Abstract
Teriflunomide is an oral, once-daily disease-modifying therapy (DMT) approved in the USA, Australia, and Argentina for the treatment of relapsing forms of multiple sclerosis (RMS). Teriflunomide reversibly limits the expansion of activated T and B cells associated with the inflammatory process purportedly involved in multiple sclerosis pathogenesis, while preserving lymphocytes for routine immune surveillance. In an extensive clinical development program, teriflunomide demonstrated consistent benefits on both clinical and magnetic resonance imaging outcomes. In long-term studies, teriflunomide treatment was associated with low rates of relapse and disability progression for up to 8 years. The safety profile of teriflunomide has been well characterized, with adverse events generally mild to moderate in nature and infrequently leading to permanent treatment discontinuation. The evidence reviewed here indicates that teriflunomide is an effective addition to the current DMTs used to treat RMS.
Keywords: disease-modifying therapy, efficacy, immunomodulator, relapsing multiple sclerosis, safety, teriflunomide
Introduction
Multiple sclerosis (MS) is a chronic, progressive autoimmune disease that can lead to neurological impairment and long-term disability [Loma and Heyman, 2011]. The goals of MS therapy include modifying the course of the disease by reducing the frequency and severity of attacks, treatment of individual attacks, and the treatment of specific MS-related symptoms [EMA, 2006]. Insight into the pathophysiology of MS has led to the development of new disease-modifying therapies (DMTs) in the hope of obtaining more effective agents that are better tolerated and safer when given long term. Teriflunomide (Aubagio®, Genzyme Corporation, Cambridge, MA, USA) was one of the first oral agents to be developed for the treatment of relapsing MS (RMS), starting with clinical trials over a decade ago. It offers a well-tolerated, efficacious, oral alternative to current DMTs.
Teriflunomide, a once-daily oral immunomodulatory DMT, has been recently approved for the treatment of patients with RMS [Genzyme Corporation, 2012]. This review provides an overview of the mechanism of action, pharmacokinetics, efficacy, safety, and tolerability of teriflunomide, as demonstrated in the extensive clinical development program.
Mechanism of action
Teriflunomide is the principal active metabolite of leflunomide (Arava®, sanofi-aventis, Bridgewater, NJ, USA), used for the treatment of active rheumatoid arthritis in adults since 1998. The chemical structures of both compounds are shown in Figure 1. Although teriflunomide and leflunomide are closely related, they are each different chemical entities with different indications.
Figure 1.
Chemical structures of leflunomide and teriflunomide.
Teriflunomide selectively and reversibly inhibits dihydroorotate dehydrogenase (DHODH), a mitochondrial enzyme necessary for de novo pyrimidine synthesis [Warnke et al. 2009], thereby limiting the expansion of stimulated T and B cells, and reducing the number of lymphocytes available to migrate into the central nervous system. The pyrimidine salvage pathway, which recycles existing substrates and fulfills the basal pyrimidine demand of resting and slowly dividing cells, is independent of DHODH and is therefore largely unaffected by teriflunomide [Gold and Wolinsky, 2011]. The effects of teriflunomide appear to be cytostatic, not cytotoxic, as teriflunomide has no significant effect on lymphocyte viability [Li et al. 2011]. In a phase III clinical study, mean reductions in lymphocyte and neutrophil counts were small in magnitude, remained within the normal range, and were reversible after treatment discontinuation, or on treatment in some cases [Comi et al. 2012]. A low incidence of serious opportunistic infections [O’Connor et al. 2011b], and the ability of subjects to mount effective immune responses to seasonal influenza vaccination [Bar-Or et al. 2012], provide further evidence for the preservation of the immune system in teriflunomide-treated patients.
Inhibition of tyrosine kinases, NF-κB activation, and COX-2 activity have been demonstrated in vitro, suggesting that teriflunomide may also affect the immune system via signal transduction, inhibition of expression of cytokines and adhesion molecules, and interleukin-2 signaling [Papadopoulou et al. 2012]. However, concentrations of teriflunomide required to inhibit tyrosine kinases were up to 10-fold higher than those needed to affect DHODH, and the inhibition of COX-2 activity may actually be reversed during inflammation, so the relevance of these in vitro observations may not translate to the in vivo situation.
Preclinical studies
In the Dark Agouti (DA) rat model of experimental autoimmune encephalomyelitis (EAE) teriflunomide was shown to reduce the functional deficits associated with inflammation, demyelination, and axonal loss [Merrill et al. 2009]. Prophylactic administration of teriflunomide (10 mg/kg orally) prevented disease development, while therapeutic dosing minimized signs of disease. Teriflunomide treatment reduced the number of T and B cells in the spinal cord and brain in DA rats with EAE [Petty et al. 2010; Ringheim et al. 2011]; these outcomes are consistent with inhibition of clonal expansion and migration of inflammatory cells as proposed above.
Pharmacokinetics
The clinical pharmacokinetic profile of teriflunomide has been well characterized. In an analysis of healthy volunteers and patients with MS, the median elimination half-life (t1/2) was 18–19 days after repeated oral doses of teriflunomide 7 mg and 14 mg, and time to steady-state concentrations was approximately 3 months. The median time to maximum plasma concentrations (tmax) was 1–4 h after oral administration [Genzyme Corporation, 2012], with absolute bioavailability approaching 100% [Limsakun et al. 2010a]. Teriflunomide is > 99% bound to plasma protein, and primarily distributed in plasma with a volume of distribution of about 11 L [Limsakun et al. 2010a].
The biotransformation pathways for teriflunomide rely on hydrolysis, oxidation, N-acetylation, and sulfate conjugation. Compared with leflunomide, teriflunomide is only moderately metabolized with minimal involvement of cytochrome P450 [Genzyme Corporation, 2012], which lowers the potential for drug–drug interactions [Ogu and Maxa, 2000].
Teriflunomide is eliminated through direct biliary excretion of unchanged drug and renal excretion of metabolites. After 21 days of oral administration in healthy volunteers, 37.5% and 22.6% of the teriflunomide dose was excreted via feces and urine, respectively. At least nine metabolites were detected in urine, including 4-trifluoromethylaniline-oxanilic acid (18.1% of the administered dose). At least three metabolites were detected in feces, although unchanged teriflunomide was the predominant component (35.7% of the administered dose) [Wang et al. 2011].
Teriflunomide is excreted by the liver into the small intestine, where it is re-absorbed by the intestinal mucosa and returned to the liver via the portal circulation, in a process known as enterohepatic recycling [Limsakun et al. 2010b]. Under normal circumstances, teriflunomide is slowly eliminated from plasma, taking an average of 8 months for drug concentrations to reach levels below 0.02 µg/ml. Due to individual variation, this can take as long as 2 years [Genzyme Corporation, 2012]. One of the distinguishing advantages of teriflunomide compared with other DMTs is the availability of an accelerated elimination procedure. Cholestyramine and activated charcoal both bind teriflunomide to form insoluble complexes that are sequestered in the small intestine and excreted in the feces. Dosing regimens of cholestyramine 8 g three times daily for 11 days, or 50 g activated charcoal twice daily for 11 days, have been shown to decrease teriflunomide plasma concentrations by more than 98% (Figure 2) [Miller et al. 2012d]. In addition, a reduced dose of cholestyramine 4 g three times daily or nonconsecutive doses can be used in cases where the 8 g three-times-daily dose is poorly tolerated. Administration of cholestyramine or activated charcoal may be useful in situations in which the accelerated elimination of teriflunomide is desirable, such as the emergence of an unexpected but potentially serious event or pregnancy.
Figure 2.
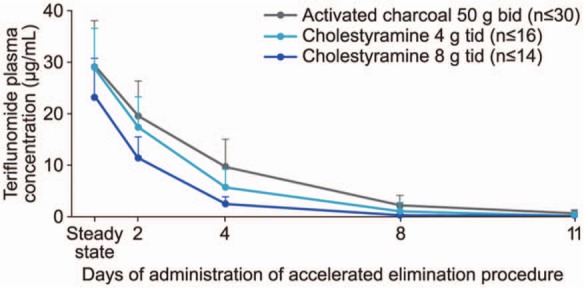
Teriflunomide plasma concentrations following an accelerated elimination procedure.
A loading dose of teriflunomide (70 mg/day for 3–4 days) was administered to achieve steady-state rapidly; this was followed by a maintenance dose of teriflunomide 14 mg/day for 8–11 days. Cholestyramine (8 or 4 g, 3 times daily) or activated charcoal (50 g, 2 times daily) was administered orally for 11 days following teriflunomide treatment.
(Adapted from Miller et al. [2012d].)
Efficacy findings from the clinical development program
The clinical development program for teriflunomide is summarized in Table 1.
Table 1.
The teriflunomide clinical program
| Study | Phase | Main objective of study | Comparator | Treatment duration | Patients randomized (n) |
|---|---|---|---|---|---|
| Monotherapy studies | |||||
| Phase II | II | Assess the effect of teriflunomide on the number of combined unique active lesions on magnetic resonance imaging | Placebo | 36 weeks | 179 |
| TEMSO | III | Evaluate the efficacy and safety of teriflunomide in reducing frequency of relapses | Placebo | 108 weeks | 1088 |
| TOWER | III | Evaluate the efficacy and safety of teriflunomide in reducing frequency of relapses | Placebo | Fixed end for all patients, 48 weeks for last patient randomized | 1096 |
| TENERE | III | Assess time to failure, defined as the first occurrence of relapse or permanent study treatment discontinuation for any cause | IFNβ-1a | Fixed end for all patients, 48 weeks for last patient randomized | 324 |
| TOPIC | III | Determine whether the effects of early intervention with teriflunomide in patients with clinically isolated syndrome prevent or delay conversion to multiple sclerosis | Placebo | 108 weeks | 618 |
| TERIVA | II | Investigate the immune response to seasonal influenza vaccination in patients treated with teriflunomide | None | Teriflunomide ≥ 6 months, influenza vaccine at day 1 | 128 |
| Adjunctive therapy studies | |||||
| Teriflunomide + IFNβ | II | Assess the safety and tolerability of teriflunomide and a stable dose of IFNβ compared with placebo and IFNβ | Placebo | 48 weeks | 118 |
| Teriflunomide + GA | II | Assess the safety and tolerability of teriflunomide and a stable dose of GA compared with placebo and GA | Placebo | 48 weeks | 123 |
| TERACLES | III | Assess the effectiveness of teriflunomide in reducing frequency of relapses in patients treated with IFNβ | Placebo | Approximately 118 weeks | 535 |
GA, glatiramer acetate; IFNβ, interferon beta.
Phase II ‘proof of concept’
This 36-week, double-blind trial [ClinicalTrials.gov identifier: NCT01487096] was the first study to evaluate the efficacy and safety of teriflunomide in subjects with RMS. Both teriflunomide doses resulted in significant reductions in the mean number of combined unique active lesions per magnetic resonance imaging (MRI) scan, the primary endpoint (mean number of lesions: 0.2 and 0.3, p < 0.03 and p < 0.01, for teriflunomide 7 mg and 14 mg, respectively) compared with placebo (0.5), representing relative reductions > 61%. There were also significant mean differences (95% confidence interval [CI]) compared with placebo observed in the number of gadolinium (Gd)-enhanced T1-weighted lesions per scan (7 mg, –1.38 [–2.25, -0.51], p < 0.04; 14 mg, -1.39 [-2.27, -0.50], p < 0.02), and the number of new or enlarging T2 lesions per scan (7 mg, -1.10 [-1.76, -0.45], p < 0.04; 14 mg, -0.81 [-1.47, -0.15], p < 0.03). Teriflunomide-treated subjects also reported lower annualized relapse rates (ARRs) than subjects receiving placebo, although the differences were not significant (mean ± standard deviation = 0.58 ± 0.85 [7 mg]; 0.55 ± 1.12 [14 mg]; 0.81 ± 1.22 [placebo]). Disability progression was significantly lower (7.4%, p < 0.04) in the 14 mg group compared with the placebo group (21.3%) [O’Connor et al. 2006]. These results paved the way for further investigation of teriflunomide in large-scale studies.
TEMSO
The Teriflunomide Multiple Sclerosis Oral trial [TEMSO; ClinicalTrials.gov identifier: NCT00134563] was the first teriflunomide phase III study, and was pivotal in the US approval of teriflunomide [Genzyme Corporation, 2012]. The full methodology has been described previously [O’Connor et al. 2011b]. Briefly, patients aged 18–55 years who met the 2001 McDonald criteria for MS [McDonald et al. 2001], and had a relapsing clinical course (two or more relapses in the previous 2 years or one or more relapses in the last year) with or without progression, were randomized to once-daily placebo, or teriflunomide 7 mg or 14 mg. The study population was well balanced across treatment groups; the mean age was 37.9 years, the majority of subjects were female (72.2%), and > 25% had been treated previously with MS medication. Roughly 35–38% of patients had >1 Gd-enhanced lesions at baseline. At the end of the 108-week treatment period, adjusted ARRs (95% CI) for teriflunomide 7 mg and 14 mg were 0.37 (0.32, 0.43) and 0.37 (0.31, 0.44), respectively, compared with 0.54 (0.47, 0.62) for placebo, representing relative risk reductions versus placebo of 31.2% and 31.5%, respectively (p < 0.001 for either dose). A significant decrease was also observed in sustained disability progression (confirmed for 12 weeks) with the 14 mg dose compared with placebo (29.8%; p = 0.03), although no significant reduction was observed with the 7 mg dose (23.7%).
Teriflunomide treatment improved several MRI outcomes in the TEMSO trial. The change from baseline in total lesion volume (the key MRI endpoint) was significantly lower in both teriflunomide groups compared with placebo (7 mg, p = 0.03; 14 mg, p < 0.001), and subjects receiving teriflunomide had significantly fewer Gd-enhanced lesions per T1-weighted scan than those receiving placebo (p < 0.001 for both teriflunomide doses); see Table 2.
Table 2.
Magnetic resonance imaging outcomes from the TEMSO trial.
| Magnetic resonance imaging outcomes | Placebo (n = 363) | Teriflunomide 7 mg (n = 365) | Teriflunomide 14 mg (n = 358) |
|---|---|---|---|
| Total lesion volume | |||
| Change from baseline (ml) | 2.21 ± 7.00 | 1.31 ± 6.80 | 0.72 ± 7.59 |
| Relative reduction versus placebo (%)* | 39.4 | 67.4 | |
| p value | 0.03 | < 0.001 | |
| Volume of hypointense lesions on T1-weighted images | |||
| Change from baseline (ml) | 0.53 ± 1.06 | 0.50 ± 1.15 | 0.33 ± 1.01 |
| Relative reduction versus placebo (%)* | 16.7 | 31.3 | |
| p value | 0.19 | 0.02 | |
| Volume of hyperintense lesion components on T2-weighted images$ | |||
| Change from baseline (ml) | 1.67 ± 6.47 | 0.81 ± 6.18 | 0.39 ± 6.90 |
| Relative reduction versus placebo (%)* | 44.0 | 76.7 | |
| p value | 0.04 | < 0.001 | |
| Gadolinium-enhanced lesions per T1-weighted scan‡ | |||
| Estimated no. (95% CI) | 1.33 (1.06–1.67) | 0.57 (0.43–0.75) | 0.26 (0.17–0.41) |
| Relative risk (95% CI) | 0.43 (0.31–0.59) | 0.20 (0.12–0.32) | |
| p value | < 0.001 | < 0.001 | |
| Absence of gadolinium-enhanced lesions on T1-weighted images | |||
| No. of patients (%)§ | 135 (39.0) | 180 (51.4) | 218 (64.1) |
| p value | < 0.001 | < 0.001 | |
| Unique active lesions per scan‡ | |||
| Estimated no. (95% CI) | 2.46 (2.10–2.89) | 1.29 (1.07–1.54) | 0.75 (0.58–0.99) |
| Relative risk (95% CI) | 0.52 (0.42–0.65) | 0.31 (0.23–0.41) | |
| p value | < 0.001 | < 0.001 | |
| Brain parenchymal fraction‖ | |||
| Change from baseline¶ | –0.004 ± 0.001 | –0.003 ± 0.001 | –0.003 ± 0.001 |
| Difference versus placebo¶ | 0.001 ± 0.001 | 0.001 ± 0.001 | |
| Relative reduction versus placebo (%) | 25.0 | 25.0 | |
| p value | 0.19 | 0.35 | |
Data are based on a mixed-effects model and repeated-measures analysis, with the use of a cube-root transformation of the volume data.
This measure is the portion of the total lesion volume that appears hyperintense on T2-weighted images (dual echo spin density and fluid-attenuation inversion recovery images), but does not appear hypointense on T1-weighted images obtained after the administration of gadolinium.
Unique active lesions were defined as the number of gadolinium-enhanced lesions on T1-weighted images, or new or enlarged lesions on T2-weighted images, without double counting. Values were calculated with the use of a Poisson regression model adjusted for treatment, Expanded Disability Status Scale score, and number of lesions at baseline and geographic region, with the log of the number of magnetic resonance imaging scans serving as an offset variable.
Data were missing for 17 patients in the placebo group, 15 patients in the lower dose teriflunomide group, and 18 patients in the higher dose teriflunomide group.
Brain parenchymal fraction was calculated as the inverse of the normalized cerebrospinal fluid volume and assessed with the use of a mixed-effects model with repeated-measures analysis.
Plus–minus values are least-square means ± standard error.
CI, confidence interval.
(Adapted from O’Connor et al. [2011b] Copyright © [2011] Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.)
An extension of TEMSO showed that the adjusted ARR remained low 5 years after initial randomization [O’Connor et al. 2011c], reflecting the outcomes observed in subjects treated for up to 8 years in a long-term extension of the phase II study [Confavreux et al. 2012].
The overall effects of teriflunomide treatment on clinical outcomes in the entire TEMSO cohort were evaluated in specific patient populations in a prospectively defined subgroup analysis. Subjects were stratified according to baseline demographics, disease characteristics, MRI parameters, and prior use of DMTs. Improvements in ARR and disability progression with teriflunomide 7 mg or 14 mg were consistent across all subgroups [Miller et al. 2012c].
An objective in the treatment of immune-mediated inflammatory diseases is sustained disease-free activity (the absence of clinical relapses, no sustained disability progression, and no MRI activity) [Havrdova et al. 2009; Giovannoni et al. 2011]. Post-hoc analyses of the TEMSO data revealed that both teriflunomide doses increased the chances of being disease-activity free compared with placebo. After 108 weeks of treatment 14.3%, 18.4%, and 22.9% of subjects were disease free in the placebo, 7 mg, and 14 mg teriflunomide groups, respectively (p = 0.0293 and p = 0.0002, for 7 mg and 14 mg versus placebo, respectively) [Freedman et al. 2012d].
Separate findings from the TEMSO study population suggest the benefits of teriflunomide on relapses extend beyond simply a reduction in relapse frequency (i.e. ARR). Severe relapses, such as those characterized by neurological sequelae, or those requiring steroid treatment or hospitalization, have a significant impact on patients and are associated with an increased consumption of healthcare resources, and thus healthcare costs [Lad et al. 2010; Naci et al. 2010; Jennum et al. 2012]. In post-hoc analyses, annualized rates of relapse leading to hospitalization were significantly reduced with teriflunomide 7 mg (36%; p < 0.05) and 14 mg (59%; p < 0.0001) compared with placebo [Miller et al. 2012b]. In addition, teriflunomide 14 mg significantly reduced relapses with neurological sequelae by 53% (p < 0.0001 versus placebo) [Miller et al. 2012b]. These findings indicate that teriflunomide has the potential to reduce relapse severity, thereby benefiting the quality of life in patients with RMS, and to reduce the healthcare costs associated with relapses. A further analysis indicated that subjects who experienced sequelae following relapses showed greater deterioration in fatigue, health-related quality of life, and disability progression than those without any relapse or with a relapse without sequelae [Miller et al. 2012a].
TOWER
The results of a second phase III study, Teriflunomide Oral in People with Relapsing–Remitting Multiple Sclerosis [TOWER; ClinicalTrials.gov identifier: NCT00751881], supported the clinical outcomes observed in TEMSO [Miller et al. 2013]. The teriflunomide 14 mg dose significantly reduced ARR (36.3%; p < 0.0001), and disability progression was sustained for 12 weeks (31.5%; p = 0.0442) compared with placebo (Table 3). The ARR was also significantly reduced in the teriflunomide 7 mg group (22.3%; p = 0.0183), although the effect of the 7 mg dose on disability progression was not significant (4.5% reduction, p = 0.7620). Full findings from the TOWER study will be reported in a forthcoming publication.
Table 3.
Key clinical efficacy outcomes from the TEMSO and TOWER studies.
| TEMSOa |
TOWERb |
|||
|---|---|---|---|---|
| Teriflunomide 7 mg (n = 365) | Teriflunomide 14 mg (n = 358) | Teriflunomide 7 mg (n = 407) | Teriflunomide 14 mg (n = 370) | |
| RRR for adjusted ARR* versus placebo | 31.2% | 31.5% | 22.3% | 36.3% |
| (p = 0.0002) | (p = 0.0005) | (p = 0.0183) | (p = 0.0001) | |
| HRR for patients with disability progression$ versus placebo | 23.7% | 29.8% | 4.5% | 31.5% |
| (p = 0.0835) | (p = 0.0279) | (p = 0.7620) | (p = 0.0442) | |
Modified intent-to-treat population.
Primary efficacy endpoint.
Key secondary efficacy endpoint, defined as a persisting increase for at least 12 weeks of ≥ 1.0 point on the EDSS from baseline (or ≥ 0.5 point on the EDSS from baseline if baseline EDSS score > 5.5).
ARR, annualized relapse rate; EDSS, Expanded Disability Status Scale; HRR, hazard risk reduction; RRR, relative risk reduction.
(aAdapted from O’Connor et al. [2011b] Copyright © [2011] Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society; bAdapted from Miller et al. [2013].)
TENERE
TENERE [ClinicalTrials.gov identifier: NCT00883337] was the first head-to-head clinical trial comparing teriflunomide treatment with interferon (IFN) β-1a 44 µg subcutaneously (Rebif®, EMD Serono, Inc., Rockland, MA, USA) in subjects with RMS [Vermersch et al. 2012]. The primary endpoint, time to failure, was defined as the first occurrence of confirmed relapse or permanent treatment discontinuation for any reason, whichever came first. No statistical superiority in time to failure was observed when comparing either teriflunomide-treatment group with the IFNβ-1a group (7 mg versus IFN, p = 0.5190; 14 mg versus IFN, p = 0.5953).The number of patients reporting failure was 44 (42.3%), 53 (48.6%), and 42 (37.8%) in the IFNβ-1a, teriflunomide 7 mg and 14 mg groups, respectively. There was also no statistical difference in ARR between teriflunomide 14 mg and IFNβ-1a (0.26 versus 0.22, respectively; p = 0.590), although IFNβ-1a was superior to the teriflunomide 7 mg dose (0.22 versus 0.41, respectively; p = 0.03). Although the primary endpoint of this superiority study was not met, there were positive outcomes for teriflunomide on secondary endpoints (patient-reported quality-of-life measurements). After 48 weeks of treatment, subjects receiving teriflunomide reported less worsening of fatigue than those receiving IFNβ-1a, and overall satisfaction with treatment was significantly higher for both doses of teriflunomide compared with IFNβ-1a as measured by the Treatment Satisfaction Questionnaire for Medication [Vermersch et al. 2012]. Outcomes from the TENERE study will be published soon.
Adjunctive therapy
Some patients continue to experience relapses and clinical deterioration while on treatment. An alternative therapeutic approach is to combine two DMTs with proven tolerability and distinct modes of action. Teriflunomide administered as an adjunctive therapy to IFNβ [Freedman et al. 2012e], or glatiramer acetate (GA) [ClinicalTrials.gov identifier: NCT00489489] [Freedman et al. 2011], was well tolerated, with safety profiles consistent with teriflunomide monotherapy. Teriflunomide added to ongoing IFNβ therapy significantly reduced the number of Gd-enhanced lesions compared with IFNβ alone (82.8%, p < 0.0001 for 14 mg and 84.6%, p < 0.0001 for 7 mg at 48 weeks). A trend toward a dose-dependent effect of teriflunomide in reducing clinical relapses was also apparent, suggestive of an additive effect of combining these two DMTs for the treatment of relapsing MS [Freedman et al. 2012e]. Teriflunomide as adjunctive therapy to GA resulted in a significant reduction in the number of Gd-enhanced lesions in the teriflunomide 7 mg group; the reduction was not significant in the 14 mg group. This outcome may have been related to higher baseline MRI disease activity in patients in the 7 mg group [Freedman et al. 2011]. The phase III TERACLES [ClinicalTrials.gov identifier: NCT01252355] study (teriflunomide in combination with IFNβ) will provide greater insight on the clinical usefulness of combination therapy [Freedman et al. 2012a].
TOPIC
The possibility that early intervention with teriflunomide in patients presenting with a first clinical episode suggestive of a demyelinating event might delay or prevent conversion to clinically definite MS (CDMS) is being investigated in a placebo-controlled phase III study (TOPIC) [ClinicalTrials.gov identifier: NCT00622700]. Conversion to CDMS was defined by the occurrence of relapse (primary outcome measure) and dissemination of lesions on MRI over time (secondary outcome measure).
TERIVA
As slowly dividing or resting cells rely more on the pyrimidine salvage pathway to meet their pyrimidine demand, which is largely unaffected by teriflunomide, it is postulated that memory lymphocytes remain available for immune surveillance. To investigate if teriflunomide might influence patients’ abilities to mount recall immune responses, antibody responses to the seasonal influenza vaccine were assessed in 82 subjects with RMS treated with teriflunomide 7 mg or 14 mg for a median duration of about 6 years (TERIVA) [ClinicalTrials.gov identifier: NCT01403376] [Bar-Or et al. 2012]. Over 77% of subjects had an effective vaccine response to all influenza strains, providing supporting evidence that teriflunomide does not significantly affect memory response to recall antigens, in this case, influenza vaccine strains.
Safety and tolerability findings from the clinical development program
Teriflunomide has a well-characterized safety profile and, to our knowledge, has the longest exposure data for an oral DMT. The incidence of treatment-emergent adverse events (TEAEs), serious TEAEs, and TEAEs leading to discontinuation of study medication was similar in the placebo and teriflunomide-treatment groups in TEMSO [O’Connor et al. 2011b]. TEAEs occurring with a greater frequency in the teriflunomide groups with an apparent dose effect were diarrhea, nausea, alopecia, and alanine aminotransferase (ALT) increase. Most cases of diarrhea and nausea were mild to moderate in nature, occurred during the first 3 months of study therapy, and resolved without corrective treatment [Freedman et al. 2012c]. No new or unexpected AEs emerged with long-term teriflunomide treatment in extension studies, and in general, teriflunomide was well tolerated during treatment of up to 9 years’ duration [Comi et al. 2011; Confavreux et al. 2011, 2012]. The relative risk of the most frequently occurring TEAEs in the teriflunomide groups from the TEMSO trial is shown in Figure 3.
Figure 3.
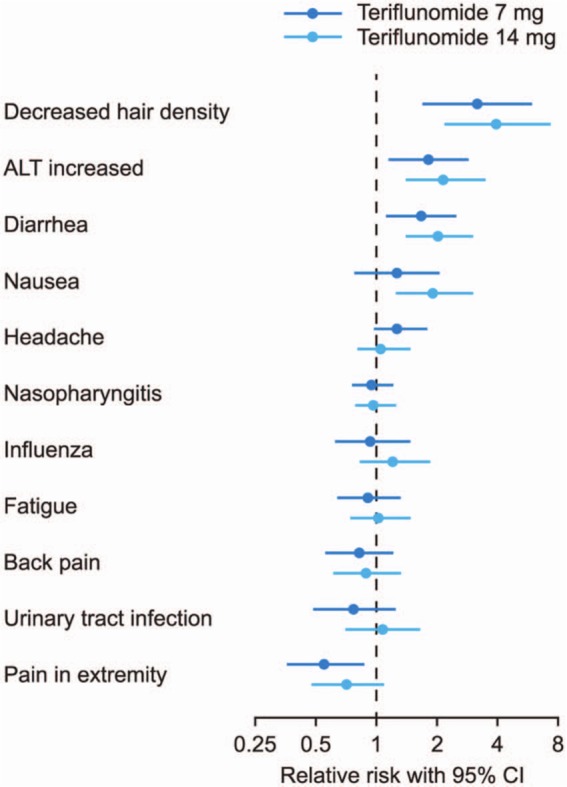
Treatment-emergent adverse events in the TEMSO trial: relative risk versus placebo.
Treatment-emergent adverse events ≥ 10% in any group by MedDRA-preferred term ranked by decreasing order of relative risk versus placebo. MedDRA-preferred term for hair thinning/decreased hair density: alopecia. The term ‘alopecia’ is used to describe any type of hair loss. Most cases of alopecia were reported as hair thinning, decreased hair density, or hair loss.
(Adapted from Miller et al. [2011].)
ALT, alanine aminotransferase; CI, confidence interval; MedDRA, Medical Dictionary for Regulatory Activities.
Seven deaths have been reported in subjects treated with teriflunomide in the clinical program to date. Four deaths occurred in long-term extension studies (phase II extension, n = 2; TEMSO extension, n = 2) [Confavreux et al. 2011; Comi et al. 2011], and three in the TOWER study [Miller et al. 2013]. Both deaths in the phase II extension study (7 mg and 14 mg) and one death in the TEMSO extension (14 mg) were of cardiac origin. The other TEMSO death (7 mg) was attributed to natural death in the context of advanced neurological disease. The three deaths in TOWER were attributed to a motorcycle accident (7 mg), suicide (14 mg), and septicemia (14 mg) due to a Gram-negative organism complicated with disseminated intravascular coagulopathy. None of these deaths were considered to be related to teriflunomide treatment.
Adverse events of special interest
An overview of TEAEs of special interest (events reported by patients or noted by the investigator) from the TEMSO trial are shown in Table 4.
Table 4.
Treatment-emergent adverse events of special interest from the TEMSO trial.
| Placebo (n = 360) | Teriflunomide 7 mg (n = 368) | Teriflunomide 14 mg (n = 358) | |
|---|---|---|---|
| Hepatic | 43 (11.9) | 71 (19.3) | 69 (19.3) |
| SAE | 9 (2.5) | 7 (1.9) | 9 (2.5) |
| Discontinuation | 15 (4.2) | 16 (4.3) | 13 (3.6) |
| Bone marrow disorders | 8 (2.2) | 36 (9.8) | 27 (7.5) |
| SAE | 0 | 0 | 2 (0.6) |
| Discontinuation | 0 | 1 (0.3) | 0 |
| Infections | 209 (58.1) | 220 (59.8) | 222 (62.0) |
| SAE | 8 (2.2) | 6 (1.6) | 9 (2.5) |
| Discontinuation | 4 (1.1) | 1 (0.3) | 3 (0.8) |
| Malignancy* | 5 (1.4) | 1 (0.3) | 2 (0.6) |
| SAE | 4 (1.1) | 0 | 1 (0.3) |
| Discontinuation | 3 (0.8) | 0 | 1 (0.3) |
| Blood pressure | 11 (3.1) | 20 (5.4) | 18 (5.0) |
| SAE | 0 | 0 | 0 |
| Discontinuation | 0 | 0 | 0 |
| Peripheral neuropathy | 11 (3.1) | 9 (2.4) | 17 (4.7) |
| SAE | 0 | 0 | 0 |
| Discontinuation | 0 | 1 (0.3) | 1 (0.3) |
| Hair thinning/decreased hair density$ | 12 (3.3) | 38 (10.3) | 48 (13.4) |
| SAE | 0 | 0 | 0 |
| Discontinuation | 0 | 2 (0.5) | 5 (1.4) |
Safety population. Events identified and retrieved for the analyses using MedDRA.
Malignant neoplasms were reported in three placebo patients (breast cancer, thyroid cancer, and cervical carcinoma), and one patient in the teriflunomide 14 mg group (cervical carcinoma with recovery following surgery).
MedDRA-preferred term for hair thinning/decreased hair density: alopecia. The term ‘alopecia’ is used to describe any type of hair loss. Most cases of alopecia were reported as hair thinning, decreased hair density, or hair loss.
MedDRA, Medical Dictionary for Regulatory Activities; SAE, serious adverse event.
Hepatic effects
Teriflunomide is contraindicated in patients with severe hepatic impairment [Genzyme Corporation, 2012], on the basis of postmarketing reports of severe liver injury, including fatal liver failure in patients with rheumatoid arthritis treated with leflunomide [sanofi-aventis, 2012]. If patients taking teriflunomide have ALT levels more than three times the upper limit of normal (ULN) on repeated testing then treatment discontinuation is recommended, and values generally normalize rapidly. Treatment discontinuation and the accelerated elimination procedure should be performed if teriflunomide is suspected as the cause of liver injury.
While the frequency of hepatic TEAEs was higher in teriflunomide-treated subjects than in those receiving placebo, incidence of serious hepatic events and the proportion of subjects discontinuing treatment because of hepatic events were low and comparable across treatment groups (Table 4). Hepatic disorders were mainly asymptomatic ALT increases that occurred within the first 6 months of treatment and often recovered even while on treatment. Figure 4 shows the percentage of subjects with serious hepatic events and ALT elevations in the TEMSO trial [O’Connor et al. 2011b]. Mild increases (ALT > 1 × ULN) were higher in the teriflunomide groups, but the frequency of ALT > 3 × ULN was low and evenly distributed across groups. Three subjects (one in each treatment group) had laboratory values in the Hy’s Law range (increased ALT ≥ 3 × ULN and total bilirubin ≥ 2 × ULN); all cases were considered as nondrug-induced liver injuries with alternative explanations provided (hepatitis C, gall bladder problems, and cytomegalovirus infection) [O’Connor et al. 2011b].
Figure 4.
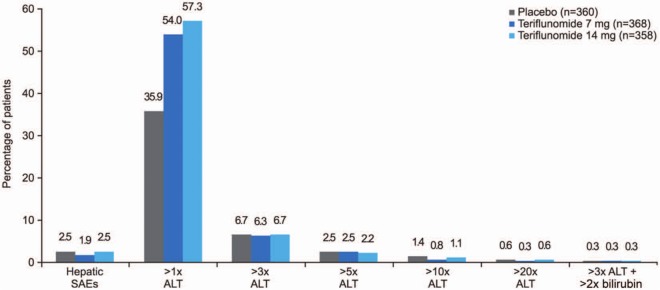
Serious hepatic adverse events and elevated alanine aminotransferase levels in the TEMSO trial.
AE, adverse event; ALT, alanine aminotransferase; SAE, serious adverse event.
Infections/bone marrow effects
In the TEMSO trial, mean reductions in neutrophil and lymphocyte counts were small in magnitude (< 15%), and remained within the normal ranges. Importantly, there was no correlation between reduced neutrophil counts and infections [Comi et al. 2012]. A low incidence of serious infections was reported in all treatment groups (placebo, 2.2%; 7 mg, 1.6%; 14 mg, 2.5%) (Table 4), and there were no cases of serious opportunistic infection, supporting the position that teriflunomide is not an immunosuppressive agent [O’Connor et al. 2011b].
In long-term extension studies, malignancies occurred within the expected range observed in the general population, with no pattern of malignancy typical of those observed in immunocompromised patients, and no lymphoproliferative disorders reported [Confavreux et al. 2011; Comi et al. 2012].
Blood pressure effects
The proportion of patients with TEAEs related to blood pressure increase was higher in the teriflunomide treatment groups (placebo, 3.1%; teriflunomide 7 mg, 5.4%; teriflunomide 14 mg, 5.0%, respectively) (Table 4), although no event was serious or led to study treatment discontinuation [O’Connor et al. 2011b].
Peripheral neuropathy
Suspected peripheral neuropathies were confirmed by electrophysiological nerve conduction studies in four patients (1.2%) in the teriflunomide 7 mg group and six patients (1.9%) in the teriflunomide 14 mg group. All reports related to peripheral neuropathy were not serious, and most were of mild and moderate intensity. Of the 10 confirmed cases, 5 were mononeuropathies related to carpal tunnel syndrome or nerve compression, 3 cases were unrelated to study drug and resolved on treatment, and 2 were ongoing at end of study [O’Connor et al. 2011b].
Hair loss/hair thinning
Pooled data from the phase II and TEMSO studies showed that the incidence of hair loss/hair thinning was higher in the teriflunomide treatment groups (7 mg, 11.4%; 14 mg, 15.2%) compared with those receiving placebo (4.3%) [Freedman et al. 2012b]. However, hair thinning in most cases was minimal in nature (76%), and resolved without sequelae (85%), often while the subjects remained on therapy. The risk of hair loss was highest during the first 6 months of treatment. Teriflunomide-related hair thinning is different in nature and severity from chemotherapy/radiotherapy-induced hair loss, which is usually acute and severe, and leads to loss of the majority of the scalp hair [Freedman et al. 2012b].
Pregnancy
Teriflunomide is contraindicated in women of childbearing potential not using reliable contraception. To minimize any potential risk, men not wishing to father a child are also advised to use contraception [Genzyme Corporation, 2012]. Despite the requirement to avoid pregnancy and to use effective contraception in the teriflunomide clinical program, pregnancies did occur. When a pregnancy was identified, the subject was advised to discontinue treatment immediately and to undergo an accelerated elimination procedure. To date, no structural or functional deficits have been reported in the 12 newborns with teriflunomide exposure [Jung Henson et al. 2013].
Safety experience in other trials
The nature and occurrence of TEAEs in the TENERE study were similar to those observed in TEMSO, with fewer discontinuations due to AEs in the teriflunomide groups than in the IFNβ treatment group [Vermersch et al. 2012]. Teriflunomide administered in addition to IFNβ or GA in the phase II combination studies was generally well tolerated, with few subjects reporting serious TEAEs (IFNβ adjunct study: teriflunomide 7 mg, 4 [10.8%]; 14 mg, 1 [2.6%]; placebo, 2 [4.9%]/GA adjunct study: teriflunomide 7 mg, 5 [11.9%]; 14 mg, 2 [4.9%]; placebo, 6 [15.0%]), or TEAEs leading to treatment discontinuation (IFNβ adjunct: teriflunomide 7 mg, 3 [8.1%]; 14 mg, 3 [7.9%]; placebo, 2 [4.9%]/GA adjunct study: teriflunomide 7 mg, 3 [7.1%]; 14 mg, 5 [12.2%]; placebo, 2 [5.0%]) [Freedman et al. 2011, 2012e].
Discussion
In clinical trials, teriflunomide reduced the frequency of clinical exacerbations and delayed the accumulation of physical disability in subjects with RMS [O’Connor et al. 2011b; Miller et al. 2013]. Beneficial effects were observed for both doses of teriflunomide on a range of MRI outcomes [O’Connor et al. 2011b]. Teriflunomide also reduced the occurrence of relapses requiring hospitalization and treatment with corticosteroids in the TEMSO trial [O’Connor et al. 2011a]. Additionally, the benefits of teriflunomide treatment were consistent across predefined subgroups stratified according to baseline demographic features or disease characteristics [Miller et al. 2012c].
Although cross-trial comparisons have obvious limitations, the relative reduction in ARR in subjects treated with teriflunomide appears to be comparable to that observed in patients with MS treated with currently available first-line injectable DMTs [IFNB Multiple Sclerosis Study Group 1993; Paty and Li, 1993; Jacobs et al. 1996; Johnson, 1996; PRISMS, 1998]. Furthermore, no significant difference in ARR was observed between teriflunomide 14 mg and IFNβ-1a in the head-to-head TENERE study.
In a phase II study, teriflunomide as adjunctive treatment to ongoing IFNβ was more effective than IFNβ alone in reducing MRI lesions, raising the possibility of teriflunomide combination therapy as an option in the treatment of patients who have a suboptimal response to current first-line monotherapy [Freedman et al. 2012e]. With greater availability of generic IFNβ, combination therapy is a realistic financial possibility.
Long-term studies have not identified new or unexpected TEAEs associated with teriflunomide treatment [Confavreux et al. 2011, 2012; Comi et al. 2011], suggesting that teriflunomide has a manageable safety profile that compares favorably to those of other oral DMTs [Giovannoni et al. 2010; Kappos et al. 2010]. Data on more than 2.3 million patient-years of exposure to leflunomide in patients with rheumatoid arthritis aids the understanding of the probable long-term safety profile of teriflunomide. In addition, the accelerated elimination procedure is a useful tool in cases of pregnancy or emerging toxicity.
These extensive studies show that teriflunomide is an effective oral monotherapy for relapsing disease. In addition, the already significant long-term safety and tolerability data concerning teriflunomide are complemented by ongoing studies that continue to collect safety information, including a pregnancy registry. All of these support teriflunomide as a first-line agent for the treatment of RMS.
Acknowledgments
Editorial assistance provided by Catherine Simonson, Fishawack Communications Ltd, was funded by Genzyme Corporation, a Sanofi company.
Footnotes
Funding: All teriflunomide studies discussed in this review were funded by Genzyme Corporation, a Sanofi company.
Conflict of interest statement: MSF has received consultancy fees from Bayer, Biogen Idec, Teva, Merck Serono, Novartis, Sanofi and Celgene; payment for lectures from Bayer, Merck Serono, Novartis, and sanofi-aventis; and payment for development of educational presentations from Novartis, Lansdowne DIME, and Medscape. His institution has received research grants from Bayer and Genzyme Corporation.
References
- Bar-Or A., Freedman M., Kremenchutzky M., Menguy-Vacheron F., Bauer D., Jodl S., et al. (2012) Effect of teriflunomide on immune responses to seasonal influenza vaccination in patients with relapsing multiple sclerosis: results from the TERIVA study. Mult Scler 18: 279–508 P925. [Google Scholar]
- Comi G., Benzerdjeb H., Wang L., Truffinet P., O’Connor P. (2012) Effect of teriflunomide on lymphocyte and neutrophil levels in patients with relapsing multiple sclerosis: results from the TEMSO study. Mult Scler 18: 279–508 P439. [Google Scholar]
- Comi G., O’Connor P., Wolinsky J., Confavreux C., Kappos L., Olsson T., et al. (2011) Extension of a phase III trial (TEMSO) of oral teriflunomide in multiple sclerosis with relapses: safety outcomes with up to 4 years of follow-up. Mult Scler 17: S182 [Google Scholar]
- Confavreux C., Li D., Freedman M., Truffinet P., Benzerdjeb H., Wang D., et al. (2012) Long-term follow-up of a phase 2 study of oral teriflunomide in relapsing multiple sclerosis: safety and efficacy results up to 8.5 years. Mult Scler 18: 1278–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Confavreux C., O’Connor P., Freedman M., Benzerdjeb H., Wang D., Bar-Or A. (2011) Long-term safety and tolerability of teriflunomide in multiple sclerosis: 9-year follow-up of a phase II study. Mult Scler 17: S409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMA (2006) Guideline on Clinical Investigation of Medicinal Products for the Treatment of Multiple Sclerosis. London: European Medicines Agency; [Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003485.pdf; accessed January 2013] [Google Scholar]
- Freedman M., Cheng S., Truffinet P., Wamil B., Confavreux C., Comi G., et al. (2012a) TERACLES study design: teriflunomide as adjunctive therapy with interferon-beta in patients with relapsing multiple sclerosis. Poster presentation at the Fourth Cooperative Meeting of CMSC and ACTRIMS, 30 May–2 June 2012, San Diego, CA, USA, P9. [Google Scholar]
- Freedman M., Confavreux C., Comi G., Kappos L., Olsson T., Miller A. et al. (2012b) Hair thinning associated with teriflunomide therapy is manageable. Poster presentation at the Fourth Cooperative Meeting of CMSC and ACTRIMS, 30 May–2 June 2012, San Diego, CA, USA, P7 [Google Scholar]
- Freedman M., Delhay J., Benamor M. (2012c) Gastrointestinal symptoms infrequently lead to discontinuation of teriflunomide therapy. Poster presentation at the Fourth Cooperative Meeting of CMSC and ACTRIMS, 30 May–2 June 2012, San Diego, CA, USA, P8 [Google Scholar]
- Freedman M., O’Connor P., Wolinsky J., Confavreux C., Comi G., Kappos L., et al. (2012d) Teriflunomide increases the proportion of patients free from disease activity in the TEMSO phase III study. Neurology 78: PD5.007 [Google Scholar]
- Freedman M., Wolinsky J., Wamil B., Confavreux C., Comi G., Kappos L., et al. (2011) Oral teriflunomide plus glatiramer acetate in relapsing multiple sclerosis. Int J MS Care 13: 17 [Google Scholar]
- Freedman M., Wolinsky J., Wamil B., Confavreux C., Comi G., Kappos L., et al. (2012e) Teriflunomide added to interferon-beta in relapsing multiple sclerosis: a randomized phase II trial. Neurology 78: 1877–1885 [DOI] [PubMed] [Google Scholar]
- Genzyme Corporation (2012) Aubagio Prescribing Information. Cambridge, MA: Genzyme Corporation [Google Scholar]
- Giovannoni G., Comi G., Cook S., Rammohan K., Rieckmann P., Soelberg Sorensen P., et al. (2010) A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med 362: 416–426 [DOI] [PubMed] [Google Scholar]
- Giovannoni G., Cook S., Rammohan K., Rieckmann P., Sorensen P., Vermersch P., et al. (2011) Sustained disease-activity-free status in patients with relapsing–remitting multiple sclerosis treated with cladribine tablets in the CLARITY study: a post-hoc and subgroup analysis. Lancet Neurol 10: 329–337 [DOI] [PubMed] [Google Scholar]
- Gold R., Wolinsky J. (2011) Pathophysiology of multiple sclerosis and the place of teriflunomide. Acta Neurol Scand 124: 75–84 [DOI] [PubMed] [Google Scholar]
- Havrdova E., Galetta S., Hutchinson M., Stefoski D., Bates D., Polman C., et al. (2009) Effect of natalizumab on clinical and radiological disease activity in multiple sclerosis: a retrospective analysis of the natalizumab safety and efficacy in relapsing–remitting multiple sclerosis (AFFIRM) study. Lancet Neurol 8: 254–260 [DOI] [PubMed] [Google Scholar]
- IFNB Multiple Sclerosis Study Group (1993) Interferon beta-1b is effective in relapsing–remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology 43: 655–661 [DOI] [PubMed] [Google Scholar]
- Jacobs L., Cookfair D., Rudick R., Herndon R., Richert J., Salazar A., et al. (1996) Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol 39: 285–294 [DOI] [PubMed] [Google Scholar]
- Jennum P., Wanscher B., Frederiksen J., Kjellberg J. (2012) The socioeconomic consequences of multiple sclerosis: a controlled national study. Eur Neuropsychopharmacol 22: 36–43 [DOI] [PubMed] [Google Scholar]
- Johnson K. (1996) Management of relapsing/remitting multiple sclerosis with copolymer 1 (Copaxone). Mult Scler 1: 325–326 [DOI] [PubMed] [Google Scholar]
- Jung Henson L., Stüve O., Kieseier B., Benamor M., Benzerdjeb H. (2013) Pregnancy outcomes from the teriflunomide clinical development program: retrospective analysis of the teriflunomide clinical trial database. Neurology 80: 1001–112005, S30.005. [Google Scholar]
- Kappos L., Radue E., O’Connor P., Polman C., Hohlfeld R., Calabresi P., et al. (2010) A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 362: 387–401 [DOI] [PubMed] [Google Scholar]
- Lad S., Chapman C., Vaninetti M., Steinman L., Green A., Boakye M. (2010) Socioeconomic trends in hospitalization for multiple sclerosis. Neuroepidemiology 35: 93–99 [DOI] [PubMed] [Google Scholar]
- Li L., Liu J., Ringheim G., Delohery T., Jones C. (2011) The effects of teriflunomide on lymphocyte subpopulations in human peripheral blood mononuclear cells. Mult Scler 17: S422. [DOI] [PubMed] [Google Scholar]
- Limsakun T., Menguy-Vacheron F. (2010a) Pharmacokinetics of oral teriflunomide, a novel oral disease-modifying agent under investigation for the treatment of multiple sclerosis. Neurology 74: P05.032 [Google Scholar]
- Limsakun T., Menguy-Vacheron F. (2010b) Effects of cholestyramine on the elimination of teriflunomide in healthy male volunteers. Mult Scler 16: P11 [Google Scholar]
- Loma I., Heyman R. (2011) Multiple sclerosis: pathogenesis and treatment. Curr Neuropharmacol 9: 409–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald W., Compston A., Edan G., Goodkin D., Hartung H., Lublin F., et al. (2001) Recommended diagnostic criteria for multiple sclerosis: guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol 50: 121–127 [DOI] [PubMed] [Google Scholar]
- Merrill J., Hanak S., Pu S., Liang J., Dang C., Iglesias-Bregna D., et al. (2009) Teriflunomide reduces behavioral, electrophysiological, and histopathological deficits in the Dark Agouti rat model of experimental autoimmune encephalomyelitis. J Neurol 256: 89–103 [DOI] [PubMed] [Google Scholar]
- Miller A., Lublin F., O’Connor P., Tanio C., Dive-Pouletty C. (2012a) Impact of relapses with sequelae on disability, health-related quality of life, and fatigue in a population with relapsing forms of multiple sclerosis (RMS) using data from TEMSO, a pivotal phase III teriflunomide trial. Neurology 78: P07.082 [Google Scholar]
- Miller A., Lublin F., O’Connor P., Wolinsky J., Comi G., Kappos L., et al. (2012b) Effect of teriflunomide on relapses with sequelae and relapse leading to hospitalization in a population with relapsing forms of multiple sclerosis: results from the TEMSO study. Neurology 78: S30.003 [Google Scholar]
- Miller A., O’Connor P., Wolinsky J., Confavreux C., Comi G., Kappos L., et al. (2011) Clinical and MRI outcomes from a phase III trial (TEMSO) of oral teriflunomide in multiple sclerosis with relapses. In American Academy of Neurology 63rd Annual Meeting, 9–16 April 2011, Honolulu, HI, S41.002 [Google Scholar]
- Miller A., O’Connor P., Wolinsky J., Confavreux C., Kappos L., Olsson T., et al. (2012c) Pre-specified subgroup analyses of a placebo-controlled phase III trial (TEMSO) of oral teriflunomide in relapsing multiple sclerosis. Mult Scler 18: 1625–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A., Turpault S., Menguy-Vacheron F. (2012d) Rapid elimination procedure of teriflunomide with cholestyramine or activated charcoal. In Fourth Cooperative Meeting of CMSC and ACTRIMS, 30 May–2 June 2012, San Diego, CA, USA, P10 [Google Scholar]
- Miller A., Kappos L., Comi G., Confavreux C., Freedman M., Olsson T., et al. (2013) Teriflunomide efficacy and safety in patients with relapsing multiple sclerosis: results from TOWER, a second pivotal phase 3 placebo-controlled study. Neurology 80: S01.004 [Google Scholar]
- Naci H., Fleurence R., Birt J., Duhig A. (2010) Economic burden of multiple sclerosis: a systematic review of the literature. Pharmacoeconomics 28: 363–379 [DOI] [PubMed] [Google Scholar]
- O’Connor P., Li D., Freedman M., Bar-Or A., Rice G., Confavreux C., et al. (2006) A phase II study of the safety and efficacy of teriflunomide in multiple sclerosis with relapses. Neurology 66: 894–900 [DOI] [PubMed] [Google Scholar]
- O’Connor P., Lublin F., Wolinsky J., Comi G., Kappos L., Freedman M., et al. (2011a) Effect of teriflunomide on relapses leading to healthcare resource use: results from the TEMSO study. Mult Scler 17(Suppl. 17): S95, P250. [Google Scholar]
- O’Connor P., Wolinsky J., Confavreux C., Comi G., Kappos L., Olsson T., et al. (2011b) Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med 365: 1293–1303 [DOI] [PubMed] [Google Scholar]
- O’Connor P., Wolinsky J., Confavreux C., Comi G., Kappos L., Olsson T., et al. (2011c) Extension of a phase III trial (TEMSO) of oral teriflunomide in multiple sclerosis with relapses: clinical and MRI data 5 years after initial randomisation. Mult Scler 17(Suppl. 17): S414, P924. [Google Scholar]
- Ogu C., Maxa J. (2000) Drug interactions due to cytochrome P450. Proc (Bayl Univ Med Cent) 13: 421–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulou A., Kappos L., Sprenger T. (2012) Teriflunomide for oral therapy in multiple sclerosis. Expert Rev Clin Pharmacol 5: 617–628 [DOI] [PubMed] [Google Scholar]
- Paty D., Li D. (1993) Interferon beta-1b is effective in relapsing–remitting multiple sclerosis. II. MRI analysis results of a multicenter, randomized, double-blind, placebo-controlled trial. UBC MS/MRI Study Group and the IFNB Multiple Sclerosis Study Group. Neurology 43: 662–667 [DOI] [PubMed] [Google Scholar]
- Petty M., Lee L., Liang J., Ying X., Torino M., Oliver J., et al. (2010) Teriflunomide treatment reduces infiltration of macrophages, T cells and B cells and increases survival of oligodendrocytes in the spinal cord of the Dark Agouti rat model of experimental allergic encephalomyelitis. Neurology 74: A415 [Google Scholar]
- PRISMS (1998) Prevention of Relapses and Disability by Interferon Beta-1a Subcutaneously in Multiple Sclerosis Study Group. Randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. Lancet 352: 1498–1504 [PubMed] [Google Scholar]
- Ringheim G., Lee L., Laws-Ricker L., Delohery T., Colletti N., Soos T., et al. (2011) Teriflunomide attentuates immunopathological changes in the Dark Agouti rat model of experimental autoimmune encephalomyelitis. Mult Scler 17: S187, P448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- sanofi-aventis (2012) Arava Prescribing Information. US LLC. 2012. Bridgewater, NJ: sanofi-aventis [Google Scholar]
- Vermersch P., Czlonkowska A., Grimaldi L., Confavreux C., Comi G., Kappos L., et al. (2012) Evaluation of patient satisfaction from the TENERE study: a comparison of teriflunomide and subcutaneous interferon beta-1a in patients with relapsing multiple sclerosis. J Neurol 259(Suppl. 1): 1–23622173952 [Google Scholar]
- Wang L., Van Horn R., Turpault S., Zeng Z. (2011) Metabolism comparison between leflunomide and teriflunomide in humans. Eur J Neurol 18(Suppl. 2): 268 [Google Scholar]
- Warnke C., Meyer zu, Horste G., Hartung H., Stuve O., Kieseier B. (2009) Review of teriflunomide and its potential in the treatment of multiple sclerosis. Neuropsychiatr Dis Treat 5: 333–340 [PMC free article] [PubMed] [Google Scholar]


