Abstract
Induction of a proinflammatory response is the hallmark of host innate defense against invading pathogens. Host recognition of influenza A virus (IAV) infection relies on pattern-recognition receptors, including Toll-like receptor 7 (TLR7) and retinoic acid inducible gene-1 (RIG-I) for the activation of innate-immune responses. Here, we show that following a physiological low dose of IAV infection, viral sensing by either TLR7 or RIG-I induces a proinflammatory program that promotes viral replication. Transfer of bronchoalveolar lavage from infected wild-type mice into the airway of mice deficient in TLR7 and RIG-I pathways was sufficient to restore viral replication efficiency. Comparison of IAV-infected cells revealed that inflammatory mediators elicited by TLR7 and RIG-I signaling recruit viral target cells to the airway, thereby enhancing viral load within the respiratory tract. Our data suggest that IAV uses physiological levels of inflammatory responses for its replicative advantage and highlight the complex interplay between viruses and the host innate-immune responses.
Keywords: inflammation, cytokine, monocytes
The innate-immune system provides the first line of defense against invading pathogens by inducing a rapid immune response upon recognition of pathogen-associated molecular patterns by pattern-recognition receptors (PRRs). Influenza A virus (IAV) infection is recognized by two sets of PRRs to induce robust type I interferon (IFN) responses, namely, through the Toll-like receptor (TLR)7 (1, 2) and TLR8 (3) in the endosome, and retinoic acid inducible gene-1 (RIG-I) in the cytosol (4, 5). Recognition of IAV by RIG-I is cell-intrinsic and relies on sensing of viral replication intermediates in infected cells, such as airway epithelial cells. In contrast, cell-extrinsic mechanisms mediated by TLR7 sensing of single-strand RNA (ssRNA) can occur in the absence of virus replication in specialized cell types, such as plasmacytoid dendritic cells (6). Both pathways converge on activation of nuclear factor kappa B (NF-κB) and IRF3/7 to induce the production of proinflammatory cytokines and type I IFNs, respectively (7). Induction of proinflammatory cytokines and IFNs is important for inducing the expression of antiviral genes that can interfere with virus replication, recruiting leukocytes and lymphocytes to the site of infection, and for providing the requisite signals for activating the adaptive immune responses for efficient viral clearance. The “cytokine burst” induced early during virus infections, however, can also contribute to clinical symptoms and immunopathology of influenza virus infection (8). In this regard, the inflammatory environment elicited by IAV has been shown to recruit CCR2+ inflammatory monocytes, which give rise to monocyte-derived dendritic cells (DCs) and macrophages in the lung and contribute to influenza-induced immune pathology (9, 10). However, the roles of physiological levels of inflammatory mediators and monocyte recruitment in antiviral defense against IAV remain unclear.
Relatively little is known about the functional interaction between TLR7- and RIG-I–mediated viral-sensing mechanisms and the contribution of these interactions to host antiviral immunity and protection from viral infections. Although it is expected that these pathways would have overlapping roles in inducing antiviral effector mechanisms (11), TLR7 and RIG-I signaling likely induces separate sets of genes (12). How these PRRs contribute to the host antiviral response and viral restriction in vivo remain poorly understood. Following intranasal infections with influenza A/New Caledonia/20/99 (H1N1) (2 × 104 pfu), WT mice and mice deficient in myeloid differentiation primary response gene 88 (MyD88) or IPS-1 (or mitochondrial antiviral signaling, MAVS) showed comparable viral titers in the lung, whereas viral titers in the lung were significantly higher in MyD88- and IPS-1–doubly deficient mice at 24 h and 6 d postinfection (dpi) (13). Consistently, another study demonstrated that WT and Myd88−/− mice showed similar viral titers in the lung at day 4 and day 6 postinfection and comparable mortality after respiratory infections with 106 pfu of influenza virus A/WSN/33 (14). Although these studies suggest that early viral replication following high doses of respiratory IAV infections is controlled by contributions from both MyD88 and RIG-I signaling pathways, the role of TLR7 and RIG-I in defense against a physiological sublethal dose of IAV remains unclear. In addition, the use of MyD88-deficient mice does not dissociate the contributions of TLR7 and interleukin 1 receptor type I (IL-1R) signaling pathways, both of which are known to contribute to anti-influenza defense (15–18). Our recent study showed that activation of CD8 T-cell response to a physiological dose of IAV does not depend on the combined signaling downstream of TLR7 and RIG-I, but depends critically on inflammasome-dependent release of IL-1β and IL-1R/MyD88 signaling (19). In this study, we examined the consequences of the combined deficiencies in both TLR7 and RIG-I–mediated host defense pathways in innate host defense against respiratory IAV infections following various doses of viral challenge. Our study revealed an unexpected finding that, following a sublethal viral challenge, IAV-induced inflammatory pathways downstream of TLR7 and RIG-I paradoxically promote viral replication by recruiting viral target cells to the airways.
Results
TLR7 and RIG-I Signaling Mediates Protection Against Lethal IAV Challenge.
To address the contributions of TLR7- and RIG-I–mediated host defense pathways in protecting against IAV infection, we first investigated virus replication in cells deficient in TLR7, MAVS, or both. Following infection with a recombinant IAV carrying a GFP reporter gene in the NS segment (PR8 NS1-GFP), Mavs−/− and Tlr7−/−Mavs−/− bone marrow-derived DCs (BMDCs) had increased frequencies of virus-infected GFP+ cells compared with WT BMDCs (Fig. S1), consistent with previous published results indicating that the RIG-I/MAVS pathway mediates antiviral responses to RNA viruses in conventional DCs (20). We next examined the contribution of TLR7 and RIG-I signaling pathways in host defense following IAV infection in vivo. To test whether the absence of TLR7- and MAVS-dependent pathways protect mice from influenza virus replication at a lethal virus challenge dose, we infected WT and Tlr7−/−Mavs−/− mice intranasally with 25 pfu of a mouse-adapted virulent strain of influenza virus A/PR8 (1 LD50) (18). As expected, Tlr7−/−Mavs−/− mice sustained a higher pulmonary viral titer in the airway compared with WT controls at 6 dpi (Fig. S2A), accompanied by robust cellular infiltration in the airways (Fig. S2B). Similarly, when mice were challenged with a lethal dose of PR8 NS1-GFP virus (2 × 106 pfu; >300 LD50) (21), we found that compared with WT infected mice, Tlr7−/−Mavs−/− mice showed enhanced recruitment of monocyte-derived DCs in the lung (Fig. S2C), and infected monocyte-derived DCs accounted for the main difference between the WT and Tlr7−/−Mavs−/− mice (Fig. S2D). When challenged with 100 pfu of A/PR8 (4 LD50), the Tlr7−/−Mavs−/− mice lost significantly more weight than WT mice at 2 and 3 dpi (Fig. S3A), but succumbed to infection, both with respect to weight loss and mortality with a similar time course as the WT mice at later timepoints (Fig. S3), suggesting that although TLR7 and MAVS pathways suppress viral replication (Fig. S2A), they are insufficient to confer survival advantage at this dose. These data indicated that activation of TLR7- and RIG-I–mediated innate host defense pathways restrict influenza virus replication in the respiratory tract at high viral challenge doses, consistent with the previous report of the increased susceptibility of Myd88−/−Mavs−/− mice to the influenza A/New Caledonia/20/99 strain (13). Moreover, we show that after lethal doses of viral challenge, a TLR7/RIG-I–independent pathway triggered the recruitment of inflammatory monocytes, which became the main target of influenza infection.
TLR7 and RIG-I Signaling Pathways Promote Virus Replication in the Airway Following a Sublethal Dose of IAV Challenge in Vivo.
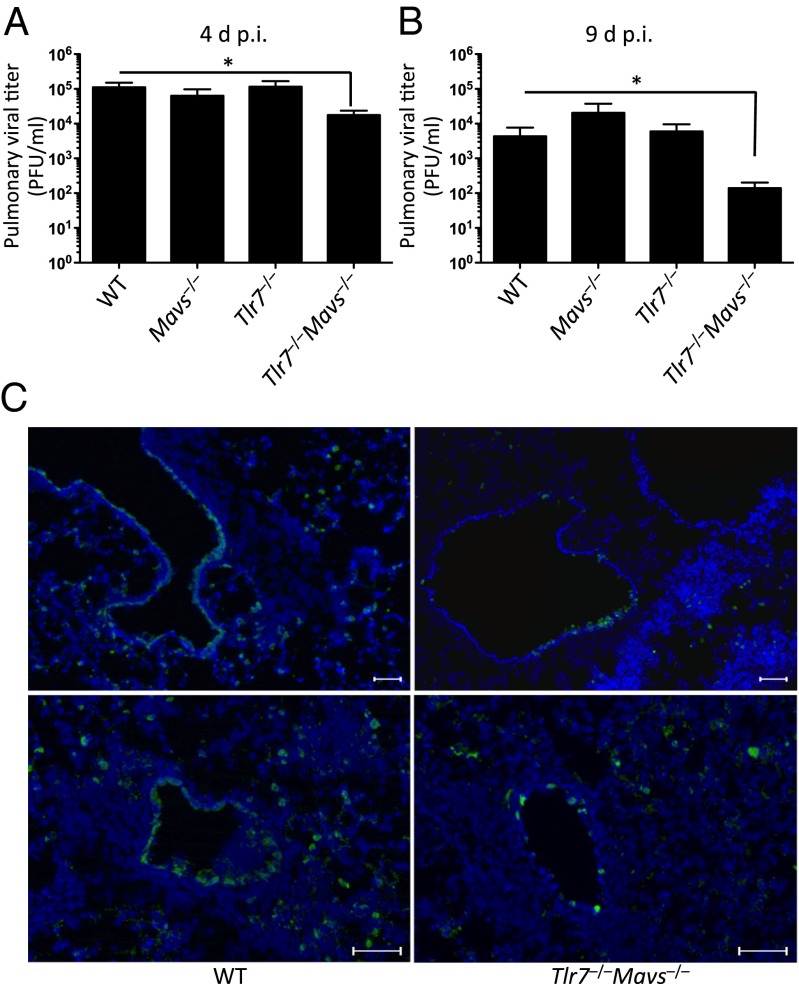
Transmission of aerosolized IAV between humans occurs at extremely low doses (22). To investigate the relative contributions of TLR7 and RIG-I signaling in defense against low and physiological IAV challenge doses, we infected WT, Tlr7−/−, Mavs−/−, and Tlr7−/−Mavs−/− double-knockout mice intranasally with a sublethal dose (10 pfu or 0.4 LD50) of A/PR8 influenza virus. In contrast to the lethal challenge doses (Fig. S2), we found that Tlr7−/−Mavs−/− mice harbored significantly lower pulmonary viral titers in the airway at 4 and 9 dpi compared with WT mice (Fig. 1 A and B). Mice deficient in TLR7 or MAVS alone supported WT levels of viral titers, indicating that combined deficiencies in both of these PRRs are required to confer resistance in these mice. There was no obvious difference in pathology observed between the infected WT and Tlr7−/−Mavs−/− mice (Fig. S4), and all of these mice survived for the duration of the experiments (Fig. S3). Immunofluorescence staining of tissue sections of the lung after removal of cells from the airway with antibody specific to influenza virus nucleoprotein (NP) revealed that virus-infected cells could be found in the respiratory epithelium of the large conducting airways and in the parenchyma in WT mice. In contrast, NP+ cells lining the conducting airways of Tlr7−/−Mavs−/− mice were more sparsely distributed six days after 10 pfu of A/PR8 influenza virus challenge, and were almost absent from the lung parenchyma (Fig. 1C). The reduction in viral titers in Tlr7−/−Mavs−/− mice is unlikely to be explained by differences in adaptive immune responses, as similar CD8 T-cell immunity develops in these different genotypes, and if anything, Tlr7−/− and Tlr7−/−Mavs−/− mice have reduced Th1 immunity compared with WT or Mavs−/− mice (19). Furthermore, impaired, but not enhanced, antibody responses to IAV have been observed in infected Tlr7−/− and Myd88−/− mice (13, 16). Thus, we focused on the innate-immune mechanism in Tlr7−/−Mavs−/− mice that confer resistance to IAV.
Fig. 1.
Tlr7−/−Mavs−/− but not Mavs−/− or Tlr7−/− mice are resistant to a sublethal dose of respiratory IAV infection. WT, Mavs−/−, Tlr7−/−, and Tlr7−/−Mavs−/− mice were infected intranasally with 10 pfu of A/PR8 influenza virus. Lung washes were collected from these mice at 4 dpi (A) and 9 dpi (B) and viral titer was determined by plaque assay. (C) Lung tissues were harvested at 6 dpi and stained for influenza NP+ cells (green) and nuclei (blue). Original magnification, 10×. (Scale bars, 100 μm.) Data represent the mean ± SEM. These results are representative of three independent experiments. *P < 0.05.
To this end, we examined the respective roles of TLR7- and RIG-I–dependent pathways in the induction of inflammatory responses in the lung. Consistent with our previous results (19), we found that the levels of proinflammatory cytokines including TNF-α, IL-12, and IFN-β secreted into the airway of Tlr7−/−Mavs−/− mice were significantly lower compared with WT, Mavs−/−, and Tlr7−/− mice at 4 dpi (Fig. S5). Collectively, our results demonstrated that despite having diminished cytokine responses, Tlr7−/−Mavs−/− mice sustained a lower pulmonary viral titer throughout the course of infection. These data indicated that, paradoxically, TLR7- and RIG-I/MAVS signaling induces redundant pathways in promoting replication and spread of IAV in the airway.
Expression of TLR7 and MAVS in Hematopoietic Cells Promotes Respiratory Influenza Virus Infection in Vivo.
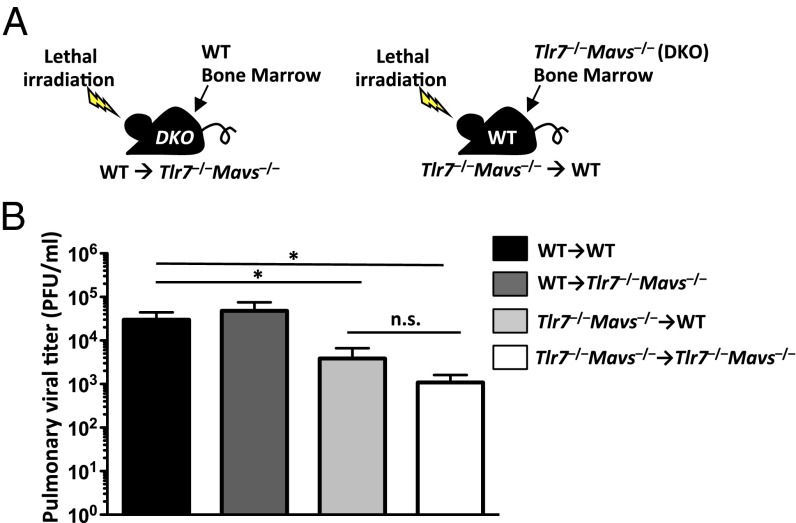
To investigate the cellular targets responsible for supporting virus replication following engagement of TLR7 and RIG-I, we generated BM chimeric mice in which either the hematopoietic (WT→Tlr7−/−Mavs−/−) or the stromal compartment (Tlr7−/−Mavs−/−→WT) express TLR7 and MAVS (Fig. 2A). Upon complete BM reconstitution, BM chimeric mice were challenged with a sublethal dose of PR8 influenza virus. Compared with WT→WT control mice, Tlr7−/−Mavs−/−→WT mice displayed significantly lower viral titer similar to Tlr7−/−Mavs−/−→Tlr7−/−Mavs−/− mice in the bronchoalveolar (BAL) fluid measured at 9 dpi (Fig. 2B). In contrast, pulmonary viral titers were similar between WT→WT mice and WT→Tlr7−/−Mavs−/− mice (Fig. 2B). These data indicated that TLR7- and MAVS-mediated signaling in the hematopoietic, but not the radioresistant, compartment is required to bring about an environment optimal for viral replication following a low dose challenge with IAV.
Fig. 2.
Expression of TLR7 and MAVS in hematopoietic compartment promotes respiratory influenza virus infection in vivo. WT→WT, WT→Tlr7−/−Mavs−/−, Tlr7−/−Mavs−/−→WT and Tlr7−/−Mavs−/−→Tlr7−/−Mavs−/− BM chimeric mice were infected intranasally with a sublethal dose (10 pfu) of A/PR8 influenza virus (A). Lung washes were collected from BM chimeric mice at 9 dpi, and viral titer was determined by plaque assay (B). Data represent the mean ± SEM. These results are representative of two independent experiments. *P < 0.05; n.s., not significant.
Intranasal Transfer of BAL Fluid from Influenza-Infected WT Mice Restores Viral Replication Efficiency in Tlr7−/−Mavs−/− Mice.
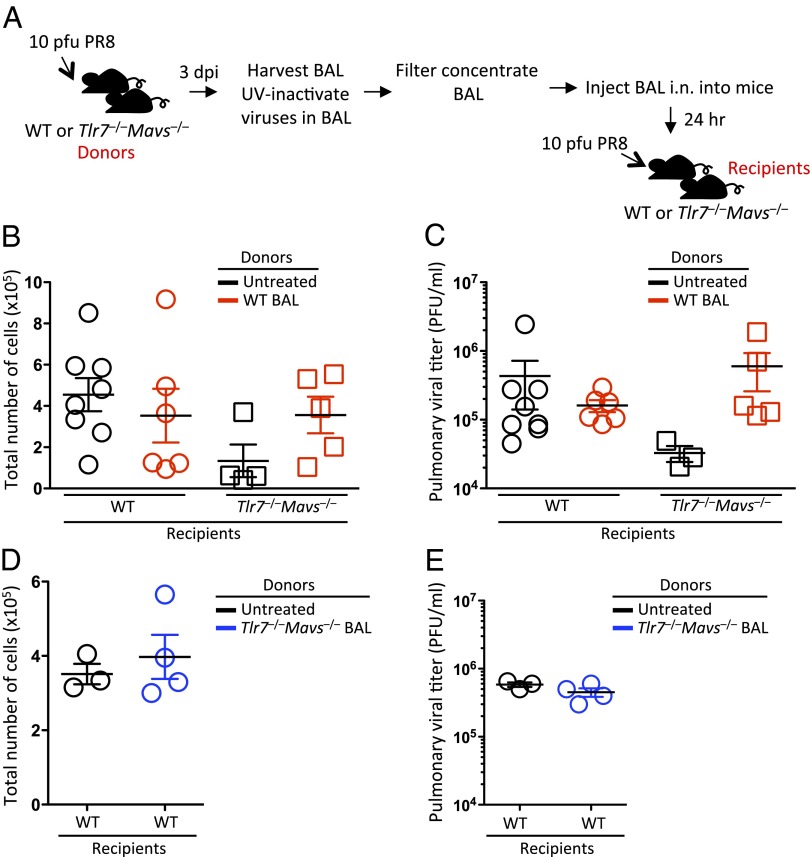
Next, we tested the ideas that (i) soluble factors released into the airway of WT mice in response to IAV infection support viral replication, or conversely, (ii) soluble factors released into the airway of Tlr7−/−Mavs−/− mice accounts for their resistance to IAV. To test the former possibility, UV-irradiated BAL fluid collected from WT mice 3 d after a sublethal dose of A/PR8 infection was applied intranasally to either WT or Tlr7−/−Mavs−/− recipients 24 h before respiratory infection with 10 pfu of A/PR8 influenza virus (Fig. 3A). Plaque assays were performed to confirm that replicating virus in the BAL was fully inactivated and failed to form any plaques after UV-irradiation. Tlr7−/−Mavs−/− mice receiving BAL from infected WT mice showed increased number of cellular infiltrates into the airway compared with untreated Tlr7−/−Mavs−/− mice, whereas cellular infiltration remained similar between BAL-treated and untreated WT mice 4 d after influenza virus infection (Fig. 3B). Notably, viral titers in the Tlr7−/−Mavs−/− mice treated with BAL from IAV-infected WT mice were restored to the level of WT mice (Fig. 3C). These data indicated that inflammatory factors released into the airway of WT IAV infected mice were sufficient to restore viral replication in the Tlr7−/−Mavs−/− mice, and that this correlated with the restoration of cellular recruitment to the airways.
Fig. 3.
Intranasal transfer of BAL isolated from the airway of WT influenza-infected mice restore viral replication efficiency in Tlr7−/−Mavs−/− mice. WT and Tlr7−/−Mavs−/− mice were infected intranasally with 10 pfu of A/PR8 influenza virus with or without intranasal administration of UV-inactivated BAL fluid from WT (red, B and C) or Tlr7−/−Mavs−/− (blue, D and E) mice 24 h before infection. Schematic representation of experimental setup is shown in A. At 4 dpi, lung washes were collected from these mice and the number of cells in the samples was enumerated (B and D). Pulmonary viral titer was determined by plaque assay (C and E). Data represent the mean ± SEM. These results are representative of three independent experiments.
Next, to test the second possibility that factors secreted into the airways of the Tlr7−/−Mavs−/− mice are sufficient to confer resistance in WT mice, UV-irradiated BAL harvested from IAV-infected Tlr7−/−Mavs−/− mice was applied intranasally to WT recipients 24 h before a sublethal dose of respiratory IAV challenge (Fig. 3A). At 4 dpi, the number of cellular infiltrates in the airway and the level of pulmonary viral titer remained similar between WT mice receiving BAL from Tlr7−/−Mavs−/− mice and their untreated WT counterparts (Fig. 3 D and E). These data indicated that BAL from Tlr7−/−Mavs−/− mice did not exert any inhibitory effect on viral growth in vivo. Collectively, these data indicated that soluble factors in the BAL of WT infected mice restored viral replication efficiency in Tlr7−/−Mavs−/− mice and suggested that the induction of inflammatory mediators downstream of TLR7 and RIG-I promoted leukocyte recruitment to the airway, enhancing viral infections in the respiratory tract.
TLR7 and RIG-I Signaling Induced by IAV Infection Leads to the Recruitment of Viral Target Cells to the Airway.
Based on the data above, we hypothesized that TLR7- and MAVS-dependent inflammatory mediators following IAV infection results in recruitment of cells into the airway that become the target of influenza virus infections. To test this hypothesis, we compared the profile of the infiltrating cell population in the BAL fluid of WT and Tlr7−/−Mavs−/− mice after 10 pfu of A/PR8 infection. Compared with WT mice, the frequency and number of monocyte-derived DCs (CD11b+Ly6c+Ly6G−CD11cint) were severely reduced in the airway of Tlr7−/−Mavs−/− mice at 4 dpi (Fig. S6). In contrast, the frequency of infiltrated CD11b+Ly6CloLy6G+ neutrophils was higher in Tlr7−/−Mavs−/− mice than in WT mice (Fig. S6A), indicating that there was no general deficiency in the recruitment of all leukocyte subsets in the Tlr7−/−Mavs−/− mice. These data indicated that the presence of TLR7 or MAVS could at least partially compensate for the loss of either viral-sensing pathway, but the absence of both pathways resulted in defective recruitment of monocytes into the airway.
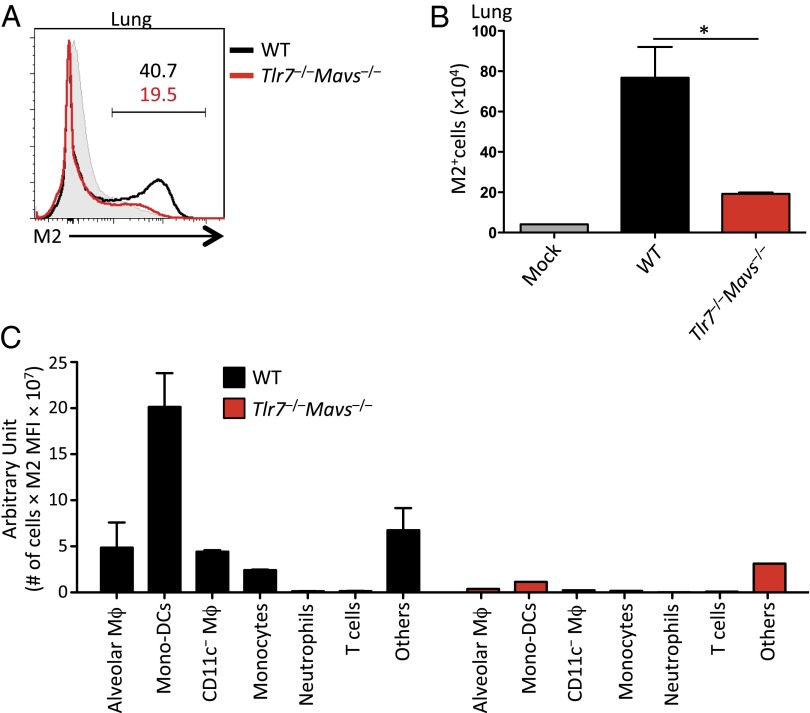
Next, we examined whether the recruited cell population could act as a target of IAV infection in the respiratory tract. Influenza virus-infected cells in the lung were visualized using an antibody against the viral Matrix 2 (M2) protein following a sublethal dose of A/PR8 infection (10 pfu). Influenza virus M2 ion channel is an integral membrane protein in the envelope of IAV and is expressed on the surface of virus-infected cells as early as 12 h postinfection (23). Six days following 10 pfu of A/PR8 infection, a significant increase in the frequency and number of virus-infected cells was found in WT mice compared with naïve controls (Fig. 4 A and B). Importantly, the lung of Tlr7−/−Mavs−/− mice contained a substantially lower frequency and number of M2+ virus-infected cells than infected WT counterparts at this time point (Fig. 4 A and B), consistent with the viral titers (Fig. 1 A and B). To assess the contribution of individual cell types in supporting IAV infection in the lung, we compared the expression level of surface M2 (median fluorescent intensity) and the total number of M2+ cells for each particular cell type present in the lung. Using this method, we found that the recruited CD11b+Ly6c+CD11c+ monocyte-derived DC population was the predominant infected cell type in the lung of WT mice (Fig. 4C). Notably, the infected inflammatory monocytes, as well as other cell types being infected with IAV in WT mice, were missing in the IAV-challenged Tlr7−/−Mavs−/− mice (Fig. 4C). Therefore, these data indicated that at the low-dose viral challenge, inflammatory cells recruited by either TLR7 or RIG-I signaling ultimately became targets of IAV infection, promoting increased viral replication in the airways.
Fig. 4.
TLR7- and MAVS-dependent signals recruit monocyte-derived DCs that become target of IAV infection. WT (black) and Tlr7−/−Mavs−/− (red) mice were infected intranasally with 10 pfu of A/PR8 influenza virus. At 6 dpi, leukocytes were isolated from the lung and stained with an antibody against influenza M2 protein. The frequency (A) and number (B) of M2+ influenza-infected cells were analyzed by flow cytometry. Shaded histograms indicate uninfected WT controls (A). In C, cell type specific contribution to influenza infections in the lung in WT (black) and Tlr7−/−Mavs−/− (red) was calculated by multiplying the median fluorescent intensity (MFI) of surface M2 expression by the total number of cells for each indicated cell type in the lung. Data represent the mean ± SEM. These results are representative of three independent experiments. *P < 0.05.
Discussion
Influenza virus infection is recognized by the host innate-immune system through TLR7 in the endosome and RIG-I in the cytoplasm. In the present study, we investigated whether the TLR7/MyD88 and RIG-I/MAVS viral recognition pathways share any redundant role in defense against respiratory IAV infection. As expected, in mice deficient in both TLR7 and RIG-I signaling pathways, lethal IAV challenge led to enhanced viral replication. In contrast, a sublethal dose of virus challenge in Tlr7−/−Mavs−/− mice unexpectedly resulted in significantly lower viral titers in the lung. Intranasal transfer of WT BAL fluid to Tlr7−/−Mavs−/− mice restored airway cellular infiltration and increased pulmonary viral titer, indicating that soluble inflammatory mediators present in the airway of WT mice are sufficient to restore viral replication efficiency in the airway of Tlr7−/−Mavs−/− mice. The apparent resistance to IAV replication in Tlr7−/−Mavs−/− mice was associated with diminished proinflammatory cytokine and IFN production and leukocyte infiltration into the airway. Recruited inflammatory monocyte-derived DCs became a major target of influenza virus infection in the respiratory tract of mice replete with TLR7 or MAVS. The TLR7 and RIG-I/MAVS-mediated viral recognition pathways appeared to be partially redundant for the recruitment of monocyte-derived DCs, because Tlr7−/−Mavs−/−, but not Tlr7−/− or Mavs−/− mice, had severely diminished recruitment of monocyte-derived DCs to the airway and consequently sustained lower viral load. Therefore, our results indicate a paradoxical effect of the innate physiological inflammatory responses leading to enhanced virus replication in the airway following respiratory IAV infections.
How might IAV co-opt host inflammatory mediators to promote their replication efficiency? Based on our results, we speculate that the recruitment of large number of leukocytes provides a target of virus infection in the respiratory tract. Previous studies have shown that IAV are able to infect both nonimmune (such as tracheal epithelial cells) and immune cells (such as macrophages and DCs) in the respiratory tract (21, 24). Our data indicated that monocyte-derived DCs were the major immune cell type infected by IAV in the lung. Thus, during low-dose IAV infection, monocyte-derived DCs recruited to the airway in a TLR7- or RIG-I–dependent manner act as targets of IAV infection and provide a way for the virus to promote its replication in the respiratory tract. In contrast, at the lethal doses of viral challenge, a robust cellular infiltration, particularly the monocyte-derived DCs, to the airway was observed in Tlr7−/−Mavs−/− mice. The recruited monocyte-derived DCs also became the main target of viral infection at the lethal doses of influenza infection. Thus, at higher viral challenge doses, compensatory mechanisms independent of TLR7 and RIG-I signaling are able to recruit viral targets, mainly the inflammatory monocyte-derived DCs, in Tlr7−/−Mavs−/− mice. Our results are consistent with published reports demonstrating that blood-derived monocytes are particularly susceptible to IAV infections and induce rapid differentiation of into DCs (25, 26), which are responsible for the pulmonary immune pathology observed in IAV infected mice (9, 10). Although these cells do become viral targets, inflammatory monocytes may assume a protective role against influenza virus infection (27). Our study showed that monocytes control viral burden at both ends of the spectrum; their recruitment requires signals from TLR7 or MAVS after a sublethal dose of influenza infection, whereas TLR7/MAVS-independent signals overcompensate for their recruitment after a lethal dose of viral challenge (Fig. S7). Future studies are needed to decipher what triggers the switch between TLR7/MAVS-dependent vs. TLR7/MAVS-independent modes of monocyte recruitment. Nevertheless, our study highlights the importance of evaluating host–virus interaction at a physiological viral challenge dose to reveal mechanisms that are otherwise obscured at high lethal doses of infection.
In addition, there may be other mechanisms that contribute to promoting IAV replication efficiency in vivo (Fig. S7, dotted arrows). Inhibition of the NF-κB and the RAF/MEK/ERK signaling pathways in airway epithelial cells by pharmacological inhibitors has been shown to reduce viral replication and secretion of proinflammatory cytokines following avian and human IAV infections (28). Several mechanisms have been proposed to explain how NF-κB supports IAV replication (reviewed in ref. 29), which include: (i) the induction of proapoptotic factors including TNF-related apoptosis-inducing ligand and Fas-L (30), and activation of caspase-3 to facilitate viral ribonucleioprotein export from the nucleus to the cytosol (31); (ii) suppression of type I IFN signaling by NF-κB induction of SOCS3 (32) or by directly binding to the promoter regions of IFN-stimulated genes (33); and (iii) regulation of influenza viral genomic RNA synthesis (34). It remains to be determined whether any of these mechanisms are responsible for TLR7- and MAVS-dependent promotion of virus propagation in vivo following a sublethal challenge.
Despite playing crucial roles in innate antiviral defense, activation of host inflammatory responses has been shown to enhance viral replication in a number of viruses. Treatment with IFNs increases susceptibility of monocyte to infections by porcine reproductive and respiratory syndrome virus by up-regulating monocyte expression of the porcine Arterivirus receptor sialoadhesin (35). Similarly, TNF-α production by macrophages infected with feline infectious peritonitis virus, a (+)ssRNA feline coronavirus, increases the expression of receptor, feline aminopeptidase N, for feline infectious peritonitis virus entry and results in increases infectivity and virus production (36). Furthermore, during HIV infections in cells of macrophage lineage, several proinflammatory cytokines, such as M-CSF, IL-6, and TNF-α, are known to enhance viral growth, whereas other cytokines, such as type I IFNs and IL-10, suppress viral replication (37). More direct evidence that pathogens use TLRs to promote replication comes from Salmonella infection, in which engagement of TLR2, -4, and -9 by the phagocytosed bacteria enables proper acidification and activation of virulence factors in macrophages to promote their replication (38). Future studies aimed at understanding the precise mechanism by which IAV co-opts host inflammatory mediators to enhance viral replication efficiency in the respiratory tract will be valuable for designing novel therapeutic approaches that target host pathways in the treatment of influenza virus infections, which do not bear the risk of emerging viral drug resistance.
Methods
Mice.
Age- and sex-matched C57BL/6 (WT) mice from the National Cancer Institute (Frederick, MD) were used as WT controls. The generations of Mavs−/− (39) and Tlr7−/− (1) have been previously described. Tlr7−/−Mavs−/− double-deficient mice were bred in the animal facility at Yale. All KO mice had been backcrossed at least nine generations onto the C57BL/6 background. All procedures used in this study complied with federal guidelines and were approved by the Yale Animal Care and Use Committee.
Virus Infections in Vivo.
A/PR8 virus (H1N1) and recombinant PR8 NS1-GFP virus (a gift from Adolfo Garcia-Sastre, Mount Sinai School of Medicine, New York, NY) used for all experiments was grown in allantoic cavities from 10- to 11-d-old fertile chicken eggs for 2 d at 35 °C. Viral titer was quantified by a standard plaque assay using Madin-Darby canine kidney (MDCK) cells and viral stock was stored at −80 °C. For intranasal infection, mice were fully anesthetized by intraperitoneal injection of ketamine and xylazine and then infected by intranasal application of 20 μL of virus suspension (10–100 pfu of A/PR8 or 1 × 106 pfu of PR8 NS1-GFP in PBS, as indicated). This procedure leads to the upper and lower respiratory tract infection.
Measurement of Virus Titers and Airway Cytokines.
BAL fluid was collected for measurement of influenza virus titer from mice, as described previously (18). The levels of airway cytokines were determined by performing ELISA with BAL fluids harvested from infected mice.
BM Chimeras.
Generation of BM chimera was carried out as previously described (18). Briefly, mice were γ-irradiated with 950 Rad and subsequently reconstituted with 4–6 × 106 BM cells of the indicated genotype and allowed to recover for 6 to 8 wk before influenza infection.
Cell Preparation and Flow Cytometry.
Single-cell suspensions of lung samples were prepared as previously described (18). For staining, fluorochrome-labeled anti-CD8α (53-6.7), anti-CD4 (RM4-5), anti-CD11b (M1/70), anti-CD11c (N418), anti-Ly6G (1A8), anti-F4/80 (BM8), and anti-CD45 (30-F11) antibodies were from BioLegend; anti-Ly6C (AL-21) antibody was from BD Biosciences; anti-MHC-II (M5/114.15.2) antibody was from eBioscience. For the detection of influenza virus M2 expression, anti-influenza A M2 antibody (14C2) was from Novus Biologicals. Cells were primary stained with an anti-M2 antibody (1:600) for 30 min on ice, followed by secondary staining with a Alexa Fluor 647 donkey anti-mouse IgG antibody (Molecular Probes) for 20 min on ice, before staining with other surface markers. Acquisition of samples was performed on a cytometer (LSR II; Becton Dickinson). Leukocytes were gated based on forward and side scatter properties, and live cells were gated based on 7-aminoactinomycin D (BioLegend) or Live/Dead fixable aqua stain (Invitrogen) exclusion. In the lung, alveolar macrophages were excluded from FACS gating by autofluorescence, high expression of CD11c, and side scatter (40). The final analysis and graphic output were performed using FlowJo software (Tree Star).
Histology.
Formalin-fixed lung tissue was paraffin embedded and sections were cut and stained with H&E (Yale University Comparative Medicine) and viewed with normal light microscopy. The sections were evaluated by a pathologist in a blinded manner.
Influenza Virus Infection in Vitro.
BMDCs, prepared as described previously (18), were incubated with indicated multiplicity of infections of PR8 NS1-GFP influenza virus in 100 μL per well of 0.1% BSA PBS at 5 × 105 cells/well in a 24-well plate for 1 h and then incubated in complete media for an additional 12 h. Cells were harvested and stained with Live/Dead fixable aqua stain (Invitrogen) and antibodies against CD11c and MHC-II, followed by detection of GFP expression by FACS.
Intranasal Transfer of BAL Fluid.
For experiments described in Fig. 3, BAL fluids were harvested from four to five influenza virus-infected mice and spun down to remove cellular debris. Live virus in the samples were inactivated by exposure to 1 J/cm2 UV light three times with Stratalinker UV cross-linker (Stratagene). Viral inactivation was confirmed by plaque assay using MDCK cells. Samples were subsequently concentrated using Amicon Ultra-4 centrifugal filter units (Millipore) and 30–40 μL were injected intranasally into four to five mice per group.
Immunohistochemistry of Frozen Lung Sections.
After harvesting the BAL, lungs were inflated with OCT media (Tissue-tek; Sakura Finetek) for the preparation of frozen sections. Immunofluorescence was performed in sections from frozen blocks. Briefly, 6- to 8-μm frozen sections were fixed in acetone and blocked with TNB buffer [3% (wt/vol) Casein in PBS; NEN Life Science Products] containing 5% (vol/vol) normal donkey serum. To block endogenous biotin, the sections were further treated with the Avidin–Biotin block (Vector Laboratories), and endogenous peroxidase activity was quenched with 1% H2O2. FITC-conjugated anti-influenza A NP monoclonal antibody (Pierce antibodies) was applied at 1:10 for 1.5 h at room temperature. At the end of the staining, slides were washed and incubated with DAPI (Molecular Probes) and mounted with Fluoromount-G (Southern Biotechnology). The stained slides were analyzed by fluorescence microscopy (Leitz Orthoplan 2) with a 10× objective lens.
Statistical Analysis.
Statistical significance was tested by Student t test using GraphPad PRISM software (Version 5; GraphPad software). Data are presented as mean ± SEM P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Dr. Alexa Siddon for expert advice, Huiping Dong for technical support, and Dr. Ellen Foxman for critical reading of the manuscript. This work was supported by National Institutes of Health Grants AI062428 and U54 AI057160 to the Midwest Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1303275110/-/DCSupplemental.
References
- 1.Lund JM, et al. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci USA. 2004;101(15):5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303(5663):1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 3.Heil F, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303(5663):1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 4.Pichlmair A, et al. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science. 2006;314(5801):997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- 5.Hornung V, et al. 5′-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314(5801):994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- 6.Iwasaki A, Medzhitov R. Regulation of adaptive immunity by the innate immune system. Science. 2010;327(5963):291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Honda K, Taniguchi T. IRFs: Master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol. 2006;6(9):644–658. doi: 10.1038/nri1900. [DOI] [PubMed] [Google Scholar]
- 8.Peiris JS, Hui KP, Yen HL. Host response to influenza virus: Protection versus immunopathology. Curr Opin Immunol. 2010;22(4):475–481. doi: 10.1016/j.coi.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aldridge JR, Jr, et al. TNF/iNOS-producing dendritic cells are the necessary evil of lethal influenza virus infection. Proc Natl Acad Sci USA. 2009;106(13):5306–5311. doi: 10.1073/pnas.0900655106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin KL, Suzuki Y, Nakano H, Ramsburg E, Gunn MD. CCR2+ monocyte-derived dendritic cells and exudate macrophages produce influenza-induced pulmonary immune pathology and mortality. J Immunol. 2008;180(4):2562–2572. doi: 10.4049/jimmunol.180.4.2562. [DOI] [PubMed] [Google Scholar]
- 11.Nish S, Medzhitov R. Host defense pathways: Role of redundancy and compensation in infectious disease phenotypes. Immunity. 2011;34(5):629–636. doi: 10.1016/j.immuni.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Negishi H, et al. Cross-interference of RLR and TLR signaling pathways modulates antibacterial T cell responses. Nat Immunol. 2012;13(7):659–666. doi: 10.1038/ni.2307. [DOI] [PubMed] [Google Scholar]
- 13.Koyama S, et al. Differential role of TLR- and RLR-signaling in the immune responses to influenza A virus infection and vaccination. J Immunol. 2007;179(7):4711–4720. doi: 10.4049/jimmunol.179.7.4711. [DOI] [PubMed] [Google Scholar]
- 14.Barchet W, et al. Dendritic cells respond to influenza virus through TLR7- and PKR-independent pathways. Eur J Immunol. 2005;35(1):236–242. doi: 10.1002/eji.200425583. [DOI] [PubMed] [Google Scholar]
- 15.Schmitz N, Kurrer M, Bachmann MF, Kopf M. Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J Virol. 2005;79(10):6441–6448. doi: 10.1128/JVI.79.10.6441-6448.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heer AK, et al. TLR signaling fine-tunes anti-influenza B cell responses without regulating effector T cell responses. J Immunol. 2007;178(4):2182–2191. doi: 10.4049/jimmunol.178.4.2182. [DOI] [PubMed] [Google Scholar]
- 17.Kaminski MM, Ohnemus A, Cornitescu M, Staeheli P. Plasmacytoid dendritic cells and Toll-like receptor 7-dependent signalling promote efficient protection of mice against highly virulent influenza A virus. J Gen Virol. 2012;93(Pt 3):555–559. doi: 10.1099/vir.0.039065-0. [DOI] [PubMed] [Google Scholar]
- 18.Ichinohe T, Lee HK, Ogura Y, Flavell R, Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med. 2009;206(1):79–87. doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pang IK, Ichinohe T, Iwasaki A. IL-1R signaling in dendritic cells replaces pattern-recognition receptors in promoting CD8+ T cell responses to influenza A virus. Nat Immunol. 2013;14(3):246–253. doi: 10.1038/ni.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kato H, et al. Cell type-specific involvement of RIG-I in antiviral response. Immunity. 2005;23(1):19–28. doi: 10.1016/j.immuni.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Manicassamy B, et al. Analysis of in vivo dynamics of influenza virus infection in mice using a GFP reporter virus. Proc Natl Acad Sci USA. 2010;107(25):11531–11536. doi: 10.1073/pnas.0914994107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alford RH, Kasel JA, Gerone PJ, Knight V. Human influenza resulting from aerosol inhalation. Proc Soc Exp Biol Med. 1966;122(3):800–804. doi: 10.3181/00379727-122-31255. [DOI] [PubMed] [Google Scholar]
- 23.Ichinohe T, Pang IK, Iwasaki A. Influenza virus activates inflammasomes via its intracellular M2 ion channel. Nat Immunol. 2010;11(5):404–410. doi: 10.1038/ni.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perrone LA, Plowden JK, García-Sastre A, Katz JM, Tumpey TM. H5N1 and 1918 pandemic influenza virus infection results in early and excessive infiltration of macrophages and neutrophils in the lungs of mice. PLoS Pathog. 2008;4(8):e1000115. doi: 10.1371/journal.ppat.1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hou W, et al. Viral infection triggers rapid differentiation of human blood monocytes into dendritic cells. Blood. 2012;119(13):3128–3131. doi: 10.1182/blood-2011-09-379479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helft J, et al. Cross-presenting CD103+ dendritic cells are protected from influenza virus infection. J Clin Invest. 2012;122(11):4037–4047. doi: 10.1172/JCI60659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao W, et al. Rapid differentiation of monocytes into type I IFN-producing myeloid dendritic cells as an antiviral strategy against influenza virus infection. J Immunol. 2012;189(5):2257–2265. doi: 10.4049/jimmunol.1200168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinto R, et al. Inhibition of influenza virus-induced NF-kappaB and Raf/MEK/ERK activation can reduce both virus titers and cytokine expression simultaneously in vitro and in vivo. Antiviral Res. 2011;92(1):45–56. doi: 10.1016/j.antiviral.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Ludwig S. Targeting cell signalling pathways to fight the flu: Towards a paradigm change in anti-influenza therapy. J Antimicrob Chemother. 2009;64(1):1–4. doi: 10.1093/jac/dkp161. [DOI] [PubMed] [Google Scholar]
- 30.Wurzer WJ, et al. NF-kappaB-dependent induction of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and Fas/FasL is crucial for efficient influenza virus propagation. J Biol Chem. 2004;279(30):30931–30937. doi: 10.1074/jbc.M403258200. [DOI] [PubMed] [Google Scholar]
- 31.Wurzer WJ, et al. Caspase 3 activation is essential for efficient influenza virus propagation. EMBO J. 2003;22(11):2717–2728. doi: 10.1093/emboj/cdg279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pauli E-K, et al. Influenza A virus inhibits type I IFN signaling via NF-kappaB-dependent induction of SOCS-3 expression. PLoS Pathog. 2008;4(11):e1000196. doi: 10.1371/journal.ppat.1000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei L, et al. NFkappaB negatively regulates interferon-induced gene expression and anti-influenza activity. J Biol Chem. 2006;281(17):11678–11684. doi: 10.1074/jbc.M513286200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar N, Xin ZT, Liang Y, Ly H, Liang Y. NF-kappaB signaling differentially regulates influenza virus RNA synthesis. J Virol. 2008;82(20):9880–9889. doi: 10.1128/JVI.00909-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delputte PL, Van Breedam W, Barbe F, Van Reeth K, Nauwynck HJ. IFN-alpha treatment enhances porcine Arterivirus infection of monocytes via upregulation of the porcine Arterivirus receptor sialoadhesin. J Interferon Cytokine Res. 2007;27(9):757–766. doi: 10.1089/jir.2007.0001. [DOI] [PubMed] [Google Scholar]
- 36.Takano T, Hohdatsu T, Toda A, Tanabe M, Koyama H. TNF-alpha, produced by feline infectious peritonitis virus (FIPV)-infected macrophages, upregulates expression of type II FIPV receptor feline aminopeptidase N in feline macrophages. Virology. 2007;364(1):64–72. doi: 10.1016/j.virol.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kedzierska K, Crowe SM, Turville S, Cunningham AL. The influence of cytokines, chemokines and their receptors on HIV-1 replication in monocytes and macrophages. Rev Med Virol. 2003;13(1):39–56. doi: 10.1002/rmv.369. [DOI] [PubMed] [Google Scholar]
- 38.Arpaia N, et al. TLR signaling is required for Salmonella typhimurium virulence. Cell. 2011;144(5):675–688. doi: 10.1016/j.cell.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun Q, et al. The specific and essential role of MAVS in antiviral innate immune responses. Immunity. 2006;24(5):633–642. doi: 10.1016/j.immuni.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Jakubzick C, Randolph GJ. Methods to study pulmonary dendritic cell migration. Methods Mol Biol. 2010;595:371–382. doi: 10.1007/978-1-60761-421-0_24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.