Abstract
Mycobacterium tuberculosis possesses agonists for several Toll-like receptors (TLRs), yet mice with single TLR deletions are resistant to acute tuberculosis. MyD88−/− mice were used to examine whether TLRs play any role in protection against aerogenic M. tuberculosis H37Rv infection. MyD88−/− mice failed to control mycobacterial replication and rapidly succumbed. Moreover, expressions of interleukin 12, tumor necrosis factor alpha, gamma interferon, and nitric oxide synthase 2 were markedly decreased in the knockout animals. These results argue that resistance to M. tuberculosis must depend on MyD88-dependent signals mediated by an as-yet-undetermined TLR or a combination of TLRs.
Studies of both experimental models and patients with genetic defects have indicated a major role for gamma interferon (IFN-γ) in host resistance to Mycobacterium tuberculosis (5, 7, 11). This typically T-lymphocyte-dependent response is thought to be driven primarily by proinflammatory cytokines such as interleukin 12 (IL-12) and IL-18 that are stimulated by innate recognition events occurring soon after initial infection. While antigen-presenting cells (APC) such as dendritic cells and macrophages are likely sources of these initiating cytokines, the receptor-mycobacterial ligand interactions responsible for APC triggering in vivo have not been clearly defined. Members of the Toll-like receptor (TLR) family are major candidates for the host receptors involved in the innate recognition of mycobacteria. TLRs are evolutionarily conserved proteins that have been shown to detect molecular patterns in nearly every class of infectious microorganism (15). Their ligation leads to proinflammatory cytokine production and up-regulated costimulatory molecule expression by APC (15). In vitro studies have shown that M. tuberculosis possesses potent agonists for a number of TLRs, including TLR2 (4, 12, 17, 28, 29) and TLR4 (1, 17). Surprisingly, mice deficient in TLR2 (18, 25), TLR4 (1, 6, 18, 23), or TLR6 (25) show no defects in acute resistance to aerogenic M. tuberculosis infections. Additionally, a recent report has shown that gene expression in macrophages infected with M. tuberculosis in vitro is largely independent of the TLR intracellular adaptor molecule myeloid differentiation factor 88 (MyD88) (22). These studies raise the question of whether TLR signaling plays any role in resistance to M. tuberculosis.
All TLRs that have so far been identified have at least one signaling pathway dependent on MyD88 (15), and thus mice deficient in MyD88 offer a system to test the hypothesis that TLR signaling is required for resistance to acute tuberculosis. Since MyD88 is also required for IL-1 receptor (IL-1R) and IL-18R signaling, the possible involvement of these receptors must also be taken into account. MyD88−/− mice have been shown to be highly susceptible to a number of pathogens, including Listeria monocytogenes (8, 21), Staphylococcus aureus (27), and Toxoplasma gondii (19), and in a recent study, we demonstrated that these animals are more susceptible to Mycobacterium avium infection than either TLR2- or TLR4-deficient mice (9).
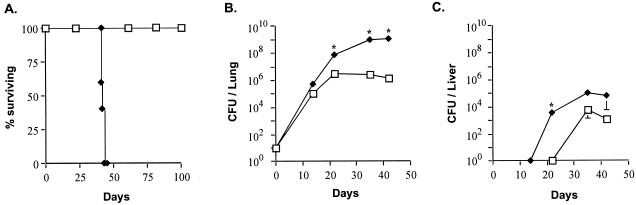
To test the requirement for MyD88 in host resistance to aerosol M. tuberculosis infection, we infected MyD88−/− mice (3) partially backcrossed on a C57BL/6 background with 20 to 50 CFU of the virulent H37Rv strain of M. tuberculosis by using a nose-only exposure chamber (CH Technologies, Westwood, N.J.) and compared their survival and immune responses to those of wild-type control (WT) mice (Taconic Farms, Germantown, N.Y.). In three separate experiments, MyD88-deficient mice succumbed to M. tuberculosis infection within only 42 days of infection, while WT mice survived for >180 days (Fig. 1A). This mortality was indistinguishable from that observed with similarly infected IFN-γ−/− mice in a separate experiment (mean survival time, 41 ± 1 days). Lungs of infected animals were removed, homogenized in phosphate-buffered saline with 0.5% Tween 80, and plated on 7H11 agar plates to enumerate the numbers of bacilli. The mycobacterial burden in the lungs of MyD88−/− mice was consistently higher than that in the WT animals at each time point examined, reaching a >3-log difference at their time of death (Fig. 1B). This increase in bacterial numbers was also evident in acid-fast stained sections of the same tissue (Fig. 2A and B). Histopathological examination at 3 weeks revealed exacerbated granulomatous inflammation and necrosis in the lungs of MyD88−/− mice relative to those of comparably infected WT animals (Fig. 2C and D), and this difference became even more pronounced at 5 weeks postinfection (data not shown). Livers from MyD88−/− mice also showed higher mycobacterial loads, particularly at 3 weeks postinfection, a time when bacterial CFU were still below the limit of detection in the WT animals (Fig. 1C). In liver tissue, sections from MyD88−/− mice showed fewer lesions (0.03 ± 0.02 granulomas/12× field) compared to those of WT animals (0.33 ± 0.18 granulomas/12× field) at the same 3 week time point (Fig. 2, compare panels E and F), despite the marked elevation in bacterial load (Fig. 1C). However, by 5 weeks, both granuloma numbers and morphology appeared comparable in the WT and MyD88−/− animals (data not shown). These findings suggest that MyD88−/− mice display both delayed initial granuloma formation as well as impaired control of bacterial growth within the mature lesions that eventually develop.
FIG. 1.
MyD88−/− mice are more susceptible than WT mice to aerogenic M. tuberculosis infection. (A) MyD88−/− mice (diamonds) and C57BL/6 × 129sv (F1) WT animals (squares), used to control for the possible influence of contaminating 129 genes, were infected in groups of 5 to 7 mice with 20 to 50 CFU of M. tuberculosis and monitored for survival. Data are representative of two separate experiments. Bacterial burdens (means ± standard errors) in the lungs (B) or livers (C) were also measured in three mice per group at the times indicated. Asterisks indicate statistically significant (P ≤ 0.05) differences determined by unpaired t test after log transformation between CFU values in MyD88−/− and WT animals. Data are representative of the results from two experiments.
FIG. 2.
MyD88−/− mice infected with M. tuberculosis exhibit more bacilli, exacerbated pathology, and reduced NOS2 expression in lungs, as well as delayed granuloma formation in liver. Formalin-fixed, paraffin-embedded lung (A to D, G, and H) and liver (E and F) tissue sections from mice 3 weeks after infection with aerosol M. tuberculosis were stained by the Kinyoun acid-fast method to detect mycobacteria (red staining) (A and B) or with hematoxylin and eosin stain (C to F). Note the absence of granulomas in panel F. NOS2 was visualized immunohistochemically in serial sections of these same tissues (G and H). Sections shown are representative of multiple fields from the organs of at least three animals per group. Original magnifications are ×63 (A and B), ×5 (C and D), ×10 (E and F), and ×20 (G and H).
We next investigated the immunologic defect(s) responsible for the striking susceptibility to M. tuberculosis exhibited by MyD88−/− animals. Total RNA was isolated from the lungs by using Trizol (Invitrogen, San Diego, Calif.), and cDNA was prepared by using Superscript reverse transcriptase (RT; Invitrogen) and subjected to quantitative real-time RT-PCR analysis with previously described primers (9) and a 7900HT sequence detection system (Applied Biosystems, Foster City, Calif.). IL-12 and IFN-γ, two cytokines required for control of M. tuberculosis in mice (10) and implicated in mycobacterial resistance in humans (5), are induced by a number of pathogens in an MyD88-dependent manner (2, 9, 19, 21). We observed significantly less IL-12p40 and IFN-γ mRNA in the lungs of M. tuberculosis-infected MyD88−/− mice than in similarly infected WT mice at both 2 and 3 weeks postinfection (Fig. 3A and B). Transcripts for tumor necrosis factor alpha (TNF-α), another proinflammatory cytokine required for resistance to M. tuberculosis infection (10), were also reduced significantly in the lungs of infected MyD88-deficient animals (Fig. 3C).
FIG. 3.
Expression of IL-12, IFN-γ, and TNF-α, as well as NOS2, is impaired in MyD88−/− mice following aerogenic M. tuberculosis infection. Real-time RT-PCR was used to quantitate IL-12p40 (A), IFN-γ (B), TNF-α (C), and NOS2 (F) mRNA expression in the lungs of infected WT (gray bars) and MyD88−/− (black bars) mice. In parallel experiments, splenocytes from infected WT and MyD88−/− mice were isolated and restimulated with purified protein derivative, and the supernatants were analyzed 3 days later for IL-12p40 (D) and IFN-γ (E) by enzyme-linked immunosorbent assay. Each bar is the mean (± standard deviation) of data from three mice. Asterisks indicate a P value of ≤0.05 determined by unpaired t test. Data are representative of results from three experiments.
The defects in the IL-12 and IFN-γ responses in the knockout (KO) animals as detected by real-time RT-PCR in vivo were investigated further in ex vivo restimulation experiments. Splenocytes were plated at 5 × 105 cells/well in 96-well plates and restimulated with purified protein derivative (Statens Seruminstitut, Copenhagen, Denmark), and IL-12p40 and IFN-γ were quantitated in supernatants 3 days later by enzyme-linked immunosorbent assay as described previously (19). At 2 and 4 weeks postinfection, splenocytes from M. tuberculosis-infected MyD88−/− mice made significantly less IL-12p40 and IFN-γ than did spleen cells from infected WT animals (Fig. 3D and E). Nevertheless, it is important to note that for each of the cytokines studied in vivo and ex vivo, a significant level of cytokine expression was evident even in the MyD88−/− animals, suggesting the existence of alternative signaling pathways for generating these responses.
IFN-γ plays a major role in the up-regulation of nitric oxide synthase 2 (NOS2) and the subsequent production of NO, a metabolite with antimycobacterial activity both in vivo and in vitro (10). We assessed whether impaired NO synthesis might contribute to the enhanced susceptibility of MyD88−/− mice to M. tuberculosis by measuring NOS2 gene expression as well as its production in situ in the lungs of infected mice. MyD88−/− mice showed markedly reduced NOS2 mRNA levels compared to those of WT animals by 3 weeks postinfection (Fig. 3F). Moreover, immunohistochemical staining for NOS2, performed as described previously (20), was greatly diminished in the lungs of the KO mice compared to those of WT animals at the same time point (Fig. 2G and H). Therefore, M. tuberculosis-induced NOS2 expression in vivo is largely dependent on MyD88, a finding which contrasts with the conclusion of in vitro studies in which the induction of NOS2 expression by M. tuberculosis was observed to be MyD88-independent (16, 22). This discrepancy may reflect additional downstream effects of MyD88 deficiency on NOS2 gene induction by M. tuberculosis in vivo versus in vitro.
These findings establish that host resistance to infection with M. tuberculosis H37Rv is dependent on MyD88 and therefore strongly implicate TLR and/or IL-1/IL-18 receptor signaling in this response. Previous studies using mice deficient in IL-1 (30), IL-1R (13), or IL-18 (14, 24) have revealed minor roles for these signaling elements in the control of M. tuberculosis relative to the role described here for MyD88. Therefore, our results argue for a major function of TLRs in host defense against this pathogen and are consistent with previous data demonstrating a requirement for MyD88 in resistance to M. avium infection (9).
Our findings are, however, in partial disagreement with a recently published study in which MyD88−/− mice aerogenically infected with the Kurono strain of M. tuberculosis showed no increase in mortality, despite developing higher bacterial loads than WT animals (26). Moreover, no significant reduction in proinflammatory or Th1 cytokine production was observed in the infected MyD88−/− animals (26). Since a similar infection protocol was used in both this and the present study, it is likely that the disparate results relate to differences in the bacterial strains employed. An alternative possibility is that the discrepancy is due to differences in the genetic background of the KO mice used in the two studies.
While MyD88 appears to regulate resistance to at least some M. tuberculosis strains, the specific TLRs involved have yet to be defined. Previous studies investigating TLR2 (18, 25), TLR4 (1, 6, 18, 23), or TLR6 (25) have revealed only a minor influence of these TLRs in the early control of M. tuberculosis. Therefore, either a TLR not yet tested or a combination of different TLRs is likely to explain the MyD88 dependency of host resistance to M. tuberculosis. Since M. tuberculosis H37Rv-infected MyD88−/− mice showed impaired IL-12, IFN-γ, TNF-α, and NOS2 responses, it is reasonable to speculate that the TLR signaling pathways involved determine host control of infection by regulating the production of these four mediators, known to be required for resistance to M. tuberculosis in mice (10). However, since MyD88 deficiency did not result in a complete elimination of IL-12, IFN-γ, TNF-α, or NOS2 expression, it is possible that other as-yet-unidentified, TLR-dependent immune responses contribute to control of this important pathogen.
Acknowledgments
We thank Shizuo Akira and Douglas Golenbach for generously providing the original MyD88−/− breeders. We are also grateful to Sandy White and Jacqueline Gonzales for technical assistance and Dragana Jankovic for critical reading of this paper.
Editor: J. F. Urban, Jr.
REFERENCES
- 1.Abel, B., N. Thieblemont, V. J. F. Quesniaux, N. Brown, J. Mpagi, K. Miyake, F. Bihl, and B. Ryffel. 2002. Toll-like receptor 4 expression is required to control chronic Mycobacterium tuberculosis infection in mice. J. Immunol. 169:3155-3162. [DOI] [PubMed] [Google Scholar]
- 2.Adachi, K., H. Tsutsui, S.-I. Kashiwamura, E. Seki, H. Nakano, O. Takeuchi, K. Takeda, K. Okumura, L. Van Kaer, H. Okamura, S. Akira, and K. Nakanishi. 2001. Plasmodium berghei infection in mice induces liver injury by an IL-12 and Toll-like receptor/myeloid differentiation factor 88-dependent mechanism. J. Immunol. 167:5928-5934. [DOI] [PubMed] [Google Scholar]
- 3.Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143-150. [DOI] [PubMed] [Google Scholar]
- 4.Brightbill, H. D., D. H. Libratey, S. R. Krutzik, R. B. Yang, J. T. Belisle, S. R. Bleharski, M. Maitland, M. V. Norgard, S. E. Plevy, S. T. Smale, P. J. Brennan, B. R. Bloom, P. J. Godowski, and R. L. Modlin. 1999. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science 285:732-736. [DOI] [PubMed] [Google Scholar]
- 5.Casanova, J. L., and L. Abel. 2002. Genetic dissection of immunity to mycobacteria: the human model. Annu. Rev. Immunol. 20:581-620. [DOI] [PubMed] [Google Scholar]
- 6.Chackerian, A. A., T. V. Perera, and S. M. Behar. 2001. Gamma interferon-producing CD4+ T lymphocytes in the lung correlate with resistance to infection with Mycobacterium tuberculosis. Infect. Immun. 69:2666-2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper, A. M., D. K. Dalton, T. A. Stewart, J. P. Griffen, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in IFN-γ gene-disrupted mice. J. Exp. Med. 178:2243-2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edelson, B. T., and E. R. Unanue. 2002. MyD88-dependent but Toll-like receptor 2-independent innate immunity to Listeria: no role for either in macrophage listericidal activity. J. Immunol. 169:3869-3875. [DOI] [PubMed] [Google Scholar]
- 9.Feng, C. G., C. A. Scanga, C. M. Collazo-Custodio, A. W. Cheever, S. Hieny, P. Caspar, and A. Sher. 2003. Mice lacking myeloid differentiation factor 88 display profound effects in host resistance and immune responses to Mycobacterium avium infection not exhibited by Toll-like receptor 2 (TLR2)- and TLR4-deficient animals. J. Immunol. 171:4758-4764. [DOI] [PubMed] [Google Scholar]
- 10.Flynn, J. L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93-129. [DOI] [PubMed] [Google Scholar]
- 11.Flynn, J. L., J. Chan, K. J. Triebold, D. K. Dalton, T. Stewart, and B. R. Bloom. 1993. An essential role for interferon-γ in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones, B. W., T. K. Means, K. A. Heldwein, M. A. Keen, P. J. Hill, J. T. Belisle, and M. J. Fenton. 2001. Different Toll-like receptor agonists induce distinct macrophage responses. J. Leukoc. Biol. 69:1036-1044. [PubMed] [Google Scholar]
- 13.Juffermans, N. P., S. Florquin, L. Camoglio, A. Verbon, A. H. Kolk, P. Speelman, S. J. H. van Deventer, and T. van der Poll. 2000. Interleukin-1 signaling is essential for host defense during murine pulmonary tuberculosis. J. Infect. Dis. 182:902-908. [DOI] [PubMed] [Google Scholar]
- 14.Kinjo, Y., K. Kawakami, K. Uezu, S. Yara, K. Miyagi, Y. Koguchi, T. Hoshino, M. Okamoto, Y. Kawase, K. Yokota, K. Yoshino, K. Takeda, S. Akira, and A. Saito. 2002. Contribution of IL-18 to Th1 response and host defense against infection by Mycobacterium tuberculosis: a comparative study with IL-12p40. J. Immunol. 169:323-329. [DOI] [PubMed] [Google Scholar]
- 15.Kopp, E., and R. Medzhitov. 2003. Recognition of microbial infection by Toll-like receptors. Curr. Opin. Immunol. 15:396-401. [DOI] [PubMed] [Google Scholar]
- 16.Means, T. K., B. W. Jones, A. B. Schromm, B. A. Shurtleff, J. A. Smith, J. Keane, D. T. Golenbock, S. N. Vogel, and M. J. Fenton. 2001. Differential effects of a Toll-like receptor antagonist on Mycobacterium tuberculosis-induced macrophage responses. J. Immunol. 166:4074-4082. [DOI] [PubMed] [Google Scholar]
- 17.Means, T. K., S. Wang, E. Lien, A. Yoshimura, D. T. Golenbock, and M. J. Fenton. 1999. Human Toll-like receptors mediate cellular activation by M. tuberculosis. J. Immunol. 163:3920-3927. [PubMed] [Google Scholar]
- 18.Reiling, N., C. Holscher, A. Fehrenbach, S. Kroger, C. J. Kirschning, S. Goyert, and S. Ehlers. 2002. Cutting edge: Toll-like receptor (TLR)2- and TLR4-mediated pathogen recognition in resistance to airborne infection with Mycobacterium tuberculosis. J. Immunol. 169:3480-3484. [DOI] [PubMed] [Google Scholar]
- 19.Scanga, C. A., J. Aliberti, D. Jankovic, F. Tilloy, S. Bennouna, E. Y. Denkers, R. Medzhitov, and A. Sher. 2002. Cutting edge: MyD88 is required for resistance to Toxoplasma gondii infection and regulates parasite-induced IL-12 production by dendritic cells. J. Immunol. 168:5997-6001. [DOI] [PubMed] [Google Scholar]
- 20.Scanga, C. A., V. P. Mohan, K. Yu, H. Joseph, K. Tanaka, J. Chan, and J. L. Flynn. 2000. Depletion of CD4+ T cells causes reactivation of murine latent tuberculosis despite continued expression of IFN-γ and NOS2. J. Exp. Med. 192:347-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seki, E., H. Tsutsui, N. M. Tsuji, N. Hayashi, K. Adachi, H. Nakano, S. Futatsugi-Yumikura, O. Takeuchi, K. Hoshino, S. Akira, J. Fujimoto, and K. Nakanishi. 2002. Critical roles of myeloid differentiation factor 88-dependent proinflammatory cytokine release in early phase clearance of Listeria monocytogenes. J. Immunol. 169:3863-3868. [DOI] [PubMed] [Google Scholar]
- 22.Shi, S., C. Nathan, D. Schnappinger, J. Drenkow, M. Fuortes, E. Block, A. Ding, T. R. Gringeras, G. Schoolnik, S. Akira, K. Takeda, and S. Ehrt. 2003. MyD88 primes macrophages for full-scale activation by interferon-γ yet mediates few responses to Mycobacterium tuberculosis. J. Exp. Med. 198:987-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shim, T. S., O. C. Turner, and I. M. Orme. 2003. Toll-like receptor 4 plays no role in susceptibility of mice to Mycobacterium tuberculosis. Tuberculosis (Edinburgh) 83:367-371. [DOI] [PubMed] [Google Scholar]
- 24.Sugawara, I., H. Yamada, H. Kaneko, S. Mizuno, K. Takeda, and S. Akira. 1999. Role of interleukin-18 (IL-18) in mycobacterial infection in IL-18-gene-disrupted mice. Infect. Immun. 67:2585-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sugawara, I., H. Yamada, C. Li, S. Mizuno, O. Takeuchi, and S. Akira. 2003. Mycobacterial infection in TLR2 and TLR6 knockout mice. Microbiol. Immunol. 47:327-336. [DOI] [PubMed] [Google Scholar]
- 26.Sugawara, I., H. Yamada, S. Mizuno, K. Takeda, and S. Akira. 2003. Mycobacterial infection in MyD88-deficient mice. Microbiol. Immunol. 47:841-847. [DOI] [PubMed] [Google Scholar]
- 27.Takeuchi, O., K. Hoshino, and S. Akira. 2000. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J. Immunol. 165:5392-5396. [DOI] [PubMed] [Google Scholar]
- 28.Thoma-Uszynski, S., S. Stenger, O. Takeuchi, M. T. Ochoa, M. Engele, P. A. Sieling, P. F. Barnes, M. Rollinghoff, P. L. Bolcskei, M. Wagner, S. Akira, M. V. Norgard, J. T. Belisle, P. J. Godowski, B. R. Bloom, and R. L. Modlin. 1999. Induction of direct antimicrobial activity through mammalian Toll-like receptors. Science 291:1544-1547. [DOI] [PubMed] [Google Scholar]
- 29.Underhill, D. M., A. Ozinsky, K. D. Smith, and A. Aderem. 1999. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc. Natl. Acad. Sci. USA 96:14459-14465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamada, H., S. Mizuno, R. Horai, Y. Iwakura, and I. Sugawara. 2000. Protective role of interleukin-1 in mycobacterial infection in IL-1α/β double-knockout mice. Lab. Investig. 80:759-767. [DOI] [PubMed] [Google Scholar]