Abstract
Placental growth factor (PlGF) remodels tumor vasculatures toward a normalized phenotype, which affects tumor growth, invasion and drug responses. However, the coordinative and spatiotemporal relation between PlGF and VEGF in modulation of tumor angiogenesis and vascular remodeling is less understood. Here we report that PlGF positively and negatively modulate tumor growth, angiogenesis, and vascular remodeling through a VEGF-dependent mechanism. In two independent tumor models, we show that PlGF inhibited tumor growth and angiogenesis and displayed a marked vascular remodeling effect, leading to normalized microvessels with infrequent vascular branches and increased perivascular cell coverage. Surprisingly, elimination of VEGF gene (i.e., VEGF-null) in PlGF-expressing tumors resulted in (i) accelerated tumor growth rates and angiogenesis and (ii) complete attenuation of PlGF-induced vascular normalization. Thus, PlGF positively and negatively modulates tumor growth, angiogenesis, and vascular remodeling through VEGF-dependent spatiotemporal mechanisms. Our data uncover molecular mechanisms underlying the complex interplay between PlGF and VEGF in modulation of tumor growth and angiogenesis, and have conceptual implication for antiangiogenic cancer therapy.
Keywords: VEGF receptor signaling, neovascularization, antiangiogenic therapy
Tumor blood vessels distinguish themselves from healthy vasculatures distributed in various tissue and organs by phenotypically and functionally exhibiting unique features. These include disorganization of the vascular architecture, lack of apparent separation of arterioles from venules, lack of appropriate coverage of perivascular (mural) cells, incomplete basement membrane, and high leakiness (1–4). These pathological features of the tumor vasculature dictate the abnormal microenvironment within tumor tissues that often produce an imbalanced ratio between angiogenic factors and inhibitors (5). Alterations of vascular structures and density are tightly associated with tumor growth, invasion, metastasis, and even responses to antiangiogenic drugs (1, 6, 7). Thus, understanding molecular mechanisms that underlie vascular remodeling in the tumor microenvironment is crucial for defining new therapeutic targets for improvement of cancer therapy by targeting tumor angiogenesis.
VEGF is one of the most frequently expressed angiogenic factors in the tumor microenvironment in which tumor cells and various nonmalignant cells contribute to high levels of VEGF production (8, 9). VEGF is a multifarious angiogenic factor that displays various vascular and nonvascular functions in tumors, including angiogenesis, vasculogenesis, intussusception, vascular fenestration, vascular permeability, inflammation, perivascular coverage, and vascular survival (8). VEGF exerts its functions via interaction with VEGF receptors (VEGFRs) VEGFR1 and VEGFR2 and neuropilins, distributed in various endothelial and nonendothelial cells (8, 10, 11). Although bulky experimental data point that VEGFR2 is the functional tyrosine receptor, which transduces angiogenic and vascular permeability signaling, functional impacts of the VEGFR1–triggered signal remain ambiguous and may serve as a decoy system for VEGF-induced angiogenesis (12, 13). Within the VEGF family, placental growth factor (PlGF) and VEGF-B are two VEGFR1 exclusive binding ligands, and their biological functions are poorly understood (12, 14–16).
We have recently demonstrated that expression of PlGF in tumor cells significantly modulates microvessel density and structures, leading to a normalized vascular phenotype (17). Moreover, PlGF-induced vascular normalization markedly increases drug sensitivity in response to anti-VEGF treatment (18). In this study, we show that depletion of VEGF from tumor cells completely abrogates PlGF-induced vascular normalization, suggesting that modulation of VEGF function is the underlying mechanism by which PlGF modulates the tumor vasculature. Inversely, accelerated angiogenesis and tumor growth have been observed in PlGF-expressing VEGF-null tumors, suggesting that PlGF homodimers might stimulate tumor angiogenesis. These findings provide mechanistic insight on complex interplay between PlGF and VEGF in regulation of tumor angiogenesis and antiangiogenic drug responses.
Results
Tumor Cell-Derived PlGF Induces Vascular Normalization.
To study the functional impact of PlGF on tumor vascularization, a mouse fibrosarcoma (T241) and lung carcinoma [Lewis lung carcinoma (LLC)] were genetically propagated to stably overexpress PlGF-1 (17–19). Expectedly, these PlGF-expressing cell lines produced a substantial amount of PlGF, and a majority of PlGF molecules became secreted into conditioned media (Table S1). Despite high expression levels of PlGF in these tumor cell lines, tumor cell growth rates in vitro were not altered (Fig. S1A), excluding the autocrine stimulatory loop on tumor cells. Conversely, implantation of these PlGF-expressing tumor cells in syngeneic mice resulted in significantly delayed tumor growth rates (Fig. S1B). These findings demonstrate that tumor cell-derived PlGF negatively modulates tumor growth.
Consistent with impaired tumor growth rates in vivo, PlGF-expressing T241 and LLC tumor contained markedly low density of microvessels relative to the nontransfected control tumors (Fig. S1 C and D). Intriguingly, PlGF-tumor vasculatures appeared as highly normalized microvessels that usually lacked branches and sprouts. Additionally, PlGF-tumor vessels also became highly dilated and covered with pericytes (Fig. S1 C and D). These findings show that expression of PlGF in tumor cells significantly reduces vascular density and remodels disorganized tumor vessels toward a normalized phenotype.
Genetic Elimination of VEGF in Tumor Cells Ablates PlGF-Induced Vascular Normalization.
We next studied the relation of PlGF-induced vascular alteration to VEGF in the tumor microenvironment. For this reason, a mouse VEGF-null fibrosarcoma cell line was generated from vegf−/− deficient mice as previously described (20). As expected, VEGF-null fibrosarcoma cells completely lacked detectable VEGF expression as assayed by a sensitive ELISA method (Table S1). In contrast, T241 fibrosarcoma expressed VEGF to a high level. We expressed PlGF-1 in VEGF-null fibrosarcoma cells and established a stable cell line that produced a relatively high amount of PlGF in the medium (Table S1). Similar to T241 and LCC tumors, overexpression of PlGF in VEGF-null tumors did not significantly affect the tumor growth rate in vitro (Fig. 1A). Surprisingly, in contrast to PlGF-T241 and PlGF-LLC tumors, implantation of PlGF-VEGF–null tumors in vivo resulted in an accelerated, rather than a delayed, tumor growth rate (Fig. 1B). These findings suggest that PlGF might promote tumor growth in malignant cells that lack of VEGF expression.
Fig. 1.
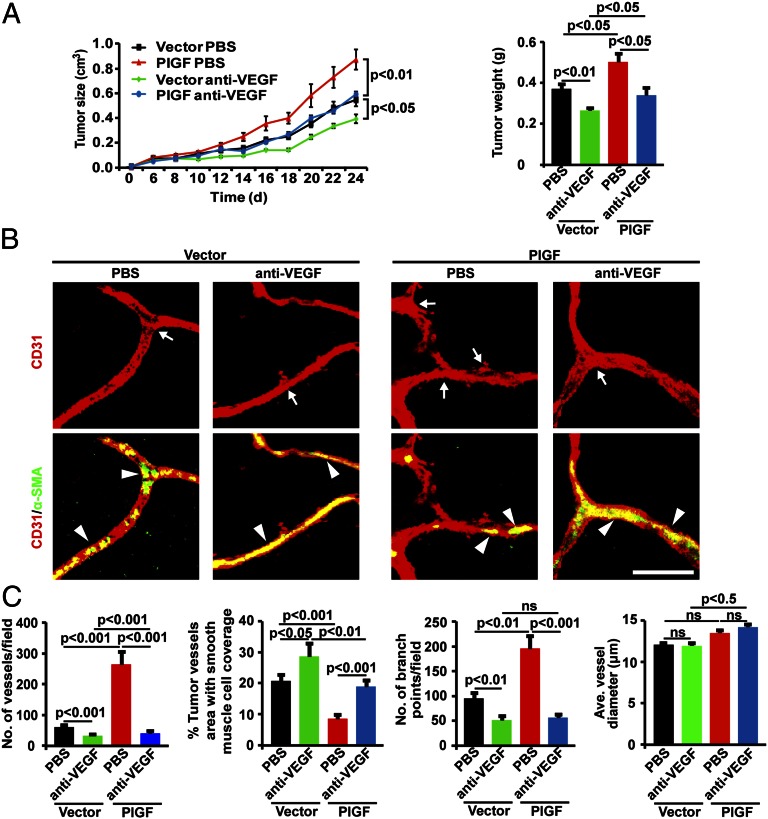
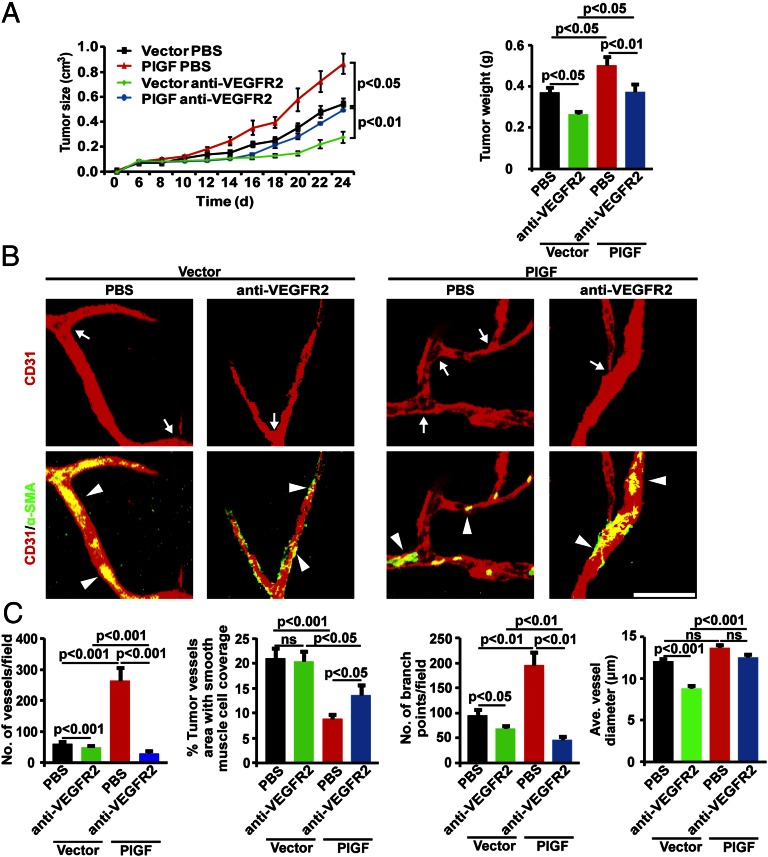
Modulation of tumor growth, angiogenesis, and vascular remodeling by PlGF in VEGF-null tumors. (A) In vitro proliferation of various cell lines (n = 6 samples per group; ns, not significant). (B) Tumor growth rates and weights (n = 8–10 mice per group). (C) Confocal images of CD31+ tumor vessels (red; arrows) and NG2+ pericyte coverage (green) or α-SMA+ smooth muscle cell coverage (green). Arrowheads point to vessel associated pericytes. (Scale bar: 50 μm). (D) Quantification of vessel numbers, pericyte coverage, numbers of vascular branching points, and vascular diameter in various tumors (n = 24 randomized fields per group). (E) Perfusion of lysinated 2,000-kDa dextran (green, Upper) in CD31+ tumor vessels (red). Arrowheads point to perfused tumor vessels (yellow). Leakiness of lysinated 70-kDa dextran (green, Lower) in CD31+ tumor vessels (red). Arrowheads point to extravasated dextran signals (green). (Scale bar: 50 μm.) (F) Quantification of vascular perfusion and extravasated dextran signals (n = 24 randomized fields per group).
In VEGF-null tumors, microvasculatures exhibited a modest normalized phenotype and tumor vessels are generally covered with α-SMA+ perivascular supportive cells (Fig. 1C). This normalized vascular phenotype in VEGF-null tumors was in general agreement with the anti-VEGF drug-induced vascular normalization (21, 22). Notably, PlGF-expressing VEGF-null tumors contained a higher density of disorganized microvascular networks relative to vector VEGF-null control tumors (Fig. 1 C and D). PlGF-expressing VEGF-null tumors also had significantly increased numbers of vascular branches and decreased perivascular cell coverage, whereas vascular diameters remained unchanged compared with those in PlGF-negative tumors.
As VEGF and PlGF form heterodimers via intermolecule disulfide bonds (23), we next measured various homo-and heterodimeric forms of VEGF and PlGF in different cell lines. Expectedly, a substantial number of VEGF molecules in PlGF-T241 and PlGF-LLC tumor cells participated in the formation of PlGF-VEGF heterodimers and only a negligible amount of VEGF remained as homodimers (Table S1). In addition to PlGF-VEGF heterodimers, a substantial amount of PlGF existed as homodimers in the conditioned media. Inversely, PlGF existed only as homodimers in VEGF-null tumors that completely lacked a detectable level of VEGF molecules (Table S1). These data show that PlGF is unable to form heterodimers with VEGF in VEGF-null tumor cells, supporting the fact that PlGF-VEGF heterodimerization occurs intracellularly.
One of the possibilities by which PlGF modulates VEGF functions is that PlGF switches macrophage subtypes and regulate their functions. In support of this view, a recent study shows that down-regulation of PlGF by histidine-rich glycoprotein leads to a switch from M2-like, angiogenic macrophages toward a proinflammatory M1 phenotype (24). Interestingly, the numbers of tumor-infiltrated macrophages and the CD206+ M2 population were slightly decreased in VEGF-null PlGF-expressing tumors relative to those in control tumors (Fig. S2 A and B). The mechanism by which PlGF in the absence of tumor cell-derived VEGF inhibits M2 macrophages remains unknown. Measurements of VEGF mRNA and protein levels showed no statistical significance between vector- and PlGF-expressing VEGF-null tumors. These findings suggest that inflammatory macrophages are unlikely to play a major role in modulation of the interactive vascular functions between PlGF and VEGF.
Alterations of Vascular Functions.
Structural changes of PlGF-induced tumor vasculatures suggested that potential functional alteration might exist in these tumors. To study the functional impact of PlGF on tumor vessels, we measured blood perfusion and vascular leakiness by using tetramethylrhodamine-labeled lysinated 2,000-kDa and 70-kDa dextran, respectively. In concordance with structural changes, the number of perfused vessels was significantly decreased in PlGF-T241 tumors relative to vector-T241 control tumors (Fig. 1 E and F). Conversely, expression of PlGF in this tumor led to marked decrease in vascular permeability (Fig. 1 E and F). These results are consistent with PlGF-mediated antiangiogenic effect and increased perivascular cell coverage in the VEGF-positive tumors. In sharp contrast to PlGF-T241 tumors, blood perfusion in PlGF-VEGF–null tumors was significantly increased relative to that of vector-VEGF–null tumors (Fig. 1 E and F). Moreover, PlGF-VEGF–null tumor microvessels showed marked increase of vascular leakiness. These functional changes of tumor vessels support the fact that PlGF display opposing effects of tumor growth, angiogenesis, and vascular remodeling in VEGF+ and VEGF− tumors.
VEGF and VEGFR2 Blockades Inhibit PlGF-Induced Angiogenesis and Tumor Growth in VEGF-Null Tumors.
To study the VEGF-dependent effect of PlGF-induced angiogenesis and tumor growth in VEGF-null tumors, an anti-mouse VEGF-specific neutralizing antibody was used in our study (18). Treatment with VEGF blockade markedly suppressed PlGF-VEGF–null tumor growth to the level of vehicle-treated vector-VEGF–null tumors (Fig. 2A). Interestingly, VEGF blockade also significantly inhibited vector-VEGF–null tumor growth, but the inhibitory effect was rather modest. Consistent with the antitumor effect, VEGF blockade also significantly suppressed tumor angiogenesis in both vector-VEGF–null and PlGF-VEGF–null tumors (Fig. 2 B and C). Of note, anti-VEGF treatment significantly increased α-SMA+ cell coverage in vector-VEGF–null and PlGF-VEGF–null tumor vasculatures. Despite anti-VEGF–induced alterations of vascular density and perivascular cell coverage, average diameter of tumor vessels remained unchanged compared with the vehicle-treated groups (Fig. 2C). Taken together, these findings demonstrate that PlGF-induced angiogenesis is dependent on VEGF and is sensitive to anti-VEGF treatment.
Fig. 2.
Therapeutic responses of PlGF-expressing VEGF-null tumors to VEGF blockade. (A) Tumor growth rates and weights in VEGF blockade-treated and nontreated tumor-bearing mice (n = 8–10 per group). (B) Confocal images of CD31+ tumor vessels (red; arrows) and α-SMA+ smooth muscle cell coverage (green). Arrowheads point to vessel-associated smooth muscle cells. (Scale bar: 50 μm.) (C) Quantification of vessel numbers, α-SMA+ smooth muscle cell coverage, numbers of vascular branching points, and vascular diameter in various tumors (n = 24 randomized fields per group).
To define the VEGFR signaling system that was responsible to PlGF-induced angiogenesis in VEGF-null tumors, specific VEGFR2 blockade (25, 26) was used for treatment. Similar to VEGF blockade, VEGFR2 blockade also significantly inhibited vector-VEGF–null and PlGF-VEGF–null tumor growth (Fig. 3A). Again, anti-VEGFR2 treatment reduced PlGF-VEGF–null tumor sizes to the similar level of vehicle-treated vector-VEGF–null tumors, validating the fact that PlGF-accelerated tumor growth was dependent on VEGF. Additionally, VEGFR2 blockade significantly inhibited tumor angiogenesis in vector-VEGF–null and PlGF-VEGF–null tumor (Fig. 3 B and C). These findings show that the VEGFR2 signaling system is responsible for PlGF-induced angiogenesis and tumor growth in VEGF-deficient tumors, which are dependent on host nonmalignant cell-derived, rather than tumor cell-derived, VEGF.
Fig. 3.
Therapeutic responses of PlGF-expressing VEGF-null tumors to VEGFR2 blockade (with the same vehicle-treated group as Fig. 3 to compare). (A) Tumor growth rates and weights in VEGFR2 blockade-treated and nontreated tumor-bearing mice (n = 8–10 mice per group). (B) Confocal images of CD31+ tumor vessels (red; arrows) and α-SMA+ smooth muscle cell coverage (green). Arrowheads point to vessel associated smooth muscle cells. (Scale bar: 50 μm.) (C) Quantification of vessel numbers, α-SMA+ smooth muscle cell coverage, numbers of vascular branching points, and vascular diameter in various tumors (n = 24 randomized fields per group).
Discussion
In the tumor microenvironment, malignant and nonmalignant cells participate in production of multiple angiogenic factors and cytokines that not only transduce signaling vertically via their specific receptors but horizontally by cross-communicating with each other (27). The complex interplay between various signaling molecules represents the factual situation of the tumor microenvironment, which relentlessly alters during tumor development and malignant progression. Consequently, the complex interaction between various angiogenic signaling pathways may determine tumor growth, metastasis, and sensitivity to drug responses. Although most of these signaling interactions are known to occur extracellularly, they may already interact with each other intracellularly because they are often synthesized in and released from the same population of cells. The intracellular interactions and their biological consequences in regulation of angiogenesis and tumor growth between various factors remain less understood. Unlike the closely related VEGF, PlGF binds to only VEGFR1 and its homodimeric molecules lack potent angiogenic ability in various angiogenic models (19, 23, 28). In agreement with this view, genetic deletion of PlGF gene in mice does not affect developmental angiogenesis in embryos and physiological angiogenesis in adults (29, 30). Why would PlGF even exist if it does not stimulate angiogenesis? Although PlGF has been proposed to modulate pathological angiogenesis, it is difficult to believe such a molecule is just made for pathological situations when it exists in various physiological tissues.
PlGF is frequently expressed in various tumor tissues and its expression has been associated with tumor growth, angiogenesis, invasion, and antiangiogenic drug responses (31, 32). However, the spatiotemporal relation between PlGF and VEGF in the tumor microenvironment is less understood. In 1996, we described that PlGF can form heterodimers with the closely related VEGF, raising the possibility of PlGF might modulate VEGF functions by the mechanism of heterodimerization (23, 28). As PlGF-VEGF heterodimers have only weak biological activities, it is likely that PlGF might down-regulate VEGF-induced angiogenesis. In support of this view, expression of PlGF in tumor cells, in which VEGF is often up-regulated, inhibits rather than stimulates tumor growth (17–19, 33–35). The mechanism of PlGF-induced suppression of tumor angiogenesis is dependent on its ability of heterodimerization with VEGF (35). Moreover, sequestration of PlGF in the endoplasmic reticulum (ER) of tumor cells by fusion with an ER retention signal peptide resulted in robust antiangiogenic and antitumor activity, owing to intracellular sequestration of VEGF by the formation of PlGF-VEGF heterodimers (35). Thus, PlGF-VEGF heterodimerization is the mechanism underlying the PlGF-induced suppression of tumor angiogenesis.
Paradoxically, PlGF has also been reported as a proangiogenic factor that promotes tumor angiogenesis and confers anti-VEGF refractoriness (31). It seems that these findings from different laboratories contradict each other on the role of PlGF in modulation of tumor angiogenesis and tumor growth. However, the spatiotemporal relation between PlGF and VEGF in the tumor microenvironment has not been investigated in these studies. By using genetically engineered VEGF-null tumor cells, we demonstrate that PlGF could positively contribute to tumor growth and angiogenesis. The possible mechanism underlying the PlGF-promoted angiogenesis may involve VEGFR1 binding competition between PlGF homodimers and nonmalignant cell-derived VEGF homodimers, allowing more VEGF molecules to interact with VEGFR2, and thus enhancing host cell-derived VEGF-induced angiogenesis and tumor growth (Fig. 4). A similar angiogenesis-enhancing mechanism may also exist in nonmalignant host cell-derived PlGF homodimers or even PlGF-VEGF heterodimers that would compete for VEGFR1 binding with tumor cell-derived VEGF. It is known that endothelial cells and other cell types including inflammatory cells and stromal cells are the rich source of PlGF (36), and these cells are major cellular components that constitute the tumor microenvironment. Thus, our findings are clinically relevant and provide mechanistic insights in the dual roles of PlGF in modulation of angiogenesis and tumor growth.
Fig. 4.
Schematic illustration of VEGF-dependent dual functions of PlGF in modulation of angiogenesis, tumor growth, and vascular remodeling. (A) In VEGF-negative tumors, tumor cell-derived PlGF homodimers compete for VEGFR1 binding with VEGF homodimers, leading to enhanced binding of VEGF homodimers to VEGFR2 that transduce angiogenesis, vascular permeability, and tumor growth signals. (B) Conversely, in PlGF- and VEGF-coexpressing tumor cells, PlGF forms heterodimers with VEGF that mainly bind to VEGFR1. The formation of PlGF-VEGF heterodimers markedly reduces the formation of functional VEGF homodimers, resulting in reduced angiogenesis and tumor growth rates. It remains unknown if PlGF-VEGF heterodimers would be able to bind to the heterodimeric VEGFR1–VEGFR2 receptor complex. Also, it is less understood what type of functional signals VEGFR1–VEGFR2 heterodimers mediate.
The VEGF-dependent mechanism of PlGF-mediated modulation of tumor angiogenesis is further supported with the use of VEGF specific blockades. In VEGF-null tumors, PlGF-driven tumor angiogenesis can be completely blocked with VEGF and VEGFR2 blockades. This finding shows the VEGF-VEGFR2–dependent effect of PlGF-induced angiogenesis. Another interesting notion is that VEGF-null tumor is still sensitive to anti-VEGF treatment, supporting the fact that nontumor cell-derived host VEGF plays a significant role in tumor neovascularization (20, 37), which can be further enhanced through activation of the VEGFR2 signaling system in the presence of VEGFR1-binding PlGF homodimers. Under this circumstance, VEGFR1 blockade would further enhance the VEGF-stimulated angiogenesis, potentially for two reasons: (i) VEGFR1 blockade neutralizes the VEGFR1-triggered negative signals for angiogenesis and (ii) there is complete blockage of interaction of the host cell-derived VEGF homodimers with VEGFR1, and thus a switch of VEGF homodimers to exclusively binding to VEGFR2. In VEGF-expressing tumors, anti-VEGFR1 could also enhance tumor angiogenesis as previously reported (18). Similarly, in PlGF-expressing tumors, anti-VEGFR1 also increases tumor angiogenesis via a similar mechanism (18).
The present study provides important therapeutic implications for cancer therapy by determining when PlGF expression should be neutralized or enhanced, depending on its cellular production relation with VEGF. If PlGF and VEGF are coexpressed in the same cell population, the formation of PlGF-VEGF heterodimers would inhibit the formation of angiogenic VEGF homodimers, leading to suppression of tumor angiogenesis and tumor growth. In this case, enhancing PlGF production would theoretically be more beneficial rather than harmful. Inversely, if PlGF and VEGF are produced in different cell populations of cells in the tumor microenvironment, PlGF homodimers might be able to compete VEGFR1 binding with VEGF, creating a situation in which VEGF preferentially interacts with VEGFR2, leading to enhancement of angiogenesis (Fig. 4). In such circumstances, neutralizing of PlGF would probably be more beneficial for cancer therapy. Another interesting issue related to anti-VEGF therapy is that inhibition of tumor angiogenesis can elevate hypoxia in tumor tissues, leading to increased levels of NADPH oxidase. In hemangiomas, for example, expression levels of PlGF, angiopoietin-2, and notch ligand Dll4 are subsequently regulated by NADPH, resulting in altered tumor angiogenesis (38, 39). Again, hypoxia-induced VEGF expression coordinately interacts with PlGF, angiopoietin-1, and Dll4 to coordinately modulate angiogenesis, vessel remodeling, and vascular functions.
The present study not only provides mechanistic insights into the complex interplay between PlGF and VEGF in modulation of angiogenesis and tumor growth, but also offers rationalized explanation of various longstanding controversial findings in regard to the enigmatic PlGF in regulation of tumor angiogenesis. Thorough analysis of the spatiotemporal relation between PlGF and VEGF expression might provide pivotal information for guiding PlGF-based therapy for treatment of cancer and other angiogenesis-dependent diseases.
Materials and Methods
All animal studies were reviewed and approved by the North Stockholm Experimental Animal Ethical Committee (Stockholm, Sweden). The data were analyzed with two-tailed Student t tests. Further details of study methods are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Simcere Pharmaceuticals for providing VEGF blockade. Y.C.’s laboratory is supported by research grants from the Swedish Research Council, the Swedish Cancer Foundation, Karolinska Institute Foundation, the Karolinska Institute Distinguished Professor Award, Torsten Söderbergs Foundation, Söderbergs Stiftelse, Tianjin Natural Science Foundation (CMM-Tianjin) Grant 09ZCZDSF04400 (for international collaboration between Tianjin Medical University and Karolinska Institute), ImClone/Eli Lilly, European Union Integrated Project of Metoxia 222741, and European Research Council Advanced ANGIOFAT Grant Project 250021.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1309629110/-/DCSupplemental.
References
- 1.Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 2.Cao Y. Tumor angiogenesis and molecular targets for therapy. Front Biosci. 2009;14:3962–3973. doi: 10.2741/3504. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438(7070):967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 4.Nagy JA, Chang SH, Dvorak AM, Dvorak HF. Why are tumour blood vessels abnormal and why is it important to know? Br J Cancer. 2009;100(6):865–869. doi: 10.1038/sj.bjc.6604929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1(1):27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 6.Cao Y, et al. Forty-year journey of angiogenesis translational research. Sci Transl Med. 2011;3(114):114rv113. doi: 10.1126/scitranslmed.3003149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358(19):2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 9.Senger DR, et al. Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer Metastasis Rev. 1993;12(3-4):303–324. doi: 10.1007/BF00665960. [DOI] [PubMed] [Google Scholar]
- 10.Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92(6):735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 11.Shalaby F, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376(6535):62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 12.Cao Y. Positive and negative modulation of angiogenesis by VEGFR1 ligands. Sci Signal. 2009;2(59):re1. doi: 10.1126/scisignal.259re1. [DOI] [PubMed] [Google Scholar]
- 13.Fong GH, Rossant J, Gertsenstein M, Breitman ML. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376(6535):66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- 14.Lee J, et al. Vascular endothelial growth factor-related protein: A ligand and specific activator of the tyrosine kinase receptor Flt4. Proc Natl Acad Sci USA. 1996;93(5):1988–1992. doi: 10.1073/pnas.93.5.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mancuso MR, et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest. 2006;116(10):2610–2621. doi: 10.1172/JCI24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olofsson B, et al. Vascular endothelial growth factor B (VEGF-B) binds to VEGF receptor-1 and regulates plasminogen activator activity in endothelial cells. Proc Natl Acad Sci USA. 1998;95(20):11709–11714. doi: 10.1073/pnas.95.20.11709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hedlund EM, Hosaka K, Zhong Z, Cao R, Cao Y. Malignant cell-derived PlGF promotes normalization and remodeling of the tumor vasculature. Proc Natl Acad Sci USA. 2009;106(41):17505–17510. doi: 10.1073/pnas.0908026106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hedlund EM, et al. Tumor cell-derived placental growth factor sensitizes antiangiogenic and antitumor effects of anti-VEGF drugs. Proc Natl Acad Sci USA. 2013;110(2):654–659. doi: 10.1073/pnas.1209310110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eriksson A, et al. Placenta growth factor-1 antagonizes VEGF-induced angiogenesis and tumor growth by the formation of functionally inactive PlGF-1/VEGF heterodimers. Cancer Cell. 2002;1(1):99–108. doi: 10.1016/s1535-6108(02)00028-4. [DOI] [PubMed] [Google Scholar]
- 20.Viloria-Petit A, et al. Contrasting effects of VEGF gene disruption in embryonic stem cell-derived versus oncogene-induced tumors. EMBO J. 2003;22(16):4091–4102. doi: 10.1093/emboj/cdg408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hormigo A, Gutin PH, Rafii S. Tracking normalization of brain tumor vasculature by magnetic imaging and proangiogenic biomarkers. Cancer Cell. 2007;11(1):6–8. doi: 10.1016/j.ccr.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winkler F, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6(6):553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 23.Cao Y, et al. Heterodimers of placenta growth factor/vascular endothelial growth factor. Endothelial activity, tumor cell expression, and high affinity binding to Flk-1/KDR. J Biol Chem. 1996;271(6):3154–3162. doi: 10.1074/jbc.271.6.3154. [DOI] [PubMed] [Google Scholar]
- 24.Rolny C, et al. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell. 2011;19(1):31–44. doi: 10.1016/j.ccr.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Cao R, et al. VEGFR1-mediated pericyte ablation links VEGF and PlGF to cancer-associated retinopathy. Proc Natl Acad Sci USA. 2010;107(2):856–861. doi: 10.1073/pnas.0911661107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xue Y, et al. Hypoxia-independent angiogenesis in adipose tissues during cold acclimation. Cell Metab. 2009;9(1):99–109. doi: 10.1016/j.cmet.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Cao Y, Zhong W, Sun Y. Improvement of antiangiogenic cancer therapy by understanding the mechanisms of angiogenic factor interplay and drug resistance. Semin Cancer Biol. 2009;19(5):338–343. doi: 10.1016/j.semcancer.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Cao Y, Linden P, Shima D, Browne F, Folkman J. In vivo angiogenic activity and hypoxia induction of heterodimers of placenta growth factor/vascular endothelial growth factor. J Clin Invest. 1996;98(11):2507–2511. doi: 10.1172/JCI119069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luttun A, et al. Loss of placental growth factor protects mice against vascular permeability in pathological conditions. Biochem Biophys Res Commun. 2002;295(2):428–434. doi: 10.1016/s0006-291x(02)00677-0. [DOI] [PubMed] [Google Scholar]
- 30.Carmeliet P, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7(5):575–583. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- 31.Fischer C, et al. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell. 2007;131(3):463–475. doi: 10.1016/j.cell.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 32.Bais C, et al. PlGF blockade does not inhibit angiogenesis during primary tumor growth. Cell. 2010;141(1):166–177. doi: 10.1016/j.cell.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 33.Xu L, et al. Placenta growth factor overexpression inhibits tumor growth, angiogenesis, and metastasis by depleting vascular endothelial growth factor homodimers in orthotopic mouse models. Cancer Res. 2006;66(8):3971–3977. doi: 10.1158/0008-5472.CAN-04-3085. [DOI] [PubMed] [Google Scholar]
- 34.Schomber T, et al. Placental growth factor-1 attenuates vascular endothelial growth factor-A-dependent tumor angiogenesis during beta cell carcinogenesis. Cancer Res. 2007;67(22):10840–10848. doi: 10.1158/0008-5472.CAN-07-1034. [DOI] [PubMed] [Google Scholar]
- 35.Björndahl M, Cao R, Eriksson A, Cao Y. Blockage of VEGF-induced angiogenesis by preventing VEGF secretion. Circ Res. 2004;94(11):1443–1450. doi: 10.1161/01.RES.0000129194.61747.bf. [DOI] [PubMed] [Google Scholar]
- 36.Fischer C, Mazzone M, Jonckx B, Carmeliet P. FLT1 and its ligands VEGFB and PlGF: Drug targets for anti-angiogenic therapy? Nat Rev Cancer. 2008;8(12):942–956. doi: 10.1038/nrc2524. [DOI] [PubMed] [Google Scholar]
- 37.Dong J, et al. VEGF-null cells require PDGFR alpha signaling-mediated stromal fibroblast recruitment for tumorigenesis. EMBO J. 2004;23(14):2800–2810. doi: 10.1038/sj.emboj.7600289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhandarkar SS, et al. Fulvene-5 potently inhibits NADPH oxidase 4 and blocks the growth of endothelial tumors in mice. J Clin Invest. 2009;119(8):2359–2365. doi: 10.1172/JCI33877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munson JM, et al. Anti-invasive adjuvant therapy with imipramine blue enhances chemotherapeutic efficacy against glioma. Sci Transl Med. 2012;4(127):127ra136. doi: 10.1126/scitranslmed.3003016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.