented the ability of Pgp to efflux a variety of opioids from the brain into the circulation (King et al., 2001). Furthermore, this transport was functionally relevant in that the analgesic activity of these centrally-administered opioids was dependent, in part, on the activation of peripheral mechanisms by the secreted drug that interacted synergically with the central ones.
Functionally, P-glycoprotein expression modulates the sensitivity to opioid analagesia through its role in the blood brain barrier (Aquilante et al., 1999;Callaghan and Riordan, 1993;Chen and Pollack, 1998;Hamabe et al., 2007;Hassan et al., 2009;Kalvass et al., 2007;King et al., 2001;Lotsch et al., 2004;Marzolini et al., 2004;Mercer and Coop, 2011;Thompson et al., 2000;Zong and Pollack, 2000). When opioids such as morphine are administered systemically, the blood-brain barrier, which includes the ABC transporters, including Pgp, impedes morphine's entry into the brain. Downregulation of Pgp thereby enhances systemic morphine analgesia. This ability of Pgp to efflux morphine from the brain to the blood leads to a very different effect when the opiate is given centrally. Although central sites remain the primary site of opioid action, peripheral systems also play a role. The importance of peripheral systems is strengthened by the presence of profound peripheral/central synergy (Kolesnikov et al., 1996b). In earlier studies, we showed that peripheral sites influenced morphine analgesia when the drug was given centrally due to the efflux of opioids into the general circulation where it activated peripheral opioid systems that synergized with the central drug actions in both mice and rats (King et al., 2001).
Like Pgp, MRP is a member of the ATP-binding cassette (ABC) or ATPase superfamily of transport proteins, conferring a pattern of drug resistance in tumor cells similar to the Pgps (Cole et al., 1994;Cole and Deeley, 1996;Cole and Deeley, 1998;Grant et al., 1994;Hipfner et al., 1999;Loe et al., 1996). Although dissimilar structurally, both MRP and Pgp transport many similar compounds. Both are upregulated in tumor cells and play a role in resistance to chemotherapeutic drugs and have a variety of physiological actions, including a functional role in maintaining the blood brain barrier. As drugs diffuse from the blood into the endothelial cells of the blood vessels, these transporters efflux the drug from the cell back into the blood, providing a “functional barrier” to supplement the structural ones associated with the blood brain barrier. MRP is expressed in the cerebellum, periaqueductal gray, and hippocampus and at lower levels in the frontal cortex and choroid plexus. Conversely, the expression of Pgp is quite high in the frontal cortex, choroid plexus and periaqueductal gray, with lower levels in cerebellum and hippocampus (King et al., 2001). In this study, we have identified MRP as a transport protein able to efflux a number of neuro-active substances from the brain into the blood, thus suggesting another important pathway for brain/body communication.
The efflux studies confirmed the transport of a number of opioids from the brain to the periphery, as previously shown (King et al., 2001). However, this transport is not limited to opioids. Other neuropeptides also are pumped into the circulation, including substance P (W. Su and G.W. Pasternak, unpublished observations). Thus, this transport system may be more general and its relevance may involve a number of different neurotransmitter/modulator systems. Down regulation of MRP impaired the secretion of all the agents tested, except for [125I]DAMGO and [125I]dynorphin A. This inability to influence [125I]DAMGO secretion, however, illustrates a difference in the selectivity of MRP from that of Pgp.
Like Pgp, MRP in the brain is functionally relevant. The role of MRP in the blood-brain barrier was clearly revealed by the increased potency of systemic morphine and oxymorphone with the downregulation of MRP by antisense in the rat model or in the MRP knockout mice. The loss of MRP presumably lowered the permeability of the blood-brain barrier to these opioids, enhancing their entry into the brain and thus their analgesic activity. The decreased potency of intracerebroventricular opioid following downregulation of MRP in the antisense or the knockout models is similar to that seen with Pgp and reflects the loss of activation of peripheral opioid sites which synergize with central ones. Even the low levels of drug seen in the circulation following intracerebroventricular administration appear sufficient to evoke this synergy. In addition to the role of the peripheral sites, it also is possible that the lack of efflux of morphine from the brain may also increase morphine concentrations in the brain. However, if morphine were acting only centrally, these increased concentrations should have enhanced morphine analgesia and not decreased it. Our current findings also suggest a role for MRP in morphine tolerance. However, it is only one of multiple systems involved with the behavioral phenomenon and its relative significance compared to the other systems is not fully worked out.
A remaining question is the relationship between the two transporters, both structurally and functionally. Each is important in the blood-brain barrier and in central-peripheral transport. At this point we do not know whether they work independently of each other or in series. However, it has been reported that Pgp is localized subapically, whereas MRP is localized basally (Rao et al., 1999). If they act in series, that might explain why downregulation of either is sufficient to lower the overall transport of the ligands and produce the same modulation of opioid analgesia. However, more information is needed to define their functional interactions.
In conclusion, MRP is another ABC transporter implicated in both the blood-brain barrier and the secretion of neuro-active compounds from the brain into the periphery. These transporters make up a unidirectional barrier – transporting compounds from the brain to the circulation. When viewed from the peripheral circulation, this transport returns compounds that diffuse into the brain back into the circulation, essentially presenting a function barrier to entry into the brain. When viewed from the brain, these transporters can secrete compounds from the brain into the peripheral circulation, giving the brain an endocrine-like function. Many of these agents, of which the opioids are only one, have well established peripheral receptors and functions, as exemplified by the brain-gut peptides, and would be anticipated to be functionally active systemically. The current studies explore opioids, but a range of studies have shown similar efflux patterns for other classes of compounds and peptides, implying a more general function. Like Pgp, the transport of these compounds by MRP is unidirectional, with all the compounds secreted into the circulation. In addition to their central actions, many neurotransmitters/modulators have receptors on peripheral tissues mediating additional physiological actions. The ability of transporters to secrete neuro-active compounds from the brain into the circulation may therefore provide a mechanism for the brain to influence a vast array of peripheral tissues in an endocrine-like manner. Understanding the role of all these transporters will be important to more fully define the actions of the brain and its potential control over the whole body.
Fig 4. Effects of DAMGO secretion from the brain to periphery by MRP antisense treatment.
[125I]DAMGO secretion from the brain to the blood in MRP antisense treated rats. Groups of rats (n ≥ 4) received saline, MRP antisense (10 μg; i.c.v.) daily for five days. On day six, rats were administered with [125I]DAMGO (8 x 106 cpm; i.c.v.) and blood samples were taken at the indicated times.
Acknowledgements
This work was supported, in part, by grants (DA06241, DA07242 and DA02615)anda Senior Scientist Award (DA00220) to GWP from the National Institute on Drug Abuse of the National Institutes of Health to GWP and a core grant (CA08748) to MSKCC from the National Cancer Institute of the National Institutes of Health.
Abbreviations
- DPDPE
[D-Pen2,D-Pen5]enkephalin
- DAMGO
[D-Ala2,MePhe4,Gly(ol)5]enkephalin
- MRP
Multidrug resistant protein
References
- Abe T, Kakyo M, Sakagami H, Tokui T, Nishio T, Tanemoto M, Nomura H, Hebert SC, Matsuno S, Kondo H, Yawo H. Molecular characterization and tissue distribution of a new organic anion transporter subtype (oatp3) that transports thyroid hormones and taurocholate and comparison with oatp2. J Biol Chem. 1998;273:22395–22401. doi: 10.1074/jbc.273.35.22395. [DOI] [PubMed] [Google Scholar]
- Aquilante CL, Letrent SP, Pollack GM, Brouwer KLR. Increased brain P-glycoprotein in morphine tolerant rats. Life Sci. 1999;66:L47–L51. doi: 10.1016/s0024-3205(99)00599-8. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ. Saturable transport of peptides across the blood-brain barrier. Life Sci. 1987;41:1319–1338. doi: 10.1016/0024-3205(87)90606-0. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ. Opposite direction of transport across the blood-brain barrier for Tyr-MIF-1 and MIF-1: Comparison with morphine. Peptides. 1994;15:23–29. doi: 10.1016/0196-9781(94)90165-1. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ. Passage of peptides across the blood-brain barrier: Pathophysiological perspectives. Life Sci. 1996;59:1923–1943. doi: 10.1016/s0024-3205(96)00380-3. [DOI] [PubMed] [Google Scholar]
- Banks WA, Kastin AJ, Ehrensing CA. Endogenous peptide Tyr-Pro-Trp-Gly-NH2 (Tyr-W-MIF-1) is transported from the brain to the blood by peptide transport system-1. J Neurosci Res. 1993;35:690–695. doi: 10.1002/jnr.490350611. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ, Pasternak GW, Mann PE, Paul D, Warren R, Donner DB. Mediation of anorexia by human, recombinant Tumor Necrosis Factor through a peripheral action in the rat. Cancer Res. 1989;49:6280–6284. [PubMed] [Google Scholar]
- Callaghan R, Riordan JR. Synthetic and natural opiates interact with P-glycoprotein in multidrug-resistant cells. J Biol Chem. 1993;268:16059–16064. [PubMed] [Google Scholar]
- Chen C, Pollack GM. Altered disposition and antinociception of [D-Penicillamine2,5] enkephalin in mdr1a-gene-deficient mice. J Pharmacol Exp Ther. 1998;287:545–552. [PubMed] [Google Scholar]
- Cole SP, Deeley RG. Multidrug resistance associated with overexpression of MRP. Cancer Treat Res. 1996;87:39–62. doi: 10.1007/978-1-4613-1267-3_2. [DOI] [PubMed] [Google Scholar]
- Cole SP, Deeley RG. Multidrug resistance mediated by the ATP-binding cassette transporter protein MRP. Bioessays. 1998;20:931–940. doi: 10.1002/(SICI)1521-1878(199811)20:11<931::AID-BIES8>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Cole SP, Sparks KE, Fraser K, Loe DW, Grant CE, Wilson GM, Deeley RG. Pharmacological characterization of multidrug resistant MRP-transfected human tumor cells. Cancer Res. 1994;54:5902–5910. [PubMed] [Google Scholar]
- Cordon-Cardo C, O'Brien JP, Rittman-Grauer L, Biedler JL, Melamed MR, Bertino JR. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-barrier sites. Proc Natl Acad Sci USA. 1989;86:695–698. doi: 10.1073/pnas.86.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drewe J, Ball HA, Beglinger C, Peng B, Kemmler A, Schächinger H, Haefeli WE. Effect of P-glycoprotein modulation on the clinical pharmacokinetics and adverse effects of morphine. Br J Clin Pharmacol. 2000;50:237–246. doi: 10.1046/j.1365-2125.2000.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B, Hagenbuch B, Kullak-Ublick GA, Benke D, Aguzzi A, Meier PJ. Organic anion-transporting polypeptides mediate transport of opioid peptides across blood-brain barrier. J Pharmacol Exp Ther. 2000;294:73–79. [PubMed] [Google Scholar]
- Goldberg IE, Rossi GC, Letchworth SR, Mathis JP, Ryan-Moro J, Leventhal L, Su W, Emmel D, Bolan EA, Pasternak GW. Pharmacological characterization of endomorphin-1 and endomorphin-2 in mouse brain. J Pharmacol Exp Ther. 1998;286:1007–1013. [PubMed] [Google Scholar]
- Goodman JC, Robertson CS, Grossman RG, Narayan RK. Elevation of tumor necrosis factor in head injury. J Neuroimmunol. 1990;30:213–217. doi: 10.1016/0165-5728(90)90105-v. [DOI] [PubMed] [Google Scholar]
- Grant CE, Valdimarsson G, Hipfner DR, Almquist KC, Cole SP, Deeley RG. Overexpression of multidrug resistance-associated protein (MRP) increases resistance to natural product drugs. Cancer Res. 1994;54:357–361. [PubMed] [Google Scholar]
- Hamabe W, Maeda T, Kiguchi N, Yamamoto C, Tokuyama S, Kishioka S. Negative relationship between morphine analgesia and P-glycoprotein expression levels in the brain. J Pharmacol Sci. 2007;105:353–360. doi: 10.1254/jphs.fp0071287. [DOI] [PubMed] [Google Scholar]
- Hassan HE, Mercer SL, Cunningham CW, Coop A, Eddington ND. Evaluation of the P-glycoprotein (Abcb1) affinity status of a series of morphine analogs: comparative study with meperidine analogs to identify opioids with minimal P-glycoprotein interactions. Int J Pharm. 2009;375:48–54. doi: 10.1016/j.ijpharm.2009.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegmann EJ, Bauer HC, Kerbel RS. Expression and functional activity of P-glycoprotein in cultured cerebral capillary endothelial cells. Cancer Res. 1992:6969–6975. [PubMed] [Google Scholar]
- Hipfner DR, Deeley RG, Cole SP. Structural, mechanistic and clinical aspects of MRP1. Biochim Biophys Acta. 1999;1461:359–376. doi: 10.1016/s0005-2736(99)00168-6. [DOI] [PubMed] [Google Scholar]
- Kalvass JC, Olson ER, Pollack GM. Pharmacokinetics and pharmacodynamics of alfentanil in P-glycoprotein-competent and P-glycoprotein-deficient mice: P-glycoprotein efflux alters alfentanil brain disposition and antinociception. Drug Metab Dispos. 2007;35:455–459. doi: 10.1124/dmd.106.011445. [DOI] [PubMed] [Google Scholar]
- King M, Su W, Chang A, Zuckerman A, Pasternak GW. Transport of opioids from the brain to the periphery by P-glycoprotein: peripheral actions of central drugs. Nat Neurosci. 2001;4:268–274. doi: 10.1038/85115. [DOI] [PubMed] [Google Scholar]
- King MA, Pan Y-X, Mei J, Chang A, Xu J, Pasternak GW. Enhanced kappa opioid analgesia by antisense targeting the σ1 receptor. Eur J Pharmacol. 1997;331:R5–R6. doi: 10.1016/s0014-2999(97)01064-9. [DOI] [PubMed] [Google Scholar]
- Kolesnikov Y, Jain S, Wilson R, Pasternak GW. Peripheral kappa 1-opioid receptor-mediated analgesia in mice. Eur J Pharmacol. 1996a;310:141–143. doi: 10.1016/0014-2999(96)00520-1. [DOI] [PubMed] [Google Scholar]
- Kolesnikov Y, Pasternak GW. Topical opioids in mice: Analgesia and reversal of tolerance by a topical N-methyl-D-aspartate antagonist. J Pharmacol Exp Ther. 1999;290:247–252. [PubMed] [Google Scholar]
- Kolesnikov YA, Jain S, Wilson R, Pasternak GW. Peripheral morphine analgesia: Synergy with central sites and a target of morphine tolerance. J Pharmacol Exp Ther. 1996b;279:502–506. [PubMed] [Google Scholar]
- Kolesnikov YA, Pan YX, Babey AM, Jain S, Wilson R, Pasternak GW. Functionally differentiating two neuronal nitric oxide synthase isoforms through antisense mapping: Evidence for opposing NO actions on morphine analgesia and tolerance. Proc Natl Acad Sci USA. 1997;94:8220–8225. doi: 10.1073/pnas.94.15.8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev. 2001;53:597–652. [PubMed] [Google Scholar]
- Letrent SP, Pollack GM, Brouwer KR, Brouwer KLR. Effect of GF120918, a potent P-glycoprotein inhibitor, on morphine pharmacokinetics and pharmacodynamics in the rat. Pharm Res. 1998;15:599–605. doi: 10.1023/a:1011938112599. [DOI] [PubMed] [Google Scholar]
- Letrent SP, Pollack GM, Brouwer KR, Brouwer KLR. Effects of a potent and specific P-glycoprotein inhibitor on the blood-brain barrier distribution and antinociceptive effect of morphine in the rat. Drug Metab Dispos. 1999;27:827–834. [PubMed] [Google Scholar]
- Loe DW, Deeley RG, Cole SP. Biology of the multidrug resistance-associated protein, MRP. Eur J Cancer. 1996;32A:945–957. doi: 10.1016/0959-8049(96)00046-9. [DOI] [PubMed] [Google Scholar]
- Lotsch J, Skarke C, Liefhold J, Geisslinger G. Genetic predictors of the clinical response to opioid analgesics: clinical utility and future perspectives. Clin Pharmacokinet. 2004;43:983–1013. doi: 10.2165/00003088-200443140-00003. [DOI] [PubMed] [Google Scholar]
- Marzolini C, Paus E, Buclin T, Kim RB. Polymorphisms in human MDR1 (P-glycoprotein): recent advances and clinical relevance. Clin Pharmacol Ther. 2004;75:13–33. doi: 10.1016/j.clpt.2003.09.012. [DOI] [PubMed] [Google Scholar]
- McClain C, Cohen D, Phillips R, Ott L, Young B. Increased plasma and ventricular fluid interleukin-6 levels in patients with head injury. J Lab Clin Med. 1991;118:225–231. [PubMed] [Google Scholar]
- Mercer SL, Coop A. Opioid analgesics and P-glycoprotein efflux transporters: a potential systems-level contribution to analgesic tolerance. Curr Top Med Chem. 2011;11:1157–1164. doi: 10.2174/156802611795371288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan YX, Xu J, Bolan EA, Abbadie C, Chang A, Zuckerman A, Rossi GC, Pasternak GW. Identification and characterization of three new alternatively spliced mu opioid receptor isoforms. Mol Pharmacol. 1999;56:396–403. doi: 10.1124/mol.56.2.396. [DOI] [PubMed] [Google Scholar]
- Rao VV, Dahlheimer JL, Bardgett ME, Snyder AZ, Finch RA, Sartorelli AC, Piwnica-Worms D. Choroid plexus epithelial expression of MDR1 P glycoprotein and multidrug resistance-associated protein contribute to the blood- cerebrospinal-fluid drug-permeability barrier. Proc Natl Acad Sci U S A. 1999;96:3900–3905. doi: 10.1073/pnas.96.7.3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regina A, Koman A, Piciotti M, Hafny BE, Center MS, Bergmann R, Couraud P-O, Roux F. Mrp1 Multidrug resistance-associated protein and P-glycoprotein expression in rat brain microvesel endothelial cells. J Neurochem. 1998;71:705–715. doi: 10.1046/j.1471-4159.1998.71020705.x. [DOI] [PubMed] [Google Scholar]
- Romero LI, Kakucska I, Lechan RM, Reichlin S. Interleukin-6 (IL-6) is secreted from the brain after intracerebroventricular injection of IL-1β in rats. Am J Physiol. 1996;270:R518–R524. doi: 10.1152/ajpregu.1996.270.3.R518. [DOI] [PubMed] [Google Scholar]
- Rossi GC, Brown GP, Leventhal L, Yang K, Pasternak GW. Novel receptor mechanisms for heroin and morphine-6β -glucuronide analgesia. Neurosci Lett. 1996;216:1–4. doi: 10.1016/0304-3940(96)12976-1. [DOI] [PubMed] [Google Scholar]
- Rossi GC, Leventhal L, Bolan EA, Pasternak GW. Pharmacological characterization of orphanin FQ/nociceptin and its fragments. J Pharmacol Exp Ther. 1997a;282:858–865. [PubMed] [Google Scholar]
- Rossi GC, Leventhal L, Pan YX, Cole J, Su W, Bodnar RJ, Pasternak GW. Antisense mapping of MOR-1 in rats: distinguishing between morphine and morphine-6beta-glucuronide antinociception. J Pharmacol Exp Ther. 1997b;281:109–114. [PubMed] [Google Scholar]
- Rossi GC, Pan Y-X, Brown GP, Pasternak GW. Antisense mapping the MOR-1 opioid receptor: Evidence for alternative splicing and a novel morphine-6β-glucuronide receptor. FEBS Lett. 1995;369:192–196. doi: 10.1016/0014-5793(95)00757-z. [DOI] [PubMed] [Google Scholar]
- Rossi GC, Pan Y-X, Cheng J, Pasternak GW. Blockade of morphine analgesia by an antisense oligodeoxynucleotide against the mu receptor. Life Sci. 1994;54:L375–L379. doi: 10.1016/0024-3205(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Rossi GC, Pasternak GW, Bodnar RJ. Synergistic brainstem interactions for morphine analgesia. Brain Res. 1993;624:171–180. doi: 10.1016/0006-8993(93)90075-x. [DOI] [PubMed] [Google Scholar]
- Schinkel AH, Mol CAAM, Wagenarr E, Van Deemter L, Smit JJM, Borst P. Multidrug resistance and the role of P-glycoprotein knockout mice. Eur J Cancer. 1995a;31A:1295–1298. doi: 10.1016/0959-8049(95)00130-b. [DOI] [PubMed] [Google Scholar]
- Schinkel AH, Smit JJ, Van TO, Beijnen JH, Wagenaar E, Van DL, Mol CA, Van Der Valk MA, Robanus Manndage EC, Riele HP. Disruption of the mouse mdrla P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell. 1994;77:491–502. doi: 10.1016/0092-8674(94)90212-7. [DOI] [PubMed] [Google Scholar]
- Schinkel AH, Wagenaar E, Mol CAAM, Van Deemter L. P-glycoprotein in the blood-barrier of mice influences the brain penetration and pharmacological activity of many drugs. J Clin Invest. 1996;97:25617–2524. doi: 10.1172/JCI118699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinkel AH, Wagenarr E, Van DL, Mol CA, Borst P. Absence of the mdrla P-Glycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone, diagoxin and cyclosporin A. J Clin Invest. 1995b;96:1698–1705. doi: 10.1172/JCI118214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standifer KM, Chien C-C, Wahlestedt C, Brown GP, Pasternak GW. Selective loss of δ opioid analgesia and binding by antisense oligodeoxynucleotides to a δ opioid receptor. Neuron. 1994;12:805–810. doi: 10.1016/0896-6273(94)90333-6. [DOI] [PubMed] [Google Scholar]
- Standifer KM, Rossi GC, Pasternak GW. Differential blockade of opioid analgesia by antisense oligodeoxynucleotides directed against various G-protein α subunits. Mol Pharmacol. 1996;50:293–298. [PubMed] [Google Scholar]
- Tatsuta T, Naito M, Oh-hara T, Sugawara I, Tsuruo T. Functional involvement of P-glycoprotein in blood-brain barrier. J Biol Chem. 1992:20383–20391. [PubMed] [Google Scholar]
- Thompson SJ, Koszdin K, Bernards CM. Opiate-induced analgesia is increased and prolonged in mice lacking P-glycoprotein. Anesthesiology. 2000;92:1392–1399. doi: 10.1097/00000542-200005000-00030. [DOI] [PubMed] [Google Scholar]
- Zong J, Pollack GM. Morphine antinociception is enhanced in mdr1a gene-deficient mice. Pharm Res. 2000;17:749–753. doi: 10.1023/a:1007546719287. [DOI] [PubMed] [Google Scholar]