Abstract
The dynamic trafficking of AMPA receptors (AMPARs) into and out of synapses is crucial for synaptic transmission, plasticity, learning, and memory. The protein interacting with C-kinase 1 (PICK1) directly interacts with GluA2/3 subunits of the AMPARs. Although the role of PICK1 in regulating AMPAR trafficking and multiple forms of synaptic plasticity is known, the exact molecular mechanisms underlying this process remain unclear. Here, we report a unique interaction between PICK1 and all three members of the protein kinase C and casein kinase II substrate in neurons (PACSIN) family and show that they form a complex with AMPARs. Our results reveal that knockdown of the neuronal-specific protein, PACSIN1, leads to a significant reduction in AMPAR internalization following the activation of NMDA receptors in hippocampal neurons. The interaction between PICK1 and PACSIN1 is regulated by PACSIN1 phosphorylation within the variable region and is required for AMPAR endocytosis. Similarly, the binding of PICK1 to the ubiquitously expressed PACSIN2 is also regulated by the homologous phosphorylation sites within the PACSIN2-variable region. Genetic deletion of PACSIN2, which is highly expressed in Purkinje cells, eliminates cerebellar long-term depression. This deficit can be fully rescued by overexpressing wild-type PACSIN2, but not by a PACSIN2 phosphomimetic mutant, which does not bind PICK1 efficiently. Taken together, our data demonstrate that the interaction of PICK1 and PACSIN is required for the activity-dependent internalization of AMPARs and for the expression of long-term depression in the cerebellum.
Keywords: syndapin, receptor trafficking
AMPA-type glutamate receptors mediate most of the fast excitatory synaptic transmission in the brain. AMPA receptors (AMPARs) are heterotetrameric assemblies of four highly homologous subunits, GluA1–4, that are highly enriched at synapses. The regulation of AMPAR density at synapses has emerged as a key regulator of synaptic plasticity, which is thought to be one of the key cellular components underlying learning and memory (1, 2). In general, an increase in the number of synaptic AMPARs leads to long-term potentiation (LTP), whereas the removal of surface AMPARs by endocytosis results in long-term depression (LTD) (1, 3). The trafficking of AMPARs into and out of synapses is highly dynamic and is regulated by various AMPAR-interacting proteins that bind to the cytoplasmic tail of the receptors (1).
Protein interacting with C-kinase 1 (PICK1) interacts directly with the C termini of the GluA2 and GluA3 subunits of AMPARs through its postsynaptic density-95/discs-large/zona occludens-1 (PDZ) domain (4, 5). PICK1 has been shown to regulate the surface expression, trafficking, and synaptic targeting of AMPARs (6), and genetic deletion of PICK1 has revealed its important role in several forms of synaptic plasticity, such as hippocampal and cerebellar LTD, hippocampal LTP, Ca2+-permeable AMPAR plasticity, mGluR LTD, and homeostatic plasticity (7–14). PICK1 also contains a central box-dependent myc-interacting protein-1 (Bin)/amphiphysin/reduced viability to nutrient starvation-homology (Rvs) (BAR) domain, which dimerizes to form a banana-shaped crescent and binds to phospholipids (15). Many BAR-domain proteins possess the ability to sense and/or generate curvature of the plasma membrane and are believed to play key roles in endocytosis and vesicle trafficking (16). Expression of a PICK1 BAR-domain mutant that is deficient in lipid binding impairs both hippocampal (15) and cerebellar LTD (8), suggesting a role for the PICK1 BAR domain in mediating the internalization, recycling, and/or intracellular retention of GluA2-containing AMPARs (1, 6). However, the molecular mechanisms by which PICK1 mediates the trafficking of AMPARs are complex and remain unclear.
Protein kinase C and casein kinase II substrate in neurons (PACSINs), also known as syndapins, belong to the feline sarcoma-Cdc42 interacting protein 4 (Fes-CIP4) homology BAR (F-BAR) subfamily within the BAR-domain superfamily of proteins, which sense and/or generate lipid tubules with a larger and shallower curvature than other BAR-domain proteins (17, 18). PACSIN1 is a neuronal-specific protein that regulates activity-dependent retrieval of synaptic vesicles in the presynaptic terminals (19–22) as well as the endocytosis of the NR3A subunit of NMDA receptors on the postsynaptic membrane (23). More importantly, PACSIN1 has been implicated in various neurodegenerative disorders, including Huntington and Alzheimer’s diseases (24, 25). PACSIN2 is ubiquitously expressed, whereas the expression of PACSIN3 is restricted to the muscle, heart, and lung (26). All three members of the PACSIN family of proteins bind to endocytic machinery such as the large GTPase dynamin and the actin-modifier neural Wiskott-Aldrich syndrome protein (N-WASP) through their conserved src-homology 3 (SH3) domains, potentially linking membrane trafficking and actin cytoskeletal rearrangement (27).
In this study, we found that PICK1 interacts with PACSINs and forms a complex with AMPARs. This interaction is regulated by PACSIN phosphorylation and is required for NMDA-induced AMPAR endocytosis and cerebellar LTD. Overall, our data provide experimental evidence supporting the functional duality of presynaptic trafficking machinery in regulating postsynaptic AMPAR trafficking and synaptic plasticity.
Results
PACSIN Interacts with PICK1 and Forms a Complex with AMPARs.
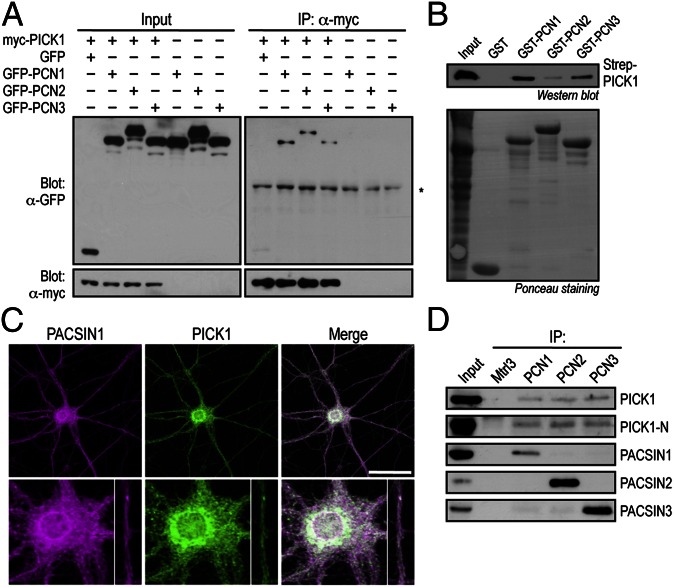
It is well established that PICK1 plays an important role in multiple forms of synaptic plasticity by modulating the subunit composition, trafficking, and surface expression of AMPARs (1). However, little is known about the detailed molecular mechanisms by which PICK1 regulates AMPAR trafficking. To investigate this we performed a yeast two-hybrid screen and discovered a unique interaction between PICK1 and PACSIN3. To validate this interaction, we first transfected HEK 293T cells with full-length constructs encoding GFP, GFP-PACSIN1−3, and myc-PICK1, either individually or in combination. GFP-PACSIN1−3, but not GFP, coimmunoprecipitated with myc-PICK1 when coexpressed in these cells and demonstrated that these coimmunoprecipitations were abolished in the absence of myc-PICK1, confirming the specificity of the interaction between PICK1 and all three PACSIN proteins in vitro (Fig. 1A). This interaction was further confirmed by the ability of recombinant GST-PACSIN1−3, but not GST alone, to pull down PICK1 when incubated with total lysates from HEK 293T cells that overexpressed Strep-tagged PICK1 (Fig. 1B). Immunofluorescence labeling of cultured rat hippocampal neurons with specific antibodies against PICK1 and PACSIN1 revealed extensive colocalization in the perinuclear region within the soma and, to a lesser extent, in the dendritic shaft, indicating that endogenous PICK1 and PACSIN1 share a similar subcellular distribution in neurons (Fig. 1C). Finally, to determine if the interaction between PICK1 and PACSINs occurs in vivo, we performed immunoprecipitation assays with total mouse brain lysates using specific antibodies against PACSIN1, 2, and 3. Western-blotting analysis revealed that PICK1 coimmunoprecipitated with PACSIN1−3, but not with anti-matrilin 3 antibody, which was used as a negative control (Fig. 1D).
Fig. 1.
PICK1 interacts with PACSIN in vitro and in vivo. (A) HEK 293T cells were transfected with GFP or GFP-PACSIN1−3 with or without myc-PICK1, lysed, and immunoprecipitated with anti-myc antibody. Bound proteins were subjected to Western blot analyses with anti-GFP and anti-myc antibodies. Asterisk denotes IgG-heavy chain. (B) Total cell lysate containing recombinant Strep-PICK1 was incubated with either GST or GST-PACSIN1−3 bound on glutathione-Sepharose beads. The binding of Strep-PICK1 was visualized by Western blotting with HRP-conjugated Strep-tactin (Upper). Equal amounts of bait proteins were used as shown by the Ponceau staining of the membrane (Lower). (C) Endogenous PICK1 and PACSIN1 colocalize in the perinuclear structure within the soma and in dendritic shafts of a cultured hippocampal neuron as shown by immunostaining with anti-PICK1 and anti-PACSIN1 antibodies. Higher-magnification images are shown in the bottom panels. (Scale bar, 50 μm.) (D) Total brain lysates were subjected to immunoprecipitation assays with anti-PACSIN1−3 antibodies cross-linked on protein-A agarose beads. Bound PICK1 was visualized by Western blot analyses with two different anti-PICK1 antibodies. Matrilin3 (Mtrl3) coimmunoprecipitation was included as a negative control.
To determine whether PACSIN associates with AMPARs, we transfected HEK 293T cells with constructs encoding HA-GluA2, myc-PICK1 and GFP-PACSIN1. Myc-PICK1 coimmunoprecipitated with HA-GluA2, but GFP-PACSIN1 only coimmunoprecipitated with HA-GluA2 when myc-PICK1 was present in the cells (Fig. 2A). These data suggest that PACSIN1 forms a complex with GluA2 in vitro via PICK1 binding. Immunoprecipitation from total mouse brain extracts using specific antibodies against PACSIN1, 2, and 3 revealed that GluA2/3 subunits of AMPARs are associated with all three PACSIN proteins in vivo (Fig. 2B). Together, these results suggest that PACSINs may play a role in the regulation of AMPAR trafficking in neurons.
Fig. 2.
PACSIN forms a complex with GluA2 in vitro and in vivo. (A) HEK 293T cells were transfected with HA-GluA2, myc-PICK1, and GFP-PACSIN1, lysed, and immunoprecipitated with anti-HA antibody. Bound proteins were blotted with antibodies against HA, GFP, and myc. Asterisk denotes IgG-heavy chain. (B) Total brain lysates were immunoprecipitated with anti-PACSIN1−3 antibodies. Bound GluA2/3 was visualized by Western blot analyses with antibodies against the GluA2/3 and GluA3 subunits of AMPARs. The immunoprecipitation with GFP antibody was included as a negative control.
PACSIN1 Loss-of-Function Impairs Activity-Dependent AMPAR Internalization.
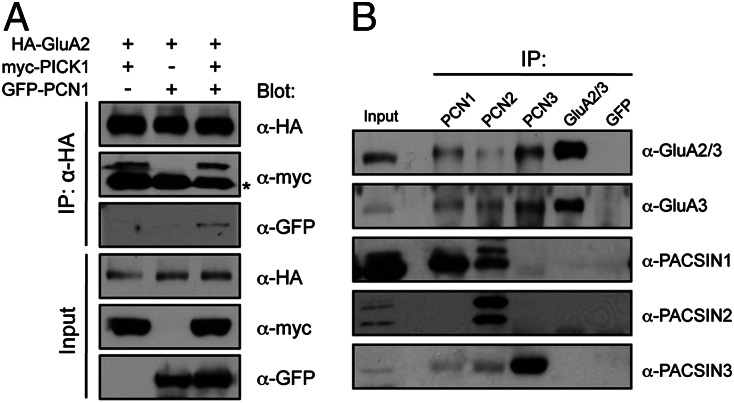
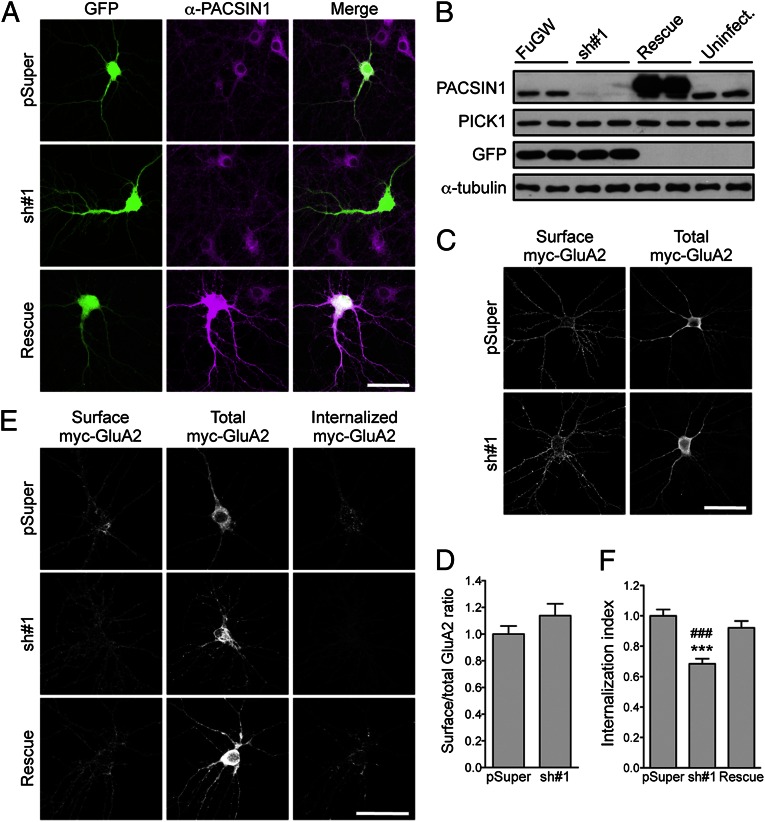
As all three PACSIN proteins bind endocytic proteins and have been implicated in endocytosis (26), we hypothesized that PACSIN might regulate AMPAR endocytosis in neurons. To test this possibility, we first cotransfected full-length PACSIN1−3 constructs with the GFP-GluA2 construct and examined the surface expression of AMPARs in hippocampal neurons. This analysis revealed that overexpression of PACSINs had no effect on the steady-state level of GFP-GluA2 surface expression (surface/total GFP-GluA2 ratio, control, 1 ± 0.02; PACSIN1, 0.97 ± 0.02; PACSIN2, 0.99 ± 0.03; PACSIN3, 0.94 ± 0.02) (Fig. S1). Next, we generated specific shRNAs against PACSIN1, the major PACSIN isoform expressed in hippocampal neurons (Fig. S2A). The PACSIN1 shRNA#1 efficiently reduced endogenous PACSIN1 expression in neurons after 3 d of expression (Fig. 3A). Western-blotting analysis of lysates from hippocampal neurons that were infected with lentiviral particles expressing GFP and PACSIN1 shRNA revealed a near-complete knockdown of PACSIN1 without affecting the level of PICK1 protein (Fig. 3B). Knockdown of PACSIN1 did not significantly alter the dendritic morphology (Fig. S2 B and C) or the steady-state level of myc-GluA2 surface expression in mature hippocampal neurons (surface/total myc-GluA2 ratio, control, 1 ± 0.06; sh#1, 1.14± 0.09) (Fig. 3 C and D). These data indicate that PACSIN1 may not regulate AMPAR trafficking under basal conditions.
Fig. 3.
PACSIN1 knockdown reduces NMDA-induced GluA2 internalization. (A) Hippocampal neurons were cotransfected with pEGFP and a pSuper empty vector, pSuper-PACSIN1-shRNA#1 or pRK5-shRNA#1-HA-PACSIN1 rescue construct, fixed, and stained with anti-PACSIN1 antibody. (Scale bar, 50 μm.) (B) Cultured hippocampal neurons were infected with lentivirus particles expressing GFP (FuGW), GFP and PACSIN1-shRNA#1, or FuW-shRNA#1-myc-PACSIN1 rescue construct at days in vitro (DIV) 7. Neurons were lysed at DIV15, and the efficacy of PACSIN1 knockdown was assessed by Western blot using specific antibodies against PACSIN1. (C) Cultured hippocampal neurons were transfected with either empty pSuper vector (control) or shRNA#1 construct, together with myc-GluA2. Representative images of surface and total myc-GluA2 in a neuron from each group are shown. (Scale bar, 50 μm.) (D) Quantification of surface/total GluA2 ratio normalized to the value of control neurons (n = 7–9 neurons per group). (E) NMDA-induced internalization of myc-GluA2 was measured by fluorescence-based antibody-feeding assay. (Scale bar, 50 μm.) (F) The internalization of myc-GluA2 was measured as the ratio of internalized/total fluorescence (internalization index), normalized to pSuper control. Data represent mean ± SEM (ANOVA, ***P < 0.001 against control cells, ###P < 0.001 against rescued cells; n = 13–21 neurons per group).
To investigate whether PACSIN1 regulates activity-dependent trafficking of AMPARs, we used the fluorescence-based antibody-feeding assay with myc antibodies to measure the degree of intracellular accumulation of endocytosed myc-GluA2 subunits in live transfected hippocampal neurons. In control neurons that were transfected with pSuper empty vector, intracellular accumulation of myc-GluA2 could be observed 15 min after NMDA treatment (50 μM NMDA + 1 μM tetrodotoxin) (Fig. 3E). The staining for internalized receptors was specific, as no staining was visible in nonpermeabilized neurons (Fig. S3). However, the amount of internalized myc-GluA2 was significantly reduced in PACSIN1 knockdown neurons compared with control neurons (internalization index, pSuper, 1 ± 0.04; sh#1, 0.68 ± 0.03) (Fig. 3 E and F). The reduction in NMDA-induced myc-GluA2 internalization caused by PACSIN1 shRNA could be fully rescued by the expression of an shRNA-resistant PACSIN1 rescue construct in the hippocampal neurons (0.92 ± 0.05) (Fig. 3 E and F). These results indicate that PACSIN1 regulates the activity-dependent trafficking of AMPARs but not their trafficking under basal conditions, demonstrating a unique role for PACSIN1 in facilitating GluA2 endocytosis.
PICK1–PACSIN1 Interaction Is Required for NMDA-Induced AMPAR Endocytosis.
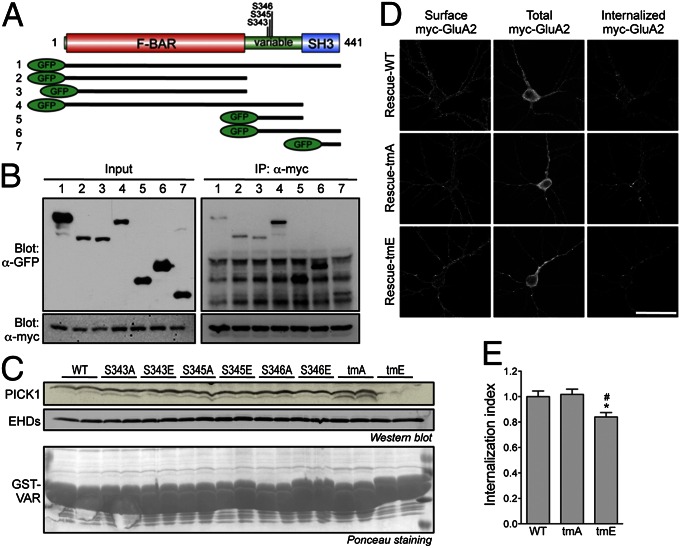
Having established a role of PACSIN1 in regulating AMPAR trafficking, we then asked whether its interaction with PICK1 was required to drive AMPAR endocytosis. To define the minimal region of PACSIN1 that interacts with PICK1, we generated a series of GFP-PACSIN1 truncation constructs and cotransfected them with myc-PICK1 into HEK 293T cells. As expected, GFP-PACSIN1 full-length and GFP-PACSIN1 F-BAR domain coimmunoprecipitated with myc-PICK1, suggesting that PICK1 can interact with PACSIN1 through heterodimerization of their BAR and F-BAR domains, respectively (Fig. 4 A and B, lanes 1–3). The interaction between PACSIN1 and PICK1 was enhanced by the SH3-domain deletion (Fig. 4B, lane 4), consistent with two earlier studies describing an intramolecular interaction between the F-BAR and the SH3 domains that inhibits PACSIN1 function (18, 28). Surprisingly, we also found that the GFP-PACSIN1-ΔF-BAR domain [variable region (VAR) + SH3] was able to bind myc-PICK1 robustly (Fig. 4B, lane 6). Furthermore, the GFP-PACSIN1–variable region alone, but not the SH3 domain, was sufficient to bind PICK1 (Fig. 4B, lanes 5 and 7), establishing the PACSIN1-variable region as the minimal sequence required for PICK1 binding in addition to the conventional BAR-domain interactions. Uniquely, the interaction between PICK1 and PACSIN1 was independent of the asparagine-proline-phenylalanine (NPF) motifs within the variable region, which are known to mediate the interaction between PACSIN and Eps15-homology-domain (EHD) proteins (Fig. S4) (29).
Fig. 4.
PICK1 binding to the PACSIN1-variable region is required for activity-dependent GluA2 internalization. (A) A schematic diagram of the domain structure of PACSIN1 and various GFP-tagged PACSIN1 truncations used in the study. (B) HEK 293T cells were cotransfected with myc-PICK1 and various GFP-PACSIN1 constructs as shown in panel 4A, lysed, and immunoprecipitated with anti-myc antibody. Bound proteins were subjected to Western blot analyses with anti-GFP and anti-myc antibodies. (C) GST-PACSIN1-VAR mutants were coupled to glutathione-Sepharose beads and incubated with total rat brain lysates. Bound proteins were subjected to Western blot analyses with anti-PICK1 and anti-EHD antibodies (Middle). Equal amounts of bait proteins were used as shown by the Ponceau staining of the membrane (Bottom). Representative blots from two independent experiments are shown. (D) Cultured hippocampal neurons were transfected with PACSIN1 rescue constructs, either WT, tmA (S343A, S345A, and S346A), or tmE (S343E, S345E, and S346E), together with myc-GluA2. NMDA-induced internalization of myc-GluA2 was measured by fluorescence-based antibody-feeding assay. (Scale bar, 50 μm.) (E) The internalization of myc-GluA2 was measured as the ratio of internalized/total fluorescence (internalization index), normalized to WT neurons. Data represent mean ± SEM (ANOVA, *P < 0.05 against WT cells, #P < 0.05 against tmA cells; n = 15–17 neurons per group).
PACSIN1 is a phosphoprotein with 15 phosphorylation sites identified through proteomics studies or candidate approaches (30–35). Interestingly, seven of these phosphorylation sites are clustered within the variable region of PACSIN1. To directly test if PICK1 binding is modulated by PACSIN1 phosphorylation, we generated a series of mutations on Ser-343, Ser-345, and Ser-346, either individually or in combination, into Ala or Glu to prevent or mimic phosphorylation on these sites. We chose these Ser residues because they have been identified as in vivo phosphorylation sites in the mouse brain (32, 34, 35). The PACSIN1-variable region, either wild type (WT) or phosphomutants, was expressed as a GST-fusion protein, immobilized on glutathione-Sepharose and incubated with total brain extract. Western-blotting analysis with specific antibodies against PICK1 revealed that the binding of PICK1 to individual nonphosphorylatable (S to A) and pseudophosphorylation (S to E) mutants of PACSIN1 had no effect on PICK1 binding (Fig. 4C). In contrast, the binding of PICK1 to a PACSIN1 triple phosphomimetic mutant (tmE) was markedly reduced, whereas PICK1 binding to the triple phosphomutant (tmA) remained intact (Fig. 4C). The interaction with EHD protein, which binds to the PACSIN1-variable region, was minimally affected, confirming the specificity of the pull-down assay (Fig. 4C). This suggests that multiple phosphorylations of PACSIN1 on Ser-343, Ser-345, and Ser-346 may inhibit its interaction with PICK1, but not with EHD proteins.
To determine the functional significance of the interaction between PACSIN1 and PICK1 in regulating AMPAR trafficking, we performed the antibody-feeding GluA2 internalization assay by applying a molecular replacement strategy. We first generated bicistronic constructs encoding shRNA#1 and HA-PACSIN1 shRNA-resistant cDNAs (either WT, tmA, or tmE) driven by H1 RNA polymerase III and CMV promoters, respectively. Transfection of these constructs into primary hippocampal neurons allows an efficient knockdown of endogenous PACSIN1 proteins and simultaneous overexpression of exogenous PACSIN1 protein. The NMDA-induced internalization of myc-GluA2 in neurons transfected with HA-PACSIN1 tmA was indistinguishable from that in neurons overexpressing HA-PACSIN1 WT (internalization index, WT, 1 ± 0.04; tmA, 1.02 ± 0.04) (Fig. 4 D and E). However, there was a significant decrease in intracellular myc-GluA2 following NMDA stimulation in neurons that were transfected with HA-PACSIN1 tmE, which had reduced binding with PICK1 (0.84 ± 0.03) (Fig. 4 D and E). These data demonstrate that PICK1 is a phosphorylation-regulated PACSIN1 partner and that their interaction is required for efficient trafficking of AMPARs following NMDA receptor activation in hippocampal neurons.
PICK1–PACSIN2 Interaction Is Required for Cerebellar LTD.
One of the phenotypes of PICK1 knockout mice is the complete absence of cerebellar LTD (8, 14). Cerebellar LTD is expressed in Purkinje cells upon coactivation of parallel fibers and climbing fibers of the cerebellar granule cells and the inferior olive, respectively. In situ hybridization data from the Allen Brain Atlas show that PACSIN2 is the major PACSIN protein expressed in the Purkinje cells of the cerebellum (36). This was further confirmed by our immunohistochemical analysis of the mouse brain stained with specific antibodies against PACSIN2 (Fig. 5A and Fig. S5). Our data demonstrated specific PACSIN2 staining in the soma and dendrites of Purkinje cells in the cerebellum, whereas its expression in the hippocampus was restricted to the CA3 and dentate gyrus (Fig. S5). Given the functional interaction between PACSIN1 and PICK1 in regulating AMPAR endocytosis in cultured hippocampal neurons, we hypothesized that PACSIN2 might serve the same role in regulating AMPAR trafficking and synaptic plasticity. No obvious abnormalities in cerebellar architecture were observed in hematoxylin- and eosin-stained brain sections of PACSIN2 knockout mice (Fig. 5A). We then measured LTD evoked by glutamate/depolarization pairing in dissociated cerebellar cultures derived from PACSIN2 knockout mice (8). We found that the delivery of a depolarization stimulus paired with a pulse of glutamate failed to induce LTD in PACSIN2 knockout Purkinje cells (107 ± 7.5% of baseline at t = 17.5 min and 117 ± 8.6% of baseline at t = 40 min, n = 7) (Fig. 5B), resembling the phenotype seen in PICK1 knockout neurons (8). Gene gun-mediated transfection with full-length GFP-PACSIN2 WT construct produced a complete rescue of LTD (54 ± 7.4% of baseline at t = 40 min, n = 8). Interestingly, the GFP-PACSIN1 construct failed to fully rescue LTD in PACSIN2 knockout neurons (84 ± 6.5% of baseline at t = 40 min, n = 13). These results demonstrate an essential role for PACSIN2 in the expression of postsynaptic LTD in the cerebellum.
Fig. 5.
PACSIN2 is required for the expression of cerebellar LTD. (A) Hematoxylin and eosin (HE)-stained sections of cerebellum from PACSIN2 WT and knockout mice, showing no gross abnormalities in the cytoarchitecture. The genotype in each case was confirmed by immunostaining with anti-PACSIN2 antibody. (Scale bar, 150 μm.) (B) Cerebellar LTD is abolished in Purkinje cells derived from PACSIN2 KO mice and is restored by acute transfection with the PACSIN2 WT or phosphodeficient mutant, but not by the PACSIN2 phosphomimetic mutant or PACSIN1 WT. Following the acquisition of baseline responses to iontophoretic pulses of glutamate, delivered at 0.05 Hz, LTD was induced in cerebellar cultures by a depolarization stimulus paired with an iontophoretic glutamate pulse at t = 0 min (six repetitions, indicated by horizontal bar). Error bars indicate the SEM. (Scale bars, 1 s, 30 pA.) (C) The amino acid sequence alignment of the PACSIN1- and PACSIN2-variable regions, highlighting the conservation of the phosphorylation sites between the two proteins. (D) HEK 293T cells were transfected with myc-PICK1 and GFP-PACSIN2, either WT or phosphodeficient and phosphomimetic mutants, lysed, and immunoprecipitated with anti-myc antibody. Bound proteins were blotted with anti-GFP and anti-myc antibodies. (E) The amount of myc-PICK1 bound to GFP-PACSIN2 mutants was quantified by densitometry analysis of Western blots. Data represent mean ± SEM (ANOVA, *P < 0.05 and **P < 0.01 against PACSIN2-WT; n = 4).
To investigate whether the interaction between PACSIN2 and PICK1 is required for the expression of cerebellar LTD, we attempted to identify a PACSIN2 mutant that decreases binding with PICK1. A protein sequence alignment of the PACSIN1- and PACSIN2-variable regions revealed that all three PACSIN1 phosphorylation sites that regulate PICK1 binding are completely conserved in PACSIN2 (Ser-372, Ser-386, and Ser-387) (Fig. 5C). We therefore generated a series of PACSIN2 nonphosphorylatable and phosphomimetic mutants on these conserved Ser residues, as well as Ser-375, which has been shown to be phosphorylated in HeLa cells (37). The binding affinity of these mutants to PICK1 was tested by coimmunoprecipitation assays in HEK 293T cell lysates expressing myc-PICK1 with either WT or mutant GFP-PACSIN2. Most of these PACSIN2 mutants had no effect on binding to PICK1, except for the S372/375A and S372/375/386/387E [quadruple glutamic acid mutant (QmE)] mutants, which had significantly increased and decreased binding to PICK1, respectively (Fig. 5 D and E). These data suggest that, like the PACSIN1–PICK1 interaction, the binding of PICK1 may also be regulated by PACSIN2 phosphorylation status on the conserved Ser residues within the variable region. Next, we transfected PACSIN2 knockout Purkinje cells with a GFP-PACSIN2 QmE cDNA construct and found that LTD evoked by glutamate/depolarization pairing was completely blocked (93 ± 7.9% of baseline at t = 40 min, n = 9) (Fig. 5B). In contrast, LTD was rescued in GFP-PACSIN2 quadruple alanine mutant (QmA)-transfected cultures (58 ± 6.9% of baseline at t = 40 min, n = 8) (Fig. 5B). Neither PACSIN2 deletion nor any of the four rescue constructs used herein produced attenuation of mGluR1 agonist-evoked Ca2+ mobilization or depolarization-evoked Ca2+ influx as measured with bis-fura-2 microfluorimetry. These results suggest that PICK1 is a phosphorylation-regulated partner for PACSIN2 and that their interaction is required for cerebellar LTD.
Discussion
PACSINs have previously been implicated in endocytosis, in particular the brain-specific PACSIN1, which interacts with dynamin to regulate activity-dependent bulk endocytosis in presynaptic terminals (21, 22, 38–40). Although it is predominantly expressed in presynaptic nerve terminals, our data indicate that PACSIN1 can also be found in the soma and dendrites, where it colocalizes with PICK1 in mature hippocampal neurons. This is consistent with a previous study reporting a postsynaptic role of PACSIN1 in mediating synaptic removal of NR3A-containing NMDA receptors in developing hippocampal neurons (23). Our biochemical data show that PACSIN1 also associates with AMPARs, most likely through its interaction with PICK1. Indeed, specific knockdown of PACSIN1 protein in neurons significantly reduced the intracellular accumulation of GluA2 following NMDA receptor activation. However, overexpression or knockdown of PACSIN1 had no effect on the steady-state surface expression of AMPARs, suggesting that PACSIN1 regulates the activity-dependent internalization of AMPARs in hippocampal neurons. We propose that PACSIN1 may serve as a scaffolding protein that links AMPARs to the endocytic machinery, such as dynamin, and facilitate the removal of AMPARs from the plasma membrane. In addition, PACSIN1 may be involved in actin cytoskeleton remodeling and/or in generating plasma-membrane curvature during endocytosis through its interaction with N-WASP and its lipid-binding F-BAR domain, respectively.
How might PICK1 and PACSIN1 work cooperatively to drive AMPAR internalization in neurons? Both PICK1 and PACSIN1 share some structural features in that they are subjected to intramolecular interactions, which allow them to exist in “open” or “closed” conformations, and they can interact with actin cytoskeleton regulators, the Arp2/3 complex, and N-WASP, respectively (18, 28, 41, 42). Our domain-mapping analysis showed that the binding to PICK1 is mediated by the variable region in PACSIN1, leaving the SH3 domain available to bind to endocytic proteins and actin-reorganizing molecules. It is plausible that when GluA2 binds PICK1 during the early stage of endocytosis, PICK1 adopts an open conformation and inhibits the activity of the Arp2/3 complex, leading to a reduction in plasma membrane tension and an easing of membrane bending (41). This subsequently leads to the recruitment of PACSIN1 in an open conformational state when bound to N-WASP and/or dynamin. At the endocytic sites, PACSIN1 may positively regulate the internalization of AMPARs by one or all of the following mechanisms: generating plasma membrane curvature during the invagination process through the F-BAR domain, facilitating vesicle fission via its interaction with dynamin, or providing mechanical forces to propel vesicles away from the plasma membrane by enhancing N-WASP-dependent actin polymerization. The fact that PICK1 binding to PACSIN1 is modulated by the phosphorylation status within the variable region indicates another layer of regulation to the intricate process of AMPAR endocytosis. Further studies will be necessary to address how phosphorylation of these serine residues is regulated by synaptic activity.
Recently, PICK1 has been shown to associate with recycling endosomes and inhibit the recycling of its binding partners from the Rab11-positive endosomes toward the plasma membrane (43). This model helps to explain the accelerated rate of GluA2 recycling observed in PICK1 knockout or knockdown neurons (11, 44) and the absence of LTD in PICK1 knockout mice (8, 10, 13, 14). In our study, the intracellular accumulation of GluA2 measured 15 min poststimulation reflects the balance between endocytosis of surface receptors and the recycling of internalized receptors back to the plasma membrane. Hence, we cannot rule out the possibility that the decrease in internalized GluA2 in PACSIN1 knockdown neurons is due to an impairment in AMPAR recycling. One of the major interacting partners of PACSIN is EHD protein, which has been shown to play a crucial role in endosomal recycling (29). Interestingly, EHD-1 has previously been implicated in the recycling of AMPARs (45). This suggests that PACSIN may also be involved in the intracellular endosomal trafficking of AMPARs via its interaction with EHD-1. Regardless of whether PACSIN1 regulates the trafficking of AMPARs at the steps of receptor internalization and/or recycling, it requires efficient binding to PICK1.
Internalization of AMPARs is believed to be a postsynaptic mechanism for the expression of LTD (1, 3, 46). Our data show that the loss of PACSIN2 function in the Purkinje cells completely eliminates cerebellar LTD in the Purkinje cells. However, given that PACSIN1 and PACSIN2 both bind PICK1, dynamin, EHD-1, and N-WASP, it was surprising that PACSIN1 only partially rescued the LTD phenotype in PACSIN2 knockout neurons, suggesting a unique function of PACSIN2 in mediating AMPAR trafficking and synaptic plasticity in Purkinje cells of the cerebellum. Although there is a high degree of conservation of the F-BAR and the SH3 domains between PACSIN1 and 2, recent structural studies have provided evidence that the level of membrane-sculpting activity and the extent of intramolecular inhibition vary between these two proteins (47, 48). Despite this structural difference, the binding of PICK1 is still regulated by the conserved phosphorylation sites within the PACSIN2-variable region. More importantly, the interaction between PACSIN2 and PICK1 is absolutely required for cerebellar LTD.
In conclusion, we have identified a unique interaction between PICK1 and the endocytic protein PACSIN and have shown that their phosphorylation-regulated interaction is required for the activity-dependent trafficking of GluA2-containing AMPARs and the expression of cerebellar LTD. Our data also provide further evidence supporting the functional duality of presynaptic trafficking machinery, such as the lipid phosphatase, synaptojanin1 (49), and the exocytic protein, complexin1 (50), in regulating the trafficking of AMPARs on the postsynaptic membrane and synaptic plasticity.
Materials and Methods
Materials.
The PACSIN1 shRNA targeting sequences were as described previously (22). The full details of DNA constructs and antibodies are described in SI Materials and Methods.
GST Pull-Down and Immunoprecipitation Assays.
GST-fusion proteins were expressed in Escherichia coli as described previously (21). GST pull-down and immunoprecipitation assays were performed on total brain extracts or transfected HEK 293T cells as described in SI Materials and Methods.
Hippocampal Neurons and Internalization Assays.
Cultured neurons were prepared from E18 rat pups as described previously (44). For the NMDA-induced internalization assays, neurons were transfected with lipofectamine and subjected to antibody-feeding assays as described in SI Materials and Methods. All experimental procedures with mice and rats were approved by the Animal Care and Use Committee of the Johns Hopkins University School of Medicine and the University of Queensland.
Electrophysiology.
Cerebellar LTD recordings from the cultured Purkinje cells were performed as described in SI Methods.
Supplementary Material
Acknowledgments
We thank Richard Johnson, Yi Lin Yu, Devorah VanNess, and Jeffrey Hanks-Thompson for technical assistance and Rowan Tweedale for helpful comments on this manuscript. This work was supported by National Institutes of Health Grants NS36715 (to R.L.H.) and MH51106 (to D.J.L.), the Deutsche Forschungsgemeinschaft (PL233/3) and the Köln Fortune program of the Medical Faculty of the University of Cologne (M.P.), and the John T. Reid Charitable Trusts (V.A.) V.A. was supported by fellowships from the International Human Frontier Science Program (LT00399/2008-L) and the Australian National Health and Medical Research Council (477108). R.L.H. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
Conflict of interest statement: R.L.H. provides antibodies to Millipore Corporation for sale and is entitled to a share of royalties received by Johns Hopkins University on sales of products described in this article. This arrangement is managed under a licensing agreement between Millipore Corporation and Johns Hopkins University, and the terms are managed by Johns Hopkins University in accordance with its conflict-of-interest policies. R.L.H. is a paid consultant to Millipore Corporation.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1312467110/-/DCSupplemental.
References
- 1.Anggono V, Huganir RL. Regulation of AMPA receptor trafficking and synaptic plasticity. Curr Opin Neurobiol. 2012;22(3):461–469. doi: 10.1016/j.conb.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- 3.Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- 4.Dev KK, Nishimune A, Henley JM, Nakanishi S. The protein kinase C alpha binding protein PICK1 interacts with short but not long form alternative splice variants of AMPA receptor subunits. Neuropharmacology. 1999;38(5):635–644. doi: 10.1016/s0028-3908(98)00230-5. [DOI] [PubMed] [Google Scholar]
- 5.Xia J, Zhang X, Staudinger J, Huganir RL. Clustering of AMPA receptors by the synaptic PDZ domain-containing protein PICK1. Neuron. 1999;22(1):179–187. doi: 10.1016/s0896-6273(00)80689-3. [DOI] [PubMed] [Google Scholar]
- 6.Hanley JG. PICK1: A multi-talented modulator of AMPA receptor trafficking. Pharmacol Ther. 2008;118(1):152–160. doi: 10.1016/j.pharmthera.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Gardner SM, et al. Calcium-permeable AMPA receptor plasticity is mediated by subunit-specific interactions with PICK1 and NSF. Neuron. 2005;45(6):903–915. doi: 10.1016/j.neuron.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 8.Steinberg JP, et al. Targeted in vivo mutations of the AMPA receptor subunit GluR2 and its interacting protein PICK1 eliminate cerebellar long-term depression. Neuron. 2006;49(6):845–860. doi: 10.1016/j.neuron.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 9.Clem RL, Anggono V, Huganir RL. PICK1 regulates incorporation of calcium-permeable AMPA receptors during cortical synaptic strengthening. J Neurosci. 2010;30(18):6360–6366. doi: 10.1523/JNEUROSCI.6276-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Volk L, Kim CH, Takamiya K, Yu Y, Huganir RL. Developmental regulation of protein interacting with C kinase 1 (PICK1) function in hippocampal synaptic plasticity and learning. Proc Natl Acad Sci USA. 2010;107(50):21784–21789. doi: 10.1073/pnas.1016103107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anggono V, Clem RL, Huganir RL. PICK1 loss of function occludes homeostatic synaptic scaling. J Neurosci. 2011;31(6):2188–2196. doi: 10.1523/JNEUROSCI.5633-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jo J, et al. Metabotropic glutamate receptor-mediated LTD involves two interacting Ca(2+) sensors, NCS-1 and PICK1. Neuron. 2008;60(6):1095–1111. doi: 10.1016/j.neuron.2008.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terashima A, et al. An essential role for PICK1 in NMDA receptor-dependent bidirectional synaptic plasticity. Neuron. 2008;57(6):872–882. doi: 10.1016/j.neuron.2008.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia J, Chung HJ, Wihler C, Huganir RL, Linden DJ. Cerebellar long-term depression requires PKC-regulated interactions between GluR2/3 and PDZ domain-containing proteins. Neuron. 2000;28(2):499–510. doi: 10.1016/s0896-6273(00)00128-8. [DOI] [PubMed] [Google Scholar]
- 15.Jin W, et al. Lipid binding regulates synaptic targeting of PICK1, AMPA receptor trafficking, and synaptic plasticity. J Neurosci. 2006;26(9):2380–2390. doi: 10.1523/JNEUROSCI.3503-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallop JL, McMahon HT. BAR domains and membrane curvature: Bringing your curves to the BAR. Biochem Soc Symp. 2005;72:223–231. doi: 10.1042/bss0720223. [DOI] [PubMed] [Google Scholar]
- 17.Itoh T, et al. Dynamin and the actin cytoskeleton cooperatively regulate plasma membrane invagination by BAR and F-BAR proteins. Dev Cell. 2005;9(6):791–804. doi: 10.1016/j.devcel.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Wang Q, et al. Molecular mechanism of membrane constriction and tubulation mediated by the F-BAR protein Pacsin/Syndapin. Proc Natl Acad Sci USA. 2009;106(31):12,700–12,705. doi: 10.1073/pnas.0902974106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qualmann B, Roos J, DiGregorio PJ, Kelly RB. Syndapin I, a synaptic dynamin-binding protein that associates with the neural Wiskott-Aldrich syndrome protein. Mol Biol Cell. 1999;10(2):501–513. doi: 10.1091/mbc.10.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plomann M, et al. PACSIN, a brain protein that is upregulated upon differentiation into neuronal cells. Eur J Biochem. 1998;256(1):201–211. doi: 10.1046/j.1432-1327.1998.2560201.x. [DOI] [PubMed] [Google Scholar]
- 21.Anggono V, et al. Syndapin I is the phosphorylation-regulated dynamin I partner in synaptic vesicle endocytosis. Nat Neurosci. 2006;9(6):752–760. doi: 10.1038/nn1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clayton EL, et al. The phospho-dependent dynamin-syndapin interaction triggers activity-dependent bulk endocytosis of synaptic vesicles. J Neurosci. 2009;29(24):7706–7717. doi: 10.1523/JNEUROSCI.1976-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pérez-Otaño I, et al. Endocytosis and synaptic removal of NR3A-containing NMDA receptors by PACSIN1/syndapin1. Nat Neurosci. 2006;9(5):611–621. doi: 10.1038/nn1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Modregger J, DiProspero NA, Charles V, Tagle DA, Plomann M. PACSIN 1 interacts with huntingtin and is absent from synaptic varicosities in presymptomatic Huntington’s disease brains. Hum Mol Genet. 2002;11(21):2547–2558. doi: 10.1093/hmg/11.21.2547. [DOI] [PubMed] [Google Scholar]
- 25.Takano M, et al. Proteomic analysis of the hippocampus in Alzheimer’s disease model mice by using two-dimensional fluorescence difference in gel electrophoresis. Neurosci Lett. 2013;534:85–89. doi: 10.1016/j.neulet.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 26.Modregger J, Ritter B, Witter B, Paulsson M, Plomann M. All three PACSIN isoforms bind to endocytic proteins and inhibit endocytosis. J Cell Sci. 2000;113(24):4511–4521. doi: 10.1242/jcs.113.24.4511. [DOI] [PubMed] [Google Scholar]
- 27.Quan A, Robinson PJ. Syndapin-a membrane remodelling and endocytic F-BAR protein. FEBS J. 2013 doi: 10.1111/febs.12343. [DOI] [PubMed] [Google Scholar]
- 28.Rao Y, et al. Molecular basis for SH3 domain regulation of F-BAR-mediated membrane deformation. Proc Natl Acad Sci USA. 2010;107(18):8213–8218. doi: 10.1073/pnas.1003478107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braun A, et al. EHD proteins associate with syndapin I and II and such interactions play a crucial role in endosomal recycling. Mol Biol Cell. 2005;16(8):3642–3658. doi: 10.1091/mbc.E05-01-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quan A, et al. Phosphorylation of syndapin I F-BAR domain at two helix-capping motifs regulates membrane tubulation. Proc Natl Acad Sci USA. 2012;109(10):3760–3765. doi: 10.1073/pnas.1108294109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schael S, et al. Casein kinase 2 phosphorylation of protein kinase C and casein kinase 2 substrate in neurons (PACSIN) 1 protein regulates neuronal spine formation. J Biol Chem. 2013;288(13):9303–9312. doi: 10.1074/jbc.M113.461293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huttlin EL, et al. A tissue-specific atlas of mouse protein phosphorylation and expression. Cell. 2010;143(7):1174–1189. doi: 10.1016/j.cell.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tweedie-Cullen RY, Reck JM, Mansuy IM. Comprehensive mapping of post-translational modifications on synaptic, nuclear, and histone proteins in the adult mouse brain. J Proteome Res. 2009;8(11):4966–4982. doi: 10.1021/pr9003739. [DOI] [PubMed] [Google Scholar]
- 34.Goswami T, et al. Comparative phosphoproteomic analysis of neonatal and adult murine brain. Proteomics. 2012;12(13):2185–2189. doi: 10.1002/pmic.201200003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strochlic TI, et al. Identification of neuronal substrates implicates Pak5 in synaptic vesicle trafficking. Proc Natl Acad Sci USA. 2012;109(11):4116–4121. doi: 10.1073/pnas.1116560109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lein ES, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445(7124):168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 37.Chen RQ, et al. CDC25B mediates rapamycin-induced oncogenic responses in cancer cells. Cancer Res. 2009;69(6):2663–2668. doi: 10.1158/0008-5472.CAN-08-3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anggono V, Robinson PJ. Syndapin I and endophilin I bind overlapping proline-rich regions of dynamin I: Role in synaptic vesicle endocytosis. J Neurochem. 2007;102(3):931–943. doi: 10.1111/j.1471-4159.2007.04574.x. [DOI] [PubMed] [Google Scholar]
- 39.Andersson F, Jakobsson J, Löw P, Shupliakov O, Brodin L. Perturbation of syndapin/PACSIN impairs synaptic vesicle recycling evoked by intense stimulation. J Neurosci. 2008;28(15):3925–3933. doi: 10.1523/JNEUROSCI.1754-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Koch D, et al. Proper synaptic vesicle formation and neuronal network activity critically rely on syndapin I. EMBO J. 2011;30(24):4955–4969. doi: 10.1038/emboj.2011.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rocca DL, Martin S, Jenkins EL, Hanley JG. Inhibition of Arp2/3-mediated actin polymerization by PICK1 regulates neuronal morphology and AMPA receptor endocytosis. Nat Cell Biol. 2008;10(3):259–271. doi: 10.1038/ncb1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kessels MM, Qualmann B. Syndapins integrate N-WASP in receptor-mediated endocytosis. EMBO J. 2002;21(22):6083–6094. doi: 10.1093/emboj/cdf604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Madsen KL, Thorsen TS, Rahbek-Clemmensen T, Eriksen J, Gether U. Protein interacting with C kinase 1 (PICK1) reduces reinsertion rates of interaction partners sorted to Rab11-dependent slow recycling pathway. J Biol Chem. 2012;287(15):12293–12308. doi: 10.1074/jbc.M111.294702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin DT, Huganir RL. PICK1 and phosphorylation of the glutamate receptor 2 (GluR2) AMPA receptor subunit regulates GluR2 recycling after NMDA receptor-induced internalization. J Neurosci. 2007;27(50):13903–13908. doi: 10.1523/JNEUROSCI.1750-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park M, Penick EC, Edwards JG, Kauer JA, Ehlers MD. Recycling endosomes supply AMPA receptors for LTP. Science. 2004;305(5692):1972–1975. doi: 10.1126/science.1102026. [DOI] [PubMed] [Google Scholar]
- 46.Wang YT, Linden DJ. Expression of cerebellar long-term depression requires postsynaptic clathrin-mediated endocytosis. Neuron. 2000;25(3):635–647. doi: 10.1016/s0896-6273(00)81066-1. [DOI] [PubMed] [Google Scholar]
- 47.Plomann M, Wittmann JG, Rudolph MG. A hinge in the distal end of the PACSIN 2 F-BAR domain may contribute to membrane-curvature sensing. J Mol Biol. 2010;400(2):129–136. doi: 10.1016/j.jmb.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 48.Goh SL, Wang Q, Byrnes LJ, Sondermann H. Versatile membrane deformation potential of activated pacsin. PLoS ONE. 2012;7(12):e51628. doi: 10.1371/journal.pone.0051628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gong LW, De Camilli P. Regulation of postsynaptic AMPA responses by synaptojanin 1. Proc Natl Acad Sci USA. 2008;105(45):17561–17566. doi: 10.1073/pnas.0809221105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ahmad M, et al. Postsynaptic complexin controls AMPA receptor exocytosis during LTP. Neuron. 2012;73(2):260–267. doi: 10.1016/j.neuron.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.