Abstract
Mammalian hair cells do not regenerate, and their loss is a major cause of deafness. We recently identified leucine-rich repeat containing, G-protein-coupled receptor 5 (Lgr5)-expressing cochlear supporting cells with the capacity for self-renewal and hair cell differentiation in vitro. We found that these cells, a subset of cochlear supporting cells, were responsive to Wnt signaling. Here we asked whether these Lgr5-positive cells, despite their lack of contribution to hair cell replacement after degenerative loss, could be driven by forced expression of β-catenin to act as hair cell progenitors in vivo. We showed that forced stabilization of β-catenin in supporting cells in neonatal animals resulted in proliferation of supporting cells and generation of hair cells. Although β-catenin expression was increased by genetic means in all supporting cells, entry to the cell cycle and differentiation to hair cells of the normally postmitotic cells was restricted to the Lgr5-positive population. Our finding suggests that Wnt/β-catenin can drive Lgr5-positive cells to act as hair cell progenitors, even after their exit from the cell cycle and apparent establishment of cell fate.
Wnt signaling is required for the maintenance of progenitor cells in tissues such as intestine, skin, the hematopoietic system, and the central nervous system (1–5). β-Catenin, the intracellular mediator of canonical Wnt signaling, enters the nucleus to activate transcription of target genes that control key decision points in proliferation and differentiation of stem cells.
Auditory hair cells are surrounded by supporting cells, and these two cell types together constitute the sensory epithelium of the organ of Corti. Supporting cells act as precursors for hair cells in lower vertebrates during hair cell regeneration (6, 7). Supporting cells in mammals share characteristics with those in lower vertebrates but do not replace hair cells when the inner ear is damaged (8). Cochlear cells, when placed in culture even from postnatal animals, have been shown to divide and form spheres with the capacity to differentiate into multiple cell types (9–11). When isolated and placed in culture, supporting cells divide and differentiate (12), and Lgr5-expressing supporting cells can be induced to divide (13, 14) and form spheres that can differentiate to hair cells (14). Wnt/Lgr5 signaling stimulated proliferation of the self-renewing Lgr5-positive progenitor cells in the spheres. Hair cell differentiation was stimulated by up-regulation of Atoh1 (14), a key gene for hair cell differentiation and a target of the Wnt pathway (15). Lgr5 is a receptor for R-spondins that activate the frizzled–Lrp5/6 complex in conjunction with Wnt and is a marker for adult stem cells in the colon, small intestine, stomach, and hair follicles (16, 17).
In the present study we found that stabilization of β-catenin in all supporting cells resulted in cell cycle reentry by these specific Lgr5-expressing supporting cells, which then proceeded through differentiation steps characteristic of hair cells.
Results
Forced Stabilization of β-catenin Expanded Pillar Cells and Greater Epithelial Ridge Cells in Vivo.
Lgr5-positive supporting cells have previously been identified as cells that were capable of cell division (13, 14). The spheres were responsive to Wnt signaling as shown by differentiation to hair cells. Using TOPGAL reporter mice, we found Wnt activity in the cochlea before hair cell and supporting cell development. The activity decreased after birth (Fig. S1). Here we tested in a gain-of-function model whether Wnt/β-catenin signals would have an effect on these cells in vivo. We reasoned that although supporting cells exit the cell cycle between E13.5 and E14.5 and remain postmitotic throughout life, exogenous stimulation by Wnt might stimulate dormant progenitor cell activity in supporting cells.
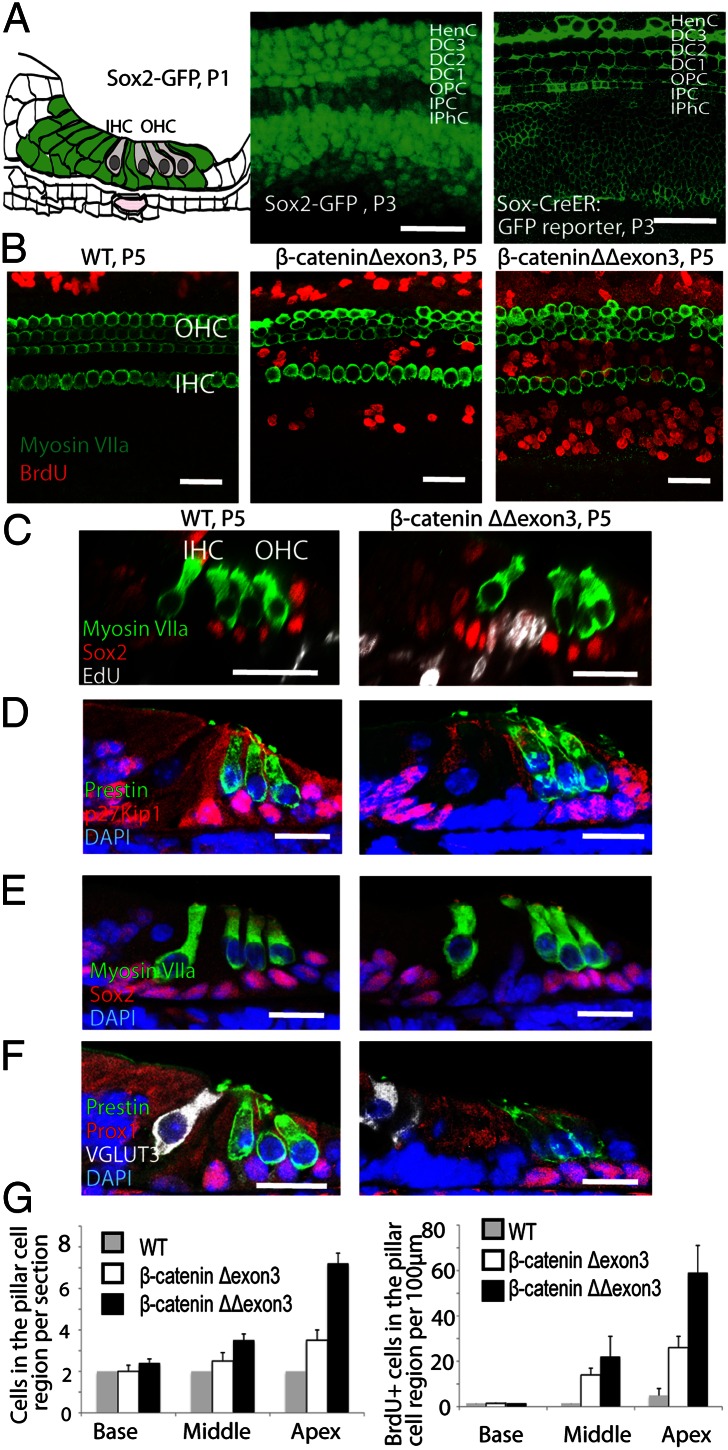
We used Sox2-Cre-ER mice to activate β-catenin constitutively in all supporting cells by crossing to a β-cateninflox(exon3) mouse, in which conditional deletion of exon3 (β-cateninΔexon3) blocks β-catenin degradation and induces accumulation of β-catenin. We first examined the Sox2-Cre-ER mice for fidelity of Cre expression and activity by comparison with mice with Sox2-GFP knocked into the same locus. Expression of the reporter in the cross between the Sox2-Cre-ER mouse and the Cre-activated GFP reporter was similar to that of Sox2-GFP (Fig. 1A). Conditional deletion of β-catenin:exon3 (β-cateninΔexon3) using the Sox2-Cre-ER mice resulted in accumulation of β-catenin in Sox2-expressing supporting cells (Fig. S2). We administered tamoxifen at P1 and dissected the cochlea at P5. Incorporation of BrdU or EdU was observed in the pillar cell region and cells in the greater epithelial ridge (GER) but not in other supporting cells (Fig. 1 B and C). In wild-type littermates, BrdU incorporation was seen only in the Hensen’s cell region peripheral to the sensory epithelium (Fig. 1 B and C). The number of BrdU-positive cells increased from the base to the apex of the cochlea, and more proliferating cells were seen in mice with two alleles deleted (β-cateninΔΔexon3) than in mice with a single deletion (β-cateninΔexon3) (Fig. 1 B and G). The number of cells in the pillar cell region increased, and expression of p27Kip1, a cell cycle inhibitor, was no longer detectable in the expanded cells (Fig. 1D), although it was still present in the intact outer pillar and Deiters’ cells. Supporting cell markers Sox2 (Fig. 1 C and E) and Prox1 (Fig. 1F) were also decreased in the expanded pillar cells but did not change in the intact outer pillar and Deiters’ cells after β-catenin overexpression, suggesting that the recently divided cells had not taken on the phenotype of a mature supporting cell.
Fig. 1.
Expansion of pillar cells and GER cells by forced in vivo expression of β-catenin. (A) The schematic illustrates the distribution of Sox2 in the organ of Corti (all supporting cells). The first image indicates the GFP distribution in supporting cells of the organ of Corti from a P3 Sox2-GFP mouse. The second image shows that the same supporting cells expressed the membrane GFP reporter when the Sox2-Cre-ER mouse was crossed to a Cre-activated membrane GFP reporter mouse and was administered tamoxifen at P1. (B) Administration of tamoxifen at P1 to Sox2-Cre-ER/β-cateninflox(exon3) mice (induction of β-catenin in supporting cells) resulted in BrdU incorporation in pillar cells and GER as viewed at P5. Labeling was greater in β-cateninΔΔexon3 than in β-cateninΔexon3 ears. No BrdU was seen in these areas in nontransgenic littermates. Hair cells were stained for myosin VII. (C) EdU was incorporated in the expanded pillar cells but not in other Sox2-positive supporting cells in cross-sections of the organ of Corti after β-catenin stabilization. (D) The newly generated cells from pillar cells but not the remaining outer pillar cells or other supporting cells in the treated ears lacked expression of p27Kip1. Outer hair cells were labeled for prestin. (E) Sox2 was down-regulated in expanded pillar cells but not in other supporting cells. Hair cells were stained for myosin VIIa. (F) Expanded pillar cells lost expression of Prox1 in the treated organ of Corti, but Deiters’ cells and outer pillar cells, whose numbers were unchanged, retained Prox1 expression. Inner and outer hair cells were labeled for VGLUT3 and prestin. (G) The number of pillar cells was increased in β-cateninΔΔexon3 relative to β-cateninΔexon3 ears and increased from base to apex, as counted in sections (n = 3 animals per group). The number of BrdU-positive cells in the pillar cell region showed a similar increase (n = 3 animals per group). Bars are SEM. IHC, inner hair cell; OHC, outer hair cells. (Scale bar, 20 μm.)
New Hair Cells Were Generated from Pillar Cells.
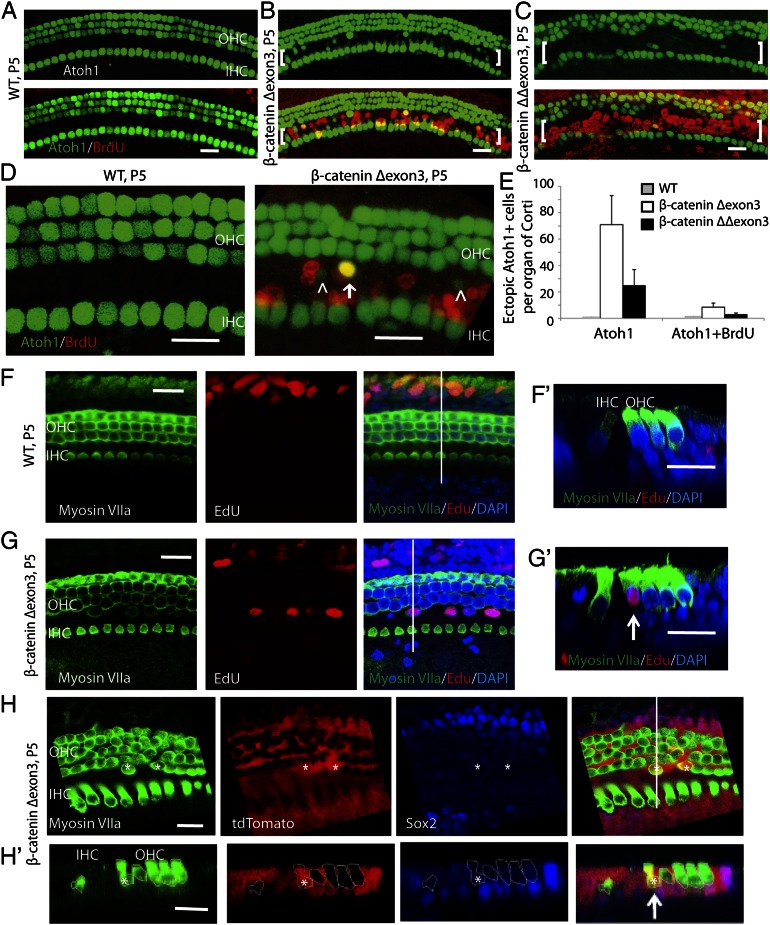
Our previous work demonstrated that overexpression of β-catenin in inner ear progenitor cells in culture increased hair cell differentiation after up-regulation of Atoh1 (14). Using the same model as above for β-catenin overexpression, we assessed pillar cell differentiation toward a hair cell fate. Because Atoh1 is expressed in hair cells but not in supporting cells in neonatal mice, we used mice with an Atoh1 reporter (Atoh1-nGFP) to determine whether the supporting cells initiated Atoh1 expression and differentiation to hair cells upon β-catenin overexpression. Atoh1-positive cells were induced in the pillar cell region by the overexpression of β-catenin based on nGFP (Fig. 2 B–D), and no ectopic Atoh1-positive cells were seen in the pillar cell region in wild-type littermates (Fig. 2 A and D). Quantitative analysis of Atoh1-expressing cells in the pillar cell region (Fig. 2E) revealed that a percentage of the Atoh1-positive cells were labeled for BrdU, indicating that β-catenin expression could generate Atoh1-positive cells whether or not cell division had occurred. Organs of Corti from β-cateninΔΔexon3 mice had less Atoh1-positive cells but more BrdU incorporation than the organs from β-cateninΔexon3 mice (Fig. 2 C and E). These results suggested that high levels of β-catenin increased cell division, whereas lower levels allowed their differentiation toward hair cells. Quantification of supporting cells in the pillar cell region throughout the cochlea revealed that 654 ± 116 (80%) had lost Sox2 expression, 102 ± 55 (10–15%) had retained Sox2 expression similar to a normal organ of Corti, and 71 ± 35 (5–10%) had become Atoh1-positive.
Fig. 2.
Induction of ectopic hair cells after in vivo overexpression of β-catenin. (A) In the cochlea, Atoh1 is expressed in hair cells, and both hair cells and supporting cells are postmitotic. No BrdU incorporation was observed in the wild-type organ of Corti. (B) In β-catenin–overexpressing littermates at P5, ectopic Atoh1 expression was induced in the pillar cell region (brackets) between inner and outer hair cells upon activation of Cre at P1 in compound mutants of Sox2-Cre-ER/β-cateninflox(exon3)/Atoh1-nGFP mice. BrdU incorporation was also seen in the pillar cell region. (C) Ectopic Atoh1 expression was observed in β-cateninΔΔexon3 mutant cells. (D) Constitutive activation of β-catenin resulted in Atoh1 expression (arrowheads) in pillar cells, some of which were positive for BrdU (arrow). Atoh1-expressing cells were not found in ectopic positions in wild-type littermates. (E) Quantification of ectopic Atoh1- and Atoh1/BrdU-positive cells in the pillar cell region in β-catenin mutants and wild-type littermates. n = 3 animals/group. Bars are SEM. (F) Myosin VIIa was used to detect hair cells in the organ of Corti, and EdU incorporation was seen outside the area of the hair cells. (F′) An x–z scan of F was performed at the position indicated by the white line. (G) In Sox2-Cre-ER/β-cateninΔ(exon3) mice, extra hair cells were seen in the pillar cell region based on myosin VIIa staining, and some of the cells had incorporated EdU. (G′) An x–z scan of G at the white line showed an ectopic myosin VIIa/EdU-positive cell (arrow). (H) Lineage tag in supporting cells (red) was seen in the myosin VIIa-expressing hair cells (asterisks). (H′) In an x–z scan of H at the white line, a lineage-tagged hair cell (arrow) appeared in the pillar cell region. Myosin VIIa-expressing hair cells are outlined. (Scale bar, 20 μm.)
We next tested for other features of hair cells in newly generated cells resulting from β-catenin overexpression. Some of the EdU-positive cells in the pillar cell region expressed the mature hair cell marker myosin VIIa (Fig. 2 F and G). In addition, they took up FM 1-43, a fluorescent dye that enters hair cells through mechanotransduction channels (Fig. S3). Expression of β-catenin thus not only pushed the cells into the cell cycle and activated Atoh1 but also induced them to differentiate to hair cells.
The source of the new hair cells was assessed by lineage tracing by crossing the Sox2-Cre-ER mouse to a floxed tdTomato reporter mouse and to the β-cateninflox(exon3) mouse. Supporting cells expressed tdTomato before expansion or differentiation induced by β-catenin overexpression. Tamoxifen was given at P4, and after staining at P7, new hair cells positive for tdTomato and myosin VIIa were seen in the pillar cell area (Fig. 2H). The new cells expressing hair cell markers had therefore arisen from supporting cells.
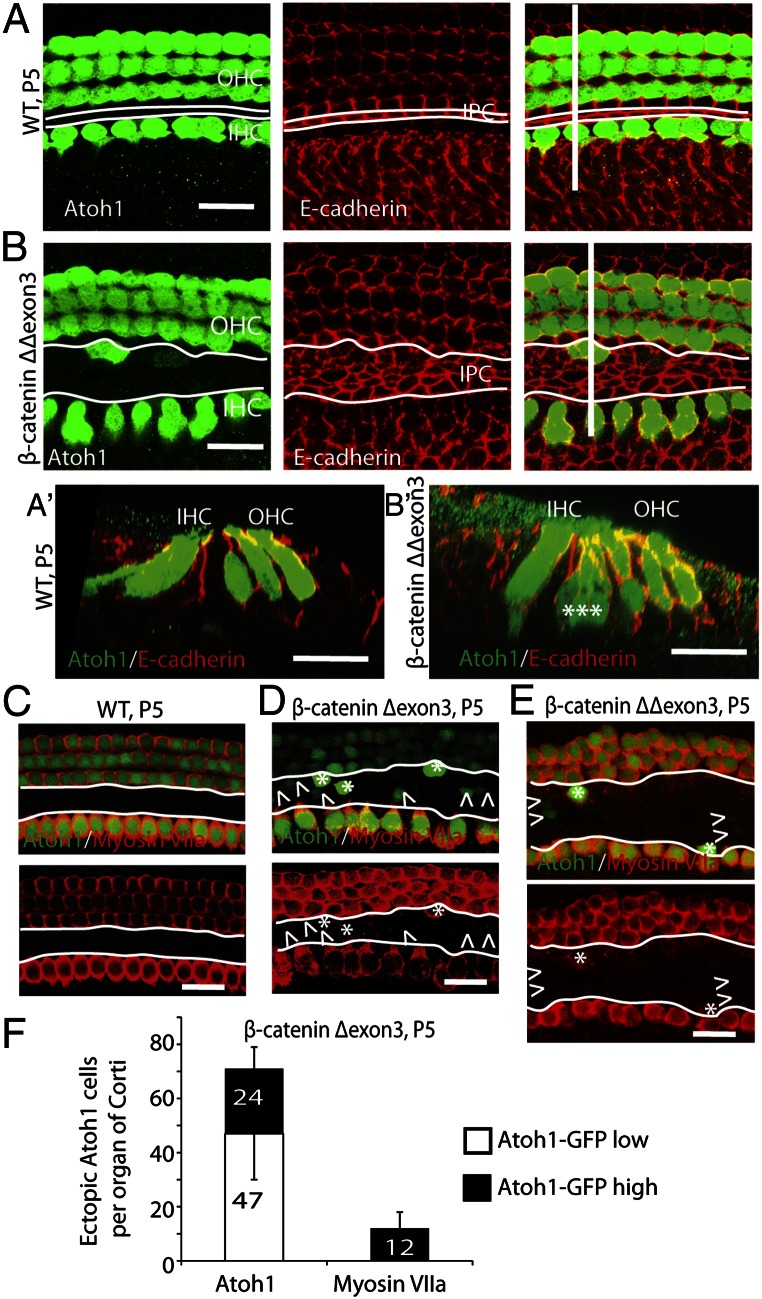
We labeled cell junctions for E-cadherin (Fig. 3 A and B) in an attempt to delineate the supporting cells obtained after β-catenin overexpression. The pillar cell region was expanded after proliferation. Ectopic Atoh1-positive cells arose from the expanded pillar cell region and often appeared as clusters (Fig. 3B). A scan in the x–z plane at the ectopic Atoh1-positive cells (Fig. 3 A′ and B′) showed that the clusters were at the level of hair cells and consisted of two to three extra cells with Atoh1 expression. The newly divided cells in the pillar cell region had varying levels of Atoh1 expression. Cells with higher GFP expression than that of preexisting hair cells constituted about one-third of the total GFP-expressing cells (Fig. 3 C–F) and accounted for all of the ectopic myosin VIIa-positive cells. Half of the cells with high GFP expression expressed myosin VIIa. Organs of Corti from β-cateninΔΔexon3 mice contained fewer Atoh1- and myosin VIIa-positive cells (Fig. 3E). When we continued the experiment to a later stage (P9), the ectopic hair cells expressed prestin (Fig. S4 A–C). It is noteworthy that a few cells expressed both prestin and VGLUT3, suggesting an unusual phenotype, likely due to incomplete signaling that would normally be present for induction of outer and inner hair cell phenotype (Fig. S4C).
Fig. 3.
Hair cells differentiate from cells that express high levels of Atoh1. (A) Atoh1 was expressed in hair cells, and E-cadherin was expressed in supporting cell junctions in a wild-type organ of Corti. The white lines along the longitudinal axis of the organ of Corti indicate inner pillar cells. (A′) An x–z scan of A was performed at the white line perpendicular to the longitudinal axis. (B) In β-catenin–overexpressing littermates the number of pillar cells increased (between the white lines), and Atoh1-positive cells appeared in the pillar cell region at P5 in mice administered tamoxifen at P1. (B′) An x–z scan of B (at the perpendicular white line). (C) Atoh1 and myosin VIIa were expressed in hair cells in a wild-type organ of Corti at P5. No Atoh1- or myosin VIIa-positive cells were seen in the pillar cell region (white outlines). (D) β-Catenin expression induced ectopic Atoh1-positive cells in the pillar cell region, some of which showed high levels of Atoh1 (asterisks) and expressed myosin VIIa. Cells with low levels of Atoh1 (arrowheads) did not express myosin VIIa. (E) In β-cateninΔΔexon3 mutants, some of the ectopic cells that expressed high levels of Atoh1 in the expanded pillar cell region expressed myosin VIIa. (F) Quantification of ectopic Atoh1- and myosin VIIa-positive cells in the pillar cell region in β-cateninΔexon3 mutants. One-third of Atoh1-positive cells showed high-level expression of Atoh1 (black bar) (n = 3). (Scale bar, 20 μm.)
We examined the potential for cell proliferation and differentiation toward a hair cell fate in the mature (P44) undamaged cochlea, using the same model as the neonates for β-catenin overexpression. No new hair cells or EdU-positive cells in the pillar cell region were observed 5 d after treatment (Fig. S5).
Inner Pillar Cells Showed Increased Lgr5 Expression and Divided in Response to β-catenin.
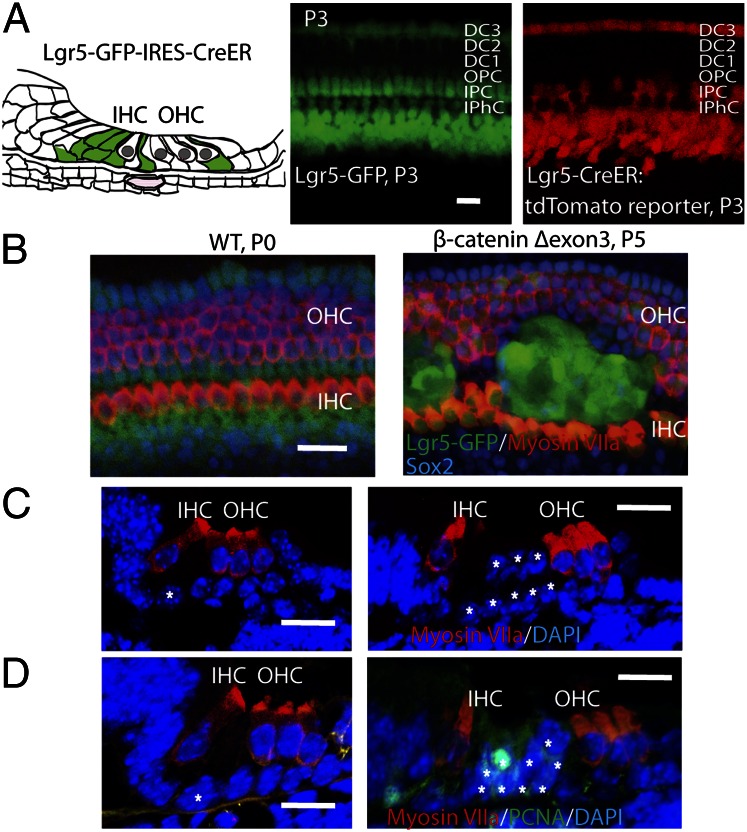
The results of E-cadherin staining suggested that the new hair cells were derived from pillar cells, and we have previously identified inner but not outer pillar cells as Lgr5-positive inner ear progenitor cells. We expected that the new cells came from inner pillar cells, but the BrdU-labeled cells were found at both sides of the pillar cell area, adjacent to either outer or inner hair cells. To test their expected origin from Lgr5-positive cells, we used a mouse with GFP and Cre in the Lgr5 locus for overexpression in the Lgr5-expressing subset of supporting cells (Fig. 4A). To follow Lgr5 expression, we used the GFP in the Lgr5 locus. The Cre mouse was crossed with the Cre-activated tdTomato reporter and to the β-cateninflox(exon3) mice. When we overexpressed β-catenin in postmitotic Lgr5-positive cells by administration of tamoxifen at E16.5 and examined the cochlea for Lgr5 expression at P0, we found an expansion of supporting cells (Fig. 4B). The locus of supporting cell expansion (between outer and inner hair cells) was the same as was found after β-catenin overexpression driven by Sox2, thus confirming that the responsive cells were inner and not outer pillar cells. The expanded supporting cells were positive for proliferating cell nuclear antigen (PCNA), suggesting that the cells had reentered the cell cycle due to the overexpression of β-catenin (Fig. 4D), whereas PCNA was not observed in the organ of Corti of a wild-type littermate. The expanded supporting cells found upon constitutive expression of β-catenin appeared as isolated clumps that extended from the base to the apex of the cochlea (an average of 76 clumps was observed; Fig. S6, n = 2), and each clump contained 30–60 cells. In cross-section, 8–10 cells were observed between inner and outer hair cells compared with 2 pillar cells in wild-type animals (Fig. 4C). The expanded clumps were Lgr5-positive (53 cells per 100 μm), and 10% of the cells expressed Sox2 (Fig. S6), confirming a supporting cell identity. Some nuclei in the clumps of new cells in the organ of Corti of the β-catenin–overexpressing mice were above the other supporting cells and appeared to have moved adjacent to the hair cell nuclei (Fig. 4C).
Fig. 4.
Wnt signaling expanded Lgr5-expressing supporting cells in vivo. (A) Lgr5 is expressed in a subset of supporting cells as illustrated. Third Deiters’ cells, inner pillar cells, and cells in the greater epithelial ridge expressed GFP in the Lgr5-GFP-IRES-Cre-ER mouse. Cre activity was seen in Lgr5-expressing cells at P3 when the mice were crossed with a Cre-activated tdTomato reporter mouse after tamoxifen administration at P1. (B) Expansion of Lgr5-positive cells at P0 upon activation of β-catenin signaling at E16.5 in Lgr5-Cre-ER mice crossed to β-cateninflox(exon3) mice, compared with wild-type mice. (C) The pillar cells were expanded in the organ of Corti cross-section. (D) The cells expanded in the pillar cell area were positive for PCNA (white asterisks indicate pillar cells). (Scale bar, 20 μm.)
Hair Cells Were Not Affected by β-catenin Expression.
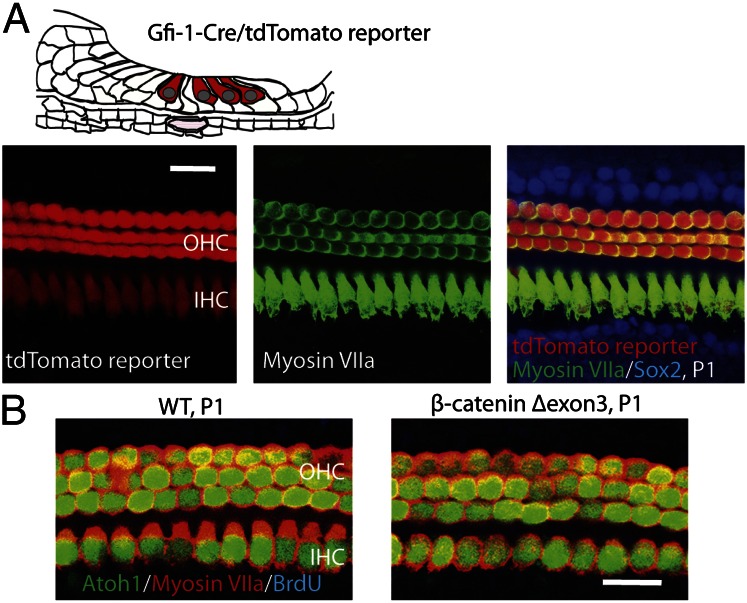
To test if hair cells were responsive to Wnt/β-catenin signaling, we expressed β-catenin in hair cells. Gfi1 is expressed in the cochlea starting from E15.5 specifically in hair cells (18). Tomato reporter was seen in all hair cells in mutants of Gfi1-Cre crossed with Cre-activated tdTomato reporter mice (Fig. 5A). β-Catenin was expressed in hair cells in Gfi1-Cre mice crossed with β-cateninflox(exon3) mice. No BrdU labeling or changes in the number of hair cells were observed in the β-catenin expression mutants at P1 (Fig. 5B). These data confirmed that β-catenin increased cell division in inner pillar cells and not hair cells.
Fig. 5.
β-Catenin expression in hair cells. (A) Gfi1 is expressed in both inner and outer hair cells as illustrated. Cre activity was seen in hair cells at P1 in a Gfi1-Cre mouse crossed to a Cre-activated tdTomato reporter mouse. (B) No BrdU incorporation or ectopic Atoh1-positive cells were observed in ears from β-catenin mutants at P1.
Discussion
Mammalian hair cells and supporting cells are postmitotic in adults, and the mammalian inner ear does not regenerate spontaneously after damage. Our data show the induction of both division and differentiation of supporting cells, which led to generation of new hair cells in the postnatal mammalian inner ear. This was achieved by forced activation of the canonical Wnt pathway mediator, β-catenin. Whereas cells isolated from the cochlea have been shown to divide and differentiate in culture (9–12), cell division and subsequent differentiation have not been previously demonstrated in vivo except in epithelial cells adjacent to hair cells in the newborn organ of Corti, where dividing cells were observed after Atoh1 up-regulation (19). Differentiation to hair cells has been observed after Notch inhibition (20–22) and manipulation of Atoh1 (19, 23–25). Proliferation in response to Atoh1 was not observed in a similar study in mouse cochlear supporting cells (26), and Atoh1 expression in the developing cochlea coincides with the cessation of cell division.
The quiescence of cochlear cells is maintained by cyclin-dependent kinase inhibitors, p27Kip1, p19Ink4d, and p21Cip1, which arrest the cells in G0 (27, 28). β-Catenin activation promoted mitosis by inhibiting p27Kip1. Cells induced by β-catenin overexpression lost Sox2 expression, as was observed after p27Kip1 knockout in the cochlea (29). Wnt function during development is cell-specific and depends on Tcf/Lef cofactor expression, the specific Wnts expressed, coexpression of Wnt inhibitors, and the resultant level of Wnt signaling. The activity of the Wnt pathway is required for diverse functions of stem cells in the regenerative response to damage. In the intestine, Wnt/β-catenin signaling specifies Atoh1-expressing secretory lineage cells, whereas Notch signaling induces Hes1/5-expressing enterocytes. In hematopoietic stem cells, Wnt signaling increases both self-renewal and differentiation in a dose-dependent fashion (30). Wnt signaling is central to the proliferative program but switches to a role in cell fate specification and differentiation during regeneration. A role for Wnt signaling in both expansion and differentiation of stem cells is an early evolutionary development that is used in regeneration in lower vertebrates (31, 32) and invertebrates (33).
Supporting cells and epithelial cells adjacent to hair cells in embryonic or newborn mammalian inner ears are converted to hair cells by Atoh1 overexpression (25). We found here that β-catenin initiated differentiation of supporting cells to Atoh1-positive cells. It also induced myosin VIIa expression and FM 1-43 uptake. We noted that only a small number of Atoh1-positive cells expressed myosin VIIa, and other Atoh1-positive cells may not adopt hair cell fates, as has been suggested in avian hair cell regeneration (34). Prestin and VGLUT3 began to be expressed at P9. Prestin was not observed in a study of Atoh1 overexpression in mouse cochlea (26), but in another study, Atoh1 overexpression induced myosin VIIa-positive cells with physiological properties reminiscent of hair cells (19). The comparison between one- and two-allele stabilization showed that the activity of β-catenin was level-dependent. Increased proliferation was seen in both cases, but cell fate determination was favored at lower levels, and proliferation took precedence over cell fate determination at higher levels. Previous work has shown that Wnt signaling is required for development of the telencephalon but that signaling modulation to a lower level is necessary for development of the head (35). Proliferation may be inhibitory for differentiation, or other signals may be needed to fully differentiate these cells. It could be speculated that Notch signaling, in the absence of damage, prevented differentiation. Wnt/β-catenin signaling could function as an initial cue for induction of the hair cell lineage, which occurs through its role in Atoh1 stimulation (15), and our recent data on hair cell differentiation after forced expression of β-catenin favor this interpretation (14). However, although we did not rule out β-catenin–independent hair cell maturation, differentiation to hair cells was not seen after supporting cell proliferation resulting from manipulation of cell cycle genes, such as p27Kip1 or Rb (26, 36).
Wnt pathway activation as a route to proliferation of supporting cells, followed by differentiation of hair cells, is consistent with a dual role for β-catenin in cochlear progenitor cells identified by the adult stem cell marker Lgr5. The marker was found in a subset of cochlear supporting cells that included inner pillar cells, the third row of Deiters’ cells, and the cells surrounding the inner hair cell and GER, and the cells selected for Lgr5 expression had an increased capacity for hair cell generation compared with unsorted supporting cells (14). Purified postmitotic supporting cells that expressed Lgr5 differentiated into hair cells at significantly higher rates than unfractionated cells, and Lgr5-negative cells did not give rise to hair cells. Lgr5 is expressed in cells outside the sensory epithelium, in the outer sulcus at P1 and at the spiral prominence at P4 (14), and may confer competence to become hair cells after Atoh1 induction (19). Lgr5 was first described as a stem cell marker for intestinal epithelial cells and has since been demonstrated as a marker of the stem cell compartment in the stomach and hair follicles (16, 17, 37–39). Lgr5 has been identified as a receptor for R-spondins, which activate the Wnt signaling pathway through an interaction between Lgr5 and frizzled–Lrp5/6. R-spondin administration to Lgr5-positive stem cells potentiated Wnt responsiveness by as much as 100-fold. Conditional deletion of Lgr5 led to the disappearance of stem cells, and expression of the Wnt agonist R-spondin-1 led to an expanded Lgr5-positive cell population in crypts (37, 40). Expansion of the β-catenin–overexpressing cells seen here is reminiscent of the phenotype seen after Wnt overexpression or activation of β-catenin in the epithelial cells lining the intestine (37). Lgr5 has been shown to be lost upon the maturation of transit-amplifying cells from intestinal epithelial stem cells (41), and the decrease of Lgr5 in the maturing cells in the cochlea was therefore consistent with Lgr5 acting as a marker for the stem cell compartment. The ability to replace lost cells by cell division and differentiation is a characteristic of adult tissue stem cells that regenerate damaged tissues.
β-Catenin expression serves to single out stem cells: indeed, in numerous tissues, forced expression of Wnt specifically expands adult stem cells, unlike knockout of other cell cycle genes, such as p27Kip1 and Rb, which induce cell cycle reentry of all of the cells in which they are silenced (27, 42). In retinal progenitor cells of the ciliary margin, progenitor populations display a selective response to β-catenin signaling even when up-regulation is induced broadly (43). To test whether Wnt pathway activation specifically affected Lgr5-expressing cells, we assessed cell cycle reentry when β-catenin was expressed in all (Sox2-positive) supporting cells. β-Catenin overexpression in Sox2-expressing cells was restricted in its effect to Lgr5-positive inner pillar cells. The correlation between expression of Lgr5 and responsiveness to β-catenin is incomplete, because inner pillar cells responded to β-catenin in our model, but the third row of Deiters’ cells was unaffected. At the base of intestinal crypts, three to four cells expressing Lgr5 are separated by Paneth cells (17), and it remains unknown whether they respond equally to Wnt (37). Our data support the identification of the Lgr5-expressing cells as progenitor cells (14). Another study that showed a proliferative effect of β-catenin on Lgr5-positive cells failed to generate new hair cells (13), likely due to use of the Lgr5-Cre-ER, which is also a heterozygous knockout of Lgr5, because the reporter and Cre disrupt the coding sequence. In addition, the irregular pattern of cell expansion in sensory epithelium after Lgr5-Cre–mediated overexpression, attributed to heterogeneity of the cells (13), was more likely due to incomplete penetrance of Cre expression in the Lgr5-Cre-ER strain used (17).
Although the current study establishes a role of Wnt signaling in hair cell generation in vivo, other pathways play important roles (20–22). Manipulation of signaling through the Wnt/β-catenin pathway may provide a way to mimic routes to progenitor cell proliferation and differentiation occurring in birds and fish, where the Wnt pathway is needed for hair cell regeneration (44). Supporting cells in the mammalian organ of Corti share markers and signaling pathways with lower-vertebrate supporting cells. The responsiveness to β-catenin overexpression was not seen beyond the perinatal period, however. This could be due to decreased expression of Lgr5 in the adult, and it will be useful to determine whether the Wnt pathway could lead to regeneration under other conditions, such as after damage, which can spontaneously activate the Wnt pathway. Stimulation of both cell division and differentiation by Wnt/β-catenin may be an avenue to replacement of cochlear hair cells, a sought-after goal for treatment of hearing loss, which is commonly caused by loss of hair cells in humans. Unlike hair cell replacement by transdifferentiation that might deplete the inner ear of supporting cells, regeneration through the up-regulation of β-catenin could replace both hair cells and supporting cells.
Materials and Methods
Animals.
β-Cateninflox(exon3) mice (45) were generously provided by Mark Taketo (Kyoto University, Kyoto, Japan); Sox2-EGFP (46) and Sox2-Cre-ER (47) mice were provided by Konrad Hochedlinger (Harvard Medical School, Boston); Atoh1-nGFP mice (48) were provided by Jane Johnson (University of Texas Southwestern Medical Center, Dallas); and Gfi1-Cre mice (18) were provided by Lin Gan (University of Rochester, Rochester, New York). Lgr5-GFP-Cre-ER mice, which contain a Lgr5-EGFP-IRES-Cre-ERT2 “knock-in” allele at the Lgr5 locus (17), were obtained from Jackson Laboratory (Stock 008875). Rosa26 reporter mice, containing a loxP-flanked STOP cassette (49), in front of EGFP (Stock 007676), or a red fluorescent protein variant (Stock 007914), were obtained from Jackson Laboratory. TOPGAL mice, in which the lacZ gene is expressed under the control of a regulatory sequence consisting of three consensus Tcf/Lef-binding motifs upstream of a minimal c-fos promoter (50), were obtained from Jackson Laboratory (Stock 004623). All animal experiments were approved by the Institutional Animal Care and Use Committee of the Massachusetts Eye and Ear Infirmary.
Constitutive Expression of β-Catenin in Vivo.
Sox2-Cre-ER mice, Lgr5-GFP-IRES-Cre-ER mice, or Gfi1-Cre mice were mated with β-cateninflox(exon3):Atoh1-nGFP mice. One hundred microliters tamoxifen dissolved in corn oil (50 mg/mL) was given to the mothers of the compound transgenic mice and passed to P1 pups via the milk to generate the β-cateninΔexon3 mice. Mouse pups were killed at P5 or P9. Ten microliters BrdU or Edu (5-ethynyl-2′-deoxyuridine, 10 mg/mL) was given to the pups by s.c. injection on the back, twice a day for 4 continuous days. A mixture of 100 μL tamoxifen (50 mg/kg in corn oil) and 0.1 μL estradiol (50 mg/kg in ethanol) was given to the pregnant mice to activate Cre in embryos at E16.5, and the mouse pups were killed at birth. Pups were genotyped for both Cre and β-cateninflox(exon3). For activation of Cre in P44 mice, tamoxifen (50 mg/mL) was given twice a day for 3 continuous days along with EdU (10 mg/mL).
Cochleae were dissected and immediately fixed for 20 min with 4% paraformaldehyde (PFA; Electron Microscopy Sciences). The organ of Corti was isolated, separated from the stria vascularis, and fixed further in 4% PFA for 30 min. Alternatively, cochleae were fixed for 2 h with 4% PFA, dehydrated overnight with 30% sucrose, and embedded in optimal cutting temperature for cryosection. Serial sections (16 μm) were mounted on Ultra-Stick Gold Seal glass slides (Becton Dickinson).
Histology and Immunostaining.
Antibodies used in this study were against myosin VIIa (1:800; Proteus), Sox2 (1:500; Santa Cruz), Prox1 (1:200; Chemicon), p27Kip1 (1:200; NeoMarkers), prestin (1:400; Santa Cruz), VGLUT3 (1:10,000; Chemicon), BrdU (1:100; ABD Serotec), β-catenin-Y489 (1:100; DSHB), and GFP (1:1,000; Invitrogen). Species-specific Alexafluor-conjugated secondary antibodies were used for detection (1:500; Invitrogen).
Cochleae of TOPGAL mice were fixed in 4% PFA or 0.5 µM glutaraldehyde for 1 h. The tissues were washed twice with 2 mM MgCl2 in PBS for 5 min each at room temperature and incubated in X-Gal and X-Gal mixer (1:40; X-Gal mixer: 5 mM K3Fe(CN)6, 5 mM K4Fe(CN)3H2O, 0.01% sodium deoxycholate, 0.2% Nonidet P-40, and 2 mM MgCl2 in 1× PBS) for 1 h at 37 °C.
To detect cell proliferation, the organ of Corti whole-mount was treated with 2 M HCl for 30 min at 37 °C before staining with BrdU and other indicated antibodies. Incorporated EdU was detected with Alexafluor azide using Click-iT EdU Image kits (Invitrogen) according to the manufacturer’s protocol before immunostaining. Sections and whole mounts were analyzed using a Leica TCS SP5 confocal microscopy. The organ of Corti was scanned in the z planes followed by the y plane at the indicated x–z line.
To detect active mechanoelectrical transduction channels, 5 μM FM 1-43 was incubated with the freshly dissected organ of Corti for 2 min. The tissue was then washed three times with PBS before fixation and confocal microscopy.
Cell Counting.
Atoh1- or myosin VIIa-positive hair cells in the pillar cell region were manually counted with Image J software (National Institutes of Health) from the entire cochlear whole mount. The number of pillar cells was counted on single sections of the cochlea. Cell counts for the β-catenin–expressing group were compared with those for littermates without β-catenin overexpression and were analyzed by the Student t test.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute on Deafness and Other Communication Disorders (R01 DC007174, P30 DC05209, and R03 DC010270), by the Shulsky Foundation and by Robert Boucai.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1219952110/-/DCSupplemental.
References
- 1.Alonso L, Fuchs E. Stem cells in the skin: Waste not, Wnt not. Genes Dev. 2003;17(10):1189–1200. doi: 10.1101/gad.1086903. [DOI] [PubMed] [Google Scholar]
- 2.Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297(5580):365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- 3.Ichii M, Frank MB, Iozzo RV, Kincade PW. The canonical Wnt pathway shapes niches supportive of hematopoietic stem/progenitor cells. Blood. 2012;119(7):1683–1692. doi: 10.1182/blood-2011-07-369199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuwabara T, et al. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat Neurosci. 2009;12(9):1097–1105. doi: 10.1038/nn.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinto D, Clevers H. Wnt, stem cells and cancer in the intestine. Biol Cell. 2005;97(3):185–196. doi: 10.1042/BC20040094. [DOI] [PubMed] [Google Scholar]
- 6.Ma EY, Rubel EW, Raible DW. Notch signaling regulates the extent of hair cell regeneration in the zebrafish lateral line. J Neurosci. 2008;28(9):2261–2273. doi: 10.1523/JNEUROSCI.4372-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stone JS, Choi YS, Woolley SM, Yamashita H, Rubel EW. Progenitor cell cycling during hair cell regeneration in the vestibular and auditory epithelia of the chick. J Neurocytol. 1999;28(10-11):863–876. doi: 10.1023/a:1007022205821. [DOI] [PubMed] [Google Scholar]
- 8.Warchol ME. Sensory regeneration in the vertebrate inner ear: Differences at the levels of cells and species. Hear Res. 2011;273(1-2):72–79. doi: 10.1016/j.heares.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Malgrange B, et al. Proliferative generation of mammalian auditory hair cells in culture. Mech Dev. 2002;112(1-2):79–88. doi: 10.1016/s0925-4773(01)00642-6. [DOI] [PubMed] [Google Scholar]
- 10.Martinez-Monedero R, Yi E, Oshima K, Glowatzki E, Edge AS. Differentiation of inner ear stem cells to functional sensory neurons. Dev Neurobiol. 2008;68(5):669–684. doi: 10.1002/dneu.20616. [DOI] [PubMed] [Google Scholar]
- 11.Oshima K, et al. Differential distribution of stem cells in the auditory and vestibular organs of the inner ear. J Assoc Res Otolaryngol. 2007;8(1):18–31. doi: 10.1007/s10162-006-0058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White PM, Doetzlhofer A, Lee YS, Groves AK, Segil N. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature. 2006;441(7096):984–987. doi: 10.1038/nature04849. [DOI] [PubMed] [Google Scholar]
- 13.Chai R, et al. Wnt signaling induces proliferation of sensory precursors in the postnatal mouse cochlea. Proc Natl Acad Sci USA. 2012;109(21):8167–8172. doi: 10.1073/pnas.1202774109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi F, Kempfle JS, Edge AS. Wnt-responsive Lgr5-expressing stem cells are hair cell progenitors in the cochlea. J Neurosci. 2012;32(28):9639–9648. doi: 10.1523/JNEUROSCI.1064-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi F, Cheng YF, Wang XL, Edge AS. Beta-catenin up-regulates Atoh1 expression in neural progenitor cells by interaction with an Atoh1 3′ enhancer. J Biol Chem. 2010;285(1):392–400. doi: 10.1074/jbc.M109.059055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barker N, Clevers H. Leucine-rich repeat-containing G-protein-coupled receptors as markers of adult stem cells. Gastroenterology. 2010;138(5):1681–1696. doi: 10.1053/j.gastro.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Barker N, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449(7165):1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 18.Yang H, et al. Gfi1-Cre knock-in mouse line: A tool for inner ear hair cell-specific gene deletion. Genesis. 2010;48(6):400–406. doi: 10.1002/dvg.20632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly MC, Chang Q, Pan A, Lin X, Chen P. Atoh1 directs the formation of sensory mosaics and induces cell proliferation in the postnatal mammalian cochlea in vivo. J Neurosci. 2012;32(19):6699–6710. doi: 10.1523/JNEUROSCI.5420-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doetzlhofer A, et al. Hey2 regulation by FGF provides a Notch-independent mechanism for maintaining pillar cell fate in the organ of Corti. Dev Cell. 2009;16(1):58–69. doi: 10.1016/j.devcel.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin V, et al. Inhibition of Notch activity promotes nonmitotic regeneration of hair cells in the adult mouse utricles. J Neurosci. 2011;31(43):15329–15339. doi: 10.1523/JNEUROSCI.2057-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mizutari K, et al. Notch inhibition induces cochlear hair cell regeneration and recovery of hearing after acoustic trauma. Neuron. 2013;77(1):58–69. doi: 10.1016/j.neuron.2012.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gubbels SP, Woessner DW, Mitchell JC, Ricci AJ, Brigande JV. Functional auditory hair cells produced in the mammalian cochlea by in utero gene transfer. Nature. 2008;455(7212):537–541. doi: 10.1038/nature07265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawamoto K, Ishimoto S, Minoda R, Brough DE, Raphael Y. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neurosci. 2003;23(11):4395–4400. doi: 10.1523/JNEUROSCI.23-11-04395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000;3(6):580–586. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- 26.Liu Z, et al. Age-dependent in vivo conversion of mouse cochlear pillar and Deiters’ cells to immature hair cells by Atoh1 ectopic expression. J Neurosci. 2012;32(19):6600–6610. doi: 10.1523/JNEUROSCI.0818-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126(8):1581–1590. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- 28.Laine H, et al. p19(Ink4d) and p21(Cip1) collaborate to maintain the postmitotic state of auditory hair cells, their codeletion leading to DNA damage and p53-mediated apoptosis. J Neurosci. 2007;27(6):1434–1444. doi: 10.1523/JNEUROSCI.4956-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oesterle EC, Chien WM, Campbell S, Nellimarla P, Fero ML. p27(Kip1) is required to maintain proliferative quiescence in the adult cochlea and pituitary. Cell Cycle. 2011;10(8):1237–1248. doi: 10.4161/cc.10.8.15301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luis TC, et al. Canonical wnt signaling regulates hematopoiesis in a dosage-dependent fashion. Cell Stem Cell. 2011;9(4):345–356. doi: 10.1016/j.stem.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 31.Lekven AC, Thorpe CJ, Waxman JS, Moon RT. Zebrafish wnt8 encodes two wnt8 proteins on a bicistronic transcript and is required for mesoderm and neurectoderm patterning. Dev Cell. 2001;1(1):103–114. doi: 10.1016/s1534-5807(01)00007-7. [DOI] [PubMed] [Google Scholar]
- 32.Stoick-Cooper CL, et al. Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development. 2007;134(3):479–489. doi: 10.1242/dev.001123. [DOI] [PubMed] [Google Scholar]
- 33.Gurley KA, et al. Expression of secreted Wnt pathway components reveals unexpected complexity of the planarian amputation response. Dev Biol. 2010;347(1):24–39. doi: 10.1016/j.ydbio.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewis RM, Hume CR, Stone JS. Atoh1 expression and function during auditory hair cell regeneration in post-hatch chickens. Hear Res. 2012;289(1-2):74–85. doi: 10.1016/j.heares.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fossat N, et al. Stringent requirement of a proper level of canonical WNT signalling activity for head formation in mouse embryo. Development. 2011;138(4):667–676. doi: 10.1242/dev.052803. [DOI] [PubMed] [Google Scholar]
- 36.Yu Y, et al. In vivo proliferation of postmitotic cochlear supporting cells by acute ablation of the retinoblastoma protein in neonatal mice. J Neurosci. 2010;30(17):5927–5936. doi: 10.1523/JNEUROSCI.5989-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barker N, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457(7229):608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 38.Fuchs E. The tortoise and the hair: Slow-cycling cells in the stem cell race. Cell. 2009;137(5):811–819. doi: 10.1016/j.cell.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaks V, et al. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat Genet. 2008;40(11):1291–1299. doi: 10.1038/ng.239. [DOI] [PubMed] [Google Scholar]
- 40.Ootani A, et al. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat Med. 2009;15(6):701–706. doi: 10.1038/nm.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Snippert HJ, et al. (2009) Prominin-1/CD133 marks stem cells and early progenitors in mouse small intestine. Gastroenterology 136(7):2187–2194. [DOI] [PubMed]
- 42.Sage C, et al. Proliferation of functional hair cells in vivo in the absence of the retinoblastoma protein. Science. 2005;307(5712):1114–1118. doi: 10.1126/science.1106642. [DOI] [PubMed] [Google Scholar]
- 43.Denayer T, et al. Canonical Wnt signaling controls proliferation of retinal stem/progenitor cells in postembryonic Xenopus eyes. Stem Cells. 2008;26(8):2063–2074. doi: 10.1634/stemcells.2007-0900. [DOI] [PubMed] [Google Scholar]
- 44.Alvarado DM, et al. An RNA interference-based screen of transcription factor genes identifies pathways necessary for sensory regeneration in the avian inner ear. J Neurosci. 2011;31(12):4535–4543. doi: 10.1523/JNEUROSCI.5456-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harada N, et al. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18(21):5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eminli S, Utikal J, Arnold K, Jaenisch R, Hochedlinger K. Reprogramming of neural progenitor cells into induced pluripotent stem cells in the absence of exogenous Sox2 expression. Stem Cells. 2008;26(10):2467–2474. doi: 10.1634/stemcells.2008-0317. [DOI] [PubMed] [Google Scholar]
- 47.Arnold K, et al. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell. 2011;9(4):317–329. doi: 10.1016/j.stem.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lumpkin EA, et al. Math1-driven GFP expression in the developing nervous system of transgenic mice. Gene Expr Patterns. 2003;3(4):389–395. doi: 10.1016/s1567-133x(03)00089-9. [DOI] [PubMed] [Google Scholar]
- 49.Madisen L, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13(1):133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vasioukhin V, Degenstein L, Wise B, Fuchs E. The magical touch: Genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc Natl Acad Sci USA. 1999;96(15):8551–8556. doi: 10.1073/pnas.96.15.8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.