Abstract
The adult mammalian heart has limited potential for regeneration. Thus, after injury, cardiomyocytes are permanently lost, and contractility is diminished. In contrast, the neonatal heart can regenerate owing to sustained cardiomyocyte proliferation. Identification of critical regulators of cardiomyocyte proliferation and quiescence represents an important step toward potential regenerative therapies. Yes-associated protein (Yap), a transcriptional cofactor in the Hippo signaling pathway, promotes proliferation of embryonic cardiomyocytes by activating the insulin-like growth factor and Wnt signaling pathways. Here we report that mice bearing mutant alleles of Yap and its paralog WW domain containing transcription regulator 1 (Taz) exhibit gene dosage-dependent cardiac phenotypes, suggesting redundant roles of these Hippo pathway effectors in establishing proper myocyte number and maintaining cardiac function. Cardiac-specific deletion of Yap impedes neonatal heart regeneration, resulting in a default fibrotic response. Conversely, forced expression of a constitutively active form of Yap in the adult heart stimulates cardiac regeneration and improves contractility after myocardial infarction. The regenerative activity of Yap is correlated with its activation of embryonic and proliferative gene programs in cardiomyocytes. These findings identify Yap as an important regulator of cardiac regeneration and provide an experimental entry point to enhance this process.
Keywords: cell cycle, cardiomyopathy, cardiac fibrosis
The adult mammalian heart has limited potential for regeneration or repair (1), and thus the loss of cardiomyocytes during injury and disease results in diminished pump function and heart failure, a primary cause of human morbidity and mortality. Therefore, there is a need for innovative approaches to replace cardiomyocytes after cardiac injury. In addition to stem cell transplantation (2, 3), recent studies have suggested strategies for repairing the adult heart through reprogramming of nonmyocytes to a cardiac cell fate using cardiogenic genes and small molecules (4–10). Because adult mammalian cardiomyocytes withdraw permanently from the cell cycle soon after birth (11, 12), approaches to stimulating cardiomyocyte proliferation represent a possible alternative means of restoring function to the injured heart.
We recently reported that the neonatal mouse heart is capable of mounting a robust regenerative response after apical resection or myocardial infarction (MI) (13, 14). The extent of neonatal heart regeneration in mice is comparable to that in zebrafish, which can regenerate diverse tissues (15–17). However, by 1 wk of life, the potential for heart regeneration is lost in mice and is replaced by a fibrotic response that generates scar tissue, which impedes regeneration and impairs cardiac contractility (13).
Heart regeneration in zebrafish and neonatal mice is correlated with global activation of cardiomyocyte proliferation throughout the injured organ (13, 18, 19). The Hippo signaling pathway is an evolutionarily ancient cascade of kinases and effector proteins that control cell proliferation and organ size (20, 21). The Hippo pathway is activated by various upstream molecular signals, including G protein-coupled receptors, cell–cell interactions, and alterations in cytoskeletal dynamics (22–29). The core components of this pathway in Drosophila include the Hippo and Warts kinases and their regulatory subunits Salvador and Mats, which culminate in phosphorylation of the transcription factor Yorkie, inhibiting its nuclear entry (20). Signals that inhibit these upstream kinases prevent phosphorylation of Yorkie, allowing its nuclear importation and association with target transcription factors to activate genes required for cell growth. Hippo, Salvador, Mats, and Yorkie are structurally and functionally orthologous to mammalian Mst1/2, Sav1, Lats1/2, and Yap/Taz, respectively, and many of these mammalian Hippo signaling components have been implicated in the control of organ size and tumorigenesis (20, 21, 30, 31).
Deletion of the Hippo pathway inhibitory upstream kinases Mst1/2 and Lats was recently shown to promote cardiomyocyte proliferation during mouse embryogenesis (32). Consistent with these findings, we and others have demonstrated that forced expression of a constitutively active form of Yap in the embryonic or postnatal mouse heart increases cardiomyocyte proliferation and heart size, and, conversely, that cardiac deletion of Yap suppresses cardiomyocyte proliferation (33, 34). Yap exerts its growth-stimulating actions in the heart by regulating genes in the insulin-like growth factor (IGF) and Wnt signaling pathways (33). Yap shares 45% amino acid identity with Taz (35), but the potential functional redundancy of these proteins has not been examined through compound loss of function mutations in mice.
In the present study, we explored the dosage requirements of Yap and Taz during heart development. We further examined whether Yap participates in neonatal heart regeneration, and whether activation of Yap in the adult heart is sufficient to improve cardiac function after MI. Our results show that Yap and Taz play redundant roles in the control of cardiac growth, and that their combined deletion leads to lethal cardiomyopathy in a gene dosage-dependent manner. Cardiac deletion of Yap impairs neonatal cardiac regeneration, whereas expression of a constitutively active form of Yap in the adult heart increases cardiomyocyte numbers, reduces scar size, and improves cardiac function post-MI. These findings suggest that manipulation of the Hippo-Yap signaling pathway may provide a means of promoting cardiac repair after injury.
Results
Requirement of Yap for Postnatal Cardiac Function.
We previously reported that deletion of a conditional Yap allele from the embryonic heart using Nkx2.5-Cre, which is expressed early in heart development, resulted in lethality by embryonic day (E) 10.5 owing to a deficiency of cardiomyocytes, revealing an essential role of Yap in embryonic heart growth (33). To investigate the role of Yap in the postnatal heart, we deleted the gene using α-myosin heavy chain (αMHC)-Cre, which is up-regulated during fetal and postnatal cardiac development (36). Cardiac-specific Yap knockout (cKO) mice generated with αMHC-Cre were viable and were obtained at predicted Mendelian ratios, indicating that Yap deletion with this Cre transgene circumvented embryonic lethality.
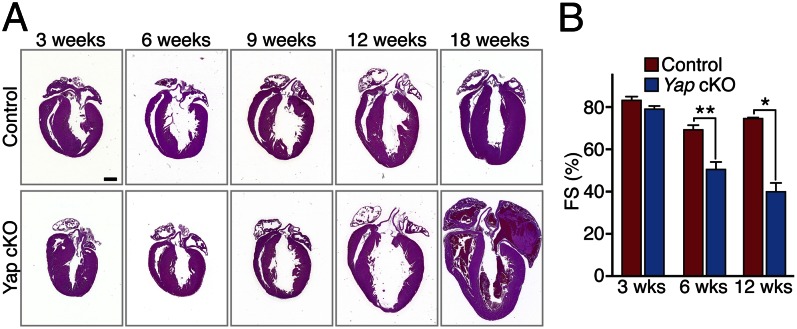
We observed no overt abnormalities in the Yap cKO mice until they became lethargic with labored breathing and died between 11 and 20 wk of age. Cardiac size and structure were normal in the Yap cKO mice at age 3 wk, but by age 9 wk, thinning of the ventricular walls and dilated cardiomyopathy were noted, which worsened with age and culminated in atrial thrombosis, fibrosis, and lethal heart failure (Fig. 1A).
Fig. 1.
Loss of Yap results in lethal cardiomyopathy. (A) Progressive dilated cardiomyopathy in Yap cKO mice. H&E-stained four-chamber view sections of mouse hearts show gradual dilation of ventricular chambers and atrial thrombosis in Yap cKO hearts, culminating in heart failure. (Scale bar: 1 mm.) (B) Progressive deterioration of cardiac function in Yap cKO mice with age, as evidenced by decreased FS. n = 4–6 per group. Data are mean ± SD. *P < 0.05; **P < 0.005.
Yap cKO hearts demonstrated normal function at age 3 wk, but exhibited mild functional deficits by age 6 wk. By age 12 wk, ventricular chamber dilation was more pronounced, as evidenced by increased left ventricular (LV) internal dimension at systole (LVIDs) and reduced fractional shortening (FS) (Fig. 1B and Fig. S1A). Heart weight (HW)-to-tibia length (TL) ratios were not significantly different in control and Yap cKO mice (Fig. S1B).
Quantitative real-time PCR (qPCR) on RNA isolated from the left ventricle of Yap cKO mice confirmed the effective Cre-mediated deletion of Yap (Fig. S1C). The residual Yap mRNA in cKO hearts is likely related to expression of Yap mRNA in cardiac fibroblasts and other nonmuscle cells in which αMHC-Cre is not expressed. In addition, Yap expression was significantly greater than Taz expression in cardiomyocytes (Fig. S1D), and, as anticipated, Taz expression was not significantly changed in Yap cKO hearts (Fig. S1C).
Gene Dosage-Dependent Cardiac Functions of Yap and Taz.
To investigate whether Taz is necessary for heart development and whether it shares essential cardiac functions with Yap, we generated a conditional Taz null allele. To do so, we introduced sites for LoxP recombination upstream and downstream of exon 3 through homologous recombination (Fig. S2). Targeting of the Taz allele was confirmed by Southern blot analysis, and deletion of Taz was validated by qPCR at the genomic level (Fig. S2).
Mice bearing the conditional Taz mutant allele were bred to CAG-Cre transgenic (Tg) mice that ubiquitously express Cre recombinase to generate mice with global deletion and confirm functionality of the targeting strategy. Taz null mice developed renal cysts and recapitulated the previously published phenotype resulting from global gene deletion (Fig. S2) (37), demonstrating effective Cre-mediated gene deletion.
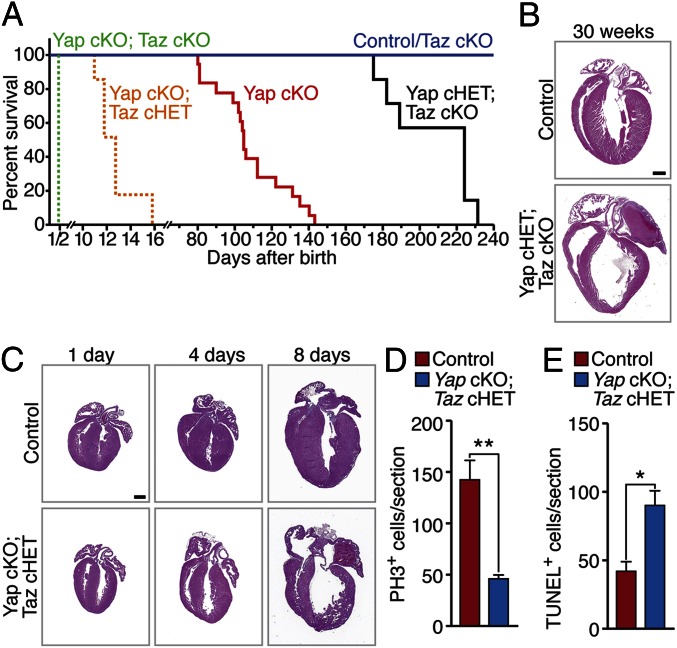
Cardiac-specific Taz knockout (Taz cKO) mice were generated by breeding mice harboring conditional Taz alleles to αMHC-Cre Tg mice. The Taz cKO mice were viable and did not display any obvious cardiac defects. Intriguingly, however, gene dosage-dependent lethal cardiomyopathy was observed when various combinations of Yap and Taz alleles were deleted in cardiomyocytes (Fig. 2A). Whereas heterozygous Yap (Yap cHET) mice and Taz cKO mice displayed no phenotypes, cardiac deletion of one copy of Yap and two copies of Taz (Yap cHET; Taz cKO) led to complete lethality by age 33 wk (Fig. 2A). Similarly, combined deletion of both Yap alleles with heterozygous deletion of Taz (Yap cKO; Taz HET) accelerated the onset of cardiac abnormalities from the complete lethality by age 20 wk seen in Yap cKO mice to lethality by age 10 d (Fig. 2A). Double genetic deletion of both Yap and Taz in the heart (Yap cKO; Taz cKO) caused the most severe phenotype, with complete lethality by 1 d after birth (Fig. 2A). The αMHC-Cre transgene is expressed as early as E10.5; thus, this severe early phenotype likely reflects essential and redundant roles of Yap and Taz in fetal cardiac growth and function. Because our primary interest was in investigating the mechanisms underlying postnatal regenerative responses of the heart, we did not further analyze potential prenatal cardiac abnormalities in the Yap cKO; Taz cKO mutant mice.
Fig. 2.
Loss of Yap and Taz results in lethal cardiomyopathy in a gene dosage-dependent manner. (A) Survival curves of cardiac Yap and Taz compound mutant mice. n = 9–21 per group. (B) H&E-stained four-chamber view sections of mouse hearts at 30 wk. (Scale bar: 1 mm.) (C) H&E-stained four-chamber view sections of mouse hearts of various ages and genotypes. (Scale bar: 1 mm.) (D) Quantification of PH3 immunostaining in control (Yapfl/fl; Tazfl/+) and Yap cKO; Taz cHET hearts at P1. (E) Quantification of TUNEL staining in control and Yap cKO; Taz cHET hearts at P4. Data are mean ± SD. *P < 0.05; **P < 0.005.
Histological analysis of the hearts lacking different numbers of Yap and Taz alleles revealed multiple cardiac defects, the severity of which were correlated with the lethality observed in mice with the compound gene mutations (Fig. 2 B and C). In Yap cHET; Taz cKO mice, hearts became grossly dilated and displayed atrial thrombi by age 30 wk, indicative of heart failure (Fig. 2B). These abnormalities were observed as early as age 8 d in Yap cKO; Taz cHET mice (Fig. 2C). The Yap cKO; Taz cKO mice exhibited extremely severe and lethal cardiac structural abnormalities at birth (Fig. S3A).
The thin myocardium of Yap cKO; Taz cHET hearts suggests a reduction in cardiomyocyte proliferation or survival. Analysis of histological sections revealed an ∼60% decrease in the number of phospho-histone H3 (PH3)-positive cardiomyocytes in mutant neonatal hearts compared with WT neonatal hearts (Fig. 2D and Fig. S3B), indicative of reduced cardiomyocyte proliferation. A dramatic increase in apoptotic cardiomyocytes in the neonatal Yap cKO; Taz cHET hearts, as measured by TUNEL staining, was detected as well (Fig. 2E and Fig. S3C).
The foregoing findings reveal an essential role of Yap in postnatal cardiac growth and function and suggest that Yap and Taz share some redundant cardiac functions in sustaining cardiomyocyte proliferation and survival. Given the dominant role and expression of Yap relative to Taz, we focused on the potential involvement of Yap in postnatal heart regeneration.
Loss of Yap Impairs Neonatal Cardiac Regeneration.
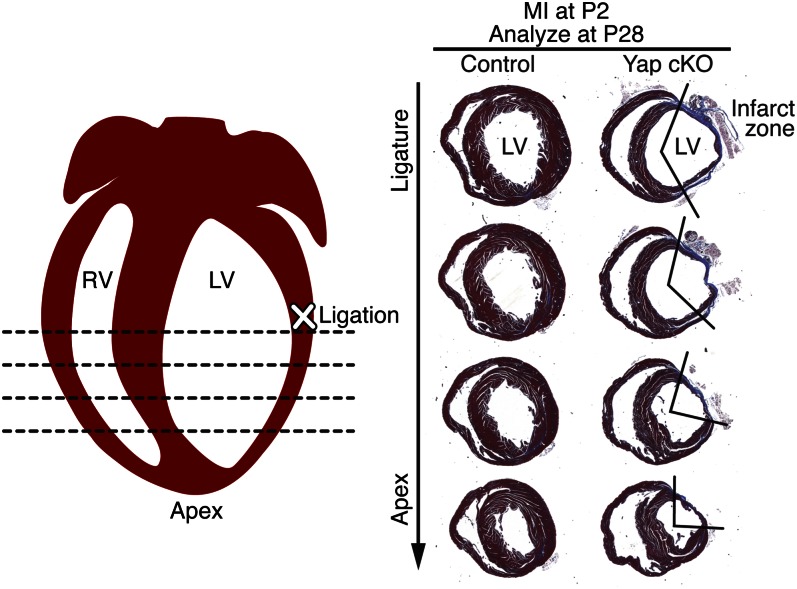
To explore the potential involvement of Yap in neonatal cardiac regeneration, we compared the regenerative responses of control and Yap cKO hearts after permanent ligation of the left anterior descending coronary artery (LAD) at postnatal day (P) 2. As reported previously (13, 14), hearts of the control mice injured at P2 were fully regenerated by P28 (Fig. 3), showing minimal scarring and healthy myocardial tissue distal to the ligature. In contrast, Yap cKO hearts displayed a gross deficiency of healthy myocardial tissue throughout the LV free wall at 26 d after MI, along with an extensive fibrotic infarct zone (Fig. 3). Considering that we detected no differences in heart size, structure, or histology between uninjured Yap cKO and control hearts until age 3 wk (Fig. 1B and Fig. S1B), the inability of Yap cKO hearts to efficiently regenerate during the neonatal period cannot be attributed to a deficiency of cardiomyocytes or other overt abnormalities during this period. We conclude that Yap is required for neonatal heart regeneration in response to injury.
Fig. 3.
Yap loss of function impairs neonatal heart regeneration. Cardiac fibrosis and scar formation were compared in control (Yapfl/fl) and Yap cKO mice. Hearts were subjected to LAD ligation at P2 and analyzed by Masson’s trichrome staining of transverse sections at P28. Shown are the site of ligation (marked with an “X”) and the four levels at which cardiac fibrosis was evaluated. Serial sections were cut at 200-μm intervals from the site of the ligature toward the apex. Three hearts of each genotype were examined; representative hearts are shown. The boundaries of the infarct zones are demarcated by the V-shaped lines on the right.
Activated Yap Increases Heart Size.
Substitution of Ser112 with Ala (S112A) in mouse Yap generates a constitutively active, nuclear form of the protein (38). To determine whether activated Yap is sufficient to promote heart regeneration postnatally, we generated Tg mice overexpressing YapS112A in the heart under control of the αMHC promoter. The Tg mice expressing YapS112A displayed a thickened myocardium (Fig. S4A) and an increased HW:TL ratio (Fig. S4B). Double immunostaining for PH3 and cardiac troponin T confirmed the enhanced proliferation of cardiomyocytes in the hearts of YapS112A Tg mice (Fig. S5 A–C) at both P7 and adulthood, although the relative percentage of PH3-positive cells decreased dramatically with age. The average cross-sectional area of cardiomyocytes and capillary density in adult YapS112A Tg hearts were comparable to those of WT littermate control hearts (Fig. S5 D–G), suggesting that the increased heart size is related mainly to proliferation rather than to hypertrophy of cardiomyocytes or increased vascularization.
Activated Yap Enhances Cardiac Regeneration.
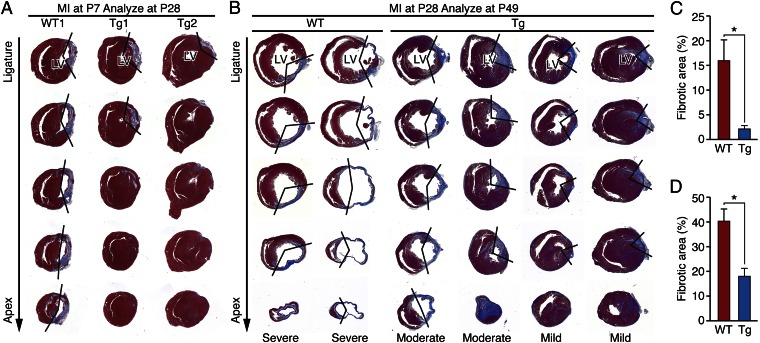
The regenerative ability of the neonatal mouse heart is lost after P7 (13, 14). To investigate whether the temporal window of regeneration can be extended by forced expression of activated Yap, we performed LAD ligation on YapS112A Tg and WT littermates at P7 and analyzed their hearts 21 d later at P28. Whereas WT mice showed extensive scarring, loss of myocardial tissue, and ventricular dilation after injury, YapS112A Tg mice hearts were regenerated with almost no fibrosis (Fig. 4 A and C). Similarly, after MI induction at P28, analysis of hearts 7 d and 21 d later revealed significantly reduced LV fibrosis and increased myocardial tissue in YapS112A Tg hearts compared with WT control hearts (Fig. 4 B and D and Fig. S6). Staining of hearts with 2,3,5-triphenyltetrazolium chloride (TTC) to visualize the ischemic zone after LAD ligation showed equivalent areas of ischemia in Yap Tg and WT hearts (Fig. S7). Assessment of the extent of cardiac injury at 21 d after MI demonstrated severe injury in all WT hearts, but injuries of varying severity, from mild to moderate, in Tg hearts. The variable degree of injury seen in the Tg hearts likely reflects, at least in part, the difference in location of the ligature. The hearts of YapS112A Tg mice consistently displayed less post-MI fibrotic scarring compared with hearts of WT mice.
Fig. 4.
Activated Yap enhances cardiac regeneration. (A) Masson’s trichrome-stained heart sections of WT and αMHC-YapS112A Tg mice at 21 d after LAD ligation performed at P7. Serial sections were cut at 200-μm intervals from the site of the ligature toward the apex. One representative WT and two Tg hearts are shown. The boundaries of the infarct zone are demarcated by the V-shaped lines on the right. (B) Masson’s trichrome-stained heart sections of WT and αMHC-YapS112A Tg mice at 21 d after LAD ligation performed at P28. Serial sections were cut at 500-μm intervals from the site of the ligature toward the apex. (C) Quantification of the fibrotic areas in heart sections shown in A. n = 3. (D) Quantification of the fibrotic areas in heart sections shown in B. n = 3 for WT; n = 4 for Tg. Fibrotic area (%) = (the sum of fibrotic area at levels 3 and 4/the sum of myocardial area in the LV at levels 3 and 4) × 100. Data are mean ± SD. *P < 0.05.
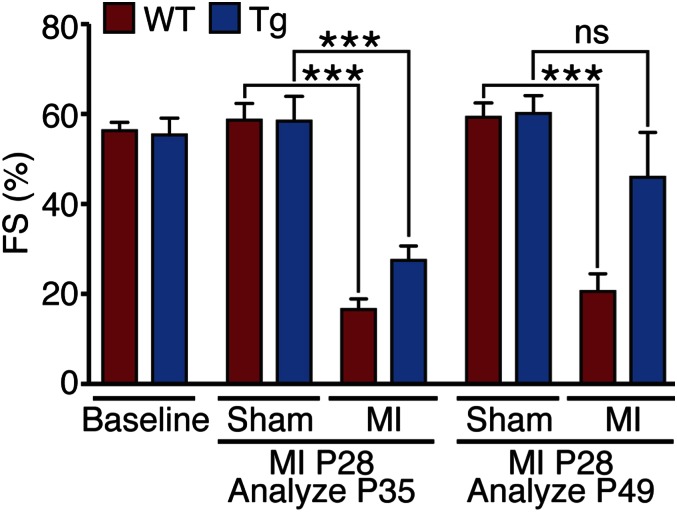
We also examined whether overexpression of YapS112A in differentiated cardiomyocytes could lead to measurable improvement in function of ischemic hearts. Cardiac function before and after MI, based on FS, ejection fraction, and stroke volume, was assessed by echocardiography. At 7 d after LAD ligation, FS and ejection fraction were decreased in all mice relative to presurgical mice; however, FS and ejection fraction were higher in the Tg hearts (Fig. 5 and Fig. S8). By 21 d post-MI, cardiac function was dramatically enhanced in Yap Tg hearts compared with WT littermate control hearts (Fig. 5 and Fig. S8). No significant differences in cardiac function between sham-operated WT and Tg mice were seen. These data indicate that expression of YapS112A in cardiomyocytes can sustain the function of injured adult hearts after MI.
Fig. 5.
Activated Yap improves function of ischemic hearts. Cardiac function of mice subjected to LAD ligation was evaluated at various time points by echocardiography. Data are mean ± SD. ***P < 0.001. ns, not statistically significant.
Activated Yap Enhances Cardiomyocyte Proliferation and Promotes Survival Post-MI.
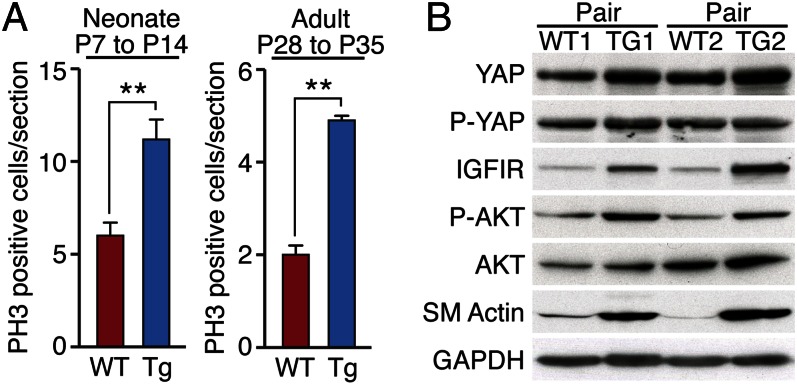
Analysis of PH3 immunostaining confirmed enhanced proliferation of cardiomyocytes in YapS112A Tg hearts at 7 d after LAD ligation at P7 (Fig. 6A and Fig. S9A). Cardiomyocyte cytokinesis, visualized by Aurora B kinase expression, was also increased in YapS112A Tg hearts at this time (Fig. S9B). Similarly, increased numbers of PH3-positive cells and decreased apoptosis, measured by PH3 and TUNEL staining, respectively, were detected in YapS112A Tg hearts at 7 d after LAD ligation at age 4 wk (Fig. 6A and Fig. S9 C–E). We conclude that YapS112A promotes cardiomyocyte proliferation and survival after cardiac injury.
Fig. 6.
Yap promotes cardiomyocyte proliferation after MI. (A) Quantification of PH3 immunostaining of WT and αMHC-YapS112A Tg hearts at 7 d post-MI performed on P7 mice (neonates) or at 7 d post-MI performed on P28 mice (adults). Three hearts were analyzed for each genotype at each time point. Data are mean ± SD. **P < 0.005. (B) Western blot analysis comparing the indicated protein levels in two pairs of WT and αMHC-YapS112A Tg adult hearts. GAPDH was detected as a loading control.
Modulation of Cardiac Gene Expression by Yap.
To further explore the mechanistic basis of the salutary effects of Yap on cardiac function post-MI, we analyzed changes in gene expression and intracellular signaling evoked by YapS112A. Consistent with previous studies demonstrating activation of IGF and Wnt signaling by Yap in embryonic heart (33), expression of the IGF-1 receptor was elevated in YapS112A Tg hearts, as was phospho-Akt, a downstream effector of IGF-1 signaling (Fig. 6B). In addition, smooth muscle α-actin, which is expressed in the fetal heart but absent from adult myocytes, was up-regulated in adult YapS112A Tg hearts (Fig. 6B and Fig. S9F). These findings suggest that YapS112A promotes adult cardiomyocyte proliferation, at least in part, by stimulating IGF-1 and Akt signaling and activation of fetal gene expression.
Discussion
Although the neonatal mouse heart displays a remarkable ability to regenerate after injury (13, 14), this regenerative potential is lost during the first week of life, such that injury thereafter results in loss of cardiomyocytes, fibrosis, and diminished pump function. During the neonatal period, cardiac injury triggers organ-wide proliferation of cardiomyocytes that supports regeneration of lost myocardium, with no apparent contribution from a stem cell population in this process. The heart’s loss of regenerative potential with age correlates temporally with the timing of irreversible withdrawal of myocytes from the cell cycle, suggesting that strategies to increase cardiomyocyte proliferation may enhance cardiac repair during adulthood.
Based on previous studies implicating the Hippo signaling pathway in the control of cardiomyocyte proliferation during embryonic development (32–34), we explored the potential of this pathway to enhance cardiomyocyte proliferation after injury to the postnatal heart. Our results demonstrate that forced expression of a constitutively active form of Yap that cannot be phosphorylated by inhibitory upstream kinases in the Hippo pathway extends the cardiac regenerative window and improves cardiac function post-MI. Although our findings clearly demonstrate that Yap can promote cardiomyocyte proliferation, multiple mechanisms likely contribute to myocardial preservation post-MI, including enhanced cardiomyocyte proliferation and survival, diminished fibrosis, and neoangiogenesis.
Activated Yap up-regulates numerous genes involved in fetal cardiac growth and stimulates phosphorylation of Akt, which can be achieved by activation of IGF signaling. IGF-1 has been shown to promote cardiomyocyte growth and suppress fibrosis (39), possibly contributing to the beneficial effects of Yap signaling in the injured heart. IGF-1 signaling may act through autocrine and paracrine pathways to promote regeneration.
The upstream signals that activate or inhibit the Hippo signaling pathway remain incompletely identified. Recently, signals from different G protein-coupled receptors were shown to exert antagonistic effects on this pathway, depending on the ligand and the coupled G protein (22). Of note in this regard, cardiac injury is accompanied by dramatic alterations in numerous G protein-coupled signaling pathways that could influence Hippo-Yap signaling. The Hippo-Yap pathway is also sensitive to changes in cell contact and dynamics of the actin cytoskeleton (23–29), which are perturbed after cardiac injury. Our findings suggest that pharmacologic or genetic inhibitors of the upstream kinases in the Hippo pathway would enhance cardiac repair after injury.
The results of this study confirm and extend those of previous studies in which various components of the Hippo signaling pathway were conditionally deleted in mice using various cardiac-specific Cre transgenes. In one study, embryonic cardiac deletion of Lats2 and Mst1/2, which function as negative regulators of Yap, resulted in excessive production of cardiomyocytes, which was been attributed to unrestrained activation of Yap (32). A similar phenotype of cardiomegaly was observed on cardiac deletion of Salvador, a WW repeat scaffolding protein that associates with activated Mst kinases, allowing phosphorylation of Lats2 (32). The importance of Wnt signaling as a mediator of Hippo-Yap signaling was underscored by the finding that deletion of β-catenin suppressed the excessive cardiac growth resulting from Lats2 and Mst1/2 deletion.
Previous studies found that deletion of a floxed Yap allele using Nkx2.5-Cre, βMHC-Cre, or Tnnt2-Cre, which are expressed during early heart development, resulted in embryonic lethality owing to a deficiency of cardiomyocytes (33, 34). In contrast, we report here that deletion of Yap using αMHC-Cre, which is activated relatively late in fetal life, allows for survival of mice and does not result in a deficiency of cardiomyocytes or cardiac dysfunction until age 11–20 wk. Through combined cardiac deletion of Yap and Taz in mice, we observed gene dosage-dependent effects of these terminal effectors in the Hippo pathway on cardiac growth and function. Deletion of Taz with αMHC-Cre did not evoke a cardiac phenotype, whereas combined deletions of Yap and Taz resulted in accelerated cardiomyocyte deficiency and heart failure. We interpret these findings to indicate a dominant role of Yap and a lesser role of Taz in maintenance of the relatively low level of postnatal cardiomyocyte proliferation that has been well documented (40–42). The absence of Yap and Taz likely results in the gradual depletion of a slowly cycling pool of postnatal cardiomyocytes. Although we have not analyzed the progrowth functions of Taz using signal-resistant mutants in cardiomyocytes, it is likely that Yap and Taz also have partially overlapping roles in the stimulation of cardiomyocyte proliferation.
The majority of the actions of Yap and Taz have been ascribed to their association with TEAD transcription factors (43, 44), and a dominant negative TEAD mutant was shown to be sufficient to block the proliferative activity of Yap in cardiomyocytes (34). However, numerous other transcription factors, including Tbx5, which plays a key role in the governance of cardiac growth and gene expression (45), have been shown to associate with Yap and Taz (46, 47). Thus, it seems likely that the regenerative activity of Yap reflects activation of multiple downstream gene programs.
Yap joins a collection of transcription factors and signaling molecules with the known ability to enhance cardiomyocyte proliferation to various degrees. For example, neuregulin signaling has been shown to be necessary and sufficient for postnatal cardiomyocyte proliferation, as has deletion of the Meis1 transcription factor (48, 49). Similarly, microRNAs have been shown to play important roles in the regulation of cardiomyocyte proliferation (50). We have shown that the miR-15 family of microRNAs, which is up-regulated in the heart postnatally, suppresses cardiomyocyte proliferation and impedes heart regeneration (14, 51). In the future, it will be of interest to directly compare the relative potencies of these various regulators with respect to promoting cardiomyocyte proliferation, to test their potential synergistic activities, and to develop pharmacologic and genetic strategies to further enhance the neonatal regenerative response as a strategy for repairing the injured adult heart.
Materials and Methods
Generation of Transgenic Mice.
Details of the generation of Tg mice are provided in SI Materials and Methods.
Creation of a Conditional Taz Mutant Allele.
Gene targeting and generation of mutant mice are described in detail in SI Materials and Methods.
Histology.
Histological analyses were performed by standard procedures. Details are provided in SI Materials and Methods.
qPCR.
qPCR was performed following standard procedures, as described in SI Materials and Methods.
Western Blot Analysis.
Western blot analysis was performed following standard procedures, as described in SI Materials and Methods.
Transthoracic Echocardiography.
Cardiac function and heart dimensions were evaluated by 2D echocardiography on conscious mice. Details are provided in SI Materials and Methods.
Neonatal and Adult MI.
MI surgeries performed on neonatal mice at P1 and P7 and on adult mice were performed as described previously (6, 14) and in SI Materials and Methods.
TTC Staining.
Details of TTC staining are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Guo Huang, Kunhua Song, Ning Liu, Young-Jae Nam, and Arin Aurora for insightful discussions; Stefanie Dimmeler and Doris Taylor for helpful comments on the manuscript; Nadine Beetz for cardiomyocyte cDNA; Ji-Hoon Li for assistance with confocal microscopy; and Jose Cabrera for graphics. This work was supported by the National Institutes of Health (Grants HL-077439, HL-111665, HL093039, and U01-HL-100401), the American Heart Association–Jon Holden DeHaan Foundation (Grant 0970518N), Foundation Leducq Networks of Excellence, the Cancer Prevention and Research Institute of Texas, and the Robert A. Welch Foundation (Grant 1-0025, to E.N.O.). M.X. was supported by a Beginning-Grant-In-Aid from the American Heart Association.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1313192110/-/DCSupplemental.
References
- 1.Laflamme MA, Murry CE. Heart regeneration. Nature. 2011;473(7347):326–335. doi: 10.1038/nature10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dimmeler S, Zeiher AM, Schneider MD. Unchain my heart: The scientific foundations of cardiac repair. J Clin Invest. 2005;115(3):572–583. doi: 10.1172/JCI24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xin M, Olson EN, Bassel-Duby R. Mending broken hearts: cardiac development as a basis for adult heart regeneration and repair. Nat Rev Mol Cell Biol. 2013;14(8):529–541. doi: 10.1038/nrm3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ieda M, et al. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142(3):375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qian L, et al. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485(7400):593–598. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song K, et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485(7400):599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aguirre A, Sancho-Martinez I, Izpisua Belmonte JC. Reprogramming toward heart regeneration: Stem cells and beyond. Cell Stem Cell. 2013;12(3):275–284. doi: 10.1016/j.stem.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Li W, Laurent T, Ding S. Small molecules, big roles—the chemical manipulation of stem cell fate and somatic cell reprogramming. J Cell Sci. 2012;125(Pt 23):5609–5620. doi: 10.1242/jcs.096032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Islas JF, et al. Transcription factors ETS2 and MESP1 transdifferentiate human dermal fibroblasts into cardiac progenitors. Proc Natl Acad Sci USA. 2012;109(32):13016–13021. doi: 10.1073/pnas.1120299109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nam YJ, et al. Reprogramming of human fibroblasts toward a cardiac fate. Proc Natl Acad Sci USA. 2013;110(14):5588–5593. doi: 10.1073/pnas.1301019110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li F, Wang X, Capasso JM, Gerdes AM. Rapid transition of cardiac myocytes from hyperplasia to hypertrophy during postnatal development. J Mol Cell Cardiol. 1996;28(8):1737–1746. doi: 10.1006/jmcc.1996.0163. [DOI] [PubMed] [Google Scholar]
- 12.Walsh S, Pontén A, Fleischmann BK, Jovinge S. Cardiomyocyte cell cycle control and growth estimation in vivo—an analysis based on cardiomyocyte nuclei. Cardiovasc Res. 2010;86(3):365–373. doi: 10.1093/cvr/cvq005. [DOI] [PubMed] [Google Scholar]
- 13.Porrello ER, et al. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331(6020):1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Porrello ER, et al. Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc Natl Acad Sci USA. 2013;110(1):187–192. doi: 10.1073/pnas.1208863110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298(5601):2188–2190. doi: 10.1126/science.1077857. [DOI] [PubMed] [Google Scholar]
- 16.Singh SP, Holdway JE, Poss KD. Regeneration of amputated zebrafish fin rays from de novo osteoblasts. Dev Cell. 2012;22(4):879–886. doi: 10.1016/j.devcel.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qin Z, et al. FGF signaling regulates rod photoreceptor cell maintenance and regeneration in zebrafish. Exp Eye Res. 2011;93(5):726–734. doi: 10.1016/j.exer.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jopling C, et al. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature. 2010;464(7288):606–609. doi: 10.1038/nature08899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kikuchi K, et al. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature. 2010;464(7288):601–605. doi: 10.1038/nature08804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19(4):491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol. 2011;13(8):877–883. doi: 10.1038/ncb2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu FX, et al. Regulation of the Hippo-YAP pathway by G-protein–coupled receptor signaling. Cell. 2012;150(4):780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halder G, Dupont S, Piccolo S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat Rev Mol Cell Biol. 2012;13(9):591–600. doi: 10.1038/nrm3416. [DOI] [PubMed] [Google Scholar]
- 24.Dupont S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474(7350):179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 25.Fernández BG, et al. Actin-capping protein and the Hippo pathway regulate F-actin and tissue growth in Drosophila. Development. 2011;138(11):2337–2346. doi: 10.1242/dev.063545. [DOI] [PubMed] [Google Scholar]
- 26.Sansores-Garcia L, et al. Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J. 2011;30(12):2325–2335. doi: 10.1038/emboj.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wada K, Itoga K, Okano T, Yonemura S, Sasaki H. Hippo pathway regulation by cell morphology and stress fibers. Development. 2011;138(18):3907–3914. doi: 10.1242/dev.070987. [DOI] [PubMed] [Google Scholar]
- 28.Zhao B, et al. Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev. 2011;25(1):51–63. doi: 10.1101/gad.2000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim M, et al. cAMP/PKA signalling reinforces the LATS-YAP pathway to fully suppress YAP in response to actin cytoskeletal changes. EMBO J. 2013;32(11):1543–1555. doi: 10.1038/emboj.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halder G, Johnson RL. Hippo signaling: Growth control and beyond. Development. 2011;138(1):9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piccolo S, Cordenonsi M, Dupont S. Molecular pathways: YAP and TAZ take the center stage in organ growth and tumorigenesis. Clin Cancer Res. 2013 doi: 10.1158/1078-0432.CCR-12-3172. in press. [DOI] [PubMed] [Google Scholar]
- 32.Heallen T, et al. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332(6028):458–461. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xin M, et al. Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Sci Signal. 2011;4(196):ra70. doi: 10.1126/scisignal.2002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Gise A, et al. YAP1, the nuclear target of Hippo signaling, stimulates heart growth through cardiomyocyte proliferation but not hypertrophy. Proc Natl Acad Sci USA. 2012;109(7):2394–2399. doi: 10.1073/pnas.1116136109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanai F, et al. TAZ: A novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J. 2000;19(24):6778–6791. doi: 10.1093/emboj/19.24.6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agah R, et al. Gene recombination in postmitotic cells: Targeted expression of Cre recombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. J Clin Invest. 1997;100(1):169–179. doi: 10.1172/JCI119509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hossain Z, et al. Glomerulocystic kidney disease in mice with a targeted inactivation of Wwtr1. Proc Natl Acad Sci USA. 2007;104(5):1631–1636. doi: 10.1073/pnas.0605266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishioka N, et al. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev Cell. 2009;16(3):398–410. doi: 10.1016/j.devcel.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 39.Padin-Iruegas ME, et al. Cardiac progenitor cells and biotinylated insulin-like growth factor-1 nanofibers improve endogenous and exogenous myocardial regeneration after infarction. Circulation. 2009;120(10):876–887. doi: 10.1161/CIRCULATIONAHA.109.852285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bergmann O, et al. Evidence for cardiomyocyte renewal in humans. Science. 2009;324(5923):98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kajstura J, et al. Cardiomyogenesis in the adult human heart. Circ Res. 2010;107(2):305–315. doi: 10.1161/CIRCRESAHA.110.223024. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Senyo SE, et al. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493(7432):433–436. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu S, Liu Y, Zheng Y, Dong J, Pan D. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev Cell. 2008;14(3):388–398. doi: 10.1016/j.devcel.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 44.Zhang L, et al. The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Dev Cell. 2008;14(3):377–387. doi: 10.1016/j.devcel.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Basson CT, et al. Mutations in human TBX5 [corrected] cause limb and cardiac malformation in Holt-Oram syndrome. Nat Genet. 1997;15(1):30–35. doi: 10.1038/ng0197-30. [DOI] [PubMed] [Google Scholar]
- 46.Murakami M, Nakagawa M, Olson EN, Nakagawa O. A WW domain protein TAZ is a critical coactivator for TBX5, a transcription factor implicated in Holt–Oram syndrome. Proc Natl Acad Sci USA. 2005;102(50):18034–18039. doi: 10.1073/pnas.0509109102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenbluh J, et al. β-Catenin–driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. 2012;151(7):1457–1473. doi: 10.1016/j.cell.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bersell K, Arab S, Haring B, Kühn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138(2):257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 49.Mahmoud AI, et al. Meis1 regulates postnatal cardiomyocyte cell cycle arrest. Nature. 2013;497(7448):249–253. doi: 10.1038/nature12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Eulalio A, et al. Functional screening identifies miRNAs inducing cardiac regeneration. Nature. 2012;492(7429):376–381. doi: 10.1038/nature11739. [DOI] [PubMed] [Google Scholar]
- 51.Porrello ER, et al. MiR-15 family regulates postnatal mitotic arrest of cardiomyocytes. Circ Res. 2011;109(6):670–679. doi: 10.1161/CIRCRESAHA.111.248880. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.