Abstract
Carbohydrate-based vaccines have shown therapeutic efficacy for infectious disease and cancer. The mushroom Ganoderma lucidum (Reishi) containing complex polysaccharides has been used as antitumor supplement, but the mechanism of immune response has rarely been studied. Here, we show that the mice immunized with a l-fucose (Fuc)-enriched Reishi polysaccharide fraction (designated as FMS) induce antibodies against murine Lewis lung carcinoma cells, with increased antibody-mediated cytotoxicity and reduced production of tumor-associated inflammatory mediators (in particular, monocyte chemoattractant protein-1). The mice showed a significant increase in the peritoneal B1 B-cell population, suggesting FMS-mediated anti-glycan IgM production. Furthermore, the glycan microarray analysis of FMS-induced antisera displayed a high specificity toward tumor-associated glycans, with the antigenic structure located in the nonreducing termini (i.e., Fucα1-2Galβ1-3GalNAc-R, where Gal, GalNAc, and R represent, respectively, D-galactose, D-N-acetyl galactosamine, and reducing end), typically found in Globo H and related tumor antigens. The composition of FMS contains mainly the backbone of 1,4-mannan and 1,6-α-galactan and through the Fucα1-2Gal, Fucα1-3/4Man, Fucα1-4Xyl, and Fucα1-2Fuc linkages (where Man and Xyl represent d-mannose and d-xylose, respectively), underlying the molecular basis of the FMS-induced IgM antibodies against tumor-specific glycans.
Keywords: mushroom polysaccharide, antitumor activity, anti-Globo H antibody
Various forms of herbal medicine polysaccharides have become valuable as health supplements worldwide (1, 2), suggesting that administration of such polysaccharides may improve innate immunity in vivo. The underlying molecular mechanisms, however, still remain ambiguous. Aberrant terminal fucosylation as well as sialylation in tumor-associated glycans is one of several glycosylation events important in cancer progression (3, 4), and such unusual glycans have recently been used for the development of anticancer vaccines (5–7). As an example, the Globo H-based glycoconjugate vaccines are currently undergoing large-scale clinical trials and have shown promise in therapeutic treatment (8, 9). Studies on the immune response to pathogenic microorganisms (such as Haemophilus influenza type B and Streptococcus pneumonia) have demonstrated that polysaccharides containing repeating antigenic units are generally T cell-independent (TI) (10, 11). Furthermore, recent findings revealed that specific B-cell subsets could establish memory for providing specific Ig synthesis in response to TI-associated polysaccharides (12–14). In an attempt to understand the biological significance of polysaccharides derived from natural sources, we previously isolated and characterized a crude extract fraction of water-soluble and l-fucose (Fuc)-containing polysaccharides (F3) from Ganoderma lucidum (Reishi) (a mushroom that has been long used as a herb medicine) (15). F3 has since been shown essential for regulation of cytokine network, IgM production, and hematopoietic cell expansion (16–19). We also identified several pattern recognition receptors that could interact with F3, including Dectin-1, DC-SIGN, Langerin, Kupffer cell receptor, macrophage mannose receptor, and Toll-like receptors (20). Notably, these results supported the idea that F3 activates the immune response likely by interacting with carbohydrate-recognizing receptors. In animal studies, F3 is reported to serve as a vaccine adjuvant and exert antitumor activities through an enhancement of the host-mediated immunity (21), leading to an interesting question of whether and how antibody-mediated immunity plays a role in the antitumor activity of F3 in mice. In the current study, Fuc-enriched F3 polysaccharides were prepared for further study, and the results showed that the induced antisera could recognize biologically relevant glycans, in particular tumor-associated glycan epitopes, supporting the hypothesis that terminal fucosylation on Reishi polysaccharides plays a critical role in the antitumor responses.
Results and Discussion
Antitumor Activity of F3.
We first conducted a study in an animal tumor model using C57BL/6J mice with implantation of murine Lewis lung carcinoma (LLC1) cells to investigate the antitumor activity of F3. Briefly, LLC1 cells were transplanted s.c. into mice, and then F3 (24, 52, 120, and 240 mg/kg body weight per mouse dissolved in PBS) was administered i.p. once every other day, and the process was repeated for 28 d. As shown in the tumor growth curves (Fig. S1A), F3 exhibited a significant inhibition against the growth of LLC1 cells in a dose-dependent manner, and the most effective inhibitory response was observed in the dosage between 120 and 240 mg/kg, which is a feasible daily dose in humans. However, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay results revealed that F3 (<200 μg/mL) had no significant effect on LLC1 cell viability compared with the untreated cells (Fig. S1B). These results suggested that F3 may suppress the LLC1 cell growth and prolong the survival rate of tumor-bearing mice via an indirect antitumor mechanism. In animal studies, the antitumor effects of polysaccharides extracted from Reishi were reported previously (22, 23). More interestingly, there was evidence that the sera from Reishi polysaccharide-treated mice markedly inhibited murine sarcoma-180 and human lung carcinoma PG cell line growth in vitro, but the pure Reishi extract alone did not induce similar effects (24, 25). Thus, we conducted a synthetic glycan microarray analysis to investigate whether F3-induced antisera could recognize biologically important glycan epitopes. The serum samples were screened at a weekly interval with 60 structurally different synthetic oligosaccharides, including several tumor-associated glycans. Given the glycan binding patterns of F3-induced antisera, there was a clear trend showing the increase in the binding affinity of IgM antibodies to Globo H and Globo H-series glycans, including the terminal tetrasaccharide (Bb4) and trisaccharide (Bb3), after 2 wk of F3 treatment compared with the control (without F3 treatment) (Fig. S2; saccharide structures are shown in Fig. S3). Contrary to IgM responses, the serum IgG had no appreciable glycan-binding effects. So far, MBr1, the IgM anti-Globo H monoclonal antibody (mAb), is one of the valuable probes for Globo H-containing glycoconjugate detection (26, 27), and it also has been known to exert complement-dependent cytotoxicity (CDC) against Globo H-positive tumors (8, 28). Thus, we examined the expression levels of Globo H antigens on the LLC1 cell surface by mAb MBr1 immunostaining (Fig. S1C). The addition of F3-induced antisera to LLC1 cells was found to trigger cell death in vitro (Fig. S1D), leading to a speculation that F3 has the potential to induce antibody-mediated antitumor activity.
Fuc-Enriched F3 Polysaccharide Fraction Induced Antibodies Recognizing Globo H-Series Structures.
Previous reports indicated that the fucosylation on polysaccharides is responsible for the immune-modulating activity of F3 (15). Because F3 is known to be a heterogeneous and high-molecular-mass polysaccharide (>100 kDa), we, therefore, purified a Fuc-enriched polysaccharide fraction from F3 (designated as FMS) by a series of chromatographic steps. Using the size-exclusion chromatography combined with multiangle-laser light-scattering system, the average molecular mass of FMS was estimated to be 35 kDa. The composition analysis showed that FMS predominantly consists of Fuc, d-xylose (Xyl), d-galactose (Gal), and d-mannose (Man) in the ratio of 2:1.5:2.5:3.5, along with a small amount of glucose and amino-sugars (such as glucosamine and galactosamine). Methylation analysis indicated that FMS is based on a 1,4-mannan backbone with side chains at the C3 position, and a 1,6-α-galactan branched at the C2 position and is highly decorated with terminal Fuc (Table S1) (29–31).
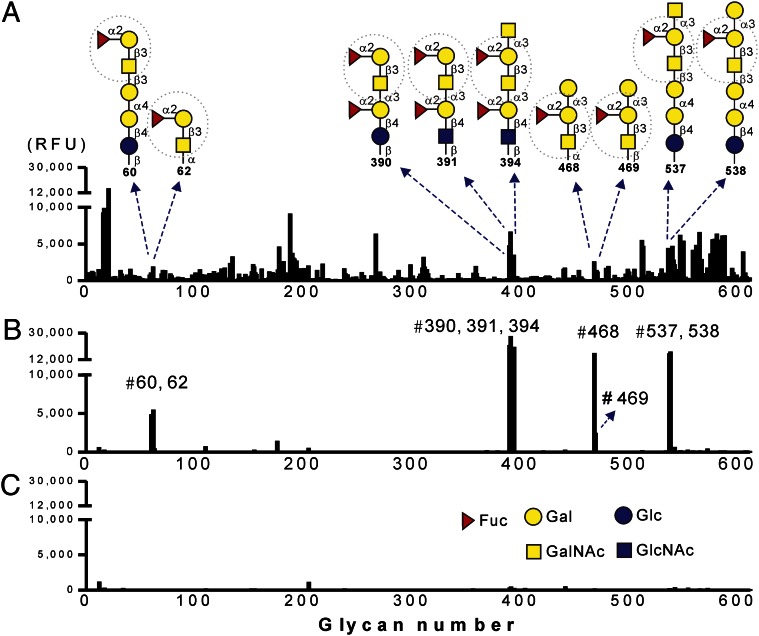
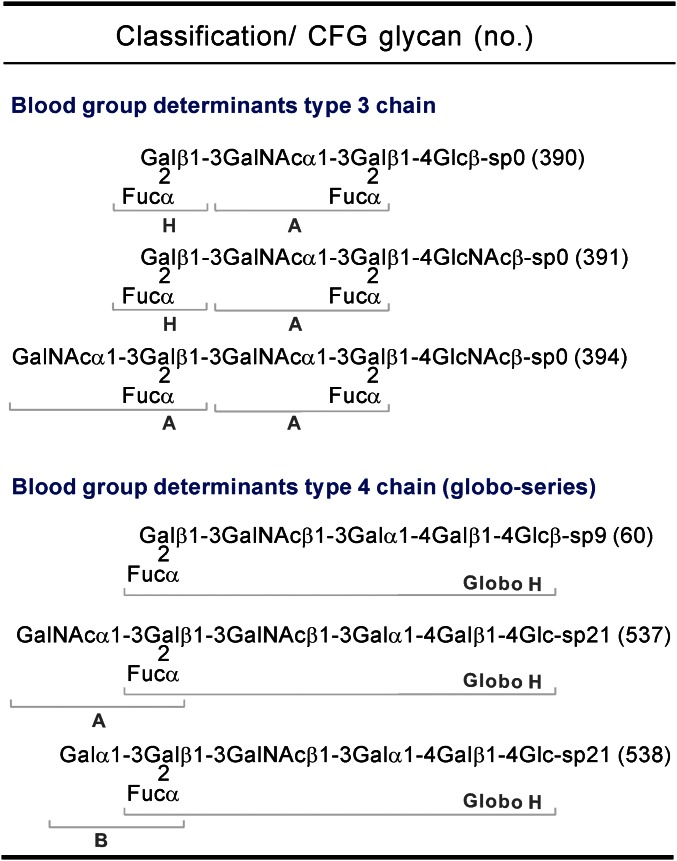
To examine the glycan-binding properties of FMS-induced antisera, a more comprehensive glycan microarray with 611 glycans from the Consortium for Functional Glycomics (CFG) Core H was used to assess the contribution of serum IgM antibodies. We examined second-week serum samples obtained from FMS- and F3-treated mice, respectively. The sera of control mice (PBS-treated) were also concurrently analyzed to determine the background of nonspecific binding. The 611 glycan-binding profiles, as indicated by relative fluorescence units (RFUs), are depicted in Fig. 1; it is evident that the anti-glycan IgM antibodies of FMS group have higher specificity and selectivity than those of the F3 group for several glycans, including glycan nos. 60, 62, 390, 391, 394, 468, 469, 537, and 538. A detailed list of the top 30 glycans bound by the F3 group is also deposited in Table S2. Although the exact antigen that affects the antibody binding avidity is not clear, it becomes apparent that these top ranked glycans bound by FMS-induced antisera all shared a common structure in the nonreducing termini (i.e., Fucα1-2Galβ1-3GalNAc-R). More interestingly, a group of glycans (nos. 390, 391, and 394) was found to display the highest antibody binding intensities, suggesting that an additional disaccharide (Fucα1-2Gal) extension in the reducing end of Fucα1-2Galβ1-3GalNAc-R improves the antisera binding affinity. Next, we asked whether any highly recognized glycans are related to endogenous human tumor-associated antigens. The identity of these glycan structures and their biological source, when further categorized, indicate that most of them represent blood group ABH determinants exclusively found in human glycosphingolipids (GSLs) (Fig. 2) (32–34). The three glycans, nos. 390, 391, and 394, have characteristic determinants that belong to terminal glycan structures of GSL neolacto series (type 3 chain). It has been reported that differences in the distribution of such GSL glycans between normal and cancerous tissues can be used for the diagnosis of human cervical carcinoma and bladder tumors (35, 36). In addition, the other three glycans that were identified as tumor-associated antigens belong to the members of GSL globo-series structures (type 4 chain), including Globo H (no. 60), Globo A (no. 537), and Globo B (no. 538). This may be the manifestation of well-known cross-reactivity of carbohydrate-specific antibodies, which led us to speculate that the glycan moiety Fucα1-2Galβ1-3GalNAcα/β (termed by H-type 3/4) is likely the antigenic determinant underlying the observed specificity of the FMS-induced IgM antibodies [i.e., the antibodies recognize Globo H and the related tumor-associated glycans (extended Globo H-series)].
Fig. 1.
Glycan-binding patterns of the serum IgM antibodies as measured by the CFG glycan microarray. Each histogram represents different sources of IgM binding to the glycan microarray, where the x axis shows the glycan number of 611 saccharides examined and the y axis is relative fluorescent units. Serum samples (tested at 1:100 dilution) from F3-treated (A), FMS-treated (B), and PBS-treated (C) mice were collected on day 14 after four dose injections and analyzed by printed array Version 5.0 of the CFG Core H. Nine of the identified glycan structures marked with glycan numbers are indicated. Dashed circles indicate the consensus glycan epitope (H-type 3/4 structure). Bars show the average RFUs [n = 4 (A and B); n = 2 (C)].
Fig. 2.
A spectrum of tumor associated-glycans highly recognized by FMS-induced antisera. Each glycan structure with chemical linker is printed on the CFG Version 5.0, which was classified into two groups. Structures of the linkers are indicated: sp0, CH2CH2NH2; sp9, CH2CH2CH2CH2CH2NH2; sp21, N(CH3)OCH2CH2NH2. Definition of blood group determinants (H, A, or B) is annotated.
Terminal Fucose of FMS Is Important for the Antibody-Mediated Antitumor Efficacy.
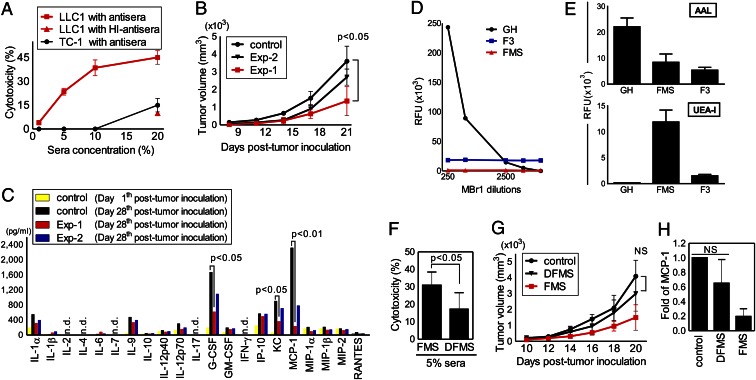
We further studied whether the FMS-mediated antibody responses to LLC1 cells could trigger cytotoxicity in vitro and whether such CDC activity is effective to Globo H-positive tumors. A Globo H-negative mouse tumor cell line TC-1 was also selected for comparison. As shown in Fig. 3A, the results of the CDC assay indicated that LLC1 cells are more sensitive than TC-1 cells to FMS-induced antisera in a concentration-dependent manner. We further investigated whether FMS could inhibit the growth of LLC1 cells in vivo. Two immunization plans were designed to assess both the preventive (Exp-1) and therapeutic (Exp-2) potentials compared with the control, PBS-treated LLC1-bearing mice (Fig. S4A). The resulting tumor growth curves suggested that pretreatment of FMS (Exp-1) could lead to a greater reduction in tumor volume (P < 0.05 versus control) (Fig. 3B). Because a close association between chronic inflammation and tumor development is often implied, we examined whether FMS dosage may regulate the production of LLC1-associated inflammatory mediators in vivo. After tumor inoculation, a multiplex cytokine profiling showed that two chemokines and one cytokine, monocyte chemoattractant protein (MCP)-1, CXCL1 (KC), and granulocyte colony-stimulating factor, respectively, were remarkably decreased in the mice pretreated with FMS (Exp-1) on day 28 (P < 0.05 versus day 28 control) (Fig. 3C), More interestingly, administration of FMS effectively lowered the serum levels of MCP-1 in LLC1-bearing mice. It is known that MCP-1 secreted from tumor cells is an important determinant in the pathogenesis of human lung cancers. Previous studies have also demonstrated that blockade of MCP-1, as mediated by neutralizing antibodies, in several animal models of non-small cell lung cancer significantly slowed the growth of primary tumors (37, 38). These results, thus, supported the notion that FMS could not only suppress the LLC1 cell growth but also attenuate relative inflammation levels in vivo.
Fig. 3.
Antitumor activities of FMS. (A) Antibody-mediated cytotoxicity (CDC) of antisera from FMS-treated mice to LLC1 and TC-1 tumor cells was determined by a lactose dehydrogenase kit. The value of antisera heated at 56 °C for 30 min (HI-antisera) is indicative of the complement depletion effect. (B) Comparison of antitumor effects between preventive (Exp-1) and therapeutic (Exp-2) FMS treatment in vivo. Control is PBS-treated mice with tumor inoculation. (C) FMS treatment suppressed tumor-associated cytokines and chemokines production in vivo. Serum samples were collected at indicated time after tumor inoculation and examined by Beadlyte mouse 21-pex kits. (D and E) Distinct binding intensities of plant lectins (AAL, 2 μg/mL; UEA-I, 10 μg/mL) and anti-Globo H mAb (MBr1, 0.5mg/mL) to Globo H (GH), FMS and F3 were determined by using the fabricated glycan microarray. (F–H) DFMS (low-Fuc content of FMS) treatment reduced CDC (F) and antitumor activities in vivo, as assessed by tumor growth curves (G) and MCP-1 production levels (H). Values show the means ± SD (n ≈ 3–5 for each experiment). n.d., not detectable; NS, no statistical significant.
The unexpected abilities of F3 and FMS serving as immunogens to induce antibodies and suppress Globo H-positive tumor growth, together with the glycan microarray analysis, suggest that the unit structure of antigen present in F3 and FMS may be fucosylated glycans. Previous studies demonstrated that the minimal epitope of mAb MBr1 is the H-type 3/4, such as the terminal trisaccharide of Globo H (Fucα1-2Galβ1-3GalNAcβ, also called Bb3), and that the terminal Fuc is essential for the antibody recognition (27, 34, 39). To examine whether Globo H-series molecules exist in our Reishi polysaccharides, we fabricated saccharide-printed slides by attachment of Globo H (100 μM), F3 (1 mg), and FMS (1 mg) onto N-hydroxysuccinimide–activated glass slides, and then the chips were interrogated with MBr1. As expected, the binding curve of the antibody to Globo H was in a dose-dependent manner. FMS and F3, nonetheless, displayed neither significant binding interaction nor dose-dependent behavior (Fig. 3D). Regarding to the antibody specificity, we previously reported that Globo H-based glycoconjugate vaccines induced antibodies more selectively for Globo H, SSEA3 (also called Gb5, Galβ1-3GalNAcβ1-3Galα1-4Galβ1-4Glcβ), and SSEA4 (Neu5Acα2-3Galβ1-3GalNAcβ1-3Galα1-4Galβ1-4Glcβ) (9). However, such cross-reactivity was not found in either F3- or FMS-induced IgM antibodies. This led us to think that Reishi polysaccharides may not contain Globo H-series antigens. This was probed with saccharide-printed slides interrogated with two α-L-Fuc–specific lectins, Ulex europaeus agglutinin-I (UEA-I) and Aleuria aurantia lectin (AAL). AAL bound to all of the samples, confirming the presence of α-fucosyl linkages. Both FMS and F3 showed significant binding intensities with lectin UEA-I (Fig. 3E), suggesting that the existence of Fucα1-2Gal disaccharide unit. The observed low binding of Globo H is consistent with the previous data showing that the lectin UEA-1 is unreactive to the H-type 3/4 structures (40, 41). Taken together, the results provide evidence that our Reishi polysaccharides contain α-l-fucosylated glycans but may differ from the Globo H-series structures.
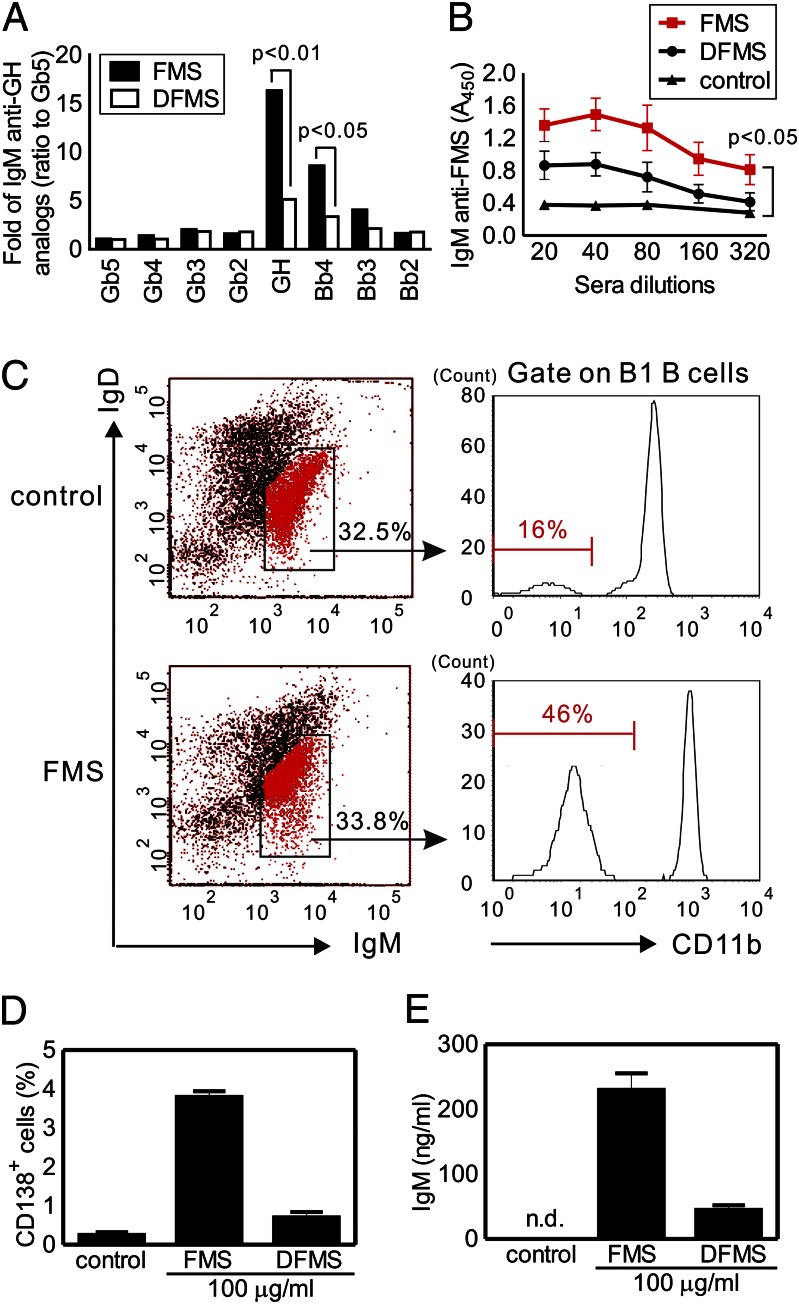
To investigate whether the α-fucosyl residues of FMS are correlated with antitumor activities, we selectively removed the terminal Fuc of FMS by a recombinant α-l-fucosidase from Bacteroides fragilis. A modified form of FMS, designated as DFMS, was obtained after enzymatic hydrolysis and subsequent purification. The Fuc content in DFMS was 50% of that in FMS, as determined by the high-performance anion-exchange chromatography with pulsed amperometric detection method. We then compared the antitumor efficacy of DFMS and FMS using the preventive immunization plan as mentioned earlier. Both the CDC activity and tumor growth analysis showed that DFMS did not display appreciable inhibition on the survival of LLC1 cells in vitro and in vivo (Fig. 3 F and G), contrary to FMS. Furthermore, there was no statistically significant difference in MCP-1 levels between DFMS-treated mice and the control mice, in contrast to the observed reduction of sera MCP-1 in FMS group (Fig. 3H). These results strongly supported a direct connection between the terminal fucosylation levels of FMS and the antitumor efficacy. To validate whether the serum antibodies are directly involved in the antitumor activity, and to study whether the antisera from different treatments have any change in the glycan-binding specificity, the Globo H-related printed glycan microarray was applied. As expected, the serum IgM against Globo H was significantly reduced in the DFMS group (P < 0.01 versus FMS group), consistent with its distinct antitumor effect (Fig. 4A and saccharide structures are shown in Fig. S3). Furthermore, we also confirmed that the FMS-induced antisera to FMS were detectable in the dilution range between 1:20–1:320, whereas the quantities of FMS-binding IgM antibodies were substantially reduced in the DFMS group, as determined by the FMS-coated 96-well plates (P < 0.05) (Fig. 4B). However, no IgG isotype in response to serological assays was detected in either study group using the same dilution factor. Because the total IgM production of each group (FMS versus DFMS) was similar, the results of these tests demonstrated that terminal fucosyl residues of FMS are critical to its immunogenicity. Although mushroom polysaccharides containing β-glucose and α-mannose have been postulated to have antitumor actions through innate carbohydrate-recognizing receptor interactions (42, 43), our results highlight the importance of terminal Fuc on Reishi polysaccharides in the antitumor activities.
Fig. 4.
Correlation between anti-glycan IgM production and B1 B cells expansion in the mice immunization with our Reishi polysaccharides. (A and B) Antisera from FMS- and DFMS-treated mice were assessed by Globo H-related printed glycan microarray (A) and FMS-coated ELISA plate (B). (A) Binding of IgM to Globo H (GH) and its truncated forms (tested at 1:100 dilution) was normalized by setting the IgM anti-Gb5 as 1. (B) Binding of IgM to FMS (tested at 1:20–1:320 dilution) was measured by detecting the absorbance at 450 nm. (C–E) Expansion of peritoneal B1 B cells upon FMS immunization. FACS profiles of B1 B cells represent FMS-treated mice and control. Additional levels of B2 B cell and macrophage are shown in Fig. S4. Numbers (%) indicate the positive cells in each gate (C). FMS induced up-regulation of plasma cell surface marker (CD138) (D) and IgM production (E) in ex vivo B1 B cells culture purified from FMS-treated mice. Means ± SD (n ≈ 3–5 for each experiment). n.d., not detectable.
Immunization of FMS Stimulates B1 B-Cell Activation.
Most anti-glycan/polysaccharide antibodies belong to the IgM isotype, which is likely produced by a subset of B cells known as B1 B cells (12, 13). Because the majority of B1 B cells reside predominantly in the peritoneal and pleural cavities of mice, we, thus, investigated whether there was any cellular change in the mice peritoneal cavity after 1 mo of FMS treatment. The result is depicted in Fig. 4C (also see Fig. S5). We found that the percentages of B1 B cells (IgMhiIgDloCD11blo) in FMS-treated mice dramatically increased (up to 46%) in comparison with the control (only 16%), whereas both B2 B cells (IgDhi) and the monocyte-macrophage (Mϕ) (CD11bhi) populations remained similar to those of the control, as indicated by flow cytometry. To further confirm whether the increased levels of peritoneal B1 B cells are directly associated with FMS-specific antibody responses, we purified both B1 B and B2 B cells from the peritoneal cavities of FMS-treated mice and cultured ex vivo in the presence of either FMS or DFMS for 3 d. As expected, the addition of FMS to the culture caused a dramatic increase of B1 B cells that were positive for CD138 expression, a surface marker for plasma cells, whereas only an insignificant amount of CD138+ B1 B cells was detected upon DFMS treatment (Fig. 4D). Additionally, we observed a considerable increase in IgM production after ex vivo culture of B1 B cells with FMS but not with DFMS treatment (Fig. 4E). However, neither FMS nor DFMS caused any noticeable effect on B2 B-cell activation. Although in vivo integrated immune responses involved in the activation of B1 B cells remain unclear, our data support that the peritoneal B1 B cells play a direct role in responding to FMS as well as TI antigens, resulting in an enhanced level of FMS-specific antibody-secreing cells (plasmablasts), along with increased IgM antibodies.
Identification of Fucosyl Glycan Moieties of FMS by an MS-Based Approach.
Based on the aforementioned sugar composition and linkage analysis, we concluded that FMS, unlike some common glycans, comprises Fuc, Gal, Man, and Xyl (Table S1). In particular, the sugar analysis supports the presence of a significant amount of terminal Fuc residues, which is of immunobiological relevance. Using a competition assay, we found that intact FMS (molecular mass, ∼35 kDa) and the small glycan fragments (molecular mass, <3 kDa) derived from FMS or algal fucoidan (FMS-H or fucoidan-H, respectively, prepared by partial acid hydrolysis) all served as competitive inhibitors to decrease the interactions of the FMS-induced antisera with Globo H-printed glycan microarray, whereas an intact algal fucoidan (Sigma; F-5631) purified from Fucus vesiculosus did not (Fig. S4B) (44). The result supports that fucosylated and/or oligofucosylated glycans, which can be released by acid hydrolysis from FMS, are the most promising small immuno-active molecules to be identified as biologics. Inexplicably, a MALDI-MS mapping of the partial hydrolysates failed to detect such convincingly fucosylated fragments among the predominant oligo-hexoses despite the apparent abundance of Fuc in the FMS (Fig. S6A). We, therefore, conducted the nano-liquid chromatography–tandem MS (nano–LC-MS/MS) analysis of the permethylated oligoglycosyl alditols, taking advantage of the highest sensitivity and selectivity afforded by a Fuc-dependent multistage MS/MS data acquisition. In this mode, as many MS2 analyses as possible were initially performed on as many detectable peaks, but only few targeted product ions would be further analyzed. In essence, the MS/MS functions would automatically sieve through hundreds of peaks and focus only on those of interest, which, in this case, are the minor components carrying terminal Fuc. It is evident from the LC-MS profiles that these fucosylated oligosaccharides were 100-fold less abundant than the hexose (Hex)-only oligosaccharides, which may constitute the structural backbone of FMS (Fig. S6B and Table S3). Among the MS2 product ions afforded by fucosylated precursors, the B ions of three distinct terminal fucosylated disaccharide epitopes, namely Fuc-Hex, Fuc-Xyl, and Fuc-Fuc at m/z 415, 371, and 385, respectively, were further isolated for MS3 analysis to confirm their identities and define their linkages. Four selected pairs of MS2/MS3 spectra are depicted in Fig. S7, which are representative of the range of fucosylated epitopes carried by FMS. Through manual interpretation of the fragment ions, it is clear that a terminal Fuc residue can indeed be directly attached to a Hex (Man or Gal), Xyl, or another Fuc, most commonly at the C4 and C2 position in each case, although it is not possible to rule out other coexisting linkages. The Fuc-Hex moiety can be further extended at the reducing end by another Hex or Xyl, whereas a Fuc-Xyl unit can be extended by another Hex. Intriguingly, a stretch of tri-Fuc can also be found, along with alternative isomers in which the Fuc residue is located internally or at the reducing end. These results may explain our observation that the possible molecular basis of the FMS-induced IgM antibodies could cross-react with H-type 3/4 glycans (30, 45–47).
Conclusion
The collective work described here demonstrates that FMS, the Fuc-enriched polysaccharide fraction from F3, exhibits unique immunogenicity, and that the mice immunized with F3 or FMS could exert effective antibody-mediated reaction against Globo H-expressing murine LLC1 cells. These findings are consistent with our previous assertion that the host immune function enhanced by Reishi polysaccharides offer great promise for the immunotherapy of Globo H-positive lung cancer patients (48). Based on our glycan structural analysis, the most likely fucosyl glycan moieties are Fucα1-2Gal-R, Fucα1-3/4Man-R, Fucα1-4Xyl-R, and Fucα1-2Fuc-R. It is likely that some of them activate the antibody responses against tumor-specific glycan epitopes, paving the way for developing complex carbohydrates for immunomodulation-based therapy. Although this study is limited to Reishi polysaccharides and mostly to lung cancer, the approach of high-throughput glycan microarray analysis and detailed structural analyses of carbohydrate antigens should be applicable to other medicinal polysaccharides, which induce different antibody-mediated biological functions.
Materials and Methods
Preparation of FMS.
The starting material is a commercial product (called F3, a crude extract fraction of water-soluble and Fuc-containing polysaccharides from Ganoderma lucidum, Reishi) manufactured by Wyntek. F3 was dissolved in 50 mM ammonium acetate and the insoluble residue was removed by centrifugation. The supernatant was fractionated by diethylaminoethyl (DEAE)–Sephadex A-50 chromatography to obtain a fraction using 50 mM ammonium acetate as the eluent. After desalting, the fraction was further purified by reversed-phase high-performance liquid chromatography using a semipreparative C8 column coupled with an Agilent 1100 series system. All runs required 0.05% trifluoroacetic acid as the eluent, and the flow-through was collected. The selected fraction was subjected to Sephadex G-50 chromatography (1.5 × 100 cm) using distilled water as eluent. The carbohydrate-containing fractions, detected using the phenol-sulfuric acid method, were lyophilized to give a polysaccharide product designated as FMS (total yield, <0.1%). Polysaccharide preparations were monitored routinely by the Limulus Amebocyte Lysate test (Associates of Cape Cod) to ensure absence of endotoxin contamination.
Glycan-Binding Analysis of Serum IgM Antibodies.
For a comprehensive glycan microarray analysis, the mice were administrated i.p. with test samples (150 mg/kg body weight per mouse) twice weekly and the serum samples were harvested on day 14 after first immunization. PBS-treated mouse sera served as control group. The glycan-binding profiling of IgM antibodies were investigated on the glycan microarray at Core H of the CFG (Emory University School of Medicine, Atlanta, GA). The serum samples were diluted by 1:100 dilution and screened using Version 5.0 of the printed array containing 611 glycans in hexaplicates. The procedure as well as all glycan structures of the referenced CFG numbers are available on the CFG Web site (www.functionalglycomics.org).
Research materials and details regarding the experimental methods, including sugar analysis, immunization schedule and mouse tumor model, serological assays, glycan-binding assays, FACS analysis, and MS analysis, etc. are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Prof. Chung-Hsuan Chen and Alice Ling-Tsing Yu for helpful discussions and technical supports. We thank the Core H of the Consortium for Functional Glycomics at Emory University School of Medicine for glycan-microarray screening. We also thank the Genomics Research Center Mass Spectrometry facility in Academia Sinica for glycomic analysis assistance. The research was supported by Academia Sinica and National Science Council of Taiwan Grants NSC 99-2320-B-010-010-MY3 and NSC 101-2627-M-010-003) and The Ministry of Education Top University Project Grant 102AC-P664 (to H.-Y.H.).
Footnotes
The authors declare no conflict of interest.
1S.-F.L. and C.-H. Liang contributed equally to this work.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1312457110/-/DCSupplemental.
References
- 1.Wasser SP, Weis AL. Therapeutic effects of substances occurring in higher Basidiomycetes mushrooms: A modern perspective. Crit Rev Immunol. 1999;19(1):65–96. [PubMed] [Google Scholar]
- 2.Vickers A. Recent advances: Complementary medicine. BMJ. 2000;321(7262):683–686. doi: 10.1136/bmj.321.7262.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hakomori S. Tumor-associated carbohydrate antigens defining tumor malignancy: Basis for development of anti-cancer vaccines. Adv Exp Med Biol. 2001;491:369–402. doi: 10.1007/978-1-4615-1267-7_24. [DOI] [PubMed] [Google Scholar]
- 4.Dube DH, Bertozzi CR. Glycans in cancer and inflammation—potential for therapeutics and diagnostics. Nat Rev Drug Discov. 2005;4(6):477–488. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- 5.Zhu J, Warren JD, Danishefsky SJ. Synthetic carbohydrate-based anticancer vaccines: The Memorial Sloan-Kettering experience. Expert Rev Vaccines. 2009;8(10):1399–1413. doi: 10.1586/erv.09.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slovin SF, Keding SJ, Ragupathi G. Carbohydrate vaccines as immunotherapy for cancer. Immunol Cell Biol. 2005;83(4):418–428. doi: 10.1111/j.1440-1711.2005.01350.x. [DOI] [PubMed] [Google Scholar]
- 7.Astronomo RD, Burton DR. Carbohydrate vaccines: Developing sweet solutions to sticky situations? Nat Rev Drug Discov. 2010;9(4):308–324. doi: 10.1038/nrd3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilewski T, et al. Immunization of metastatic breast cancer patients with a fully synthetic globo H conjugate: A phase I trial. Proc Natl Acad Sci USA. 2001;98(6):3270–3275. doi: 10.1073/pnas.051626298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang YL, et al. Carbohydrate-based vaccines with a glycolipid adjuvant for breast cancer. Proc Natl Acad Sci USA. 2013;110(7):2517–2522. doi: 10.1073/pnas.1222649110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siber GR. Pneumococcal disease: Prospects for a new generation of vaccines. Science. 1994;265(5177):1385–1387. doi: 10.1126/science.8073278. [DOI] [PubMed] [Google Scholar]
- 11.Lesinski GB, Westerink MA. Vaccines against polysaccharide antigens. Curr Drug Targets Infect Disord. 2001;1(3):325–334. doi: 10.2174/1568005014605964. [DOI] [PubMed] [Google Scholar]
- 12.Foote JB, Kearney JF. Generation of B cell memory to the bacterial polysaccharide alpha-1,3 dextran. J Immunol. 2009;183(10):6359–6368. doi: 10.4049/jimmunol.0902473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14(5):617–629. doi: 10.1016/s1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- 14.Alugupalli KR, et al. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 2004;21(3):379–390. doi: 10.1016/j.immuni.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 15.Wang YY, et al. Studies on the immuno-modulating and antitumor activities of Ganoderma lucidum (Reishi) polysaccharides: Functional and proteomic analyses of a fucose-containing glycoprotein fraction responsible for the activities. Bioorg Med Chem. 2002;10(4):1057–1062. doi: 10.1016/s0968-0896(01)00377-7. [DOI] [PubMed] [Google Scholar]
- 16.Lin KI, et al. Reishi polysaccharides induce immunoglobulin production through the TLR4/TLR2-mediated induction of transcription factor Blimp-1. J Biol Chem. 2006;281(34):24111–24123. doi: 10.1074/jbc.M601106200. [DOI] [PubMed] [Google Scholar]
- 17.Chen WY, Yang WB, Wong CH, Shih DT. Effect of Reishi polysaccharides on human stem/progenitor cells. Bioorg Med Chem. 2010;18(24):8583–8591. doi: 10.1016/j.bmc.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 18.Hua KF, et al. Ganoderma lucidum polysaccharides enhance CD14 endocytosis of LPS and promote TLR4 signal transduction of cytokine expression. J Cell Physiol. 2007;212(2):537–550. doi: 10.1002/jcp.21050. [DOI] [PubMed] [Google Scholar]
- 19.Chen HS, et al. Studies on the immuno-modulating and anti-tumor activities of Ganoderma lucidum (Reishi) polysaccharides. Bioorg Med Chem. 2004;12(21):5595–5601. doi: 10.1016/j.bmc.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Hsu TL, et al. Profiling carbohydrate-receptor interaction with recombinant innate immunity receptor-Fc fusion proteins. J Biol Chem. 2009;284(50):34479–34489. doi: 10.1074/jbc.M109.065961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai CY, et al. Immunomodulatory and adjuvant activities of a polysaccharide extract of Ganoderma lucidum in vivo and in vitro. Vaccine. 2010;28(31):4945–4954. doi: 10.1016/j.vaccine.2010.05.037. [DOI] [PubMed] [Google Scholar]
- 22.Gao Y, et al. Antitumor activity and underlying mechanisms of ganopoly, the refined polysaccharides extracted from Ganoderma lucidum, in mice. Immunol Invest. 2005;34(2):171–198. [PubMed] [Google Scholar]
- 23.Lu H, Kyo E, Uesaka T, Katoh O, Watanabe H. A water-soluble extract from cultured medium of Ganoderma lucidum (Rei-shi) mycelia suppresses azoxymethane-induction of colon cancers in male F344 rats. Oncol Rep. 2003;10(2):375–379. [PubMed] [Google Scholar]
- 24.Wang PY, Zhu XL, Lin ZB. Antitumor and immunomodulatory effects of polysaccharides from broken-spore of Ganoderma lucidum. Front Pharmacol. 2012;3:135. doi: 10.3389/fphar.2012.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao QZ, Lin ZB. Antitumor and anti-angiogenic activity of Ganoderma lucidum polysaccharides peptide. Acta Pharmacol Sin. 2004;25(6):833–838. [PubMed] [Google Scholar]
- 26.Mènard S, Tagliabue E, Canevari S, Fossati G, Colnaghi MI. Generation of monoclonal antibodies reacting with normal and cancer cells of human breast. Cancer Res. 1983;43(3):1295–1300. [PubMed] [Google Scholar]
- 27.Bremer EG, et al. Characterization of a glycosphingolipid antigen defined by the monoclonal antibody MBr1 expressed in normal and neoplastic epithelial cells of human mammary gland. J Biol Chem. 1984;259(23):14773–14777. [PubMed] [Google Scholar]
- 28.Slovin SF, et al. Carbohydrate vaccines in cancer: Immunogenicity of a fully synthetic globo H hexasaccharide conjugate in man. Proc Natl Acad Sci USA. 1999;96(10):5710–5715. doi: 10.1073/pnas.96.10.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ye L, et al. Purification, NMR study and immunostimulating property of a fucogalactan from the fruiting bodies of Ganoderma lucidum. Planta Med. 2008;74(14):1730–1734. doi: 10.1055/s-2008-1081354. [DOI] [PubMed] [Google Scholar]
- 30.Alquini G, Carbonero ER, Rosado FR, Cosentino C, Iacomini M. Polysaccharides from the fruit bodies of the basidiomycete Laetiporus sulphureus (Bull.: Fr.) Murr. FEMS Microbiol Lett. 2004;230(1):47–52. doi: 10.1016/S0378-1097(03)00853-X. [DOI] [PubMed] [Google Scholar]
- 31.Usui T, Hosokawa S, Mizuno T, Suzuki T, Meguro H. Investigation of the heterogeneity of heterogalactan from the fruit bodies of Fomitopsis pinicola, by employing concanavalin A-Sepharose affinity chromatography. J Biochem. 1981;89(4):1029–1037. [PubMed] [Google Scholar]
- 32.Hakomori S. Structure, organization, and function of glycosphingolipids in membrane. Curr Opin Hematol. 2003;10(1):16–24. doi: 10.1097/00062752-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Clausen H, Levery SB, Nudelman E, Tsuchiya S, Hakomori S. Repetitive A epitope (type 3 chain A) defined by blood group A1-specific monoclonal antibody TH-1: Chemical basis of qualitative A1 and A2 distinction. Proc Natl Acad Sci USA. 1985;82(4):1199–1203. doi: 10.1073/pnas.82.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clausen H, Holmes E, Hakomori S. Novel blood group H glycolipid antigens exclusively expressed in blood group A and AB erythrocytes (type 3 chain H). II. Differential conversion of different H substrates by A1 and A2 enzymes, and type 3 chain H expression in relation to secretor status. J Biol Chem. 1986;261(3):1388–1392. [PubMed] [Google Scholar]
- 35.Cui Y, et al. Human cervical epidermal carcinoma-associated intracellular localization of glycosphingolipid with blood group A type 3 chain. Jpn J Cancer Res. 1993;84(6):664–672. doi: 10.1111/j.1349-7006.1993.tb02027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurimoto S, et al. Detection of a glycosphingolipid antigen in bladder cancer cells with monoclonal antibody MRG-1. Histochem J. 1995;27(3):247–252. [PubMed] [Google Scholar]
- 37.Fridlender ZG, et al. Monocyte chemoattractant protein-1 blockade inhibits lung cancer tumor growth by altering macrophage phenotype and activating CD8+ cells. Am J Respir Cell Mol Biol. 2011;44(2):230–237. doi: 10.1165/rcmb.2010-0080OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stathopoulos GT, et al. A central role for tumor-derived monocyte chemoattractant protein-1 in malignant pleural effusion. J Natl Cancer Inst. 2008;100(20):1464–1476. doi: 10.1093/jnci/djn325. [DOI] [PubMed] [Google Scholar]
- 39.Wang CC, et al. Glycan microarray of Globo H and related structures for quantitative analysis of breast cancer. Proc Natl Acad Sci USA. 2008;105(33):11661–11666. doi: 10.1073/pnas.0804923105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baldus SE, et al. Characterization of the binding specificity of Anguilla anguilla agglutinin (AAA) in comparison to Ulex europaeus agglutinin I (UEA-I) Glycoconj J. 1996;13(4):585–590. doi: 10.1007/BF00731446. [DOI] [PubMed] [Google Scholar]
- 41.Mollicone R, et al. Recognition of the blood group H type 2 trisaccharide epitope by 28 monoclonal antibodies and three lectins. Glycoconj J. 1996;13(2):263–271. doi: 10.1007/BF00731501. [DOI] [PubMed] [Google Scholar]
- 42.Lombard Y, Giaimis J, Makaya-Kumba M, Fonteneau P, Poindron P. A new method for studying the binding and ingestion of zymosan particles by macrophages. J Immunol Methods. 1994;174(1-2):155–165. doi: 10.1016/0022-1759(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 43.Wasser SP. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl Microbiol Biotechnol. 2002;60(3):258–274. doi: 10.1007/s00253-002-1076-7. [DOI] [PubMed] [Google Scholar]
- 44.Bilan MI, et al. Structure of a fucoidan from the brown seaweed Fucus evanescens C.Ag. Carbohydr Res. 2002;337(8):719–730. doi: 10.1016/s0008-6215(02)00053-8. [DOI] [PubMed] [Google Scholar]
- 45.Miyazaki T, Nishijima M. Studies on fungal polysaccharides. XXVII. Structural examination of a water-soluble, antitumor polysaccharide of Ganoderma lucidum. Chem Pharm Bull (Tokyo) 1981;29(12):3611–3616. doi: 10.1248/cpb.29.3611. [DOI] [PubMed] [Google Scholar]
- 46.Ye L, et al. Structural elucidation of the polysaccharide moiety of a glycopeptide (GLPCW-II) from Ganoderma lucidum fruiting bodies. Carbohydr Res. 2008;343(4):746–752. doi: 10.1016/j.carres.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 47.Axelsson K, Björndal H, Svensson S, Hammarström S. Polysaccharides elaborated by Fomes annosus (Fr.) Cooke. II. Neutral polysaccharides from the fruit bodies. Isolation and purification of a fucoxylomannan by precipitation with the H-agglutinin from eel-serum. Acta Chem Scand. 1971;25(10):3645–3650. doi: 10.3891/acta.chem.scand.25-3645. [DOI] [PubMed] [Google Scholar]
- 48.Gao Y, et al. Effects of water-soluble Ganoderma lucidum polysaccharides on the immune functions of patients with advanced lung cancer. J Med Food. 2005;8(2):159–168. doi: 10.1089/jmf.2005.8.159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.