Abstract
Arrhythmias, especially supraventricular arrhythmias, often complicate the clinical course during autologous hematopoietic cell transplantation (AHCT). We undertook this study to determine the incidence and risk factors for cardiac arrhythmias during AHCT. The study included 983 patients who underwent AHCT between August 2006 and December 2010 at a single institution, and in whom all the relevant medical records were available for review. The median age was 58 years (range; 19–77); 61% were male. AHCT was done for plasma cell disorders in 58% patients and for lymphoma or leukemia in the remaining. Overall, 92 (9.4%) patients developed a supraventricular tachyarrhythmia at a median of 9 days post transplant (range; 0, 18) and with a median duration of <1 day (range; <1 to 17 days). Atrial fibrillation (AF) was the most common and seen in 71 (7%) patients, followed by atrial flutter and supraventricular tachycardia in 12 (1%) and 8 (1%) patients respectively. In multivariate analysis, age > 63 years, presence of premature supraventricular complexes or Atrio-ventricular conduction delay on pre-transplant ECG, and history of any prior arrhythmia increased the risk of arrhythmia. Development of arrhythmia resulted in longer outpatient follow up after AHCT, with the median follow-up for those developing an arrhythmia of 22 days compared with 19 days for the rest; P < 0.001. In conclusion, 9% of patients undergoing ASCT develop supraventricular arrhythmias post transplant and this risk is elevated among the older patients, those with a prior history of arrhythmias, and those with pre-transplant ECG abnormalities.
Keywords: Arrhythmia, autologous stem cell transplantation, atrial fibrillation, anti-arrhythmics Autologous hematopoietic cell transplant (AHCT), electrocardiographic (EKG)
INTRODUCTION
Autologous hematopoietic cell transplantation (AHCT) has become the standard of care for a variety of malignancies and is also increasingly being used for congenital as well as acquired disorders. Among more than 50,000 first HSCTs reported for 2006 worldwide; the leading indications were lymphoproliferative disorders (54.5%), leukemia (33.8%), solid tumors (5.8%) followed by nonmalignant disorders (5.1%) [1]. The majority of these transplants were done in either Europe (48%) or North America (36%), with AHCT constituting the majority. In the United States, AHCT is performed most commonly for multiple myeloma followed by the lymphomas. Although the process has become considerably safer over the past two decades, high dose chemotherapy and AHCT can be associated with significant morbidity related to a variety of side effects [2–4].
One of the more serious complications of AHCT remains cardiac toxicity. With the use of conventional pre-AHCT clinical cardiac evaluation, major cardiotoxic events such as heart failure, cardiac tamponade, and life-threatening cardiac arrhythmias have been reported to be uncommon, occurring in <1% of patients [5]. Arrhythmias, especially supraventricular arrhythmias, however more commonly complicate the clinical course of these patients [6–9]. This has been clear since the initial report in 1998 by Olivieri et al, who reported five cases of atrial fibrillation in stem cell transplantation recipients following high dose melphalan [10]. Subsequent studies reported the occurrence of supraventricular arrhythmias in 4–10% of bone marrow transplant recipients [6–9]. However factors predisposing to such arrhythmias have not been well studied to date.
While these arrhythmias are easily treatable in the majority of patients, they can still result in prolongation of hospital stay as well as an increased rate of intensive care admissions. We undertook this study to determine the incidence and risk factors for cardiac arrhythmias during AHCT.
PATIENTS AND METHODS
Following the approval from the Mayo Clinic Institutional Review Board, we retrospectively analyzed the medical records of 1000 consecutive patients who underwent AHCT for various malignancies between August 2006 and December 2010. All patients had given informed consent for the use of their records for research purposes. Seventeen patients who had AHCT for non-hematological malignancies and those who had incomplete information regarding various parameters described below were excluded leaving 983 patients who met the criteria. Twenty-two of the 983 patients received a combination of marrow and Peripheral Blood Stem Cell (PBSC) graft and all other patients received PBSC only. These patients were managed by multidisciplinary team on an outpatient basis and were admitted to hospital as and when indicated [11]. Patients were seen on a daily basis or more often if needed, with daily monitoring of CBC and chemistry with replacement of electrolytes and transfusions as clinically indicated. Patients were not routinely placed on cardiac monitoring of any nature irrespective of prior cardiac history.
For all patients, the medical records were reviewed for age, sex, height, weight, and clinical risk factors for atrial arrhythmia including presence of systemic hypertension, diabetes mellitus, coronary artery disease, valvular heart disease and thyroid diseases. Information regarding history of arrhythmia was obtained from the medical records. Additionally, we also reviewed the electrocardiographic (EKG) records in our Mayo EKG Database for any reports of arrhythmias or conduction abnormalities. All patients also underwent transthoracic echocardiography before transplant. The echocardiographic data were electronically retrieved and reviewed for parameters that could predispose to arrhythmias such as LV diastolic dysfunction, LV ejection fraction, indexed LA volume index and valvular lesions.
Logistic regression was used to identify the best cut-point for continuous variables, associated with the maximum risk of developing an arrhythmia. The Kaplan Meier method was used to estimate the median time to onset of any arrhythmia, with patients not developing any arrhythmia censored at the time of dismissal home post AHCT. The Cox proportional hazards model was used to estimate the risk associated with various baseline factors. Patient related variables included age, gender, diagnosis, history of coexisting illnesses such as coronary artery disease (CAD), hypertension, diabetes, prior history of arrhythmias, antiarrhythmic medications, or beta-blocker use. Cardiac parameters examined included LV ejection fraction, left atrial size, or presence of valvular abnormalities on echocardiogram, PR interval, QTc interval, presence of supraventricular complexes or Atrio-ventricular (AV) conduction delays/blocks which included 1 st and 2 nd degree AV blocks on pre-transplant ECG. Transplant related factors examined include position of central line tip (right atrium vs. superior vena cava), conditioning regimen, neutropenic fever, hypokalemia in the post transplant period, development of mucositis, and diarrhea. All the baseline variables were evaluated, and those which were found to be significantly associated with arrhythmia at a P level of <0.05 on univariate analysis were then considered for multivariate analyses. All analyses were performed using JMP® 9.03 software (SAS Institute Inc., North Carolina).
RESULTS
The median age of 983 patients who underwent AHCT between August 2006 and December 2010 was 58 years (range; 19–77), of which 61% were male. Among these patients, 41% had myeloma and together with amyloidosis and POEMS, plasma cell disorders were the indication for transplant in 58% of patients, followed by lymphomas (40%) and acute leukemia (2%) (Table 1). Eighty-two (8.3%) patients had a prior history of arrhythmia and 38 (3.9%) had a history of arrhythmia during the month prior to transplant. Eighteen (0.2%) of the patients had atrial fibrillation or flutter at the start of transplant. Two hundred seven (21.1%) patients were on a beta-blocker and 24 (2.4%) were on verapamil or diltiazem at the time of AHCT. The median estimated duration to dismissal from outpatient area to home hematologist (home-dismissal) post transplant was 19 days (95% CI; 19, 20). Overall, 29 (3%) patients died during the 100 days post transplant due to various reasons, including infectious causes, multi-organ failure, pulmonary complications, and progressive disease in 15 patients and infection, multi-organ failure syndrome, ARDS and or thromboembolic complications in the remaining 14 patients.
Table 1.
Baseline characteristics
| N | % | |
|---|---|---|
| No. of patients | 983 | – |
| Age (range) | 58 years | (19–77) |
| Gender: Male | 603 | 61% |
| Disease | ||
| Acute Leukemia (%) | 10 | 2% |
| Amyloidosis (%) | 140 | 14% |
| Hodgkin Disease (%) | 63 | 6% |
| Myeloma (%) | 404 | 41% |
| NHL (%) | 337 | 34% |
| POEMS (%) | 29 | 3% |
| Conditioning | ||
| BEAM | 395 | 40% |
| Busulfan + Cytoxan | 3 | 0.3% |
| Cytoxan + TBI | 10 | 1% |
| Melphalan | 565 | 58% |
| ThioTEPA/BCNU | 1 | 0.1% |
| Zevalin/Melphalan | 8 | 0.8% |
| Medical comorbidities | ||
| Hypertension | 372 | 37.8% |
| CAD | 75 | 7.6% |
| DM | 105 | 10.6% |
| Hypothyroidism | 114 | 11.6% |
| Hyperthyroidism | 2 | 0.2% |
| Renal insufficiency | 152 | 15.5% |
| COPD | 27 | 2.7% |
| Obstructive Sleep Apnea | 70 | 7.1% |
Overall, 92 (9.4%) patients developed a symptomatic supraventricular arrhythmia during the stem cell transplant course, at a median of 9 days post-transplant (range; 0, 18). The cumulative incidence of symptomatic arrhythmia in the post transplant period is as shown in Figure 1 (Kaplan Meier estimate). Atrial fibrillation was the most common and was seen in 71 (7%) patients, followed by atrial flutter in 12 (1%) and supraventricular tachycardia in 8 (1%) (Table 2). One patient developed multifocal atrial tachycardia. The rhythm had normalized in 81 (88%) patients at the time of dismissal post-transplant, with a median duration of arrhythmia of <1 day (range; <1 to 17 days). 82 (89%) of patients developing arrhythmia required treatment with most of them receiving a beta-blocker and/or calcium channel blocker. 36% of the patients with an arrhythmia developed hypotension, but only 14% required vasopressor support and 8% were electrically cardioverted during the peri-transplant period. 23 patients (25%) had recurrence of their arrhythmia before dismissal at a median time of 12.5 days (range, 5–21). The median time to dismissal after transplant for patients developing an arrhythmia was 22 days as compared to 19 days in those who did not; P < 0.001 (Figure 2).
Figure 1. Time to onset of arrhythmia post-transplant.
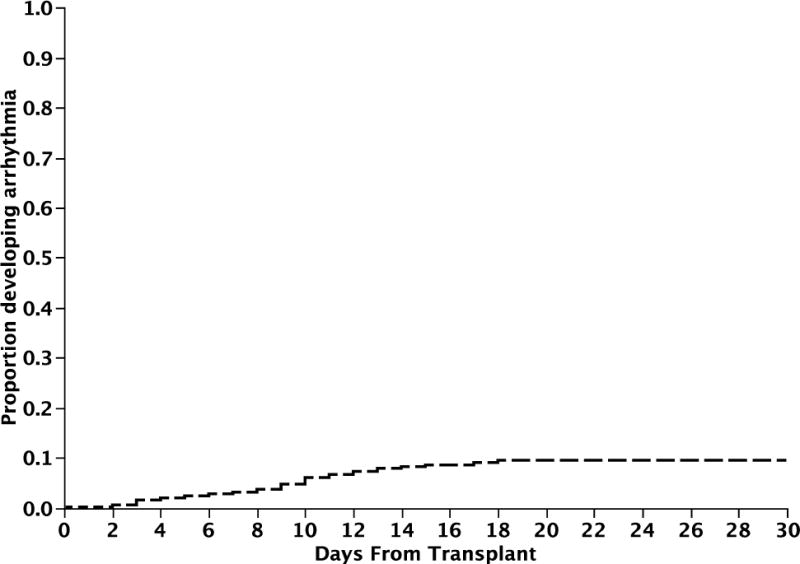
Figure 1 depicts the median time to onset of arrhythmia post transplant (Kaplan Meier estimate). The median estimated time was 9 days (95% CI; 8, 10).
Table 2.
Arrhythmia characteristics (n=92)
| Arrhythmia onset, BMT day (range) | 9 | 0–18 |
|---|---|---|
| Duration, mean (range) | <1 day | (<1 to 17 days) |
| Type of arrhythmia | ||
| Atrial Fibrillation | 71 | 78% |
| Atrial Flutter | 12 | 13% |
| SVT | 8 | 1% |
| MAT | 1 | <1% |
| Management | ||
| Treatment required | 82 | 89% |
| Beta blockers | 68 | 74% |
| Calcium channel Blockers | 40 | 43% |
| DC Cardioversion | 7 | 8% |
| Outcome | ||
| Hypotension | 33 | 36% |
| Vasopressors | 13 | 14% |
| Relapse | 23 | 25% |
Figure 2. Time to dismissal home after transplant.
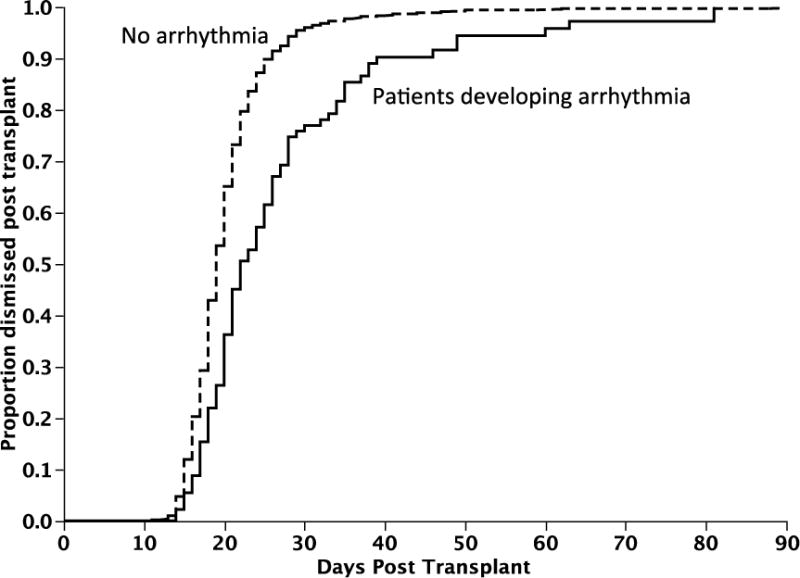
Figure 2 depicts the median time to dismissal home following transplant (Kaplan Meier estimate). The median time to dismissal after transplant for patients developing an arrhythmia was 22 days as compared to 19 days in those who did not; P < 0.001.
We then examined various pre and peri-transplant clinical and laboratory parameters to identify risk factors for onset of supraventricular arrhythmias. In a univariate analysis, older age, presence of supraventricular complexes or AV conduction delays such as 1 st or 2 nd degree AV block on pre-transplant ECG, presence of any valvular abnormality, presence of premature atrial complexes on ECG pre-transplant, increased atrial size, history of hypertension, history of CAD, any prior history of arrhythmia, or being on a beta blocker or an antiarrhythmic agent all increased the risk of developing a supraventricular arrhythmia following transplant (Table 3). We specifically examined the relation between amyloid heart disease and risk of developing arrhythmia. While there was a trend towards increased risk in the presence of amyloid heart disease, this was not significant (p=0.08). Using logistic regression, the best cutoff for age and for atrial size in terms of risk of developing arrhythmia was 63 years and 33 cc/m2. However, in a multivariate analysis, only age > 63 years, presence of supraventricular complexes or AV conduction delays on pre-transplant ECG, and history of any prior arrhythmia, increased the risk of arrhythmia during transplant. Among the patients with age > 63 years, presence of supraventricular complexes or AV conduction delays on pre-transplant ECG, and history of any prior arrhythmia, 20%, 26% and 23% respectively developed an arrhythmia compared to 4%, 8% and 8% among those without the risk factors, respectively (P < 0.0001 for each of the three comparisons) Patients with two or more risk factors, and those with one risk factor present were at 12.7 (95%CI; 6.7, 24) and 6.4 (95% CI; 3.8, 11.3) fold higher risk of developing arrhythmia compared to those with no risk factors (Figure 3). None of the underlying hematological malignancy or medical co-morbidities impacted the risk of developing arrhythmia.
Table 3.
Univariate Comparisons of patients with or without arrhythmia
| Characteristics | Arrhythmia (n=92) | Controls (n=891) | Risk Ratio (95% CI) | P-value | ||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Age > 63 years | 62 | 67% | 246 | 28% | 4.8 (3.2, 7.6) | < 0.0001 |
| Prior history of arrhythmia | 19 | 20% | 63 | 7% | 3.2 (1.9, 5.2) | <0.0001 |
| History of Hypertension | 47 | 51% | 325 | 36% | 1.7 (1.1, 2.6) | 0.01 |
| History of CAD | 15 | 16% | 60 | 7% | 2.5 (1.4, 4.2) | 0.003 |
| Beta blockers/antiarrhythmic at transplant | 31 | 34% | 183 | 21% | 1.9 (1.2, 3.0) | 0.005 |
| ECG features | ||||||
| AV conduction delay or Premature supraventricular complexes | 17 | 18.5% | 49 | 5.5% | 3.2 (1.8, 5.4) | 0.0002 |
| ECHO characteristics | ||||||
| Atrium >33 cc/m2 | 49 | 54% | 304 | 35% | 2 (1.3, 3.1) | 0.0008 |
| Presence of valvular abnormalities | 12 | 13% | 55 | 6% | 2.2 (1.1, 3.9) | 0.02 |
• Cox proportional hazards models
• Bolded rows indicate variables significant in multivariate analysis
Figure 3. Risk factors predicting development of arrhythmia post transplant.
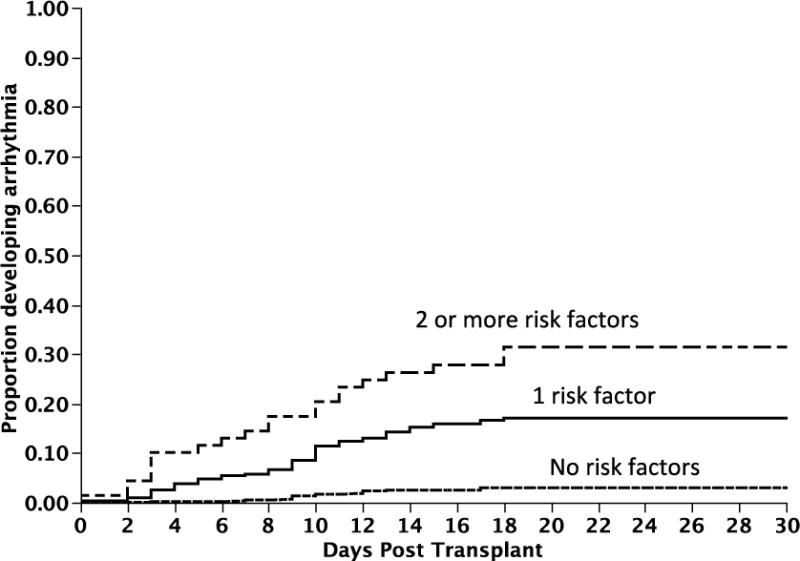
Figure 3 provides the cumulative incidence curves for onset of arrhythmia following transplant. Patients with two or more risk factors, and those with one risk factor present were at 12.7 (95%CI; 6.7, 24) and 6.4 (95% CI; 3.8, 11.3) fold higher risk of developing arrhythmia compared to those with no risk factors.
DISCUSSION
Supraventricular arrhythmias are a well-recognized complication after hematopoietic stem cell transplantation [6–9]. The incidence of these complications was 9% in this study, which is consistent with the previously reported incidence of 4–10% [6–9]. However, none of the studies so far including ours had patients on continuous cardiac monitors, so the true incidence may be even higher.
Atrial fibrillation was the most common arrhythmia and was present in about three-quarters (77%) of patients developing an arrhythmia. The median time of onset of arrhythmias was 9 days after AHCT and this is slightly later than the onset of between 3–7 days as reported by earlier studies [6, 8, 9]. Most arrhythmias were however short lasting with median duration of less than a day. Interestingly, one fourth of these patients had a recurrence of the arrhythmia at a median of 12.5 days but still most patients (88%) were discharged in sinus rhythm. Though a difference in mortality was not seen, these arrhythmias were associated with an increase in the median time to dismissal home from outpatient follow up by 3 days.
Though well known, studies evaluating risk factors for these arrhythmias are scarce. Most studies done so far have implicated older age and the presence of cardiac dysfunction in predisposition to this risk [6, 7, 9]. Even in the general population, the risk of developing AF increases with age, with approximately 70% of patients with AF being between age 65 and 85 years [12]. Statistical analysis of our results showed age >63 as a major risk factor with a risk ratio of 4.7.
Another predisposing factor emphasized in the multiple studies is the use of chemotherapeutic agents. Amongst the agents which are currently in use in transplant patients, the association of melphalan with AF is most well established and has been implicated in a significant proportion of patients including those without any structural or functional cardiac defects as well as those who are young [10]. Studies have shown melphalan use to be associated with AF in 6.6–11% of bone marrow transplant patients, which is significantly higher than any other chemotherapeutic agent [9, 13–15]. Our study cohort was mainly composed of patients with plasma cell disorders or lymphoma as the indication for transplant in most and thus melphalan free regimens were given in only a few patients. Hence the role of melphalan could not be ascertained from our study.
History of arrhythmias in the past was another predisposing factor we observed in this study. Surprisingly, being on an antiarrhythmic agent was shown as a risk factor but only on univariate analysis. This may reflect identification of those with prior history of arrhythmias. A predominance of older patients in our study with multiple co-morbidities requiring the use of these agents may explain this finding. Similarly, factors such as increased atrial size, history of hypertension or CAD were found significant on univariate model but not on the multivariate model. It is possible that all of these risk factors are inter related and may be predisposing to atrial arrhythmias even before the transplant.
More than half of our study group patients had a plasma cell disorder. Whether the presence of cardiac amyloidosis in these patients predisposes to arrhythmias, has always been a question. We found that even though a higher percentage of patients developing a supraventricular arrhythmia had evidence of cardiac amyloidosis by echo than the controls, the results were not statistically significant. However at the same time it must be kept in mind that sensitivity of echocardiograms to detect early cardiac involvement in amyloidosis tends to be low even with recent advances [16–18].
EKG changes such as AV conduction delays (1st or 2 nd degree blocks) and PACs were present in about 7% patients during pre-transplant period and their presence independently predicted atrial arrhythmias post-transplant. Both frequent PACs and AV conduction abnormalities have been previously shown to predict new AF in general population [19, 20]. Several studies have demonstrated the association between delayed intra-atrial or inter-atrial conduction and increased risk of AF [21–23]. Although first-degree AV block usually involves conduction delay in the atrioventricular node, it is frequently accompanied by abnormalities in other parts of the conduction system. In addition, a prolonged PR interval can lead to delayed and ineffective mitral valve closure and diastolic mitral regurgitation, especially when the PR interval exceeds 230 milliseconds. Early asymptomatic cardiac amyloidosis predisposing to AV conduction problems as well as post-transplant AF could be a possibility that will need further investigation [24–26]. Meanwhile, the presence of asymptomatic conduction blocks or SVC on EKG even prior to stem cell transplants should not be disregarded.
In conclusion, among patients undergoing autologous peripheral blood stem cell transplantation, the risk of developing a supraventricular arrhythmia in the peri-transplant period is approximately 9%. The risk is higher among older patients, those with a prior history of arrhythmias and those with ECG abnormalities suggesting AV conduction delay and premature supraventricular complexes. Though no association with increase in mortality has been shown, these arrhythmias may warrant additional management and can increase the time to discharge, thereby increasing the cost of care. Identification of these high-risk patients may allow development of specific interventions in future.
Acknowledgments
This work is supported in part by: Mayo Clinic Hematological Malignancies Program, Paul Calabresi K12 Award (CA96028).
Disclosures: SKK has research support for clinical trials from Celgene, Millennium, Novartis, and Genzyme and is a consultant for Merck. AD and MQL received clinical trial support from Celgene. None of these funding was used for the current study.
Abbreviations
- AF
Atrial fibrillation
- AFl
Atrial flutter
- AHCT
Autologous hematopoietic cell transplantation
- AV
Atrio-ventricular
- CAD
Coronary artery disease
- EKG
Electrocardiogram/electrocardiographic
- HSCT
Hematopoietic stem cell transplantation
- LA
Left atrium
- LV
left ventricle
- PBSC/PBSCT
Peripheral Blood Stem Cell/Peripheral blood stem cell transplantation
- SVC/PACs
Supraventricular complexes/Premature atrial contractions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: AS and SKK designed the study, collected and analyzed the data, and wrote the manuscript, WJH, SMA, FKB, DD, AD, DAG, MAG, SRH, DJI, PBJ, MQL, MRL, INM, and LFP contributed patients and was involved in writing the manuscript.
Financial Disclosures: None
References
- 1.Gratwohl A, Baldomero H, Aljurf M, et al. Hematopoietic stem cell transplantation: a global perspective. JAMA. 2010;303:1617–1624. doi: 10.1001/jama.2010.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones JA, Qazilbash MH, Shih YC, Cantor SB, Cooksley CD, Elting LS. In-hospital complications of autologous hematopoietic stem cell transplantation for lymphoid malignancies: clinical and economic outcomes from the Nationwide Inpatient Sample. Cancer. 2008;112:1096–1105. doi: 10.1002/cncr.23281. [DOI] [PubMed] [Google Scholar]
- 3.Afessa B, Abdulai RM, Kremers WK, Hogan WJ, Litzow MR, Peters SG. Risk Factors and Outcome of Pulmonary Complications After Autologous Hematopoietic Stem Cell TransplantHematopoietic Stem Cell Transplant Complications. CHEST Journal. 2012;141:442–450. doi: 10.1378/chest.10-2889. [DOI] [PubMed] [Google Scholar]
- 4.Gertz MA, Gastineau DA, Lacy MQ, et al. SCT without growth factor in multiple myeloma: engraftment kinetics, bacteremia and hospitalization. Bone Marrow Transplant. 2011;46:956–961. doi: 10.1038/bmt.2010.233. [DOI] [PubMed] [Google Scholar]
- 5.Murdych T, Weisdorf DJ. Serious cardiac complications during bone marrow transplantation at the University of Minnesota, 1977–1997. Bone Marrow Transplant. 2001;28:283–287. doi: 10.1038/sj.bmt.1703133. [DOI] [PubMed] [Google Scholar]
- 6.Hidalgo JD, Krone R, Rich MW, et al. Supraventricular tachyarrhythmias after hematopoietic stem cell transplantation: incidence, risk factors and outcomes. Bone Marrow Transplant. 2004;34:615–619. doi: 10.1038/sj.bmt.1704623. [DOI] [PubMed] [Google Scholar]
- 7.Fatema K, Gertz MA, Barnes ME, et al. Acute weight gain and diastolic dysfunction as a potent risk complex for post stem cell transplant atrial fibrillation. Am J Hematol. 2009;84:499. doi: 10.1002/ajh.21459. [DOI] [PubMed] [Google Scholar]
- 8.Peres E, Levine JE, Khaled YA, et al. Cardiac complications in patients undergoing a reduced-intensity conditioning hematopoietic stem cell transplantation. Bone Marrow Transplant. 2010;45:149–152. doi: 10.1038/bmt.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feliz V, Saiyad S, Ramarao SM, Khan H, Leonelli F, Guglin M. Melphalan-induced supraventricular tachycardia: incidence and risk factors. Clin Cardiol. 2011;34:356–359. doi: 10.1002/clc.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olivieri A, Corvatta L, Montanari M, et al. Paroxysmal atrial fibrillation after high-dose melphalan in five patients autotransplanted with blood progenitor cells. Bone Marrow Transplant. 1998;21:1049. doi: 10.1038/sj.bmt.1701217. [DOI] [PubMed] [Google Scholar]
- 11.Gertz MA, Ansell SM, Dingli D, et al. Autologous stem cell transplant in 716 patients with multiple myeloma: low treatment-related mortality, feasibility of outpatient transplant, and effect of a multidisciplinary quality initiative. Mayo Clin Proc. 2008;83:1131–1138. doi: 10.4065/83.10.1131. [DOI] [PubMed] [Google Scholar]
- 12.Fuster V, Ryden LE, Cannom DS, et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines developed in partnership with the European Society of Cardiology and in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. J Am Coll Cardiol. 2011;57:e101–98. doi: 10.1016/j.jacc.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Moreau P, Milpied N, Mahé B, et al. Melphalan 220 mg/m2 followed by peripheral blood stem cell transplantation in 27 patients with advanced multiple myeloma. Bone Marrow Transplant. 1999;23:1003. doi: 10.1038/sj.bmt.1701763. [DOI] [PubMed] [Google Scholar]
- 14.Sirohi B, Powles R, Treleaven J, et al. The role of autologous transplantation in patients with multiple myeloma aged 65 years and over. Bone Marrow Transplant. 2000;25:533. doi: 10.1038/sj.bmt.1702188. [DOI] [PubMed] [Google Scholar]
- 15.Phillips GL, Meisenberg B, Reece DE, et al. Amifostine and autologous hematopoietic stem cell support of escalating-dose melphalan: a phase I study. Biol Blood Marrow Transplant. 2004;10:473–483. doi: 10.1016/j.bbmt.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Rahman JE, Helou EF, Gelzer-Bell R, et al. Noninvasive diagnosis of biopsy-proven cardiac amyloidosis. J Am Coll Cardiol. 2004;43:410–415. doi: 10.1016/j.jacc.2003.08.043. [DOI] [PubMed] [Google Scholar]
- 17.Selvanayagam JB, Hawkins PN, Paul B, Myerson SG, Neubauer S. Evaluation and management of the cardiac amyloidosis. J Am Coll Cardiol. 2007;50:2101–2110. doi: 10.1016/j.jacc.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 18.Banypersad SM, Moon JC, Whelan C, Hawkins PN, Wechalekar AD. Updates in Cardiac Amyloidosis: A Review. Journal of the American Heart Association. 2012;1 doi: 10.1161/JAHA.111.000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chong BH, Pong V, Lam KF, et al. Frequent premature atrial complexes predict new occurrence of atrial fibrillation and adverse cardiovascular events. Europace. 2011 doi: 10.1093/europace/eur389. [DOI] [PubMed] [Google Scholar]
- 20.Perez MV, Dewey FE, Marcus R, et al. Electrocardiographic predictors of atrial fibrillation. Am Heart J. 2009;158:622. doi: 10.1016/j.ahj.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Cheng S, Keyes MJ, Larson MG, et al. Long-term outcomes in individuals with prolonged PR interval or first-degree atrioventricular block. JAMA. 2009;301:2571–2577. doi: 10.1001/jama.2009.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dilaveris PE, Gialafos EJ, Sideris SK, et al. Simple electrocardiographic markers for the prediction of paroxysmal idiopathic atrial fibrillation. American heart journal. 1998;135:733–738. doi: 10.1016/s0002-8703(98)70030-4. [DOI] [PubMed] [Google Scholar]
- 23.Agarwal YK, Aronow WS, Levy JA, Spodick DH. Association of interatrial block with development of atrial fibrillation. The American journal of cardiology. 2003;91:882. doi: 10.1016/s0002-9149(03)00027-4. [DOI] [PubMed] [Google Scholar]
- 24.Reisinger J, Dubrey SW, Lavalley M, Skinner M, Falk RH. Electrophysiologic abnormalities in AL (primary) amyloidosis with cardiac involvement. J Am Coll Cardiol. 1997;30:1046–1051. doi: 10.1016/s0735-1097(97)00267-2. [DOI] [PubMed] [Google Scholar]
- 25.Murtagh B, Hammill SC, Gertz MA, Kyle RA, Tajik AJ, Grogan M. Electrocardiographic findings in primary systemic amyloidosis and biopsy-proven cardiac involvement. Am J Cardiol. 2005;95:535–537. doi: 10.1016/j.amjcard.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 26.Dubrey S, Hawkins P, Falk R. Amyloid diseases of the heart: assessment, diagnosis, and referral. Heart. 2011;97:75–84. doi: 10.1136/hrt.2009.190405. [DOI] [PubMed] [Google Scholar]


