Abstract
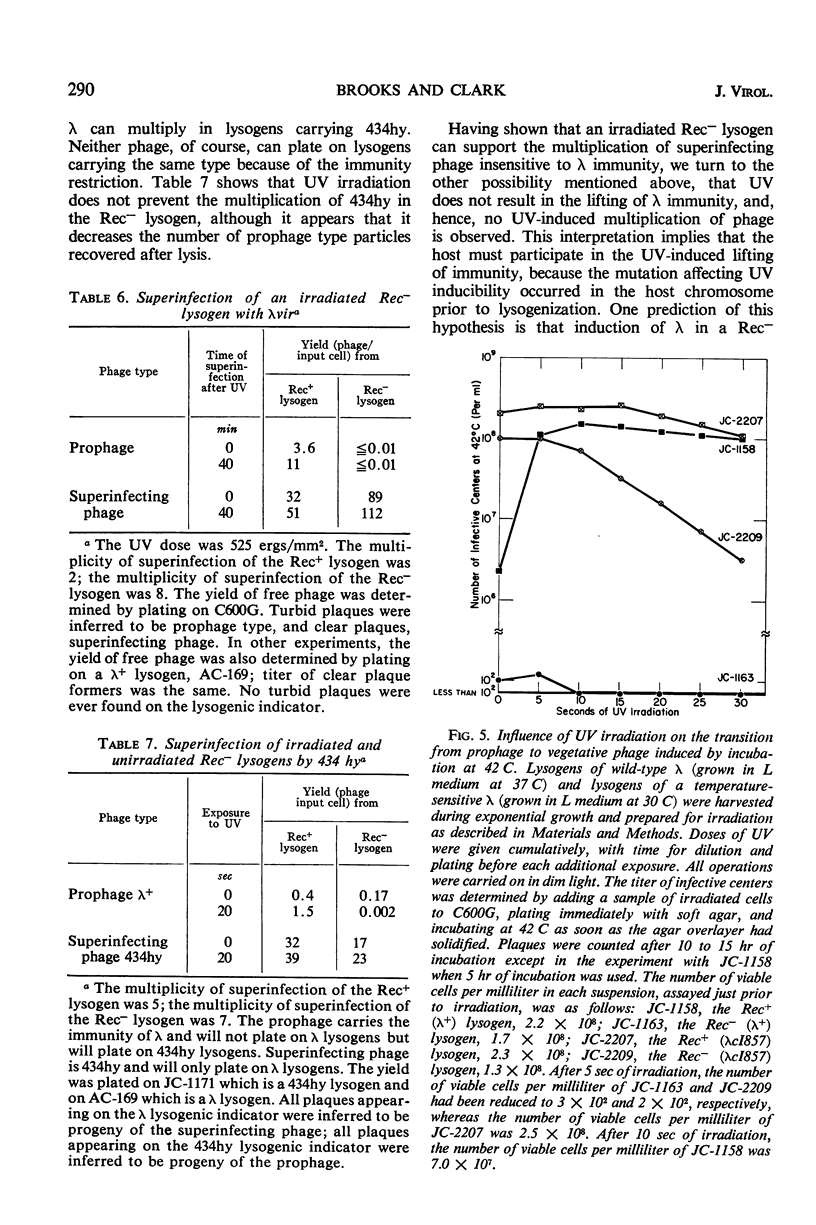
The behavior of λ phage in the Rec− strain JC-1569 is compared with that in the Rec+ strain JC-1557. No difference deemed significant was noted in the adsorption rate, latent period, burst size, frequency of lysogenization, and frequency of vegetative phage recombination. The location of the prophage and its mode of insertion in the Rec− lysogen of wild-type λ (λ+) were inferred to be normal from the results of conjugational crosses. Spontaneous and ultraviolet (UV) irradiation induction of λ+ were markedly reduced in the Rec− lysogen. On the other hand, thermal induction of a mutant lambda (λcI857) lysogen of the Rec− strain was not reduced and was only slightly affected by UV irradiation. Phage subject to inhibition by λ immunity failed to multiply in UV-irradiated cells of the Rec− λ+ lysogen, whereas those not inhibited by this immunity did multiply. It was concluded that the failure of UV to induce λ+ in the Rec− lysogen was not due to damage to the prophage, but rather to the inability of the irradiated cells to respond by lifting immunity. Preliminary evidence indicates that a single mutation confers recombination deficiency and the inability to lift immunity after UV irradiation. Possible relationships between recombination and the lifting of immunity are enumerated.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPLEYARD R. K., MCGREGOR J. F., BAIRD K. M. Mutation to extended host range and the occurrence of phenotypic mixing in the temperate coliphage lambda. Virology. 1956 Aug;2(4):565–574. doi: 10.1016/0042-6822(56)90012-5. [DOI] [PubMed] [Google Scholar]
- CAMPBELL A. Sensitive mutants of bacteriophage lambda. Virology. 1961 May;14:22–32. doi: 10.1016/0042-6822(61)90128-3. [DOI] [PubMed] [Google Scholar]
- CLARK A. J. Genetic analysis of a "double male" strain of Escherichia coli K-12. Genetics. 1963 Jan;48:105–120. doi: 10.1093/genetics/48.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK A. J., MARGULIES A. D. ISOLATION AND CHARACTERIZATION OF RECOMBINATION-DEFICIENT MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1965 Feb;53:451–459. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDTHWAIT D., JACOB F. SUR LE M'ECANISME DE L'INDUCTION DU D'EVELOPPEMENT DU PROPHAGE CHEZ LES BACT'ERIES LYSOG'ENES. C R Hebd Seances Acad Sci. 1964 Jul 20;259:661–664. [PubMed] [Google Scholar]
- Howard-Flanders P., Theriot L. Mutants of Escherichia coli K-12 defective in DNA repair and in genetic recombination. Genetics. 1966 Jun;53(6):1137–1150. doi: 10.1093/genetics/53.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAISER A. D., JACOB F. Recombination between related temperate bacteriophages and the genetic control of immunity and prophage localization. Virology. 1957 Dec;4(3):509–521. doi: 10.1016/0042-6822(57)90083-1. [DOI] [PubMed] [Google Scholar]
- KAISER A. D. Mutations in a temperate bacteriophage affecting its ability to lysogenize Escherichia coli. Virology. 1957 Feb;3(1):42–61. doi: 10.1016/0042-6822(57)90022-3. [DOI] [PubMed] [Google Scholar]
- MONOD J., COHEN-BAZIRE G., COHN M. Sur la biosynthèse de la beta-galactosidase (lactase) chez Escherichia coli; la spécificité de l'induction. Biochim Biophys Acta. 1951 Nov;7(4):585–599. doi: 10.1016/0006-3002(51)90072-8. [DOI] [PubMed] [Google Scholar]
- Radding C. M. Nuclease activity in defective lysogens of phage lambda. Biochem Biophys Res Commun. 1964 Feb 18;15(1):8–12. doi: 10.1016/0006-291x(64)90093-2. [DOI] [PubMed] [Google Scholar]
- Radding C. M., Shreffler D. C. Regulation of lambda exonuclease. II. Joint regulation of exonuclease and a new lambda antigen. J Mol Biol. 1966 Jul;18(2):251–261. doi: 10.1016/s0022-2836(66)80244-9. [DOI] [PubMed] [Google Scholar]
- SUSSMAN R., JACOB F. [On a thermosensitive repression system in the Escherichia coli lambda bacteriophage]. C R Hebd Seances Acad Sci. 1962 Feb 19;254:1517–1519. [PubMed] [Google Scholar]
- TAYLOR A. L., THOMAN M. S. THE GENETIC MAP OF ESCHERICHIA COLI K-12. Genetics. 1964 Oct;50:659–677. doi: 10.1093/genetics/50.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessman I. Genetic recombination of phage S13 in a recombination-deficient mutant of Escherichia coli K12. Biochem Biophys Res Commun. 1966 Jan 24;22(2):169–174. doi: 10.1016/0006-291x(66)90427-x. [DOI] [PubMed] [Google Scholar]
- van de Putte P., Zwenk H., Rörsch A. Properties of four mutants of Escherichia coli defective in genetic recombination. Mutat Res. 1966 Oct;3(5):381–392. doi: 10.1016/0027-5107(66)90048-0. [DOI] [PubMed] [Google Scholar]



