Abstract
Introduction
The outcome of patients with CML who discontinue 2G-TKI initial therapy is unknown. We analyzed the characteristics of patients in whom treatment with first-line 2G-TKIs had failed.
Patients and Methods
A total of 218 patients with CML were treated with dasatinib (n = 101) or nilotinib (n = 117; 12 in AP). After a median follow-up of 23 months, 40 patients (18%) discontinued therapy: 25 initially treated with nilotinib (21% of all treated with nilotinib; 6 treated in AP) and 15 (15%) initially treated with dasatinib. Median age of the patients was 47 (range, 19–79) years, and they had received therapy for a median of 8 (range, 0–62) months.
Results
Reasons for treatment discontinuation include: toxicity, 16 patients; resistance in CP, 5 patients; transformation to blast phase, 4 patients (2 treated in AP); and other reasons, 15 patients. Subsequent treatment was imatinib in 11 patients, nilotinib in 7, dasatinib in 4, ponatinib in 2, chemotherapy plus dasatinib in 3, stem cell transplant in 2, bafetinib in 1, and unknown or none in 8 patients. A complete cytogenetic response was achieved in 19 patients, including 17 with major molecular response. Fourteen of the patients who achieved a complete molecular response or major molecular response with subsequent TKIs were in CP at the time of 2G-TKI discontinuation.
Conclusion
We conclude that treatment failure after first-line therapy with 2G-TKIs is mostly associated with toxicity or patient preference, and these patients respond well to alternative TKIs.
Keywords: Dasatinib, Nilotinib, CML, TKIs, Discontinuation
Introduction
Imatinib has been used as the standard first-line therapy for the management of patients with chronic myeloid leukemia (CML) for nearly a decade. With standard-dose imatinib, a complete cytogenetic response (CCyR) is achieved in approximately 85% of CML patients, with a major molecular response (MMR) in approximately 60%.1–7 However, more than 30% of patients do not have a favorable long-term outcome using imatinib, including 15% to 20% of patients who never achieve a CCyR, 10% to 15% who achieve CCyR but eventually lose it, and 4% to 8% who are intolerant to imatinib.7 In view of this, second-generation tyrosine kinase inhibitors (TKIs; nilotinib, dasatinib, bosutinib) were developed. These agents are more potent inhibitors of Bcr-Abl kinase activity and might overcome resistance by most Bcr-Abl mutants associated with resistance to imatinib. With these agents, CCyR is achieved in approximately 50% of patients in whom previous imatinib therapy had failed.8–11 More recently, second-generation TKIs have been used as initial therapy for patients with CML in chronic phase (CP).12–14 High rates of CCyR and MMR have been achieved at early time points, and randomized trials demonstrated improved response rates and decreased rate of transformation compared with imatinib.15–17 Dasatinib and nilotinib have recently received regulatory approval for first-line therapy in chronic-phase CML. Despite the success of using these agents, in some patients therapy fails using second-generation TKIs as initial therapy. One concern about the widespread use of dasatinib and nilotinib as initial therapy is the uncertainty regarding treatment options for patients who received these agents as initial therapy and required subsequent therapy. There is currently no available information on the outcome and management of these patients. The objective of this analysis is to provide the first description of the management and outcome of patients after they discontinue nilotinib or dasatinib as initial therapy for their disease.
Patients and Methods
From June 2005 to August 2011, 218 patients were enrolled in 2 parallel studies of second-generation TKIs as initial therapy for CML in early CP conducted at a single institution, 1 with nilotinib (n = 117) and 1 with dasatinib (n = 101). Patients in accelerated phase (AP) who had never received previous therapy were eligible for the nilotinib trial (n = 12). A total of 40 patients (18%) were eventually taken off therapy and constitute the focus of this report, including 34 patients who were in CP and 6 in AP at the start of frontline therapy. Among these 40 patients, 25 had received initial therapy with nilotinib (6 in AP at the start of therapy) representing 21% of all patients treated with this agent, and 15 initially treated with dasatinib (representing 15% of all patients treated with dasatinib). Subsequent therapy on discontinuation of the initial agent was determined by their treating physician. The medical records were reviewed to determine the patient characteristics, subsequent therapy, and outcome with such therapy. Patients were included in a retrospective chart review protocol for patients with CML approved by the Institutional Review Board.
Chronic myeloid leukemia phases were classified according to standard definitions used in clinical trials for TKIs.12,13,18 Response to therapy was defined as previously reported.19 Briefly, complete hematologic response (CHR) required a white blood cell and platelet count within normal limits with normal differential and absence of signs or symptoms of disease. Cytogenetic response was classified according to the best response determined by cytogenetic analysis using G-banding in 20 metaphases. A CCyR was defined as 0% of metaphases with the Philadelphia chromosome (ie, 0% Ph+ metaphases), a partial cytogenetic response (PCyR) as 1% to 35% Ph+ metaphases, a minor cytogenetic response (minCyR) as 36% to 95% Ph+ metaphases, and no response with > 95% Ph+ metaphases. Molecular response was assessed using real-time polymerase chain reaction of peripheral blood. An MMR was defined as a Bcr-Abl ratio of ≤ 0.1% on the International Scale; a complete molecular response (CMR) was defined as undetectable transcript levels in a sample with sensitivity of at least 5 logs.
Statistical Analysis
The distribution of time-to-event end points, overall survival (OS), event-free survival (EFS), failure-free survival (FFS), and transformation-free survival (TFS), were estimated using the Kaplan-Meier method. OS was measured from the time the patients discontinued their first TKI to the date of death from any cause at any time or date of last follow-up. EFS was measured from the time of start of first therapy after discontinuation of first-line therapy to the date of any of the following events, whichever occurred first: death from any cause during therapy, loss of CHR or loss of minCyR during second therapy, or progression to AP or blast phase (BP). TFS was measured from the time of start of first therapy after discontinuation of first-line therapy to the date of transformation to AP or BP during second therapy or the date of the last follow-up. FFS was measured from the time of start of first therapy after discontinuation of first-line therapy to the date of any of the following events: loss of CHR or loss of CCyR during second therapy, failure to achieve CCyR within 18 months of second therapy, discontinuation of second therapy because of toxicity or lack of efficacy, progression to AP or BP, and death from any cause during therapy.
Results
After a median follow-up of 23 months (range, 0–72 months) from start of initial therapy with dasatinib or nilotinib for the 218 patients treated in the 2 parallel studies, 40 patients (18%) had discontinued therapy, 15 (15%) initially treated with dasatinib and 25 (21%) initially treated with nilotinib (including 6 of the 12 patients in AP at the time of their initial TKI therapy). The median age for these 40 patients was 47 years (range, 19–79 years), and they had received nilotinib or dasatinib therapy for a median of 8 months (range, 0–62 months) (Table 1). Eighteen (45%) of the 40 patients had required treatment interruptions at some time during the course of therapy with the first TKI and 35% had required at least 1 dose reduction. The best response to first-line treatment with nilotinib or dasatinib was CMR in 3 patients (8%), MMR in 13 (32%), CCyR in 8 (20%), PCyR in 3 (8%), minCyR in 1 (2%), CHR with no cytogenetic response in 10 (25%), and 2 (5%) patients were not evaluable because of short duration of therapy. The response status at the time of treatment discontinuations were CMR in 1 patient, MMR in 11 patients, CCyR in 5, PCyR in 3, minCyR in 1, CHR in 10, AP determined by clonal evolution in 2 and by platelet count in 1, BP in 4, and 2 were nonevaluable because of very short duration of first TKI therapy.
Table 1.
Characteristics of Patients at the Time of Discontinuation of Their First TKI
| Characteristic | Value |
|---|---|
| Median Age (Range), y | 49 (19–81) |
| Male, n (%) | 22 (55) |
| Sokal Score at Start (Patients in CP), n (%) | |
| Low risk | 26 (65) |
| Intermediate risk | 9 (23) |
| High risk | 5 (12) |
| Initial TKI, n (%) | |
| Dasatinib | 15 (38) |
| Nilotinib | 25 (62) |
| Median Duration of First Therapy, mo (Range) | 8 (0–62) |
| Patients With Dose Interruptions, n (%) | 18 (45) |
| Patients With Dose Reductions, n (%) | 14 (35) |
| Median Follow-up From Failure to First TKI, mo (Range) | 13 (0–72) |
| Disease Stage, n | |
| CP | 33 |
| AP | 3 |
| BP | 4 |
Abbreviations: AP = accelerated phase; BP = blast phase; CP = chronic phase; TKI = tyrosine kinase inhibitor.
The reasons for treatment discontinuation are summarized in Table 2. The most common reason was adverse events in 16 (40%; 7 nilotinib and 9 dasatinib) patients. Adverse events leading to treatment discontinuation are described in Table 2. Patients who discontinued treatment because of adverse events had been treated for a median of 10 months (range, 1–72 months). The next most common reason for treatment discontinuation was personal reasons in 15 (38%) patients, including lack of compliance (n = 7), insurance reasons (ie, lack of coverage for standard follow-up procedures [drugs were provided to study participants at no cost], n = 3), and patient choice (n = 5). Of the latter group, 1 patient who opted to discontinue therapy with dasatinib died of metastatic pancreatic cancer shortly after discontinuation. Nine patients discontinued therapy because of loss of response, including 4 who transformed to BP (all 4 in lymphoid BP [LyBP]; all receiving nilotinib). Two of these patients had been initially treated in AP. Among the other 5 patients who discontinued because of loss of response, 2 never achieved cytogenetic response after 6.5 and 7 months of initial therapy with nilotinib and 3 had lost CCyR (1 was in AP and the other 2 in CP with PCyR and minCyR at the time treatment was discontinued). At the time of treatment discontinuation 8 of the patients who had lost response were screened for mutations using direct sequencing. A mutation was identified in 5 patients (1 patient had T315I, and 2 each had Y253H and E255K), all initially treated with nilotinib. In addition, 2 of the 5 patients who had lost response developed clonal evolution at the time of treatment discontinuation, 1 in AP and the other in BP. In both instances, clonal evolution was represented by a double Philadelphia chromosome. One patient who discontinued therapy because of adverse events attributed to nilotinib, developed BP after 1 month of imatinib therapy as the second TKI; at the time BP was identified, she had clonal evolution and an F359C mutation.
Table 2.
Reason for Treatment Discontinuation Among the 40 Patients Included in the Analysis
| Cause | n |
|---|---|
| Nilotinib Group | |
| Personala | 11 |
| Blast phase | 4 |
| Loss of response | 3 |
| Toxicityb | 7 |
| Dasatinib Group | |
| Personala | 4 |
| Toxicityc | 9 |
| Loss of response | 2 |
Personal reasons include: lack of compliance, lack of insurance coverage, and patient choice.
Nilotinib toxicity: liver (n = 2), pancreatitis (n = 2), acute renal failure (n = 1), pericardial effusion (n = 1), hyperbilirubinemia (n = 1), atrial fibrillation (n = 1); 1 patient had concomitant pancreatitis and atrial fibrillation.
Dasatinib toxicity: pleural effusion (n = 3), thrombocytopenia (n = 1), asthma (n = 1), gastrointestinal bleeding (n = 1), bone pain (n = 1), headache (n = 1), and congestive heart failure (n = 1).
Treatment and Outcome After Discontinuation of First TKI
The second treatment of patients in CP (or AP according to clonal evolution alone) at the time of discontinuation of the first TKI is described in Tables 3 and 4. Among the 16 patients who discontinued therapy because of intolerance, 7 patients received nilotinib, 5 imatinib, 3 dasatinib, and 1 bafetinib (INNO-406) as their next therapy. The starting dose was what is considered standard for these agents in 9 patients (for bafetinib, 480 mg twice a day was given, with 240 mg twice a day identified as recommended phase 2 dose) and 7 started at reduced doses. After a median second therapy time of 9 months (range, 1–39 months), 9 patients achieved or maintained MMR (including 4 with CMR). The best response for the other 6 patients was CCyR in 1 (12 months), PCyR in 1 (20 months), minCyR in 2 (1 and 2 months, respectively), and CHR in 2 (1 month and 4 months each using nilotinib). This represented a loss of response in 3 patients from MMR to minCyR, from CCyR to minCyR, and from minCyR to BP. All had received nilotinib as first-line therapy. One patient in this cohort was lost to follow-up after he was switched to imatinib. Five of the other 15 patients who discontinued the first TKI because of toxicity required treatment interruptions of their second TKI but only 2 required dose reductions and none discontinued the second TKI because of toxicity. After a median of 10 months (range, 3–46 months) from discontinuation of the first TKI, 14 patients are still in CP and 12 of them remain taking a second TKI. Three patients have received additional therapy after the second TKI, including imatinib plus hyperfractionated cyclophosphomide, vincristine, adriamycin and dexamethasone (for BP), dasatinib (for loss of response during second therapy with imatinib), and imatinib (for failure to achieve an acceptable response during second therapy with bafetinib). One patient in this group was diagnosed with a concomitant advanced esophageal cancer, acquired severe pneumonia, developed respiratory failure, and died in CP after second TKI treatment. His second treatment (imatinib) was put on hold 8 months before his death because of his worsening comorbidities. He had achieved CCyR 3 months before he died. One patient transformed to BP shortly after discontinuation because of toxicity (described later in text).
Table 3.
Outcome After Second Treatment in Patients Who Discontinued First-line Therapy for Adverse Events or Personal Reasons
| Reason for Treatment Discontinuation of First TKI | Disease Status at Start of Second Therapy | Second Therapy | Best Response | Duration of Best Response (mo)a |
|---|---|---|---|---|
| Adverse Events (n= 16) | ||||
| MMR, n = 7 | Nilotinib | CMR | 12+ | |
| Nilotinib | CMR | 7+ | ||
| Nilotinib | CMR | 9+ | ||
| Dasatinib | MMR | 15.5+ | ||
| Dasatinib | MMR | 9.5+ | ||
| Bafetinib | minCyR | 1.5 | ||
| Imatinib | NA | NA | ||
| CCyR, n = 5 | Imatinib | MMR | 17.5+ | |
| Nilotinib | MMR | 38.5+ | ||
| Nilotinib | CCyR | 9.5 | ||
| Nilotinib | NA | b | ||
| Imatinib | minCyR | 1 | ||
| PCyR, n = 2 | Imatinib | MMR | 38.5+ | |
| Dasatinib | CMR | 3+ | ||
| CHR, n = 2 | Imatinib | PCyR | 4 | |
| Nilotinib | CHR | 4+ | ||
| Personal (n = 15) | ||||
| CMR, n = 1 | Unknown | NA | NA | |
| MMR, n = 4 | Died before second therapy | NA | NA | |
| Unknown, n = 3 | NA | NA | ||
| CHR, n = 5 | Allogeneic SCT | CMR | 36.5+ | |
| Imatinib, n = 3 | NA | NA | ||
| Unknown, n = 1 | NA | NA | ||
| Accelerated phase, n = 2 | Imatinib | MMR | 7.5+ | |
| Dasatinib | NA | NA | ||
| Nonevaluable, n = 3 | Imatinib | MMR | 12+ | |
| Unknown | NA | NA | ||
| Unknown | NA | NA |
Abbreviations: CCyR = complete cytogenetic response; CHR = complete hematologic response; CMR = complete molecular response; minCyR = minor cytogenetic response; MMR = major molecular response; NA = not available; PCyR = partial cytogenetic response; SCT = stem cell transplantation; TKI = tyrosine kinase inhibitor.
“+” Indicates the duration continued past the indicated time period.
Too early to evaluate.
Table 4.
Outcome in Patients (All Chronic Phase) Who Discontinued First-line Therapy Because of Resistance to First-line TKI
| Patient | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Age (y) | 28 | 40 | 49 | 67 | 55 |
| First-line Therapy | Dasatinib | Dasatinib | Nilotinib | Nilotinib | Nilotinib |
| Dose at the Time Resistance Documented (mg/d) | 100 | 100 | 300 b.i.d. | 400 b.i.d. | 400 b.i.d. |
| Sokal Score | Low risk | Low risk | Intermediate risk | Intermediate risk | Low risk |
| Response to First-line Therapy at 3 mo | PCyR | CCyR | CHR | CCyR | CHR |
| Best Response to First-line Therapy | CCyR | CCyR | CHR | MMR | minCyR |
| Time to Resistance (mo) | 36 | 18 | 8 | 20 | 6 |
| %Ph at Time of Resistance | 10 | 70 | 97 | 32 | 73 |
| Mutation at Resistance | None | None | None | Y253H | T315I |
| Second Therapy | Imatinib | Unknown | Ponatinib | Ponatinib | Allogeneic SCT |
| Dose of Second Therapy (mg/d) | 400 | na | 45 | 45 | na |
| Response to Second Therapy at 3 mo | CHR | na | CCyR | MMR | CMR |
| Best Response to Second Therapy | CHR | na | CCyR | CMR | CMR |
| Duration of Best Response (mo)a | 4 | na | 6 | 11+ | 3+ |
| Third Therapy | Allogeneic SCT | na | Allogeneic SCT | None | None |
| Status | Alive at 24 mo (CP) | Alive at 50 mo (CP) | Alive at 4 mo (CP) | Alive at 13 mo (CP) | Alive at 4 mo (CP) |
Abbreviations: b.i.d. = twice per day; CCyR = complete cytogenetic response; CHR = complete hematologic response; CMR = complete molecular response; CP = chronic phase; minCyR = minor cytogenetic response; MMR = major molecular response; na = not available; PCyR = partial cytogenetic response; %Ph = percentage of Philadelphia chromosome-positive metaphases; SCT = stem cell transplantation; TKI = tyrosine kinase inhibitor.
“+” Indicates the duration continued past the indicated time period.
The 15 patients who discontinued therapy for personal reasons had received the first TKI for a median of 8 months (range, 0.1–50 months). One patient received no further therapy and died from newly diagnosed metastatic pancreatic cancer 7 weeks after discontinuation of the first TKI therapy, and 8 were lost to follow-up on discontinuation of the first TKI therapy. Among the others, 4 received imatinib therapy, 1 received dasatinib therapy, and 1 received an allogeneic stem cell transplant (SCT). Two patients have achieved a MMR (both taking imatinib), 1 (SCT) achieved CMR, and 1 who had progressed to AP because of noncompliance is now taking dasatinib (less than 1 month of therapy; too early to assess response). Three other patients were lost to follow-up after treatment change. Survival information is available regarding the death of 1 patient for unknown reason in the latter group.
The 5 patients who discontinued the first TKI in CP because of resistance had received initial therapy using dasatinib in 2 and nilotinib in 3 (Table 4). Both patients taking dasatinib had achieved CCyR and later lost it. One patient had no further response in follow-up after discontinuation, but is known to be alive 50 months after discontinuation. The second patient lost hematologic response while taking dasatinib. No mutations were identified in the Abl kinase domain but clonal evolution was identified with der(7), ins(7;?)(p13;?), inv(7)(p11.2p15), and del(8)(p12). The patient received imatinib as second therapy and achieved only CHR. She later underwent an allogeneic SCT and achieved a sustained CMR ongoing 24 months after the transplant. Of the 3 patients taking nilotinib, 1 achieved MMR and then lost it. She was found to have a Y253H mutation and received ponatinib as second therapy and achieved a sustained CMR ongoing for 11 months. The other 2 taking nilotinib never achieved CCyR; 1 was found to have T315I and underwent an allogeneic SCT with CMR in 3 months, and the other (no mutations identified), received ponatinib as second therapy, achieved CCyR in 6 months, and later received an allogeneic SCT.
Outcome of Patients in BP at the Time of Treatment Discontinuation
Four patients were in BP at the time of discontinuation of therapy with the first TKI. All 4 had received nilotinib for a median of 6 months (range, 2.2–10 months), 2 of them in AP at the start of therapy (criteria for AP: peripheral blood blast 11% and 15%, respectively). The first patient was a 35-year-old man who achieved CCyR 3 months after the start of therapy with nilotinib but developed LyBP 6 months later with a mutation E255K. He was treated with Hyper CVAD plus dasatinib, achieved a remission, and then received an allogeneic SCT, and had a sustained CMR 3 months after the SCT. Eventually his disease relapsed without response to the next line of treatments and he died 36 months after first treatment discontinuation. The second patient was a 56-year-old man who achieved only CHR using nilotinib but rapidly developed LyBP 2 months after the start of therapy. He died before receiving further therapy. ABL sequencing was not performed. The third patient was a 53-year-old man who started nilotinib in CP, achieved PCyR at 3 months, and developed LyBP 5 months after the start of initial therapy. He had a Y253H mutation at the time of treatment failure along with clonal evolution with −7, −15, i(17q), and −20. He received Hyper CVAD plus dasatinib, achieved MMR followed by allogeneic SCT. Twelve months after transplantation he had a sustained MMR. The fourth patient was a 25-year-old woman who developed to LyBP after 10 months of initial therapy with nilotinib and CCyR as best response. She had a E255K mutation at the time of BP development. She received Hyper CVAD plus dasatinib and achieved CMR in 1 month and then underwent allogeneic SCT, sustaining CMR 6 months after transplantation.
Of interest, 1 patient who was taking nilotinib and achieved CCyR at 3 months, discontinued nilotinib because of liver toxicity in CP, and soon after she started taking imatinib developed LyBP. An F359C mutation was identified at the time of LyBP and the cytogenetic analysis demonstrated clonal evolution with t(1;2)(q44; q31). The patient received imatinib plus Hyper CVAD and achieved CMR at 2 months. She then underwent an allogeneic SCT that induced CMR, which was ongoing after 67 months.
Long-term Outcome
The median follow-up from the time of discontinuation of first TKI for patients in CP or with clonal evolution was 13 months (range, 0–72 months). Ten patients were lost to follow-up on discontinuation of first TKI or immediately after treatment change and are therefore not evaluable for long-term survival outcomes. For 2 additional patients only survival information is available after discontinuation of first TKI. The median EFS for the remaining 24 patients is 44 months. Among these patients, 3 (13%) progressed to accelerated or BP, for a survival free from transformation at 36 months of 86%. Only 3 patients have died. The causes of death were metastatic pancreatic cancer, esophageal cancer with concomitant respiratory failure because of pneumonia, and unknown, respectively. The OS at 36 months is 75%. Among the 4 patients in BP at the time of treatment discontinuation, 2 are alive after a median of 14 months of follow-up (both after SCT). Long-term outcomes are illustrated in Figures 1–4.
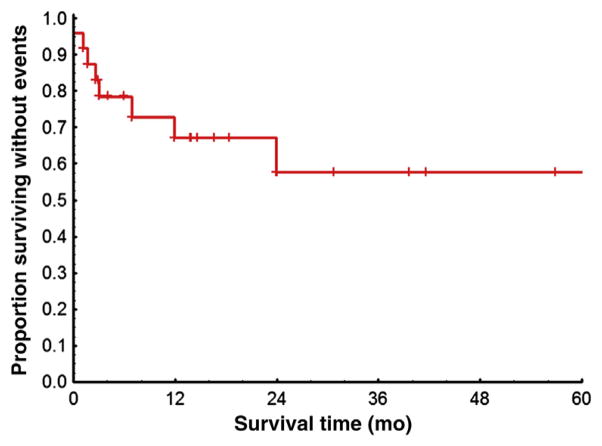
Figure 1. Kaplan-Meier Estimates of Event-Free Survival Rates Among 24 Patients in CP at the Time of First TKI Discontinuation.
Excluded are 10 patients lost to follow-up and 2 patients with only survival information available. Events were defined as death, loss of CHR or loss of minCyR, and progression to AP or BP during second therapy from the time of the first TKI discontinuation.
Abbreviations: AP = accelerated phase; BP = blast phase; CHR = complete hematologic response; CP = chronic phase; minCyR = minor cytogenetic response; TKI = tyrosine kinase inhibitor.
Figure 4. Kaplan-Meier Estimates of Overall Survival Rates Among 26 Patients in CP at First TKI Discontinuation.
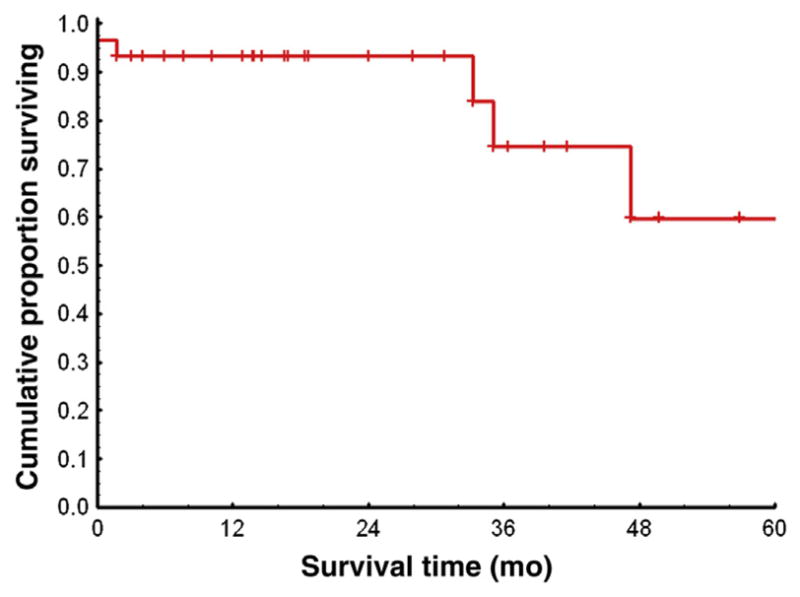
Excluded are 10 patients lost to follow-up.
Abbreviations: CP = chronic phase; TKI = tyrosine kinase inhibitor.
Discussion
Excellent results have been reported when using second-generation TKIs as initial therapy for CML in early CP. Data from phase II studies have demonstrated high response rates at early time points, with CCyR rates of > 90% as early as 3 months from the start of therapy, and MMR > 60% at 12 months.12–14 Randomized studies have demonstrated that results using nilotinib and dasatinib are superior to those using imatinib for rates of CCyR, and these 2 agents and bosutinib in terms of MMR and rate of transformation, albeit with short follow-up.15–17 These results have led not only to regulatory approval of dasatinib and nilotinib as initial therapy for patients with CML in CP, but also to the increased use of them in a growing number of patients at the time CML is confirmed. One of the concerns about the widespread use of these agents as initial therapy is the options that might be available for patients who do not have a favorable outcome. When imatinib is used as initial therapy, this concern is alleviated by the availability of 2 agents, dasatinib and nilotinib, that have proven efficacy and safety in the setting of imatinib failure,8–10 and are widely available in many parts of the world. Dasatinib and nilotinib have been used as initial therapy for CML in CP (in clinical trials) since 2005 and, to our knowledge, this is the first report on the management of patients who discontinue therapy with these agents for any reason.
Treatment discontinuation has been uncommon, occurring in only 40 (18%) of the 218 patients treated. This rate compares favorably with the 20% to 25% reported from large randomized trials.20,21 A common cause of treatment discontinuation was for personal and other reasons. This included patients who decided early during the course of therapy to abandon the trial and receive imatinib therapy, frequently out of concern of the novelty of the approach at the time they were enrolled, or because third parties would not cover the cost of monitoring the patient for CML as required during a clinical trial. As expected, most of these patients responded well to imatinib.
Among the 16 patients who discontinued because of intolerance, 9 have achieved or maintained an MMR (8 already in MMR and 1 in CCyR at the time of treatment discontinuation), though the others had less than a CCyR. It is possible that the response might still improve in some of these patients because the follow-up is still short. Subsequent treatment has been compromised in few of these patients because of the need for subsequent treatment interruptions because of adverse events but none have discontinued therapy with the second TKIs because of intolerance. Cross-intolerance has been reported to be minimal for patients who experience intolerance to imatinib and receive subsequent therapy with dasatinib or nilotinib.22 Whether the same is true when these agents are used in reverse order (ie, imatinib after intolerance to dasatinib or nilotinib) or when one second-generation agent is changed to the next has not been reported to date, and this series represents a glimpse into this option. Some adverse events are less prone to cause cross-intolerance, but others, particularly myelosuppression, might recur more frequently despite a change to the next agent.22 Only 6% of patients have discontinued nilotinib or dasatinib as first-line therapy because of intolerance in our studies, with few discontinuing because of myelosuppression. This rate is similar to what has been reported for imatinib in the IRIS (International Randomized Study of Interferon Versus STI571) trial (approximately 4%–8%).1–3 None of the patients in our series discontinued the second TKI therapy and only 2 patients reduced their second TKI dose because of intolerance, suggesting minimal cross-intolerance.
As expected, in view of the excellent results reported with dasatinib and nilotinib as initial therapy, very few patients switched therapy because of resistance. Of the 5 who had been treated in CP, 1 was lost to follow-up and the other 4 have had a favorable outcome after SCT or third TKIs. When dasatinib or nilotinib are used as second-line therapy, the use of the alternative second-generation TKI yields limited, and generally short duration, responses.23 More experience is required in this setting when these drugs are used as first-line therapy to be able to make firmer recommendations. However, it is reasonable to consider that a patient who experiences resistance to a second-generation TKI used as initial therapy for CML should be considered for SCT. New agents are being developed that offer promise for patients in whom second-generation TKI therapy has failed. Notably, results of a phase I study of ponatinib (AP24535) have suggested a minCyR in 66% of patients, most of whom had already received at least 3 TKIs.24 Two of our patients have already experienced excellent responses to ponatinib after nilotinib therapy had failed. Omacetaxine, formerly known as homoharringtonine, has also shown evidence of clinical activity in this setting, with cytogenetic responses in nearly 30% of patients.25 Using bosutinib, another second-generation TKI, minCyR rates of approximately 30% have been reported.26
One important question regarding patients treated with second-generation TKIs as initial therapy is whether this could exert a selection that could increase the frequency of detection of T315I. In the present series, only 1 of 8 patients who were sequenced has been identified to have a T315I mutation according to direct sequencing. Other studies have reported this mutation in a minority of patients after resistance to nilotinib or dasatinib.20,21 Taken together, there does not seem to be clear evidence of an increase in the frequency of this mutation among patients treated with dasatinib or nilotinib as initial therapy for CML. However, more patients and additional follow-up are required to understand the true effect of the use of second-generation TKIs in the possible selection of T315I or other mutations.
Conclusion
Treatment discontinuation of dasatinib and nilotinib used as initial therapy for CML is uncommon and most frequently because of intolerance or patient preference. In this setting, a significant number of patients who discontinue initial therapy with second-generation TKIs will have a favorable outcome. Additional data are needed to better understand the outcome of the few patients who discontinued because of resistance. These results, together with the excellent overall results reported for patients using these agents as first-line therapy, suggest that most patients with CML CP will benefit from a therapeutic strategy that involves initial use of dasatinib or nilotinib at the time of diagnosis.
Clinical Practice Points
Second-generation TKIs, dasatinib and nilotinib, have been approved as first-line therapy for CML patients because they induce sustained high rates of CCyR and MMR at earlier time points than imatinib.
Management of patients in whom first-line treatment with second-generation TKIs has failed is a challenge because there are no data available on outcomes of such patients.
A favorable outcome is observed among most patients who discontinue second-generation TKI therapy. Intolerance and patient preference are the main cause of therapy discontinuation and switching to other TKIs in this group of patients leads to a sustained acceptable response.
There are encouraging novel treatments along with SCT for patients who discontinue first-line therapy with second-generation TKIs because of resistance.
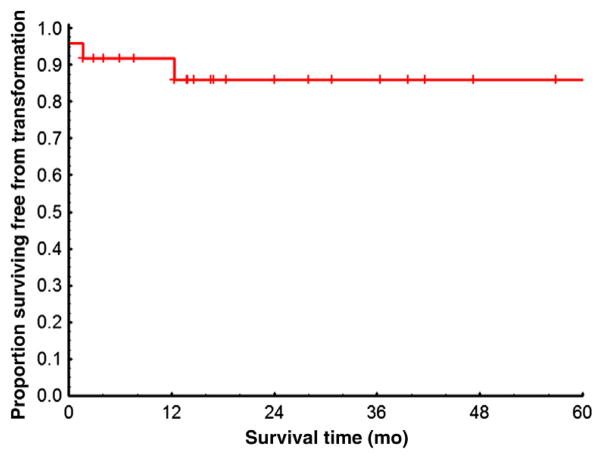
Figure 2. Kaplan-Meier Estimates of Transformation-Free Survival Rates Among 24 Patients in CP at the Time of First TKI Discontinuation.
Excluded are 10 patients lost to follow-up and 2 patients with only survival information available. Transformation-free survival was measured from the time of first TKI discontinuation to the date of AP or BP transformation during the second therapy or the date of the last follow-up.
Abbreviations: AP = accelerated phase; BP = blast phase; CP = chronic phase; TKI = tyrosine kinase inhibitor.
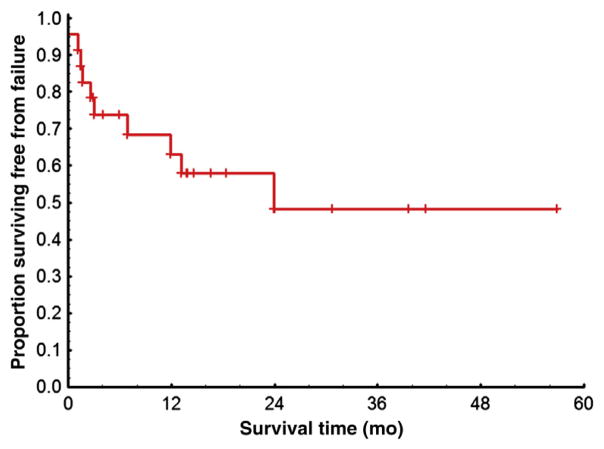
Figure 3. Kaplan-Meier Estimates of Failure-Free Survival Rates Among 23 Patients in CP at First TKI Discontinuation.
Excluded are 10 patients lost to follow-up, 2 patients with only survival information available, and 1 patient who died before initiation of second therapy. The failure was considered as loss of CHR or CCyR, discontinuation of second therapy for toxicity or lack of efficacy, progression to AP or BP, or death during second therapy.
Abbreviations: AP = accelerated phase; BP = blast phase; CCyR = complete cytogenetic response; CHR = complete hematologic response; CP = chronic phase; TKI = tyrosine kinase inhibitor.
Acknowledgments
Dr. Cortes’ participation in this study was supported in part by MD Anderson Cancer Center Support Grant CA016672 and Award Number P01 CA049639 from the National Cancer Institute.
Footnotes
Presented in part at the American Society of Hematology annual meeting, December 2010, Orlando, FL
Disclosure
J.C. received research support from Ariad, BMS, Chemgenex, Novartis and Pfizer, and is a consultant for Ariad, Pfizer and Teva. BMS, and Novartis. H.K. received research support from Pfizer, BMS, and Novartis. The remaining authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 2.de Lavallade H, Apperley JF, Khorashad JS, et al. Imatinib for newly diagnosed patients with chronic myeloid leukemia: incidence of sustained responses in an intention-to-treat analysis. J Clin Oncol. 2008;26:3358–63. doi: 10.1200/JCO.2007.15.8154. [DOI] [PubMed] [Google Scholar]
- 3.Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–17. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 4.Hochhaus A, O’Brien SG, Guilhot F, et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia. 2009;23:1054–61. doi: 10.1038/leu.2009.38. [DOI] [PubMed] [Google Scholar]
- 5.Hughes TP, Kaeda J, Branford S, et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med. 2003;349:1423–32. doi: 10.1056/NEJMoa030513. [DOI] [PubMed] [Google Scholar]
- 6.Kantarjian HM, Talpaz M, O’Brien S, et al. Survival benefit with imatinib mesylate versus interferon-alpha-based regimens in newly diagnosed chronic-phase chronic myelogenous leukemia. Blood. 2006;108:1835–40. doi: 10.1182/blood-2006-02-004325. [DOI] [PubMed] [Google Scholar]
- 7.O’Brien SG, Guilhot F, Goldman JM, et al. International Randomized Study of Interferon Versus STI571 (IRIS) 7-year follow-up: sustained survival, low rate of transformation and increased rate of major molecular response (MMR) in patients (pts) with newly diagnosed chronic myeloid leukemia in chronic phase (CMLCP) treated with imatinib (IM) ASH Annual Meeting Abstracts. 2008:112. (abstract 186) [Google Scholar]
- 8.Hochhaus A, Baccarani M, Deininger M, et al. Dasatinib induces durable cytogenetic responses in patients with chronic myelogenous leukemia in chronic phase with resistance or intolerance to imatinib. Leukemia. 2008;22:1200–6. doi: 10.1038/leu.2008.84. [DOI] [PubMed] [Google Scholar]
- 9.Kantarjian HM, Giles FJ, Bhalla KN, et al. Nilotinib is effective in patients with chronic myeloid leukemia in chronic phase after imatinib resistance or intolerance: 24-month follow-up results. Blood. 2010;117:1141–5. doi: 10.1182/blood-2010-03-277152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shah NP, Kim DW, Kantarjian H, et al. Potent, transient inhibition of BCR-ABL with dasatinib 100 mg daily achieves rapid and durable cytogenetic responses and high transformation-free survival rates in chronic phase chronic myeloid leukemia patients with resistance, suboptimal response or intolerance to imatinib. Haematologica. 2011;95:232–40. doi: 10.3324/haematol.2009.011452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cortes JE, Kantarjian HM, Brummendorf TH, et al. Safety and efficacy of bosutinib (SKI-606) in chronic phase Philadelphia chromosome-positive chronic myeloid leukemia patients with resistance or intolerance to imatinib. Blood. 2011;118:4567–76. doi: 10.1182/blood-2011-05-355594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cortes JE, Jones D, O’Brien S, et al. Nilotinib as front-line treatment for patients with chronic myeloid leukemia in early chronic phase. J Clin Oncol. 2010;28:392–7. doi: 10.1200/JCO.2009.25.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cortes JE, Jones D, O’Brien S, et al. Results of dasatinib therapy in patients with early chronic-phase chronic myeloid leukemia. J Clin Oncol. 2010;28:398–404. doi: 10.1200/JCO.2009.25.4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosti G, Palandri F, Castagnetti F, et al. Nilotinib for the frontline treatment of Ph(+) chronic myeloid leukemia. Blood. 2009;114:4933–8. doi: 10.1182/blood-2009-07-232595. [DOI] [PubMed] [Google Scholar]
- 15.Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362:2251–9. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 16.Cortes JE, Kim DW, Kantarjian HM, et al. Bosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: results from the BELA trial. J Clin Oncol. 2012;30:3486–92. doi: 10.1200/JCO.2011.38.7522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362:2260–70. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- 18.Talpaz M, Silver RT, Druker BJ, et al. Imatinib induces durable hematologic and cytogenetic responses in patients with accelerated phase chronic myeloid leukemia: results of a phase 2 study. Blood. 2002;99:1928–37. doi: 10.1182/blood.v99.6.1928. [DOI] [PubMed] [Google Scholar]
- 19.Baccarani M, Saglio G, Goldman J, et al. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108:1809–20. doi: 10.1182/blood-2006-02-005686. [DOI] [PubMed] [Google Scholar]
- 20.Kantarjian HM, Shah NP, Cortes JE, et al. Dasatinib or imatinib in newly diagnosed chronic-phase chronic myeoid leukemia: 2-year follow-up from a randomized phase 3 trial (DASISION) Blood. 2012;119:1123–9. doi: 10.1182/blood-2011-08-376087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saglio F. The incidence of BCR-ABL mutations in patients (pts) with newly diagnosed chronic myeloid leukemia (CML) in chronic phase (CP) treated with nilotinib or imatinib in ENESTnd: 24-month follow-up. J Clin Oncol. 2011;29(suppl):abstract 6502. [Google Scholar]
- 22.Cortes JE, Hochhaus A, le Coutre PD, et al. Minimal cross-intolerance with nilotinib in patients with chronic myeloid leukemia in chronic or accelerated phase who are intolerant to imatinib. Blood. 2011;117:5600–6. doi: 10.1182/blood-2010-11-318949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garg R, Kantarjian HM, O’Brien S, et al. The use of nilotinib or dasatinib after failure to 2 prior tyrodine kinase inhibitors: long term follow-up. Blood. 2009;114:4361–8. doi: 10.1182/blood-2009-05-221531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cortes JE, Kantarjian HM, Shah NP, et al. Ponatinib in refractory Philadelphia chromosome-positive leukemias. N Engl J Med. 2012;367:2075–88. doi: 10.1056/NEJMoa1205127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cortes JE, Lipton JH, Rea D, et al. Phase 2 study of subcutaneous omacetaxine mepesuccinate after TKI failure in patients with chronic-phase CML with T315I mutation. Blood. 2012;120:2573–80. doi: 10.1182/blood-2012-03-415307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khoury HJ, Cortes JE, Kantarjian HM, et al. Bosutinib is active in chronic pahse chronic myleoid leukemia after imatinib and dasatinib and/or nilotinib therspy failure. Blood. 2012;119:3403–12. doi: 10.1182/blood-2011-11-390120. [DOI] [PMC free article] [PubMed] [Google Scholar]