Abstract
Monocyte recruitment to inflamed arterial endothelium initiates plaque formation and drives progression of atherosclerosis. Three distinct monocyte subsets are detected in circulation (CD14++CD16−, CD14++CD16+, and CD14+CD16++), and each may play distinct roles during atherogenesis and myocardial infarction. We studied a range of subjects that included otherwise healthy patients with elevated serum triglyceride levels to patients presenting with acute myocardial infarction. Our objective was to correlate an individual’s risk with the activation state of each monocyte subset as a function of changes in adhesion receptor expression using flow cytometric quantitation of integrins and l-selectin membrane expression. A microfluidic-based laboratory-on-a-chip was developed to quantify the adhesion efficiency of monocytes sheared in whole blood on vascular cell adhesion molecule-1, while characterizing adhesion receptor expression and topography on captured monocytes. CD14++CD16+ monocytes adhered with sevenfold higher efficiency than other subsets, and in patients with myocardial infarction the capture efficiency of this subset was double that for healthy subjects. In patients with hypertriglyceridemia, this increase in monocyte adhesion was attributable to CD14++CD16+ uptake of triglyceride-rich lipoproteins and subsequent signaling via a Phospholipase C–dependent mechanism to increase CD11c expression, very late antigen–4 function, and integrin coclustering within focal adhesive sites on vascular cell adhesion molecule-1. In summary, we introduce a unique laboratory-on-a-chip method for quantifying the activation state of monocyte subsets. These experiments reveal that CD11c/CD18 is an inducible integrin whose expression correlates with a monocyte inflammatory state in subjects at risk for atherogenesis and in patients with myocardial infarction.
Keywords: B2-integrin, cell arrest, shear flow
Monocyte activation and adhesion to inflamed endothelium is an obligate step in the initiation of atherosclerosis (1). Despite the accepted paradigm of atherosclerosis as an inflammatory disease, population-wide studies that correlate established biomarkers (e.g., monocyte chemoattractant protein–1, myeloperoxidase, c-reactive protein) with the progression of coronary artery disease (CAD) and during acute myocardial infarction (MI) fail to provide a personalized quantitative measure of activation at the level of the participating immune cells (2). Monocyte recruitment to sites of atherogenesis is associated with accumulation of lipoproteins and differentiation of monocytes into foamy macrophages, but the exact mechanisms through which circulating lipoproteins interact with blood monocytes remain unclear. Although low-density lipoprotein (LDL) is a clinically important marker used to predict onset of CAD, serum triglyceride level and its content in triglyceride-rich lipoproteins (TGRLs) are emerging as metabolically driven biomarkers for predicting acute cardiovascular events (3). In this context, TGRLs containing apolipoprotein C-III remain in circulation due to diminished hepatic clearance (4), are readily taken up by monocytes in blood (5), and are endocytosed by endothelial cells, resulting in vascular cell adhesion molecule-1 (VCAM-1) up-regulation and enhanced recruitment of monocytes in shear flow (6). Activation of monocytes and their increased influx to coronary arteries correlate with plaque progression and instability, and inflammatory monocytes contribute to infarct size (7).
Monocytes in the circulation represent a heterogeneous population, from naïve myeloid progenitors in the bone marrow to differentiated subsets that are stimulated during transport through microvasculature of the spleen and adipose tissue (8). Three major subsets of monocytes have been identified based on relative expression of the lipopolysaccharide coreceptor CD14 and the FcγRIII receptor CD16 (9). The “classical” monocyte (CD14++CD16−, Mon1) accounts for 80–90% of cells in circulation, whereas the “intermediate” (CD14++CD16+, Mon2) and “nonclassical” (CD14+CD16++, Mon3) subsets account for the remaining 10–20%. The relative distribution of monocytes observed in the circulation is dynamic and changes as a function of the inflammatory and metabolic drivers that influence differentiation between the subsets and as a function of the extent of CAD. For example, Mon2 and Mon3 are more prevalent in subjects with elevated triglycerides and are reported as proinflammatory cytokine-producing subsets whose numbers are elevated in the circulation of patients with atherosclerosis and CAD (10). Changes in expression of integrins [CD11b, very late antigen–4 (VLA-4)] and l-selectin provide a gross measure of monocyte inflammatory activation in blood, but these methods do not gauge the relative level of monocyte activation from resting to maximally activated and poised for recruitment to inflamed arteries.
Functional studies of monocyte recruitment indicate that Mon2 has high potential to participate in atherosclerosis based on differential expression of chemokine and adhesion receptors that orchestrate their recruitment to vasculature with elevated VCAM-1 expression and interstitial MCP-1 (11). We have identified CD11c/CD18 as a functional receptor expressed on an inflammatory subset of monocytes (5). CD11c/CD18 is up-regulated in apolipoprotein E–deficient mice made dyslipidemic on a high-fat diet, and genetic depletion of CD11c abrogated the development of atherosclerosis (12). CD11c/CD18 and CD49d/CD29 (VLA-4) were identified as the principal receptors necessary for facilitating monocyte arrest on VCAM-1 (12). In humans with hypertriglyceridemia, expression of CD11c is up-regulated on circulating monocytes within hours of consumption of a high-fat meal (5). This up-regulation of CD11c correlated with the uptake of TGRL, appearance of foam cells in blood, and enhanced efficiency of monocytes to recruit to VCAM-1 in shear flow. In vitro studies confirmed that uptake of TGRLs by monocytes in blood is mediated by LDL receptor–1 (LRP-1) (5), however the mechanism linking hypertriglyceridemia to CD11c up-regulation and increased VLA-4 function remains unclear. These findings prompted our current focus on the mechanism by which up-regulation of CD11c expression during hypertriglyceridemia and cardiovascular disease is related to the increased capacity of monocytes to activate VLA-4 and recruit on VCAM-1.
Although previous studies have suggested an important role for Mon2 and CD11c in atherosclerotic cardiovascular disease, no prior study has integrated phenotypic characterization of monocyte subsets with a functional readout of cell activation state. We developed a microfluidic-based laboratory-on-a-chip assay that facilitates interrogation of monocytes capturing on VCAM-1 under fluid shear stress to mimic exposure to focal sites of arterial inflammation. Through these experiments, we show that Mon2 uptake of TGRL via LRP-1 leads to increased CD11c expression and VLA-4 function via a Phospholipase C (PLC)-dependent mechanism that involves integrin activation and colocalization within focal adhesive sites on VCAM-1. Cooperative regulation of Mon2 integrin function makes these cells a dynamic population that responds to perturbations in lipid uptake (among patients with high triglycerides) and local inflammation at the level of the coronary plaque (in MI).
Results
CD11c on Inflammatory Monocytes Along with Serum Triglycerides Are Elevated in the Postprandial State.
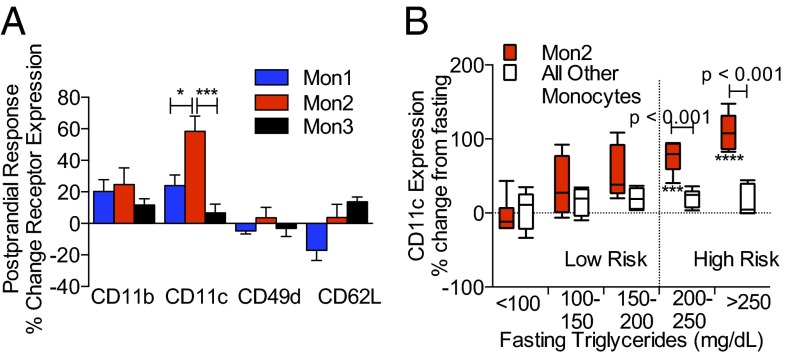
Monocyte adhesion receptor expression is rapidly regulated in response to uptake of TGRL, thereby facilitating recruitment to inflamed aortic endothelium during hypercholesterolemia-driven atherogenesis (5, 12). This observation motivated the measurement of acute changes in expression of integrins CD11b/CD18, CD11c/CD18, and CD49d/CD29 (VLA-4), as well as CD62L (l-selectin) on monocyte subsets to detect their activation state in blood samples from subjects during the fasting (Fig. S1) and postprandial state (Fig. 1). In response to a high-fat meal, CD11c expression increased by 60% on Mon2, whereas expression of other adhesion receptors did not change significantly on Mon2 or other subsets (Fig. 1A). We next assessed whether a subject’s fasting triglyceride (fTG) level was correlated with the extent of CD11c up-regulation on monocyte subsets. There was a trend for increased CD11c with triglyceride that reached significance at levels above 200 mg/dL only on Mon2, whereas CD11c expression on the other subsets did not change postprandial (linear regression of CD11c on Mon2 over range of fTG; Pearson r = 0.7563, P < 0.0001) (Fig. 1B). These data suggest that up-regulation of CD11c on Mon2 is associated with increased baseline cardiovascular risk in hypertriglyceridemic subjects.
Fig. 1.
Adhesion receptor expression on monocyte subsets in a fasting and postprandial state. (A) Receptor expression on monocyte subsets as labeled in postprandial subjects 3.5 h after consumption of a high-fat meal. Data are expressed as the percent change in receptor expression relative to fasting state. Significance was determined using a one-way ANOVA with a Tukey posttest, *P < 0.05, ***P < 0.005. (B) CD11c receptor expression on Mon2 versus Mon1 and Mon3 plotted as a function of fTGs. Subjects were categorized into low-risk (n = 15) or high-risk (n = 11) groups based on fTGs and postprandial change in CD11c. Subjects with fTGs ≥200 mg/dL were grouped as high risk (right of horizontal dashed line). Data are representative of 26 subjects (10 female). Significant changes in CD11c expression were determined using a repeated measures ANOVA with Dunnets posttest, ***P < 0.005, ****P < 0.001. Significance comparing Mon2 to other monocytes was determined using a one-way ANOVA with a Tukey posttest.
CD11c Up-Regulation Tracks in Time with Triglyceride Levels on Mon2 in High-Risk Subjects.
Increased fructose consumption raises a subject’s serum triglyceride levels significantly following a 2-wk fructose-supplemented diet (13). We evaluated changes in CD11c expression on circulating monocytes from subjects participating in a 2-wk high-fructose diet and tracked CD11c levels with serum triglyceride concentration over a 24 h feeding study (Fig. 2). Subjects were categorized into low- or high-risk groups based on fTG levels, and CD11c expression was measured on blood monocytes at intervals throughout the day. Among high-risk subjects, Mon2 exhibited a steady increase in expression of CD11c rising 100% above fasting levels, whereas no significant change in CD11c was observed on the other monocyte subsets or on those from low-risk subjects (Fig. 2A). Triglycerides increased threefold more for high-risk versus low-risk subjects (Fig. 2B). The change in CD11c on Mon2 tracked closely in time with the rise and fall in triglyceride concentration for high-risk subjects over 24 h, suggesting that these monocytes respond to acute changes in metabolic state.
Fig. 2.
Kinetics of CD11c expression on monocyte subsets tracked with serum triglyceride over a 24 h feeding study. (A) Monocyte CD11c expression and serum triglyceride levels plotted versus time for low-risk subjects (fTG <200 mg/dL, n = 4). (B) Monocyte CD11c expression and serum triglyceride levels plotted versus time for high-risk subjects (fTG >200 mg/dL, n = 5). Three meals were administered at 9:00 AM, 1:00 PM, and 6:00 PM, as indicated by black arrows. The percent change in CD11c expression from fasting was calculated at 1:00 PM, 3:00 PM, 6:00 PM, 12:00 AM, and 8:00 AM (the following day). Statistics were measured against fasting values, and significance was determined using a repeated measures ANOVA with Dunnets posttest, *P < 0.05, **P < 0.01, ***P < 0.005.
CD11c Expression on Mon2 Correlates with Biomarkers of MI Severity.
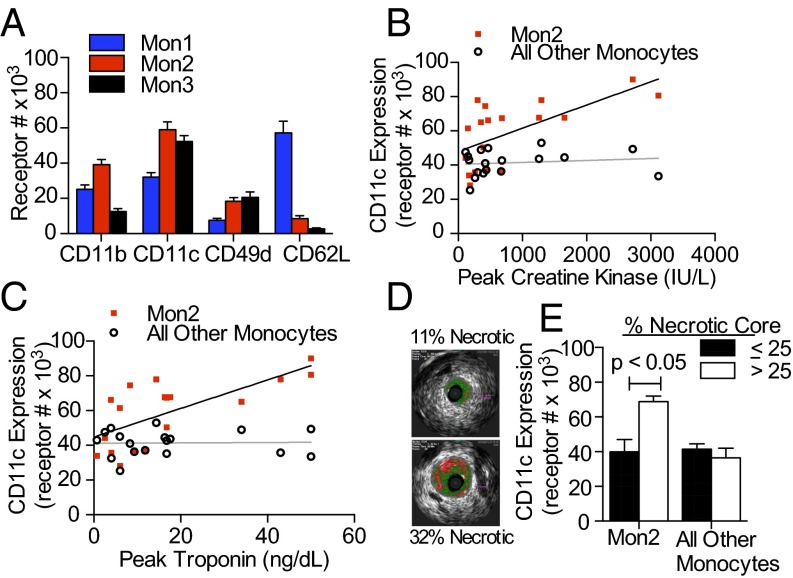
Monocytes are the principal inflammatory cells that initiate and participate in arterial plaque progression and instability, which motivated measurement of acute changes in adhesion molecule expression to quantify a monocyte activation state in blood. Receptor expression of CD11c and VLA-4 was ∼twofold higher on Mon1 and Mon2 from MI patients compared with those of fasting subjects in the feeding study, whereas there was no significant difference in adhesion molecule expression on Mon3 (Fig. 3A). Mon1 from MI patients had increased expression of CD62L compared with fasting subjects, and because MI patients have on average twice as many circulating Mon1 compared with healthy subjects (14), these data suggest mobilization of inactivated monocytes into the circulation in response to MI. To evaluate the relationship between CD11c expression and the severity of MI, we assessed the number of CD11c receptors on monocytes versus circulating levels of creatine kinase (Fig. 3B) (Pearson r = 0.6298, R2 = 0.39, P = 0.005) and troponin levels (Fig. 3C) (Pearson r = 0.6919, R2 = 0.48, P = 0.002), each of which is associated with myocardial infarct size. CD11c receptor number on Mon2 positively correlated with peak levels of both troponin and creatine kinase, whereas no significant correlation was observed for Mon1 or Mon3 subsets. We also evaluated the relationship between culprit artery plaque characteristics as imaged by intravascular virtual histology, rendered from the ultrasound-derived echogenic properties of the obstructed coronary artery. MI patients were segregated based on necrotic plaque volume and compared for receptor expression of CD11c on their blood monocytes (Fig. 3D). Patients with a necrotic plaque volume ≥25% registered a significantly greater CD11c receptor number only on Mon2 and not other monocyte subsets. These data reveal that CD11c is elevated on Mon2 in MI patients compared with high-risk healthy subjects and that it increases in proportion with elevated clinical correlates of MI severity.
Fig. 3.
CD11c receptor number on Mon2 correlates with severity of MI and coronary artery plaque characteristics. (A) Receptor expression on monocyte subsets from fasting patients with MI on day of cardiac catheterization (n = 18). (B and C) Correlation of CD11c expression on Mon2 and all other monocytes with peak troponin and peak creatine kinase. (D) Representative images taken by intravascular ultrasound with virtual histology used to quantify a coronary arterial cross-sectional area (total colored area) and necrotic core percentage (red colored area), which was then integrated over the total plaque length to calculate volume. (E) Expression of CD11c on Mon2 versus Mon1 and Mon3 for two groups of MI patients. MI patients were grouped based on necrotic core volume percentage of <25 (18.4 ± 6.15, mean ± SD, n = 5) and >25 (30.7 ± 1.15, n = 3). Significance was determined by a Student t test.
CD11c Expression and VLA-4 Affinity Are Increased on Mon2 Following TGRL Uptake in a PLC-Dependent Manner.
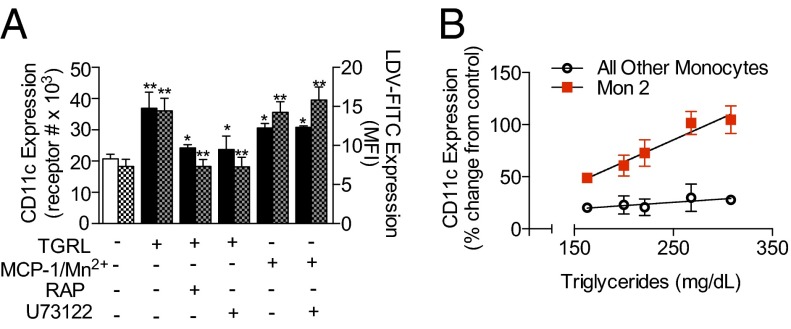
We have previously reported that TGRL uptake is mediated through LRP-1 and blocked with the LRP-1 antagonist receptor-associated protein (RAP). To elucidate the mechanism through which hypertriglyceridemia leads to increased CD11c expression on monocytes, side scatter (SSC) profile and CD11c expression were measured by FACS on freshly isolated monocytes incubated with TGRL isolated from postprandial blood of five subjects fed a high-fat meal. Mon1 and Mon2 exhibited a significant 30% increase in SSC following incubation with TGRL, and this correlated with uptake of apolipoprotein particles and formation of lipid droplets. To confirm the specificity of lipid endocytosis, we pretreated monocytes with RAP, which blocked lipid uptake by 54% for Mon1 and 100% for Mon2 (Fig. S2 B and C). Concomitant with lipid uptake, we observed a 70% increase in CD11c expression on Mon2, a level equivalent to that measured ex vivo in postprandial blood. Integrin up-regulation was also inhibited down to baseline levels with RAP (Fig. S2 A and B) pretreatment (Fig. 4A). There was no significant change in CD11c expression on Mon1 or Mon3 (Fig. 4B).
Fig. 4.
TGRL uptake increases membrane CD11c and VLA-4 affinity on Mon2. (A) CD11c expression (left axis, solid colors) and LDV-FITC expression (right axis, checkered boxes) on Mon2 following incubation with postprandial TGRL. Significance for TGRL alone, MCP-1 (30 nM), or Mn2+ (1 mM) treated monocytes was compared versus control (open white box). Blocking with RAP or PLC was compared against TGRL alone. Significance was determined by one-way ANOVA with Tukey posttest, *P < 0.05, **P < 0.01. (B) Percent change from baseline levels of CD11c receptor expression on Mon2 and all other monocytes plotted as a function of the mean triglyceride concentration of TGRL particles incubated with monocytes.
Because ligation of LRP-1 triggers PLC activation, which leads to a rise in intracellular Ca2+, activation of PKC, and subsequent degranulation (15, 16), we hypothesized that CD11c up-regulation following TGRL uptake is dependent on this signaling pathway. To test this hypothesis, we pretreated monocytes with the PLC inhibitor U73122 and measured lipid uptake and CD11c expression. PLC inhibition did not reduce the capacity of Mon2 to endocytose TGRL but did prevent CD11c up-regulation in the presence of TGRL; these effects reveal a specific pathway, as they were not observed in response to stimulation with MCP-1 (Fig. 4A, Fig. S2B). To assess Mon2 sensitivity to the triglyceride content of the TGRL, we plotted the extent of CD11c up-regulation against triglyceride concentration, which yielded a tight correlation (Pearson r = 0.9767, R2 = 0.9539, P = 0.004) (Fig. 4B). In contrast, Mon1 and Mon3 showed no significant increase in CD11c with triglyceride content (Pearson r = 0.8435, R2 = 0.7144, P = 0.07).
To quantify changes in VLA-4 function in response to uptake of TGRL and inside-out signaling, or allosteric activation by addition of Mn2+, we used 4-[(N′-2-methylphenyl)ureido]-phenylacetyl-L-leucyl-L-α-aspartyl-L-valyl-L-prolyl-L-alanyl-L-alanyl-L-lysine - FITC (LDV-FITC) as a reporter of conversion of the integrin to the high-affinity conformation. Mon2 exhibited the highest expression of LDV-FITC at baseline compared with Mon1 and Mon3 (Fig. S2 D and E), and following uptake of TGRL or activation via Mn2+ doubled its expression. Signaling via TGRL uptake was blocked by pretreatment with RAP or the PLC inhibitor U73122, whereas extracellular activation via Mn2+ was unaffected (Fig. 4A).
Lab-on-a-Chip Phenotyping of Monocytes Recruited on VCAM-1 Under Shear Flow.
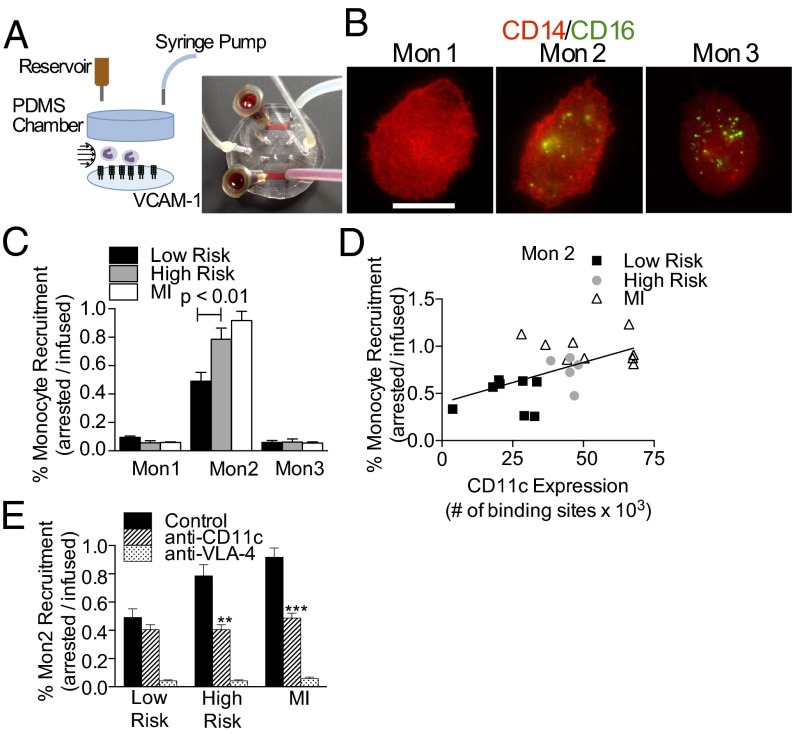
Monocyte recruitment from the blood stream to a nascent site of arterial plaque formation expressing VCAM-1 is an early step in atherogenesis (1). Our strategy was to characterize the integrin dependence of monocyte recruitment using a custom laboratory-on-a-chip microfluidic channel that recapitulates arterial hydrodynamic transport (Fig. 5A) (17). Whole blood was sheared over a glass coverslip functionalized with recombinant VCAM-1, and arrested monocyte subsets were discriminated based on the relative immunofluorescence of antibodies to CD14 and CD16 (Fig. 5B and Fig. S3). Mon2 exhibited a sevenfold greater propensity to arrest on VCAM-1 compared with the other subsets (Fig. 5C). Discrimination of Mon2 in this assay is highlighted by the absence of a significant difference in the percent of Mon1, Mon3, or neutrophils (∼0.05%) recruited from blood samples of MI patients versus low- and high-risk subjects. In contrast, Mon2 from MI patients exhibited twice the capacity to capture under shear stress than monocytes from low-risk subjects. Moreover, a smaller albeit significant increase in Mon2 recruitment was detected in the high- versus low-risk subjects (Fig. 5C). Thus, adhesion of Mon2 exhibited a hierarchy in recruitment capacity among subjects with increased triglycerides and in response to acute MI, whereas Mon1 and Mon3 did not. Plotting recruitment capacity as a function of CD11c expression on Mon2 revealed a linear correlation (Pearson r = 0.5649, R2 = 0.3191, P = 0.006) between CD11c receptor number and Mon2 recruitment that discriminated the different patient populations (Fig. 5D).
Fig. 5.
Lab-on-a-chip analysis of monocyte recruitment on VCAM-1 under hydrodynamic shear. (A) Schematic and image of the arterial mimetic device detailed in SI Materials and Methods. (B) Arrested monocytes identified by anti-CD14 (red) and anti-CD16 (green) acquired using wide-field immunofluorescence (images are representative of 150–200 monocytes phenotyped per subject). (Scale bar, 10 μm.) (C) Postprandial monocyte recruitment efficiency for subjects categorized as low (fTG <200 mg/dL, n = 8) and high risk (fTG >200 mg/dL, n = 5) and MI patients (n = 8). (D) Recruitment efficiency plotted as a function of CD11c expression for postprandial study subjects and MI patients. A regression curve was fit to the means of low-risk, high-risk, and MI groups (Pearson r = 0.9957, R2 = 0.9915, P = 0.0587) (E) Integrin specificity of monocyte recruitment studied by application of blocking antibodies against CD11c and VLA-4 depicted for Mon2 and other subsets (Fig. S4). Statistics were measured against samples treated with the isotype antibody control for each group, and significance was determined by one-way ANOVA with Tukey posttest, **P < 0.01, ***P < 0.005.
CD11c and VLA-4 cooperate in mediating arrest of monocytes on VCAM-1 in response to postprandial elevation of triglycerides and during atherogenesis (5, 12). We evaluated the integrin specificity during arrest by addition of function-blocking antibodies before shearing blood over a VCAM-1 substrate. Pretreatment of blood samples with antibody 496K, which binds to an allosteric site on the metal-ion–dependent adhesion site (MIDAS) domain and inactivates high-affinity CD11c binding function (18), reduced the arrest fraction of Mon2 for high-risk and MI subjects to levels equivalent to that of low-risk subjects (Fig. 5E). Inactivation of CD11c did not further diminish the already low recruitment efficiency of Mon1 and Mon3 (Fig. S4 A and B). In contrast, treatment of blood with function-blocking antibody to VLA-4 abrogated monocyte arrest on VCAM-1 that was equivalent to blocking with anti-VCAM-1 (Fig. S4C). These data confirm that Mon2 adhesion on VCAM-1 in shear is a cooperative process partly dependent on the conformation of CD11c, but predominantly dependent on VLA-4.
Monocyte Capture on VCAM-1 Is Regulated by Activation of CD11c and VLA-4.
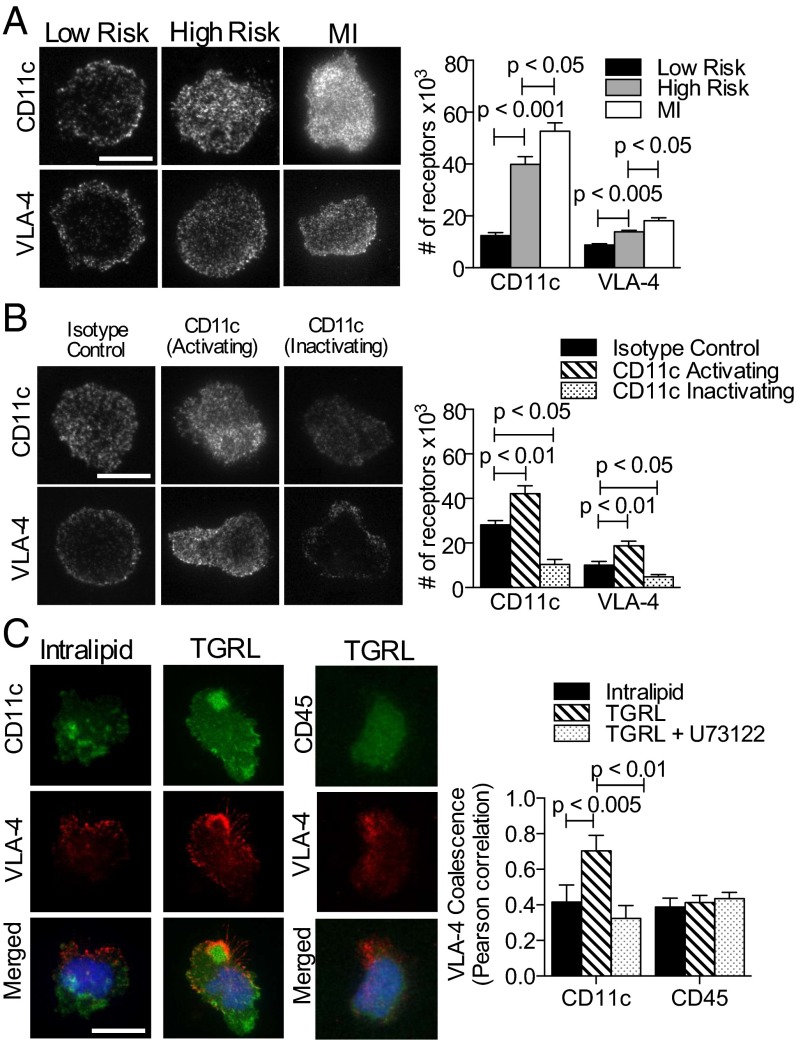
To characterize the integrin binding mechanisms underlying the hierarchy in Mon2 recruitment efficiency observed between postprandial subjects and MI patients, we used total internal reflection fluorescence (TIRF) microscopy, which provides optical discrimination of receptor enrichment within the plane of adhesive contact with VCAM-1. Blood samples obtained from meal study subjects and MI patients were sheared across a substrate of VCAM-1 in the microfluidic flow channels, and integrin receptor interactions were imaged on adherent monocytes after labeling with fluorescent antibodies against CD11c or VLA-4 (Fig. 6A). CD11c receptor number increased threefold within the contact region for high- versus low-risk subjects, and this was augmented by 25% in samples from MI patients (Fig. 6A).
Fig. 6.
CD11c and VLA-4 density and topography on monocytes at adhesive contact with VCAM-1. (A) TIRF images of anti-CD11c and anti–VLA-4 reveal receptors in contact with VCAM-1 acquired from postprandial study subjects; low-risk (n = 4), high-risk (n = 5), and MI patients (n = 5) (images are representative of 20–30 monocytes per subject). (Scale bar, 10 μm.) Receptor number was quantified using calibration beads as described in SI Materials and Methods. (B) TIRF images of CD11c and VLA-4 on monocytes treated with allosteric antibody 496B-activation, 496K-inactivation, or an isotype control antibody (images are representative of 20–30 monocytes per condition). (Scale bar, 10 μm.) Number of CD11c and VLA-4 receptors on arrested monocytes from healthy donors (n = 5). Monocytes in blood were treated with 496B, 496K, or isotype control prior to the adhesion assay. (C) Integrin colocalization on VCAM-1 was imaged by TIRF microscopy on monocytes incubated with TGRL or control Intralipid, and a group was pretreated with the PLC inhibitor U73122 (images are representative of 15–20 monocytes per condition). VLA-4 (red) coalescence with CD11c (green) or VLA-4 with CD45 at the contact site was quantified using Pearson’s coefficient. Significance was determined by one-way ANOVA with Tukey posttest.
To determine whether CD11c affinity state influences integrin binding to VCAM-1 and the efficiency of monocyte recruitment in shear, blood was pretreated with allosteric antibodies that stabilize the inactivated (496K) and activated (496B) ligand-binding conformation of CD11c (18). Fresh blood obtained from fasting subjects was treated with allosteric or isotype control antibody and subsequently sheared on VCAM-1 and compared for VLA-4 receptor number and focal clustering on captured monocytes (Fig. 6B). Allosteric activation elicited a ∼onefold enrichment in CD11c number in contact with VCAM-1, and this translated to a concomitant increase in VLA-4, which rose to a level equivalent to that supporting recruitment of Mon2 from the MI subject’s blood (Fig. 6 A and B). In contrast, stabilizing CD11c in an inactive state significantly diminished integrin accumulation to levels below the detection limit of ∼100 sites/μm2 as assessed by labeling with isotype control antibody. This was accompanied by a diminished capacity for both integrins to organize into focal clusters on VCAM-1.
To determine the mechanism underlying increased CD11c and VLA-4 expression and clustering at the contact site, we assessed the topography of CD11c and VLA-4 on monocytes activated by uptake of TGRL. Two-color TIRF images revealed that treatment with TGRL, and not a control emulsion (intralipid), elicited a doubling in the number of VLA-4 colocalized with CD11c on arrested monocytes (Fig. 6C). In contrast, CD45 receptor topography remained unaltered in response to TGRL, confirming specificity in the synergistic redistribution of CD11c and VLA-4 within focal clusters. To gauge the importance of signaling via PLC, monocytes were treated with TGRL along with the inhibitor U73122. A decrease in the coalescence of VLA-4 with CD11c to baseline was observed. Taken together, these data indicate that CD11c numbers exceed that of VLA-4 within the contact region and that the organization and activation state of CD11c within sites of focal adhesion is synergistic with VLA-4 in mediating firm arrest of monocytes on VCAM-1.
Discussion
Inflammatory monocytes in the circulation precipitate atherosclerosis and are associated with the onset of MI, yet functional biomarkers that reliably predict their participation in disease initiation and progression are lacking (19). By interrogating monocyte receptor number and adhesion function in blood samples soon after venipuncture or cardiac catheterization, we identified CD11c on Mon2 as a reliable biomarker that increased with the extent of a subject’s hypertriglyceridemia and in proportion to the severity of myocardial injury in MI patients. After shearing blood across a VCAM-1 substrate in a unique microfluidic device, we observed (i) a ∼sevenfold higher recruitment efficiency for Mon2 over Mon1 or Mon3, (ii) a hierarchy in capture efficiency that was ∼50% higher for MI patients than for postprandial hypertriglyceridemic subjects at high risk of cardiovascular disease (CVD), (iii) that CD11c mediates Mon2-specific adhesion efficiency and is up-regulated via LRP-1–mediated uptake of TGRL and signaling through PLC, and (iv) that CD11c activation state regulates the number of VLA-4 enrichment with the contact region that supports shear-resistant monocyte capture on VCAM-1.
Acute Changes in Monocyte Phenotype Correlate with Dyslipidemia and Atherosclerosis.
CD11c expression and function increase on monocytes in the blood of humans and mice that take up very-low-density lipoprotein (vLDL) particles in vitro (5, 12). Here we report that CD11c expression on Mon2 in postprandial blood samples was up-regulated up to threefold more than conventional measures of acute monocyte activation, including increases in CD11b, CD49d, and shedding of CD62L. CD11c expression level also discriminated low- versus high-risk subjects based on fTGs of these individuals. Analysis of Mon2 in the high-fructose feeding study confirmed that this subset, and not monocytes in general, provides a dynamic measure of systemic metabolic stress among high-risk subjects. We also confirmed in an in vitro assay that incubating monocytes with TGRLs elicited an increase in membrane CD11c at levels equivalent to that measured in hypertriglyceridemic subjects ex vivo. LRP-1–mediated uptake and integrin up-regulation were dependent on PLC, as both VLA-4 conversion to high affinity and adhesion to VCAM-1 increased with TGRL uptake. Whether monocyte activation reflects changes in a single population induced in the circulation, or a change in lineage dynamics during chronic inflammation, requires further investigation.
Discrimination of a Mon2 Activation State in Blood Samples of High-Risk and MI Patients.
Inflammatory monocytes are an independent risk factor for CAD even after adjusting for other confounding conditions such as diabetes, hypertension, and hyperlipidemia (20). Direct evidence that inflammatory monocytes contribute to CVD derives from monocyte subset immunophenotyping, which has shown that inflammatory monocytes express CCR2, CX3CR1, and CCR5; up-regulate CD11b receptor expression; and bind VCAM-1 (21). Our studies of adhesion receptor changes on postprandial monocytes and on those from MI patients confirmed the trend for CD11b up-regulation, but changes in CD11c expression and adhesion function correlated more closely with measures of cardiovascular disease risk and markers of damage due to MI. Remarkably, CD11c receptor number on Mon2 increased steadily from low- to high-risk subjects as a function of serum triglyceride level and was highest on patients with MI.
Microfluidic devices have proven to be effective tools for quantifying the adhesion potential of leukocytes as they flow across endothelial monolayers or substrates presenting recombinant adhesion molecules (22). Shearing blood over a recombinant VCAM-1 substrate resulted in enrichment of Mon2 with an efficiency of capture that exceeded Mon1 and Mon3. Although Mon3 had the highest baseline expression of CD11c relative to other subsets, Mon2 exhibited a ∼sevenfold greater recruitment to VCAM-1 under shear flow that was dependent on high-affinity CD11c and VLA-4. Mon2 was the most responsive subset to hypertriglyceridemia and MI injury as indicated by significant increases in CD11c expression and capacity to arrest on VCAM-1. A positive linear regression emerged when comparing CD11c expression and recruitment efficiency of Mon2, allowing distinction between low-risk, high-risk, and MI cohorts. These findings suggest that CD11c expression and VLA-4 avidity for binding to VCAM-1 on Mon2 could provide a subject-specific measure of cardiovascular risk, although larger scale studies incorporating other clinical characteristics will be necessary to confirm the clinical utility of such an approach.
CD11c and VLA-4 Cooperate During Recruitment of Mon2 on VCAM-1.
A shift in integrin conformation from low to high affinity and clustered distribution facilitates leukocyte arrest on inflamed sites in the vasculature (23). Using our laboratory-chip device, we observed that monocyte arrest was dependent on recognition and binding of VLA-4 to VCAM-1 and this was modulated by CD11c affinity state. Importantly, the hierarchy in recruitment of Mon2 for high-risk subjects and MI patients was accounted for by increased surface expression and function of the activated conformation of CD11c. The synergistic relationship between CD11c activation and VLA-4 binding to VCAM-1 was dependent on activation of PLC, a signaling molecule that mediates Ca2+ flux and redistribution and stabilization of focal clusters of high-affinity VLA-4 that support monocyte adhesion strengthening at arterial levels of shear stress (24). TIRF optics, which excite only membrane receptors in contact with the substrate, revealed that CD11c number increased fourfold on monocytes from high-risk subjects and MI patients, and this correlated with a 60% enrichment in VLA-4 bound to VCAM-1. Focal clustering of VLA-4 is a mechanism associated with adhesion strengthening of recruited monocytes under shear flow (25) and is cooperative with β2 integrin activation to a high-affinity state. Monocyte arrest on VCAM-1 in this laboratory-chip assay required VLA-4, as indicated by abrogation of recruitment in the presence of function-blocking antibody. VCAM-1 can serve as a ligand for high-affinity CD11c, and we observed a threefold enrichment of CD11c over VLA-4 redistributed within the contact region upon activation in vitro with TGRL or detected in ex vivo blood samples in high-risk and in MI patients. We also found that stabilizing CD11c at low affinity with the allosteric antibody 496K abrogated the increase in Mon2 arrest observed in high-risk and MI subjects. Thus, the molecular mechanisms underlying CD11c cooperation with VLA-4 remain unknown, but may involve CD11c shift to high affinity in circulation, subsequent recognition of focally expressed VCAM-1 on inflamed endothelium stabilization, and localization of high-affinity VLA-4 within lipid rafts that are enriched in cytoplasmic paxillin, talin, and Src family kinases (SFKs) (26). Current studies in our laboratory are focused on quantifying the spatial and temporal dynamics of CD11c and VLA-4 redistribution and their possible colocalization with adapter proteins and SFKs during adhesion to VCAM-1 under shear flow.
Lab-on-a-Chip Assay for Assessing the Severity of CVD.
In this study, we report that CD11c and VLA-4 receptor expression on Mon2 and relative capacity to recruit on VCAM-1 in shear flow provide a sensitive readout of perturbations in the inflammatory and metabolic status of an individual. CD11c and VLA-4 receptor expression levels on Mon2 increased by ∼25% and ∼5%, respectively, from fasting to the postprandial state, whereas receptor numbers of both integrins increased by 100% in patients with MI. Mon2 recruitment on VCAM-1 proved to be the best discriminator of activation, increasing by 60% from low- to high-risk (P < 0.01) and 87% from low-risk to MI patients (P < 0.005). Our portable and personalized laboratory-chip assay within minutes provides a rapid measure of monocyte activation state that can resolve differences between subjects with risk factors for atherosclerosis or with established coronary disease such as acute MI. In the case of early atherosclerosis, identification of Mon2 activation may prompt a clinician to prescribe more intensive lifestyle modifications that are specific to predisposing risk factors, such as dietary lipids or high blood pressure. Among patients with MI, increased Mon2–CD11c activation may identify patients at elevated risk for recurrent cardiovascular events. Specific interventions to abrogate Mon2–CD11c activation could also limit infarct size by limiting Mon2 localization during an acute MI. Further longitudinal characterization of Mon2–CD11c levels and their contribution to atherosclerosis could lead to development of unique risk markers and therapeutics for patients with cardiovascular disease.
Materials and Methods
Subjects.
Twenty-six healthy subjects (10 female) were recruited for the meal study, nine subjects (three female) were recruited for the fructose feeding study, and an additional 18 fasting patients (four female) with acute MI were recruited from the University of California Davis Medical Center. All experiments were approved by institutional review board protocols at the University of California, Davis. Participating subjects and patients provided informed consent as approved by institutional review board protocols at the University of California, Davis. Clinical characteristics for the recruited cohorts are available in Tables S1–S4.
Antibodies.
Commercially available antibodies are listed in SI Materials and Methods. CD11c monoclonal antibodies 496K and 496B, which inactivate or activate CD11c, respectively, were obtained from Eli Lilly.
Flow Cytometry.
Whole blood was drawn via venipuncture and immediately cooled to 4 °C. After labeling with fluorescent antibodies for 30 min, red blood cells were removed with lysis buffer (eBiosciences). LDV-FITC (Tocris Bioscience) was used to label high-affinity VLA-4 at 30 nM. Data were acquired on a BD FACScan cytometer within 2 h of venipuncture. The gating scheme for identification of monocyte subsets is shown in Fig. S5.
Whole Blood Adhesion Assay.
Design and assembly of the microfluidic device and the whole blood adhesion assay are described in SI Materials and Methods. Arrested monocytes were discriminated into subsets using quantitative immunofluorescence (Fig. S6). Quantification of receptor topography is described in SI Materials and Methods.
Statistics.
Data are reported as mean ± SEM. Multiple groups were compared using one-way ANOVA with Tukey posttest. Multiple time points were compared using a repeated measures one-way ANOVA with Dunnets posttest. All analyses were carried out using Graph Pad Prism version 5.0d for Mac.
Supplementary Material
Acknowledgments
The authors thank Kristine Kikly (Eli Lilly) for providing antibodies and Christina Edwards, Vivien Lee, and Hazel Lam for their invaluable assistance as clinical coordinators. This work was supported by NIH HL082689, HL098839 (to S.I.S.), AHA 11CRP7260031 (to E.J.A.), NIH K12 HD051958 (to K.L.S.), and NIH HL091333, HL107256 (to P.J.H.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1300651110/-/DCSupplemental.
References
- 1.Cybulsky MI, Gimbrone MA., Jr Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991;251(4995):788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- 2.Libby P, Ridker PM, Hansson GK. Leducq Transatlantic Network on Atherothrombosis Inflammation in atherosclerosis: From pathophysiology to practice. J Am Coll Cardiol. 2009;54(23):2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller M, et al. American Heart Association Clinical Lipidology, Thrombosis, and Prevention Committee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Cardiovascular Nursing; Council on the Kidney in Cardiovascular Disease Triglycerides and cardiovascular disease: A scientific statement from the American Heart Association. Circulation. 2011;123(20):2292–2333. doi: 10.1161/CIR.0b013e3182160726. [DOI] [PubMed] [Google Scholar]
- 4.Mendivil CO, Zheng C, Furtado J, Lel J, Sacks FM. Metabolism of very-low-density lipoprotein and low-density lipoprotein containing apolipoprotein C-III and not other small apolipoproteins. Arterioscler Thromb Vasc Biol. 2010;30(2):239–245. doi: 10.1161/ATVBAHA.109.197830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gower RM, et al. CD11c/CD18 expression is upregulated on blood monocytes during hypertriglyceridemia and enhances adhesion to vascular cell adhesion molecule-1. Arterioscler Thromb Vasc Biol. 2011;31(1):160–166. doi: 10.1161/ATVBAHA.110.215434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang YI, et al. Endothelial inflammation correlates with subject triglycerides and waist size after a high-fat meal. Am J Physiol Heart Circ Physiol. 2011;300(3):H784–H791. doi: 10.1152/ajpheart.01036.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsujioka H, et al. Impact of heterogeneity of human peripheral blood monocyte subsets on myocardial salvage in patients with primary acute myocardial infarction. J Am Coll Cardiol. 2009;54(2):130–138. doi: 10.1016/j.jacc.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 8.Swirski FK, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325(5940):612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ziegler-Heitbrock L, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116(16):e74–e80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 10.Schlitt A, et al. CD14+CD16+ monocytes in coronary artery disease and their relationship to serum TNF-alpha levels. Thromb Haemost. 2004;92(2):419–424. doi: 10.1160/TH04-02-0095. [DOI] [PubMed] [Google Scholar]
- 11.Swirski FK, et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117(1):195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu H, et al. Functional role of CD11c+ monocytes in atherogenesis associated with hypercholesterolemia. Circulation. 2009;119(20):2708–2717. doi: 10.1161/CIRCULATIONAHA.108.823740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stanhope KL, et al. Consumption of fructose and high fructose corn syrup increase postprandial triglycerides, LDL-cholesterol, and apolipoprotein-B in young men and women. J Clin Endocrinol Metab. 2011;96(10):E1596–E1605. doi: 10.1210/jc.2011-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tapp LD, Shantsila E, Wrigley BJ, Pamukcu B, Lip GYH. The CD14++CD16+ monocyte subset and monocyte-platelet interactions in patients with ST-elevation myocardial infarction. The CD14++CD16+ Monocyte Subset and Monocyte-Platelet Interactions. 2012;10(7):1231–1241. doi: 10.1111/j.1538-7836.2011.04603.x. [DOI] [PubMed] [Google Scholar]
- 15.Misra UK, Gawdi G, Pizzo SV. Ligation of low-density lipoprotein receptor-related protein with antibodies elevates intracellular calcium and inositol 1,4, 5-trisphosphate in macrophages. Arch Biochem Biophys. 1999;372(2):238–247. doi: 10.1006/abbi.1999.1521. [DOI] [PubMed] [Google Scholar]
- 16.Chun J, Auer KA, Jacobson BS. Arachidonate initiated protein kinase C activation regulates HeLa cell spreading on a gelatin substrate by inducing F-actin formation and exocytotic upregulation of beta 1 integrin. J Cell Physiol. 1997;173(3):361–370. doi: 10.1002/(SICI)1097-4652(199712)173:3<361::AID-JCP8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 17.Tsou JK, et al. Spatial regulation of inflammation by human aortic endothelial cells in a linear gradient of shear stress. Microcirculation. 2008;15(4):311–323. doi: 10.1080/10739680701724359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sadhu C, Hendrickson L, Dick KO, Potter TG, Staunton DE. Novel tools for functional analysis of CD11c: Activation-specific, activation-independent, and activating antibodies. J Immunoassay Immunochem. 2008;29(1):42–57. doi: 10.1080/15321810701735062. [DOI] [PubMed] [Google Scholar]
- 19.Rogacev KS, et al. CD14++CD16+ monocytes independently predict cardiovascular events: A cohort study of 951 patients referred for elective coronary angiography. J Am Coll Cardiol. 2012;60(16):1512–1520. doi: 10.1016/j.jacc.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 20.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: Their role in infection and inflammation. J Leukoc Biol. 2007;81(3):584–592. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 21.Tacke F, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117(1):185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stott SL, et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc Natl Acad Sci USA. 2010;107(43):18392–18397. doi: 10.1073/pnas.1012539107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imhof BA, Aurrand-Lions M. Adhesion mechanisms regulating the migration of monocytes. Nat Rev Immunol. 2004;4(6):432–444. doi: 10.1038/nri1375. [DOI] [PubMed] [Google Scholar]
- 24.Hyduk SJ, et al. Phospholipase C, calcium, and calmodulin are critical for alpha4beta1 integrin affinity up-regulation and monocyte arrest triggered by chemoattractants. Blood. 2007;109(1):176–184. doi: 10.1182/blood-2006-01-029199. [DOI] [PubMed] [Google Scholar]
- 25.Chan JR, Hyduk SJ, Cybulsky MI. Alpha 4 beta 1 integrin/VCAM-1 interaction activates alpha L beta 2 integrin-mediated adhesion to ICAM-1 in human T cells. J Immunol. 2000;164(2):746–753. doi: 10.4049/jimmunol.164.2.746. [DOI] [PubMed] [Google Scholar]
- 26.Manevich E, Grabovsky V, Feigelson SW, Alon R. Talin 1 and paxillin facilitate distinct steps in rapid VLA-4-mediated adhesion strengthening to vascular cell adhesion molecule 1. J Biol Chem. 2007;282(35):25338–25348. doi: 10.1074/jbc.M700089200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.