Abstract
More than 5 million deaths a year are attributable to tobacco smoking, but attempts to help people either quit or reduce their smoking often fail, perhaps in part because the intention to quit activates brain networks related to craving. We recruited participants interested in general stress reduction and randomly assigned them to meditation training or a relaxation training control. Among smokers, 2 wk of meditation training (5 h in total) produced a significant reduction in smoking of 60%; no reduction was found in the relaxation control. Resting-state brain scans showed increased activity for the meditation group in the anterior cingulate and prefrontal cortex, brain areas related to self-control. These results suggest that brief meditation training improves self-control capacity and reduces smoking.
Keywords: addiction, anterior cingulate cortex, brain state, integrative body–mind training, mindfulness
Smoking harms nearly every organ of the body, causing many diseases and compromising smokers’ health (1). Despite the negative consequences, many smokers have difficulty quitting or even reducing tobacco use (2). In addition, many teenagers are added to the smoking roll each year and may be at risk for abuse of other substances (2). Because tobacco use is often thought of as a gateway to other drug use, reducing smoking might reduce the vulnerability of youths to cocaine and other drugs (3). Although public health campaigns may have decreased the number of smokers, current methods for aiding those who persist in smoking have had limited success (4, 5). These failures may be a result of the inability to relieve withdrawal symptoms, stress, and cue-induced cravings, which often leads to drug seeking and taking (6–10). This urgent need calls for a short-term, effective intervention for reducing smoking behavior and cravings (2).
One reason for addiction to tobacco may involve a deficit in self-control. Self-control is important because the level of childhood self-control predicts long-term outcomes, including mental health, substance abuse, financial independence, and criminal behavior (11). Individuals at risk for substance abuse typically have deficits in self-control (12–16). Dysfunction of the prefrontal cortex (PFC), including dorsolateral PFC, anterior cingulate cortex (ACC), and medial orbitofrontal cortex, play a key role in addiction (12, 17, 18). In cigarette smokers, regional cerebral blood flow was reduced in the left dorsal ACC, and this correlated with a decrease in craving after smoking the first cigarette of the day (19). These reports raise the question of whether impaired self-control could be ameliorated and strengthened with intervention, and thus potentially change smoking behavior.
There is emerging evidence that mindfulness meditation has the potential to ameliorate negative outcomes resulting from deficits in self-control (20–25). Although preliminary findings suggest mindfulness training shows some proof of efficacy in substance abuse, these studies are replete with limitations, including a lack of adequate control conditions, failure to randomize participants, and lack of assessment of biological markers of change. Thus, more rigorous and randomized controlled studies are warranted (26–29). In a series of randomized controlled trials, it was found that a form of mindfulness meditation, integrative body–mind training (IBMT; Materials and Methods), reduces stress, increases positive emotion, and improves attention and self-control after a few hours of practice compared with the same amount of relaxation training (RT). Moreover, these positive changes were accompanied by increased brain changes of ACC and parasympathetic activity associated with a brain state of increased self-control (20, 30–33).
Because addictions, including smoking, involve ACC and adjacent PFC function related to self-control (12, 17), we hypothesize that improved self-control through short-term IBMT would reduce craving and smoking. To test this hypothesis, we advertised for volunteers wishing to reduce stress and improve performance. Among those who responded were 27 cigarette smokers and 33 nonsmokers. We then randomly assigned both smokers and nonsmokers to IBMT or RT groups. Both groups received 2 wk of training for a total of 5 h (Materials and Methods).
Results
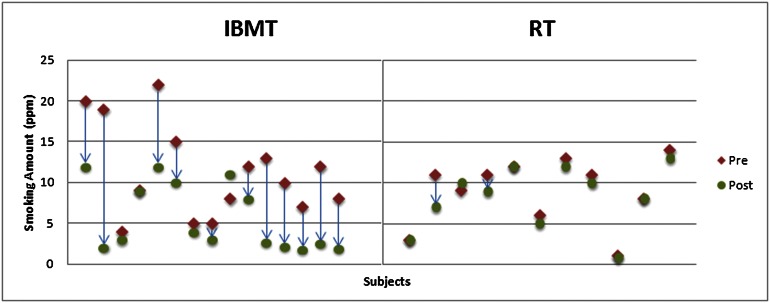
We used objective measures of smoking amount (carbon monoxide level in parts per million), and ANOVAs were conducted with group (IBMT and RT) and training session (before and after) as factors. Before training, no differences in smoking amount were found among smokers in the two groups (P > 0.05). After training, the main effect of the training session was significant [F(1,24) = 16.635; P = 0.000], and the group–session interaction was also significant [F(1,24) = 9.099; P = 0.006]. Subsequent t tests indicated there was significant smoking reduction in the IBMT group (P < 0.01) but no significant reduction in the RT group (P > 0.05). Fig. 1 shows the amount of smoking reduction after 2 wk of IBMT and RT for each smoker.
Fig. 1.
Demonstration of smoking change (parts per million, PPM) after 2 wk of IBMT and RT. After 2 wk of training, there were significant smoking reduction in the IBMT group (but not in the RT group). PPM is an index of the exhaled CO level.
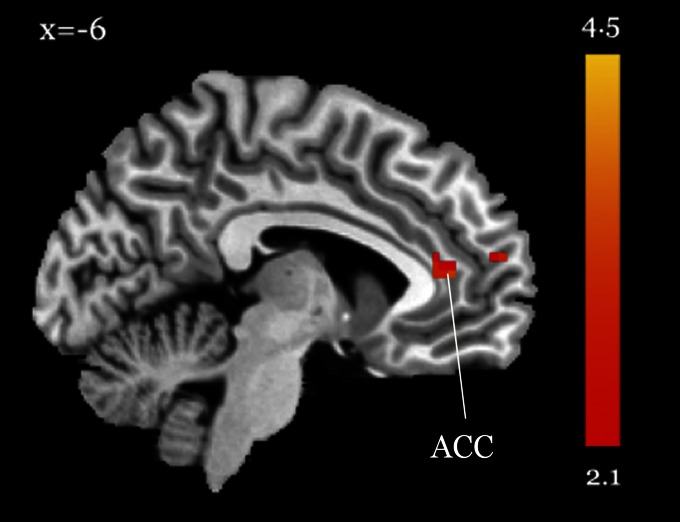
To identify brain mechanisms underlying the observed smoking reductions, we used fractional amplitude of low-frequency fluctuation (fALFF), an index of intrinsic resting-state activity, which has been widely used in studies of addiction, posttraumatic stress disorder, attention deficit hyperactivity disorder, mild cognitive impairment, and early-onset Alzheimer’s disease (34–39). We measured whole-brain fALFF, using resting-state functional MRI, before and after 2 wk of training. Before training, we compared smokers and nonsmokers and found that smokers had reduced activity in ACC, left lateral PFC, and other areas during rest (Pcorrected < 0.05), indicating impaired self-control. This is in accord with previous findings showing that smokers often had lower PFC and ACC activity at rest than nonsmokers (12, 17). After 2 wk of IBMT, we found significantly increased activity at ACC/medial PFC and inferior frontal gyrus/ventrolateral PFC (Pcorrected < 0.05), but no significant change was detected after the same amount of RT (P > 0.05). In comparison with the RT group, the IBMT group showed significantly decreased activity at posterior cingulate cortex (PCC)/precuneus, cerebellum, and other regions after training (Pcorrected < 0.05); see Table 1 for activity details with Brodmann areas, coordinates, t values, and cluster sizes in two groups before and after training. Fig. 2 illustrates the increased ACC activity after 2 wk of IBMT.
Table 1.
Global fALFF changes in smokers and nonsmokers before and after training
| Area | MNI coordinates | T value* | Cluster size, mm3 |
| Pre | |||
| Smoker > nonsmoker | |||
| ACC/BA 24 | −3 −3 33 | −3.53 | 189 |
| BA 44 | −57 15 15 | −3.09 | 351 |
| Post | |||
| IBMT > RT | |||
| PCC/PC/BA 31 | 24 −75 15 | −3.91 | 540 |
| Cerebellum | 15 −54 −9 | −4.10 | 1,404 |
| IBMT post > pre | |||
| BA 10 | 12 63 15 | 5.79 | 783 |
| ACC/BA32 | −9 39 14 | 3.66 | 675 |
| BA 44 | −54 9 12 | 4.99 | 459 |
| BA 46 | −45 33 18 | 4.28 | 459 |
| RT post > pre | |||
| Nonsignificant |
BA, Brodmann area; MNI, Montreal Neurological Institute; PC, precuneus. Pcorrected < 0.05, corrected for multiple comparisons.
A positive T value indicates increased activity. A negative T value indicates decreased activity.
Fig. 2.
Increased ACC activity after 2 wk of IBMT. After 2 wk of IBMT, we found significantly increased activity at ACC/medial PFC, orbitofrontal cortex, and inferior frontal gyrus/ ventrolateral PFC (displayed at Pcorrected < 0.05).
In the present study, self-report craving did not differ in the two groups before training (P > 0.05). After training, the main effect of the training session had a significant effect on craving reports [F(1,23) = 14.710; P = 0.001], and the group–session interaction was marginally significant [F(1,23) = 3.935; P = 0.059]. Comparing before and after training, t tests showed a significant decrease in craving in the IBMT group (P < 0.01), but not the RT group (P > 0.05).These results demonstrate that short-term IBMT practice can significantly reduce craving.
Discussion
Mindfulness meditation involves a systematic training of attention and self-control with an attitude of acceptance and openness to internal and external experiences (22–25). Given the core clinical symptoms of drug addiction [intoxication (impaired self-awareness), bingeing (loss of control), withdrawal, and craving] (12), mindfulness meditation may be helpful for coping with these addiction symptoms and with the accompanied negative emotion and stress reactivity. Prior studies have shown the preliminary efficacy of mindfulness meditation in treating several forms of addiction including alcohol, cigarettes, cocaine, amphetamines, marijuana, and opiates (26–28); however, as recent reviews have pointed out, the lack of randomization and active control groups indicate that these findings should be interpreted with caution (26, 27).Our current study used a randomized controlled design with an active relaxation control (RT), as did our previous studies (20, 30–32), and we found a significant reduction in smoking and craving after 2 wk of IBMT.
Our previous work has shown that ACC activity and the efficiency (as measured by fractional anisotropy, using diffusion tensor imaging MRI) of white matter connectivity surrounding ACC can be increased by short-term IBMT more than by RT (30–32). In this study, before training, smokers had less activity in the ACC and PFC than nonsmokers. After training, ACC and PFC activity increased and smoking decreased only in the IBMT group, not in the RT group. In one sense, training seems to have moved the smokers to more normal activity, but the exact brain areas reduced in smokers before training are somewhat different from the areas in which training produced improvement. Moreover, we did not note significant correlations between the extent of brain changes in ACC and PFC and the amount of reduced smoking or craving. Nonetheless, we think that increased ACC and PFC activity with training are most likely associated with the observed reduction in smoking.
We also found significantly reduced activity in PCC and cerebellum in the IBMT group in comparison with the RT group. These results are in line with some previous findings. For example, in one study, a stroke that produced a lesion of the PCC resulted in an immediate cessation of smoking (40). More generally, studies of addicted smokers have shown increased activity in PCC and cerebellum in comparison with those of nonsmokers (12, 17, 19). Thus, both increased activity in ACC and PFC and reduced activity in PCC and cerebellum may be important neural correlates of reduced smoking (12, 17, 19).
We also tested the follow-up effects: After ending the 2 wk of IBMT, five smokers responded to our 2 and 4 wk of follow-up, using measures of objective carbon monoxide (CO) level and self-reported Fagerström Test for Nicotine Dependence (FTND). They all maintained reduced smoking.
Because the number was so small, we do not yet know exactly how long the reduction will last; this warrants further investigation. Nevertheless, our preliminary finding suggested that continued practice may not be needed to maintain the smoking reduction for at least few weeks.
A major problem in overcoming tobacco use is craving. Craving is also a significant factor that can lead to relapse during attempts to quit smoking (8, 41) and that is associated with some degree of resisting the urge to smoke: Trying to resist is almost always accompanied by some degree of craving (42). IBMT does not force participants to resist craving or quit smoking; instead, it focuses on improving self-control capacity to handle craving and smoking behavior, indicating a unique way to treat addiction.
Intention is often thought to be important to achieve a goal and change behavior. However, intention to avoid a thought often leads to thinking about the very thought one hopes to suppress (43, 44). Moreover, attempts to suppress thoughts about using substances may actually lead to increases in substance use (45). Unlike most interventions to reduce smoking, in this study we did not recruit participants who had an expressed intention of quitting smoking. To test whether intention related to the reduction of smoking, we measured intention using self-report questionnaires (Materials and Methods) and found that intention did not make a significant difference in smoking reduction (P > 0.05). Furthermore, our results indicate that the participants who reported an intention to quit did not outperform those without an intention, suggesting that changing smoking behavior through IBMT intervention may relate to unconscious processing (46–48).
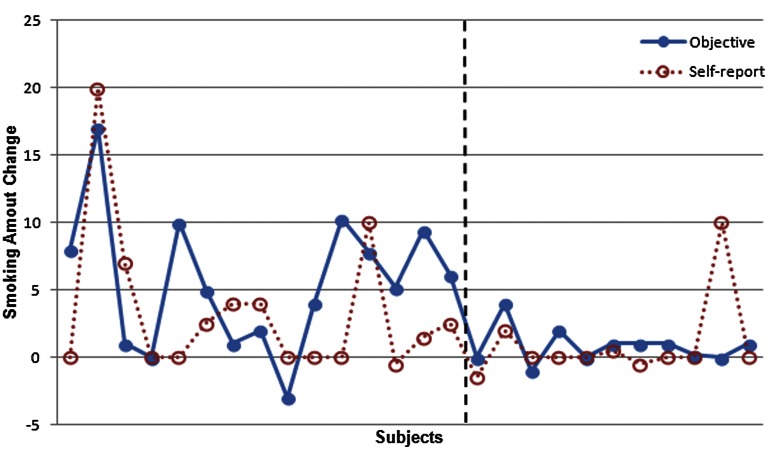
Consistent with the objective CO results, the self-report FTND result also showed a significant smoking reduction for the IBMT group (P < 0.05), but not the RT group. However, the self-report of smoking reduction before compared with after training did not match well with the objective measurement of CO level (Fig. 3). A clue to this mismatch may be provided by the account of one participant in our sample who reduced smoking significantly from 20 to 10 cigarettes per day after 5 h of IBMT during a 2-wk period. He stated, “I was not aware of my smoking reduction while filling out the self-report questionnaires. I usually consumed one pack with 20 cigarettes each day before training. But after I thought carefully and checked my pocket, actually I only needed half pack per day recently. It started around after 1 wk [of] training naturally, but I didn't know why.” This evidence suggests that the smoker was not aware of the dramatic reduction and that the unconscious change of smoking behavior and habit spontaneously happened after short-term IBMT practice (47).
Fig. 3.
Comparison between changes in self-report and objective CO measure of smoking. There is a mismatch between self-report of daily cigarettes using FTND and objective CO measure of smoking amount. (Left) IBMT group. (Right) RT group.
Stress is also important in substance use. Cumulative stress decreases self-control, increases impulsivity, and increases the risk for current cigarette smoking (49). Mindfulness training has shown promise for stress-related maladies and substance use (50), and in particular, IBMT has been shown to reduce stress only after few hours of training (20, 51). It is possible that one mechanism by which IBMT reduces craving and smoking is through stress reduction (22, 52).
Our results to date suggest it may be possible to reduce smoking and craving, even in those who have no intention to quit smoking. This low-cost and short-term intervention may influence a common anatomical pathway to substance abuse and reduce possible risk for drug use in youth.
Materials and Methods
Participants.
Healthy college students were recruited through campus advertisements for learning meditation/relaxation to reduce stress and improve cognitive performance. Participants with the goal of quitting smoking were not included. Among those who responded, we found there were 27 cigarette smokers and 33 nonsmokers (mean ages, 21.46 ± 3.08 y). We randomly assigned both smokers and nonsmokers to either the IBMT group or the RT group. There were 15 smokers (11 men) in the IBMT group and 12 smokers (8 men) in the RT group, and 18 nonsmokers in the IBMT group and 15 nonsmokers in the RT group. The smokers used tobacco without other drugs, with an average of 10 cigarettes per day. Three heavier smokers were placed in the IBMT group by chance, but the IBMT and RT groups showed no significant difference in smoking before training. One smoker in the RT group did not finish the whole study and was excluded, and one smoker in the IBMT group did not have usable craving data and was excluded from the craving analysis. All participants had no prior experience in meditation or RT. The experiment was approved by the local institutional review board at Texas Tech University, and informed consent was obtained from each participant.
Self-Report Measurement.
Among those who responded, we screened and selected smokers and nonsmokers using the FTND, a 6-item validated scale. Participants also rated the severity of their cravings to smoke using a 5-point Likert scale and FTND (53). These self-report measures were taken before and after 2 wk of training. We measured intention using a 10-point Likert scale (sample items included “mark the number that shows how you feel about quitting”; “in the past year, how many times have you made a serious attempt to quit smoking?”; and “are you seriously thinking about quitting smoking in the next 30 days?”; score: from 0 = no thought of quitting to 10 = ready to quit now). These self-report measures have been commonly used in the smoking field (8, 41, 54).
Objective Measurement.
To validate the self-report smoking behavior and craving, we used a CO monitor (Micro+ Smokerlyzer, Bedfont Instruments) to measure the exhaled CO level as an objective indicator of smoker’s addiction to nicotine. This noninvasive measure has been widely used in smoking research (55, 56) and provides CO level in parts per million and percentage carboxyhemoglobin (%COHb) in smokers’ lungs and blood. We used the objective CO level in parts per million, shown on the monitor screen as a converted index of the actual number of cigarettes smoked.
Training/Intervention.
IBMT is a form of mindfulness meditation that involves body relaxation, mental imagery, and mindfulness training accompanied by selected music background. Cooperation between the body and the mind is emphasized in facilitating and achieving a meditative state. The trainees concentrated on achieving a balanced state of body and mind guided by an IBMT coach and a compact disc. The method stresses no effort to control thoughts but, instead, a state of restful alertness that allows a high degree of awareness of body, mind, and environment (20, 30–33).
RT involves the relaxing of different muscle groups over the face, head, shoulders, arms, legs, chest, back, abdomen, and so on, guided by a RT coach and compact disc. With eyes closed and in a sequential pattern, one is forced to concentrate on the sensation of relaxation, such as the feelings of warmth and heaviness. This progressive training helps the participant achieve physical and mental relaxation and calmness (20, 31). We trained all participants (smokers and nonsmokers) together but divided them into small groups (33). The participants received 30-min of IBMT or RT group practice every night for 10 consecutive sessions, for a total of 5 h of training (32).
Imaging Data Acquisition and Analysis.
All data were collected using a 3-Telsa Siemens Skyra scanner at the Texas Tech Neuroimaging Institute. 3D T1-weighted anatomical images were acquired using the MPRAGE sequence (TR = 1,780 ms; TE = 2.36 ms; slice thickness = 1.0 mm). A 6-min resting-state functional scan (T2* weighted images) was obtained for each participant before and after 2 wk of training with gradient echo planar sequence (Repetition time = 2,000 ms; Echo time = 27 ms; flip angle = 80°; field of view = 256 mm × 256 mm; matrix size = 64 × 64; slice thickness = 4 mm; Axial direction, 36 slices). Participants looked at a crosshair shown on a screen and did not think of anything in particular; head movement was minimized, using individually custom-made foam padding (57).
Functional data were processed using the Data Processing Assistant for Resting-State fMRI (www.restfmri.net), which is based on Statistical Parametric Mapping (www.fil.ion.ucl.ac.uk/spm) and Resting-State fMRI Data Analysis Toolkit (58). For each participant, the subsequent standard procedures included slice timing, motion correction, and spatial normalization of images into the Montreal Neurological Institute template with a resampling voxel size of 3 × 3 × 3 mm. Finally, a Gaussian filter of 5 mm full-width at half-maximum was applied to the dataset for spatial smoothing.
An improved approach of the ALFF method, fractional ALFF (fALFF) (34), was used for detecting regional signals change of spontaneous activity by taking the ratio of power spectrum of low-frequency (0.01–0.08 Hz) to that of the entire frequency range. Similar to the procedures of previous literature (34–39), the time series of each voxel was transformed to a frequency domain after the linear trend was removed without band-pass filtering. The square root was then calculated at each frequency of the power spectrum, and finally the sum of amplitude across 0.01–0.08 Hz was divided by that across the entire frequency range (0–0.25 Hz) to obtain fALFF. The fALFF maps of nonsmokers and smokers before training were compared using two sample t tests, and the fALFF maps of smokers in the IBMT and the RT groups after training also were compared using two sample t tests. The fALFF maps of smokers in IBMT and RT groups before and after the training were compared using paired t test. All results were corrected for multiple comparisons (Pcorrected < 0.05), based on Monte Carlo stimulation (34).
Acknowledgments
We thank Elliot Stein for insightful comments. We also thank laboratory members for assistance with data collection and Chao-Gan Yan for resting analysis assistance using the Data Processing Assistant for Resting-State fMRI. This work was supported by Grant R21DA030066, Grant 973 Program 2012CB518200, and the Office of Naval Research.
Footnotes
The authors declare no conflict of interest.
References
- 1. Centers for Disease Control and Prevention (2004). The health consequences of smoking: A report of the surgeon general (Office on Smoking and Health, Atlanta) [PubMed]
- 2. Centers for Disease Control and Prevention (2006) Sustaining state programs for tobacco control: State data highlights (Office on Smoking and Health, Atlanta)
- 3. Levine A, et al. (2011) Molecular mechanism for a gateway drug: Epigenetic changes initiated by nicotine prime gene expression by cocaine. Sci Transl Med 3(107):107ra109. [DOI] [PMC free article] [PubMed]
- 4.Hajek P, Stead LF, West R, Jarvis M, Lancaster T. Relapse prevention interventions for smoking cessation. Cochrane Database Syst Rev. 2009;1(1):CD003999. doi: 10.1002/14651858.CD003999.pub3. [DOI] [PubMed] [Google Scholar]
- 5.Fiore MC, et al. Methods used to quit smoking in the United States. Do cessation programs help? JAMA. 1990;263(20):2760–2765. [PubMed] [Google Scholar]
- 6.Alessi SM, Badger GJ, Higgins ST. An experimental examination of the initial weeks of abstinence in cigarette smokers. Exp Clin Psychopharmacol. 2004;12(4):276–287. doi: 10.1037/1064-1297.12.4.276. [DOI] [PubMed] [Google Scholar]
- 7.Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, et al. Neural substrates of abstinence-induced cigarette cravings in chronic smokers. J Neurosci. 2007;27(51):14035–14040. doi: 10.1523/JNEUROSCI.2966-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lerman C, Patterson F, Berrettini W. Treating tobacco dependence: State of the science and new directions. J Clin Oncol. 2005;23(2):311–323. doi: 10.1200/JCO.2005.04.058. [DOI] [PubMed] [Google Scholar]
- 10.Bough KJ, et al. Biomarkers for smoking cessation. Clin Pharmacol Ther. 2013;93(6):526–538. doi: 10.1038/clpt.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moffitt TE, et al. A gradient of childhood self-control predicts health, wealth, and public safety. Proc Natl Acad Sci USA. 2011;108(7):2693–2698. doi: 10.1073/pnas.1010076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: Neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12(11):652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baler RD, Volkow ND. Drug addiction: The neurobiology of disrupted self-control. Trends Mol Med. 2006;12(12):559–566. doi: 10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Ersche KD, et al. Abnormal brain structure implicated in stimulant drug addiction. Science. 2012;335(6068):601–604. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- 15.Nigg JT, et al. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. J Am Acad Child Adolesc Psychiatry. 2006;45(4):468–475. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- 16.Tarter RE, et al. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. Am J Psychiatry. 2003;160(6):1078–1085. doi: 10.1176/appi.ajp.160.6.1078. [DOI] [PubMed] [Google Scholar]
- 17.Hong LE, et al. Association of nicotine addiction and nicotine’s actions with separate cingulate cortex functional circuits. Arch Gen Psychiatry. 2009;66(4):431–441. doi: 10.1001/archgenpsychiatry.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Posner MI, Rothbart MK, Sheese BE, Tang YY. The anterior cingulate gyrus and the mechanism of self-regulation. Cogn Affect Behav Neurosci. 2007;7(4):391–395. doi: 10.3758/cabn.7.4.391. [DOI] [PubMed] [Google Scholar]
- 19.Zubieta JK, et al. Regional cerebral blood flow responses to smoking in tobacco smokers after overnight abstinence. Am J Psychiatry. 2005;162(3):567–577. doi: 10.1176/appi.ajp.162.3.567. [DOI] [PubMed] [Google Scholar]
- 20.Tang YY, et al. Short-term meditation training improves attention and self-regulation. Proc Natl Acad Sci USA. 2007;104(43):17152–17156. doi: 10.1073/pnas.0707678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lutz A, Slagter HA, Dunne JD, Davidson RJ. Attention regulation and monitoring in meditation. Trends Cogn Sci. 2008;12(4):163–169. doi: 10.1016/j.tics.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tang YY, Posner MI. Attention training and attention state training. Trends Cogn Sci. 2009;13(5):222–227. doi: 10.1016/j.tics.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Holzel BK, et al. How does mindfulness meditation work? Proposing mechanisms of action from a conceptual and neural perspective. Perspect Psychol Sci. 2011;6:537–559. doi: 10.1177/1745691611419671. [DOI] [PubMed] [Google Scholar]
- 24.Tang YY. Mechanism of integrative body-mind training. Neurosci Bull. 2011;27(6):383–388. doi: 10.1007/s12264-011-1141-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang YY, Yang L, Leve LD, Harold GT. Improving Executive Function and Its Neurobiological Mechanisms Through a Mindfulness-Based Intervention: Advances Within the Field of Developmental Neuroscience. Child Dev Perspect. 2012;6:361–366. doi: 10.1111/j.1750-8606.2012.00250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Black DS. Mindfulness and substance use intervention. Subst Use Misuse. 2012;47(3):199–201. doi: 10.3109/10826084.2011.635461. [DOI] [PubMed] [Google Scholar]
- 27.Chiesa A, Serretti A. Are mindfulness-based interventions effective for substance use disorders? A systematic review of the evidence. Subst Use Misuse. 2013 doi: 10.3109/10826084.2013.770027. [DOI] [PubMed] [Google Scholar]
- 28.Zgierska A, et al. Mindfulness meditation for substance use disorders: A systematic review. Subst Abus. 2009;30(4):266–294. doi: 10.1080/08897070903250019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang YY, Posner MI. Tools of the trade: Theory and method in mindfulness neuroscience. Soc Cogn Affect Neurosci. 2013;8(1):118–120. doi: 10.1093/scan/nss112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang YY, et al. Central and autonomic nervous system interaction is altered by short-term meditation. Proc Natl Acad Sci USA. 2009;106(22):8865–8870. doi: 10.1073/pnas.0904031106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang YY, et al. Short-term meditation induces white matter changes in the anterior cingulate. Proc Natl Acad Sci USA. 2010;107(35):15649–15652. doi: 10.1073/pnas.1011043107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang YY, Lu Q, Fan M, Yang Y, Posner MI. Mechanisms of white matter changes induced by meditation. Proc Natl Acad Sci USA. 2012;109(26):10570–10574. doi: 10.1073/pnas.1207817109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang YY. Exploring the Brain, Optimizing the Life. Beijing: Science Press; 2009. [Google Scholar]
- 34.Zou QH, et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: Fractional ALFF. J Neurosci Methods. 2008;172(1):137–141. doi: 10.1016/j.jneumeth.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han Y, et al. Frequency-dependent changes in the amplitude of low-frequency fluctuations in amnestic mild cognitive impairment: A resting-state fMRI study. Neuroimage. 2011;55(1):287–295. doi: 10.1016/j.neuroimage.2010.11.059. [DOI] [PubMed] [Google Scholar]
- 36.Lui S, et al. High-field MRI reveals an acute impact on brain function in survivors of the magnitude 8.0 earthquake in China. Proc Natl Acad Sci USA. 2009;106(36):15412–15417. doi: 10.1073/pnas.0812751106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bing X, et al. Alterations in the cortical thickness and the amplitude of low-frequency fluctuation in patients with post-traumatic stress disorder. Brain Res. 2013;1490:225–232. doi: 10.1016/j.brainres.2012.10.048. [DOI] [PubMed] [Google Scholar]
- 38.Yu R, et al. Frequency-specific alternations in the amplitude of low-frequency fluctuations in schizophrenia. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, et al. Altered fronto-striatal and fronto-cerebellar circuits in heroin-dependent individuals: A resting-state FMRI study. PLoS ONE. 2013;8(3):e58098. doi: 10.1371/journal.pone.0058098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jarraya B, et al. Disruption of cigarette smoking addiction after posterior cingulate damage. J Neurosurg. 2010;113(6):1219–1221. doi: 10.3171/2010.6.JNS10346. [DOI] [PubMed] [Google Scholar]
- 41.Killen JD, Fortmann SP. Craving is associated with smoking relapse: Findings from three prospective studies. Exp Clin Psychopharmacol. 1997;5(2):137–142. doi: 10.1037//1064-1297.5.2.137. [DOI] [PubMed] [Google Scholar]
- 42.Hartwell KJ, et al. Neural correlates of craving and resisting craving for tobacco in nicotine dependent smokers. Addict Biol. 2011;16(4):654–666. doi: 10.1111/j.1369-1600.2011.00340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wegner DM. White bears and other unwanted thoughts: Suppression, obsession, and the psychology of mental control. New York: Viking; 1989. [Google Scholar]
- 44.Wegner DM. Setting free the bears: Escape from thought suppression. Am Psychol. 2011;66(8):671–680. doi: 10.1037/a0024985. [DOI] [PubMed] [Google Scholar]
- 45.Bowen S, Witkiewitz K, Dillworth TM, Marlatt GA. The role of thought suppression in the relationship between mindfulness meditation and alcohol use. Addict Behav. 2007;32(10):2324–2328. doi: 10.1016/j.addbeh.2007.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bargh JA, Gollwitzer PM, Lee-Chai A, Barndollar K, Trötschel R. The automated will: Nonconscious activation and pursuit of behavioral goals. J Pers Soc Psychol. 2001;81(6):1014–1027. [PMC free article] [PubMed] [Google Scholar]
- 47.Custers R, Aarts H. The unconscious will: How the pursuit of goals operates outside of conscious awareness. Science. 2010;329(5987):47–50. doi: 10.1126/science.1188595. [DOI] [PubMed] [Google Scholar]
- 48.Dijksterhuis A, Aarts H. Goals, attention, and (un)consciousness. Annu Rev Psychol. 2010;61:467–490. doi: 10.1146/annurev.psych.093008.100445. [DOI] [PubMed] [Google Scholar]
- 49.Ansell EB, Gu P, Tuit K, Sinha R. Effects of cumulative stress and impulsivity on smoking status. Hum Psychopharmacol. 2012;27(2):200–208. doi: 10.1002/hup.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brewer JA, et al. Mindfulness training and stress reactivity in substance abuse: Results from a randomized, controlled stage I pilot study. Subst Abus. 2009;30(4):306–317. doi: 10.1080/08897070903250241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fan Y, Tang YY, Posner MI. Cortisol level modulated by integrative meditation in a dose-dependent fashion. Stress Health. 2012 doi: 10.1002/smi.2497. in press. [DOI] [PubMed] [Google Scholar]
- 52.Tang YY, Rothbart MK, Posner MI. Neural correlates of establishing, maintaining, and switching brain states. Trends Cogn Sci. 2012;16(6):330–337. doi: 10.1016/j.tics.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Radzius A, et al. A factor analysis of the Fagerström Test for Nicotine Dependence (FTND) Nicotine Tob Res. 2003;5(2):255–240. doi: 10.1080/1462220031000073289. [DOI] [PubMed] [Google Scholar]
- 54.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 55.Middleton ET, Morice AH. Breath carbon monoxide as an indication of smoking habit. Chest. 2000;117(3):758–763. doi: 10.1378/chest.117.3.758. [DOI] [PubMed] [Google Scholar]
- 56.Deveci SE, Deveci F, Açik Y, Ozan AT. The measurement of exhaled carbon monoxide in healthy smokers and non-smokers. Respir Med. 2004;98(6):551–556. doi: 10.1016/j.rmed.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 57.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 58.Song XW, et al. REST: A toolkit for resting-state functional magnetic resonance imaging data processing. PLoS ONE. 2011;6(9):e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]