Abstract
A flea-to-mouse transmission model was developed for use in testing new candidate vaccines for the ability to protect against flea-borne plague. The model was used to evaluate a recombinant fusion protein vaccine consisting of the Yersinia pestis F1 and V antigens. After one to three challenges with Y. pestis-infected fleas, 14 of 15 unvaccinated control mice developed plague, with an average septicemia level of 9.2 × 108 Y. pestis CFU/ml. None of 15 vaccinated mice developed the disease after similar challenges, and serological testing indicated that transmitted bacteria were eliminated by the immune system before extensive replication and systemic infection could occur. The transmission and development of disease in control mice correlated with the number of bites by blocked fleas but not with the total number of fleabites. The model provides a means to directly assess the efficacy of new vaccines to prevent naturally acquired bubonic plague and to study events at the vector-host interface that lead to dissemination and disease.
Yersinia pestis, the bacterial agent of plague, remains an international public health concern. Recent plague outbreaks in India and parts of East Africa, where the disease had been dormant for decades, have raised fears of a new resurgence (6, 33). More troubling was the isolation of multidrug-resistant strains of Y. pestis during the ongoing plague epidemic in Madagascar (10, 12). Added to these concerns is the recognized potential of Y. pestis as a bioterrorism agent (19). The potential threat of plague outbreaks caused by naturally occurring or deliberately released antibiotic-resistant strains increases the urgency of possessing an effective plague vaccine, but none is available; the previously used killed whole-cell vaccine is no longer being produced.
Y. pestis is transmitted primarily by fleabite among its many rodent reservoir hosts. Transmission by direct contact or ingestion of infected tissues can occur in some cases, but the maintenance of plague in its natural environment is thought to depend on rodent-flea-rodent transmission cycles, and most human cases of plague also result from fleabites (21). During the first week after transmission, the bacteria disseminate from the peripheral fleabite site to the regional lymph nodes and produce a severe acute lymphadenitis, characterized by a swollen, painful lymph node called a bubo. Bubonic plague can be treated successfully with an appropriate antibiotic. If not treated, however, a fulminant, high-density, and usually fatal septicemia rapidly develops. Hematogenous spread to the lungs causes secondary pneumonic plague in ∼5% of human cases (5).
New second-generation bivalent recombinant plague vaccines consisting of the Y. pestis F1 capsular and V antigens have shown promise in trials using rodent and primate animal models (13, 20). To evaluate protection against bubonic plague, vaccinated animals were challenged by subcutaneous (s.c.) or other parenteral injection of bacterial suspensions prepared from in vitro-grown cultures. There are important differences between this challenge model and natural challenge by fleas, however. To produce a transmissible infection in the flea, Y. pestis blocks the proventriculus, a valve that connects the esophagus to the midgut, by forming a large aggregate that is embedded within an extracellular matrix. When these blocked or partially blocked fleas attempt to feed, blood containing Y. pestis from the blocking mass is refluxed into the bite site (3, 15). It is in this flea-specific context, which is not duplicated by artificial challenge models, that Y. pestis exits the flea and enters the mammal.
For other arthropod-borne pathogens, it is known that the route of transmission can affect the initial interaction with the host immune system and vaccine efficacy. For example, the saliva of blood-feeding arthropods contains immunomodulatory components that inhibit complement activation, phagocytosis, T-cell proliferation, and cytokine secretion (24, 26). Notably, a vaccine that fully protected mice against needle-injected Plasmodium sporozoites was much less protective against mosquito challenge (31). Because of the unique phenotype and microenvironment of Y. pestis present at transmission and its potential to influence the immune response, we developed a model to explicitly evaluate the abilities of candidate plague vaccines to protect against fleabite challenge.
MATERIALS AND METHODS
Animal immunization.
Outbred, immunocompetent, hairless Crl:SKH1-hrBR mice (Charles River Laboratories, Wilmington, Mass.) were used in this study. Hairless mice were chosen because infected fleas could be more easily recovered from them after challenges. The F1-V recombinant fusion protein vaccine developed at the United States Army Medical Research Institute of Infectious Diseases (13) was evaluated in the flea challenge model. Fifteen 11-week-old female mice were immunized by s.c. injection of 0.2 ml of phosphate-buffered saline (PBS) containing 10 μg of recombinant F1-V fusion protein adsorbed to 0.5 mg of aluminum hydroxide gel adjuvant (Alhydrogel; HCI Biosector, Frederikssund, Denmark). The vaccine was prepared by mixing 8.56 ml of PBS containing 0.56 mg of F1-V protein with 2.64 ml of 2% Alhydrogel overnight at 8°C. Adsorption of the F1-V protein to the adjuvant was checked by protein quantitation (Bio-Rad, Hercules, Calif.) of a portion of the supernatant of the vaccine preparation before and after desorption with 1 M Na2CO3 (1). A second group of 15 11-week old female mice were injected s.c. with 0.2 ml of adjuvant alone. The adjuvant-only control inoculum was prepared similarly by mixing 8.56 ml of PBS without added vaccine and 2.64 ml of 2% Alhydrogel. Six weeks after the initial s.c. injections, the mice received a second identical injection of vaccine or adjuvant control.
Flea infection.
Xenopsylla cheopis fleas were infected with the virulent Y. pestis strain 195/P by using a previously described artificial feeding system (15, 17). To prepare the infectious-blood meal, brain heart infusion broth cultures of Y. pestis were incubated at 37°C for 16 to 18 h without aeration. A volume of culture containing ∼109 bacteria was centrifuged, and the cell pellet was resuspended in 1 ml of PBS. The bacterial suspension was added to 5 ml of fresh heparinized mouse blood, which was placed on a stretched mouse skin that was secured to the bottom of the bell-shaped, water-jacketed blood feeder (32). Fleas were allowed to feed for 90 min. Those that took an infectious-blood meal, as evidenced by the presence of fresh red blood in the midgut, were kept at 21°C and 75% relative humidity and fed on uninfected mice on days 2 and 6 after the infectious-blood meal before they were used for challenges.
Fleabite challenges.
During the period between 7 and 15 weeks after the second immunization, vaccinated and control mice received one to three challenges with infected fleas. Individual mice were anesthetized by intraperitoneal injection of 0.2 ml of a 17-mg/ml ketamine-0.7-mg/ml xylazine solution and confined in a restraint tube within a high-walled container. Beginning 9 to 10 days after the infectious-blood meal, cohorts of infected fleas were split and used to challenge equal numbers of vaccinated and control mice. Ten to 60 fleas were applied to each restrained mouse and allowed to feed ad libitum for 60 min. All of the fleas were then recovered and individually examined under a dissecting microscope to determine how many had taken a normal blood meal (indicated by the presence of fresh red blood filling the midgut) and how many were blocked (indicated by the presence of fresh red blood only in the esophagus, anterior to the proventriculus) (17). The fleas were then combined, returned to the 21°C incubator, and used to challenge other mice in the same way every 2 to 3 days for 2 to 3 weeks.
After each challenge, the mice were returned to their cages and examined three times daily for disease onset, evidenced by lethargy, hunched posture, and reluctance to respond to external stimuli. Mice were euthanized upon the appearance of these symptoms, and blood and/or spleens were collected to verify infection. Mice that survived the first challenge were rechallenged once or twice more at intervals of 2 to 4 weeks. The surviving mice were euthanized 4 to 5 weeks after the final challenge, blood was collected for serology, and spleens were collected for bacteriological analysis. All experiments were performed at biosafety level 3 and approved by the Rocky Mountain Laboratories, National Institute of Allergy and Infectious Diseases, National Institutes of Health Biosafety and Animal Care and Use Committees in accordance with National Institutes of Health guidelines.
Bacteriological and serological testing.
One week before the first challenge, 0.25 ml of blood was collected from each mouse by retroorbital bleeding, and the anti-F1 and anti-V titers in the sera were determined by direct immunoglobulin G (IgG) enzyme-linked immunosorbent assay in which 96-well plates were coated with soluble F1 and V proteins, as previously described (1).
For immunoblots, proteins in whole-cell lysates of Y. pestis grown at 37°C in brain heart infusion were first separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (12.5% polyacrylamide) and then electrophoretically transferred to nitrocellulose membranes using equipment and protocols from Bio-Rad. The blots were blocked with 5% (wt/vol) nonfat dry milk in Tris-buffered saline containing 0.05% Tween 20 (TBST). The membranes were probed with a 1:200 dilution of mouse serum at room temperature for 2 h, washed five times in TBST, and incubated with horseradish peroxidase-conjugated goat anti-mouse IgG (Pierce, Rockford, Ill.), following the manufacturer's protocol. Immunoreactive proteins were visualized by using the SuperSignal West Pico Chemiluminescent Luminol/Enhancer reagent (Pierce).
Of the 14 symptomatic mice, heparinized blood was collected from 7, the spleens were collected from 4, and both blood and spleens were collected from 3 immediately after euthanasia. Both blood and spleen were also collected from the one surviving control mouse. The spleens were completely triturated through wire mesh into sterile PBS, pH 7.4, and dilutions of the blood samples and spleen triturates were spread on Yersinia selective agar plates (Difco). CFU were counted after 48 to 72 h of incubation at 28°C.
Statistical analyses.
Correlation between the total number of fleabites, or the number of bites by blocked fleas, and outcome was done by chi square calculated from contingency tables charting the number of bites versus survival or disease. Correlation between the time to onset of symptoms and the total number of fleabites, or the number of bites by blocked fleas, was evaluated by linear regression.
RESULTS
Vaccination in the hairless-mouse model.
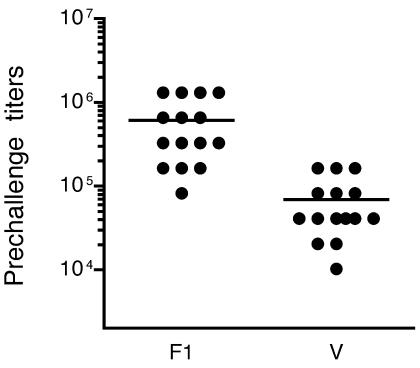
Successful vaccination was verified by determining the anti-F1 and anti-V enzyme-linked immunosorbent assay total IgG titers of mice prior to challenge (Fig. 1). The average anti-F1 titer in the 15 vaccinated mice was 606,208 (range, 81,920 to 1,310,720; median, 327,680), and the average anti-V titer was 68,949 (range, 10,240 to 163,840; median, 40,960). The antibody titers in the SKH1-hrBR hairless mice were as high as those measured in inbred Smith Webster mice used for s.c. injection trials with the same vaccine and immunization protocol (13). The relative responses of the outbred hairless mice to the two antigens were different, however, in that anti-F1 titers were higher than anti-V titers. Sera from the 15 adjuvant-only control mice did not contain detectable antibody to either the F1 or V protein.
FIG. 1.
Prechallenge anti-F1 and anti-V total IgG antibody titers in vaccinated mice. Average titers are indicated by the horizontal lines.
Challenge model.
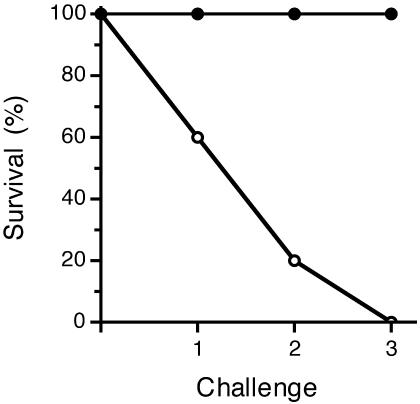
Given the inability to control the infectious dose delivered by the fleas, the first objective in establishing the model was to determine a level of challenge that would adequately test vaccine efficacy. Before being used for challenges, the fleas were fed on nonexperimental mice for the first week after the infectious-blood meal. This prechallenge incubation period was to allow sufficient time for a transmissible infection to develop in the fleas. Our previous experience, and that of other investigators, had shown that proventricular blockage, which correlates with maximal transmission efficiency, does not develop in the flea until at least 1 week after infection (4, 9, 15). A single challenge with infected fleas resulted in successful transmission and disease development in 6 of the 15 control mice (Fig. 2). Six of the nine control mice that survived a single challenge developed disease after a second challenge. Two of the three control mice that survived two challenges were challenged a third time, and both developed disease. Each challenge consisted of 12 to 48 fleabites, which included 0 to 6 bites by blocked fleas. In summary, the challenges resulted in plague in 14 of 15 (93.3%) control mice (Table 1).
FIG. 2.
Survival rates of vaccinated mice (solid circles) and control mice (open circles) after consecutive challenges with infected fleas. One control that survived two challenges (mouse C11 [Table 1]) was not given a third challenge.
TABLE 1.
Incidence of plague in control mice after fleabite challenge
| Mouse | No. of challenges | Total no. of fleabites (cumulative) | No. of blocked fleabites (cumulative) | Time to onset of symptoms (h) |
|---|---|---|---|---|
| C1 | 1 | 12 | 0 | 101 |
| C2 | 2 | 51 | 3 | 55 |
| C3 | 2 | 56 | 3 | 50 |
| C4 | 1 | 13 | 0 | 53 |
| C5 | 1 | 18 | 3 | 56 |
| C6 | 1 | 22 | 5 | 77 |
| C7 | 1 | 24 | 2 | 74 |
| C8 | 1 | 16 | 0 | 74 |
| C9 | 3 | 79 | 6 | 49 |
| C10 | 3 | 87 | 8 | 75 |
| C11 | 2 | 47 | 3 | Survived |
| C12 | 2 | 61 | 5 | 99 |
| C13 | 2 | 55 | 5 | 66 |
| C14 | 2 | 58 | 6 | 72 |
| C15 | 3 | 96 | 7 | 56 |
| Mean ± SD | 1.8 ± 0.8 | 46.3 ± 27.8 | 3.7 ± 2.5 | 68 ± 17 |
Indicators of flea-borne transmission.
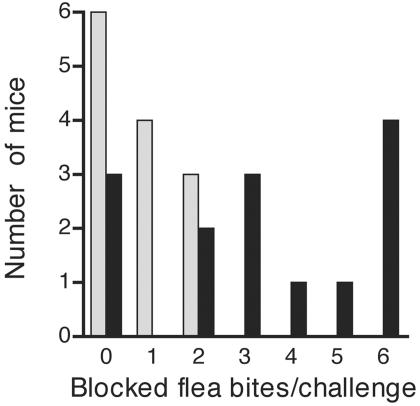
Three control mice developed plague after a single challenge of 12 to 16 fleabites in which none of the fleas was blocked. On the other hand, the one surviving control mouse was challenged twice and had been fed upon by 47 fleas, 3 of which were blocked. Despite the inherent flea-to-flea variability in transmission, development of disease correlated with the number of bites by blocked fleas (P < 0.05) but not with the total number of fleabites (P = 0.46) per challenge (Fig. 3). When plague did occur, it developed rapidly in the control mice (50 to 101 h after challenge). The time to onset of symptoms did not correlate with either the total number of fleabites (P = 0.37) or the number of bites by blocked fleas (P = 0.60). Following euthanasia, Y. pestis levels in the peripheral blood, the spleen, or both tissues were determined for each symptomatic mouse. The average septicemia was 9.2 × 108 Y. pestis CFU/ml (range, 2.2 × 104 to 5.6 × 109; median, 2.4 × 108), and the average bacterial burden in the spleen was 6.9 × 109 Y. pestis CFU (range, 6.0 × 104 to 4.0 × 109; median, 1.0 × 108).
FIG. 3.
Frequency distribution of numbers of control mice that survived (shaded bars) or developed disease (solid bars) after individual challenges that included zero to six bites by blocked fleas.
Protective efficacy of F1-V vaccine.
The 15 vaccinated mice were challenged concurrently with the control mice, using the same cohorts of infected fleas. Vaccinated mice received two or three challenges until each had experienced a minimum of four bites by blocked fleas and on average were challenged with more total bites and more bites from blocked fleas than the controls (Table 2). None of the vaccinated mice developed disease or showed any symptoms. The 15 vaccinated mice and the one surviving control mouse were euthanized 4 to 5 weeks after the final challenge. Cultures of blood and spleen from the control mouse, and of spleens from the 15 vaccinated mice, were all negative.
TABLE 2.
Protection of vaccinated mice against fleabite challenge
| Mouse | No. of challenges | Total no. of fleabites (cumulative) | No. of blocked fleabites (cumulative) | Time to onset of symptoms (h) |
|---|---|---|---|---|
| V1 | 3 | 107 | 11 | Survived |
| V2 | 3 | 97 | 7 | Survived |
| V3 | 3 | 101 | 6 | Survived |
| V4 | 3 | 87 | 8 | Survived |
| V5 | 2 | 67 | 6 | Survived |
| V6 | 2 | 45 | 4 | Survived |
| V7 | 2 | 43 | 11 | Survived |
| V8 | 3 | 91 | 13 | Survived |
| V9 | 3 | 69 | 7 | Survived |
| V10 | 2 | 66 | 7 | Survived |
| V11 | 3 | 103 | 13 | Survived |
| V12 | 2 | 61 | 6 | Survived |
| V13 | 2 | 60 | 5 | Survived |
| V14 | 2 | 75 | 9 | Survived |
| V15 | 2 | 60 | 4 | Survived |
| Mean ± SD | 2.5 ± 0.5 | 75.5 ± 20.9 | 7.8 ± 3.0 |
Pre- and postchallenge sera from three surviving vaccinated mice were used for immunoblots of whole-cell Y. pestis lysates. The sera used for Fig. 4 were from mouse V1 (Table 2), which was bitten by 24 fleas in the first challenge, none of which were blocked; by 52 fleas, 5 of which were blocked, in a second challenge 5 weeks later; and by 31 fleas, 6 of which were blocked, in the final challenge 2 weeks after the second. Thus, this mouse experienced bites from 11 blocked fleas, indicating a high probability of transmission. As expected, the paired sera both reacted with V and F1 antigens, the vaccine components. Few additional Y. pestis antigens were recognized in the postchallenge sera, collected 4 weeks after the final challenge.
FIG. 4.

Anti-Y. pestis antibodies present in prechallenge (A) and postchallenge (B) paired sera from vaccinated mouse V1 (Table 2) that survived three challenges with infected fleas over a 7-week period. Immunoblots of whole-cell lysates of Y. pestis grown at 37°C are shown. The asterisks indicate the positions of the 37-kDa V antigen and the 17.6-kDa F1 antigen.
DISCUSSION
Potential new vaccine formulations designed to prevent disease caused by arthropod-transmitted pathogens are not often tested by natural infection routes, but there are good reasons for doing so. Transmission and subsequent infection caused by these agents depends on a complex coevolved interaction among the pathogen, vector, and vertebrate host that has not been well characterized for any arthropod-borne disease (23). However, vector-specific attributes are known to influence the outcome of infection, immune response, and vaccine efficacy for some arthropod-borne pathogens. For example, a component of sandfly saliva greatly enhances the infectivity of Leishmania, and normal infectivity of an arbovirus depends on both vector saliva and host factors at the bite site (8, 30). The 50% infective dose of Plasmodium berghei sporozoites recovered from infected mosquitoes and injected intravenously has also been shown to be 10 to 100 times greater than that of sporozoites delivered by mosquito bite. More importantly from the standpoint of vaccinology, the neutralizing ability of protective antibody and vaccine efficacy were greatly reduced in mosquito-borne compared to needle and syringe transmission models (31). Because the phenotype of the pathogen in the vector and the microenvironment of the deposition site in the skin are usually unknown and impossible to duplicate by needle and syringe inoculation of artificially cultured organisms, it seems prudent to include the natural transmission route in evaluating the efficacies of potential vaccines for arthropod-borne pathogens. Relying on artificial challenge alone may rule out candidate vaccines that may be perfectly adequate in the natural situation or falsely sanction others that are not, even though artificial challenge models indicate efficacy.
Previous trials of the recombinant FI-V fusion protein vaccine used in this study have demonstrated complete protection for vaccinated mice challenged by s.c. injection with >106 wild-type Y. pestis organisms, a dose which is 105 times greater than the 50% lethal dose (13). Few data are available that address the number of Y. pestis organisms transmitted per fleabite, but an estimate of 11,000 to 24,000 bacteria transmitted by a single blocked X. cheopis flea has been reported (4). From the standpoint of sheer numbers, therefore, the challenge inocula delivered by the natural flea-borne route of transmission can be easily matched experimentally by needle injection, but factors specific to the bacteria-vector-host transmission triad are not. Arthropod-borne bacterial pathogens exhibit a specific phenotype in their vectors and produce vector-specific antigens. For example, Borrelia burgdorferi spirochetes transmitted by ticks display different surface antigens and induce a different immune response than do in vitro-grown, needle-inoculated spirochetes (11, 27). Y. pestis upregulates the expression of certain transmission-related genes in its flea vector that are downregulated or not expressed in the mammal (14, 21, 28). These genes enable Y. pestis to grow in a flea-specific biofilm-like phenotype in which a dense bacterial aggregate embedded within an extracellular matrix blocks the proventriculus (15, 18). The composition of the extracellular matrix associated with the biofilm-like growth of Y. pestis in the flea is unknown, but it is complex and appears to contain flea gut components in addition to bacterially derived material (B. J. Hinnebusch, unpublished data). The extracellular matrix associated with Y. pestis as it exits the flea and enters the mammal might provide initial protection against uptake or killing by phagocytes, as has been demonstrated for the extracellular matrices of other bacterial biofilms (7).
The feeding mechanism of fleas determines the initial site of infection and the ensuing dissemination route of Y. pestis. The mouthparts of X. cheopis are ∼400 μm in length, which precludes s.c. injection. Flea saliva introduced into the intradermal bite site contains apyrase, an enzyme which acts to inhibit platelet and neutrophil aggregation, and may contain other immunomodulatory factors, as is true for the saliva of other blood-feeding arthropods (24-26).
Because of the unique phenotype and microenvironment in which Y. pestis is presented to the host by fleas, and its potential to affect immune surveillance and vaccine-induced protection, we developed a model to evaluate the abilities of plague vaccines to protect against natural challenge. Our results provide direct evidence that the F1-V recombinant fusion vaccine is able to protect against bubonic plague transmission in a real-world context. In this trial, the vaccine proved 100% effective in preventing flea-borne plague in mice after they had received challenges greater than those which resulted in disease in adjuvant-only control mice. Immunoblots of pre- and postchallenge sera demonstrated little evidence of seroconversion to other Y. pestis antigens in the vaccinated mice (Fig. 4), indicating that immunization eliminated the bacteria before they could extensively replicate, produce a disseminated infection, and stimulate a generalized immune response.
Several factors contributed to establishing a practical model. The mouse strain used was highly susceptible to plague and rapidly developed terminal septicemia, making it a sensitive animal model. The major disadvantage of natural challenge is that the dose cannot be controlled. Thus, an important aspect of the model was to define the parameters of a sufficient challenge. Transmission by fleas is inherently variable, as has been noted consistently by previous investigators. Nearly half of the fleas clear themselves of infection even after feeding on a highly septicemic blood meal, and less than half of the fleas that are successfully colonized by Y. pestis develop proventricular blockage (4, 17, 22). Then, individual blocked X. cheopis fleas that attempt to feed on a susceptible host transmit plague only ∼50% of the time (4), despite the fact that blocked X. cheopis fleas contain 105 to 107 Y. pestis organisms (16). The use of hairless mice made it feasible to simultaneously challenge with large numbers of infected fleas, which helped to overcome individual flea-to-flea variability and to increase the probability of transmission. The data reinforce the importance of the proventricular-blockage phenomenon in transmission by X. cheopis. Successful transmission to control mice correlated strongly with the number of bites by blocked fleas but not with the total number of fleabites (Fig. 3). Complete proventricular blockage was not absolutely required, however, because 3 of 15 (20%) control mice acquired plague even though none of the challenge fleas appeared to be blocked. This could be due to missed diagnosis of blockage or transmission by partially blocked fleas, which are also considered to be transmission competent (2). In our model, the vaccinated mice experienced bites from a minimum of four blocked fleas to ensure a high probability of transmission.
The natural challenge model provides a new tool to evaluate future second- and third-generation plague vaccines. In addition, very little is known about the early events after flea-borne transmission that lead to systemic invasion and disease. It has been hypothesized that Y. pestis is ingested and transported from the dermis to the regional lymph nodes by macrophages (29), but this has not been examined directly. The vector-borne transmission model should be useful in investigating how the flea-specific phenotype of Y. pestis and the microenvironment of the fleabite site influence the initial encounter with the host.
Acknowledgments
We thank James Musser for suggesting the hairless mouse strain, Donald Lodmell, Roberto Rebeil, and David Erickson for critical reading of the manuscript, and Christopher Bolt for technical assistance.
This work was supported in part by a New Scholars Award in Global Infectious Diseases to B.J.H. from the Ellison Medical Foundation.
Editor: J. B. Bliska
REFERENCES
- 1.Anderson, G. W., Jr., S. Leary, E. D. Williamson, R. W. Titball, S. L. Welkos, P. L. Worsham, and A. M. Friedlander. 1996. Recombinant V antigen protects mice against pneumonic and bubonic plague caused by F1-capsule-positive and -negative strains of Yersinia pestis. Infect. Immun. 64:4580-4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bacot, A. W. 1915. Further notes on the mechanism of the transmission of plague by fleas. J. Hyg. Plague 14(Plague Suppl. 4):774-776. [PMC free article] [PubMed] [Google Scholar]
- 3.Bacot, A. W., and C. J. Martin. 1914. Observations on the mechanism of the transmission of plague by fleas. J. Hyg. Plague 13(Plague Suppl. 3):423-439. [PMC free article] [PubMed] [Google Scholar]
- 4.Burroughs, A. L. 1947. Sylvatic plague studies. The vector efficiency of nine species of fleas compared with Xenopsylla cheopis. J. Hyg. 45:371-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Butler, T. 1983. Plague and other Yersinia infections. Plenum Press, New York, N.Y.
- 6.Chanteau, S., L. Ratsifasoamanana, B. Rasoamanana, L. Rahalison, J. Randriambelosoa, J. Roux, and D. Rabeson. 1998. Plague, a reemerging disease in Madagascar. Emerg. Infect. Dis. 4:101-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donlan, R. M., and J. W. Costerton. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 15:167-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards, J. F., S. Higgs, and B. J. Beaty. 1998. Mosquito feeding-induced enhancement of Cache Valley virus (Bunyaviridae) infection in mice. J. Med. Entomol. 35:261-265. [DOI] [PubMed] [Google Scholar]
- 9.Eskey, C. R., and V. H. Haas. 1940. Plague in the western part of the United States. Public Health Bulletin 254. U. S. Public Health Service, Washington, D.C.
- 10.Galimand, M., A. Guiyoule, G. Gerbaud, B. Rasoamanana, S. Chanteau, E. Carniel, and P. Courvalin. 1997. Multidrug resistance in Yersinia pestis mediated by a transferable plasmid. N. Engl. J. Med. 337:677-680. [DOI] [PubMed] [Google Scholar]
- 11.Golde, W. T., and M. C. Dolan. 1995. Variation in antigenicity and infectivity of derivatives of Borrelia burgdorferi, strain B31, maintained in the natural, zoonotic cycle compared with maintenance in culture. Infect. Immun. 63:4795-4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guiyoule, A., G. Gerbaud, C. Buchrieser, M. Galimand, L. Rahalison, S. Chanteau, P. Courvalin, and E. Carniel. 2001. Transferable plasmid-mediated resistance to streptomycin in a clinical isolate of Yersinia pestis. Emerg. Infect. Dis. 7:43-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heath, D. G., G. W. J. Anderson, J. M. Mauro, S. L. Welkos, G. P. Andrews, J. Adamovicz, and A. M. Friedlander. 1998. Protection against experimental bubonic and pneumonic plague by a recombinant capsular F1-V antigen fusion protein vaccine. Vaccine 16:1131-1137. [DOI] [PubMed] [Google Scholar]
- 14.Hinnebusch, B. J. 2003. Transmission factors: Yersinia pestis genes required to infect the flea vector of plague. Adv. Exp. Med. Biol. 529:55-62. [DOI] [PubMed] [Google Scholar]
- 15.Hinnebusch, B. J., E. R. Fischer, and T. G. Schwan. 1998. Evaluation of the role of the Yersinia pestis plasminogen activator and other plasmid-encoded factors in temperature-dependent blockage of the flea. J. Infect. Dis. 178:1406-1415. [DOI] [PubMed] [Google Scholar]
- 16.Hinnebusch, B. J., K. L. Gage, and T. G. Schwan. 1998. Estimation of vector infectivity rates for plague by means of a standard curve-based competitive polymerase chain reaction method to quantify Yersinia pestis in fleas. Am. J. Trop. Med. Hyg. 58:562-569. [DOI] [PubMed] [Google Scholar]
- 17.Hinnebusch, B. J., R. D. Perry, and T. G. Schwan. 1996. Role of the Yersinia pestis hemin storage (hms) locus in the transmission of plague by fleas. Science 273:367-370. [DOI] [PubMed] [Google Scholar]
- 18.Hinnebusch, B. J., M.-L. Rosso, T. G. Schwan, and E. Carniel. 2002. High-frequency conjugative transfer of antibiotic resistance genes to Yersinia pestis in the flea midgut. Mol. Microbiol. 46:349-354. [DOI] [PubMed] [Google Scholar]
- 19.Inglesby, T. V., D. T. Dennis, D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. D. Fine, A. M. Friedlander, J. Hauer, J. F. Koerner, M. Layton, J. McDade, M. T. Osterholm, T. O'Toole, G. Parker, T. M. Perl, P. K. Russell, M. Schoch-Spana, and K. Tonant. 2000. Plague as a biological weapon. JAMA 283:2281-2290. [DOI] [PubMed] [Google Scholar]
- 20.Jones, S. M., F. Day, A. J. Stagg, and E. D. Williamson. 2001. Protection conferred by a fully recombinant sub-unit vaccine against Yersinia pestis in male and female mice of four inbred strains. Vaccine 19:358-366. [DOI] [PubMed] [Google Scholar]
- 21.Perry, R. D., and J. D. Fetherston. 1997. Yersinia pestis—etiologic agent of plague. Clin. Microbiol. Rev. 10:35-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pollitzer, R. 1954. Plague. World Health Organization, Geneva, Switzerland.
- 23.Randolph, S. E., and P. A. Nuttall. 1994. Nearly right or precisely wrong? Natural versus laboratory studies of vector-borne diseases. Parasitol. Today 10:458-462. [DOI] [PubMed] [Google Scholar]
- 24.Ribeiro, J. M. C. 1995. How ticks make a living. Parasitol. Today 11:91-93. [DOI] [PubMed] [Google Scholar]
- 25.Ribeiro, J. M. C., J. A. Vaughan, and A. F. Azad. 1990. Characterization of the salivary apyrase activity of three rodent flea species. Comp. Biochem. Physiol. B 95:215-219. [DOI] [PubMed] [Google Scholar]
- 26.Schoeler, G. B., and S. K. Wikel. 2001. Modulation of host immunity by haematophagous arthropods. Ann. Trop. Med. Parasitol. 95:755-771. [DOI] [PubMed] [Google Scholar]
- 27.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 92:2909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Straley, S. C., and R. D. Perry. 1995. Environmental modulation of gene expression and pathogenesis in Yersinia. Trends Microbiol. 3:310-317. [DOI] [PubMed] [Google Scholar]
- 29.Titball, R. W., J. Hill, and K. A. Brown. 2003. Yersinia pestis and plague. Biochem. Soc. Trans. 31:104-107. [DOI] [PubMed] [Google Scholar]
- 30.Titus, R. G., and J. M. C. Ribeiro. 1988. Salivary gland lysates from the sand fly, Lutzomyia longipalpis, enhance Leishmania infectivity. Science 239:1306-1308. [DOI] [PubMed] [Google Scholar]
- 31.Vaughan, J. A., L. F. Scheller, R. F. Wirtz, and A. F. Azad. 1999. Infectivity of Plasmodium berghei sporozoites delivered by intravenous inoculation versus mosquito bite: implications for sporozoite vaccine trials. Infect. Immun. 67:4285-4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wade, S. E., and J. R. Georgi. 1988. Survival and reproduction of artificially fed cat fleas, Ctenocephalides felis Bouché (Siphonaptera: Pulicidae). J. Med. Entomol. 25:186-190. [DOI] [PubMed] [Google Scholar]
- 33.Walker, D. H., A. G. Barbour, J. H. Oliver, R. S. Lane, J. S. Dumler, D. T. Dennis, D. H. Persing, A. F. Azad, and E. McSweegan. 1996. Emerging bacterial zoonotic and vector-borne diseases. Ecological and epidemiological factors. JAMA 275:463-469. [PubMed] [Google Scholar]