Abstract
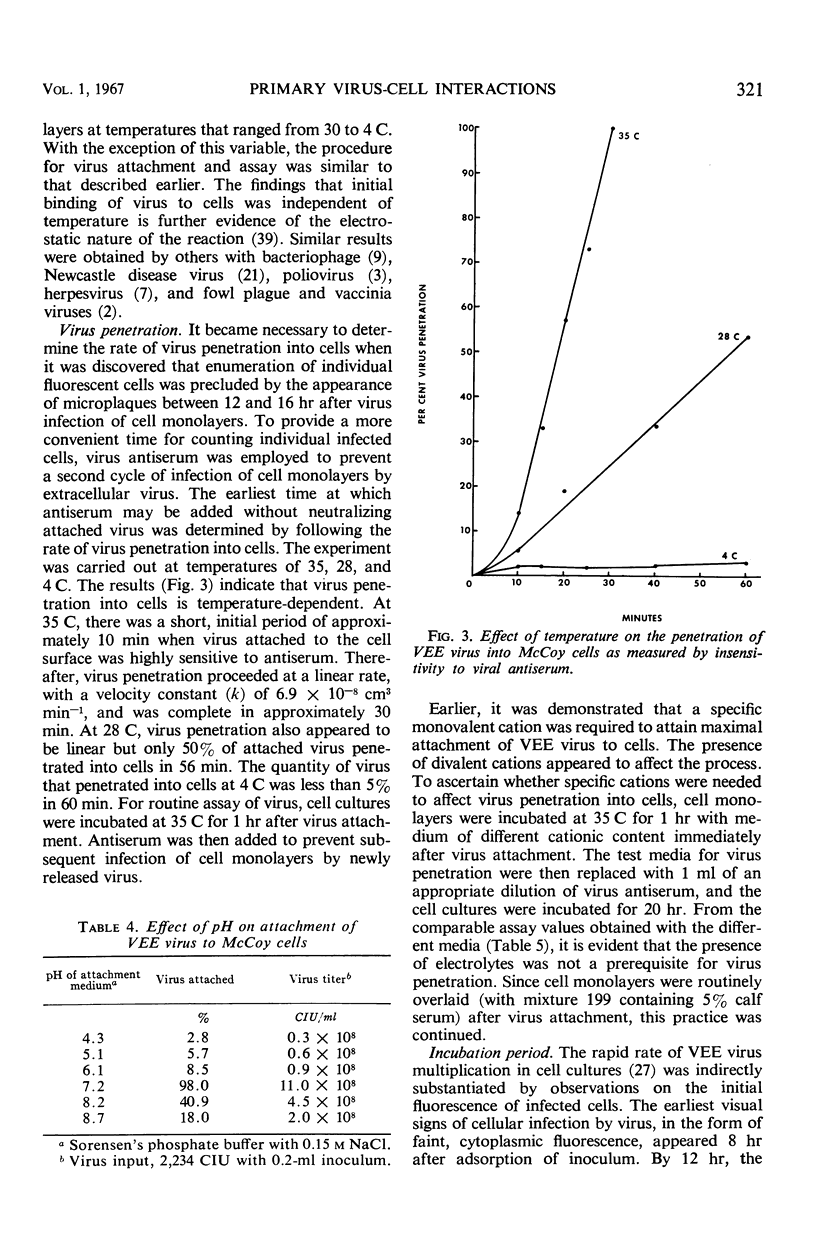
The conditions under which Venezuelan equine encephalomyelitis (VEE) virus attached to host cells markedly influenced the assay of virus by the fluorescent cell-counting technique. When virus inoculum was centrifuged onto McCoy cell monolayers, approximately 97% of virus was attached to cells within 10 min, in contrast to 34% after stationary incubation at 35 C for 2 hr. Maximal binding of virus occurred only in the presence of 0.1 to 0.15 m NaCl. This salt requirement, added to evidence of pH dependence and temperature independence of VEE virus attachment to cells, indicated that the initial union involved electrostatic forces. Virus penetration, measured by the insensitivity of virus-cell complexes to viral antiserum, was complete in 30 min at 35 C. The process was temperature-dependent and un-affected by the ionic content of medium. For assay of VEE virus by the fluorescent cell-counting technique, infected cells may be enumerated as early as 12 hr after infection of cell monolayers. The relationship between virus concentration and cell-infecting units was linear; the distribution of fluorescent cells was random. The virus assay was equivalent in sensitivity but more precise and rapid than that of intracerebral inoculation of mice.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACKERMANN W. W., ISHIDA N. Growth characteristics of influenza virus; properties of the initial cell-virus complex. J Exp Med. 1956 Oct 1;104(4):501–515. doi: 10.1084/jem.104.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALLISON A. C., VALENTINE R. C. Virus particle adsorption, III. Adsorption of viruses by cell monolayers and effects of some variables on adsorption. Biochim Biophys Acta. 1960 Jun 3;40:400–410. doi: 10.1016/0006-3002(60)91380-9. [DOI] [PubMed] [Google Scholar]
- ALLISON A. C., VALENTINE R. C. Virus particle adsorption. II. Adsorption of vaccinia and fowl plague viruses to cells in suspension. Biochim Biophys Acta. 1960 Jun 3;40:393–399. doi: 10.1016/0006-3002(60)91379-2. [DOI] [PubMed] [Google Scholar]
- BACHTOLD J. G., BUBEL H. C., GEBHARDT L. P. The primary interaction of poliomyelitis virus with host cells of tissue culture origin. Virology. 1957 Dec;4(3):582–589. doi: 10.1016/0042-6822(57)90087-9. [DOI] [PubMed] [Google Scholar]
- CARTER G. B. THE RAPID DETECTION, TITRATION, AND DIFFERENTIATION OF VARIOLA AND VACCINIA VIRUSES BY A FLUORESCENT ANTIBODY-COVERSLIP CELL MONOLAYER SYSTEM. Virology. 1965 Apr;25:659–662. doi: 10.1016/0042-6822(65)90094-2. [DOI] [PubMed] [Google Scholar]
- DARNELL J. E., Jr, SAWYER T. K. The basis for variation in susceptibility to poliovirus in HeLa cells. Virology. 1960 Aug;11:665–675. doi: 10.1016/0042-6822(60)90113-6. [DOI] [PubMed] [Google Scholar]
- FARNHAM A. E., NEWTON A. A. The effect of some environmental factors on herpes virus grown in HeLa cells. Virology. 1959 Apr;7(4):449–461. doi: 10.1016/0042-6822(59)90073-x. [DOI] [PubMed] [Google Scholar]
- FERNANDES M. V. Irradiation of cells in tissue culture. VII. Studies on the susceptibility to bluetongue virus on radiation induced giant cells in vitro. Z Zellforsch Mikrosk Anat. 1959;50:433–443. [PubMed] [Google Scholar]
- GAREN A., PUCK T. T. The first two steps of the invasion of host cells by bacterial viruses. II. J Exp Med. 1951 Sep;94(3):177–189. doi: 10.1084/jem.94.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAHON N., NAKAMURA R. M. QUANTITATIVE ASSAY OF PSITTACOSIS VIRUS BY THE FLUORESCENT CELL-COUNTING TECHNIQUE. Virology. 1964 Jun;23:203–208. doi: 10.1016/0042-6822(64)90283-1. [DOI] [PubMed] [Google Scholar]
- HARDY F. M., HEARN H. J., Jr The formation of plaques by two strains of Venezuelan equine encephalomyelitis virus. Am J Hyg. 1961 May;73:258–262. doi: 10.1093/oxfordjournals.aje.a120184. [DOI] [PubMed] [Google Scholar]
- HEARN H. J., Jr A variant of Venezuelan equine encephalomyelitis virus attenuated for mice and monkeys. J Immunol. 1960 Jun;84:626–629. [PubMed] [Google Scholar]
- HOLLAND J. J., HOYER B. H. Early stages of enterovirus infection. Cold Spring Harb Symp Quant Biol. 1962;27:101–112. doi: 10.1101/sqb.1962.027.001.013. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J. Irreversible eclipse of poliovirus by HeLa cells. Virology. 1962 Feb;16:163–176. doi: 10.1016/0042-6822(62)90292-1. [DOI] [PubMed] [Google Scholar]
- HOLLAND J. J., McLAREN L. C. The mammalian cell-virus relationship. II. Adsorption, reception, and eclipse of poliovirus by HeLa cells. J Exp Med. 1959 May 1;109(5):487–504. doi: 10.1084/jem.109.5.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahon N. Assay of variola virus by the fluorescent cell-counting technique. Appl Microbiol. 1965 Nov;13(6):865–871. doi: 10.1128/am.13.6.865-871.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahon N., Cooke K. O. Assay of Coxiella burnetii by enumeration of immunofluorescent infected cells. J Immunol. 1966 Oct;97(4):492–497. doi: 10.21236/ad0482256. [DOI] [PubMed] [Google Scholar]
- Hahon N. Fluorescent cell-counting assay of yellow fever virus. J Infect Dis. 1966 Feb;116(1):33–40. doi: 10.1093/infdis/116.1.33. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K., DARNELL J. E., Jr The adsorption and early fate of purified poliovirus in HeLa cells. Virology. 1961 Apr;13:439–447. doi: 10.1016/0042-6822(61)90275-6. [DOI] [PubMed] [Google Scholar]
- LEVINE S., SAGIK B. P. The interactions of Newcastle disease virus (NDV) with chick embryo tissue culture cells: attachment and growth. Virology. 1956 Feb;2(1):57–68. doi: 10.1016/0042-6822(56)90076-9. [DOI] [PubMed] [Google Scholar]
- MANDEL B. Studies on the interactions of poliomyelitis virus, antibody, and host cells in tissue culture system. Virology. 1958 Oct;6(2):424–447. doi: 10.1016/0042-6822(58)90092-8. [DOI] [PubMed] [Google Scholar]
- MANDEL B. The use of sodium dodecyl sulfate in studies on the interaction of poliovirus and HeLa cells. Virology. 1962 Jun;17:288–294. doi: 10.1016/0042-6822(62)90119-8. [DOI] [PubMed] [Google Scholar]
- METZGER J. F., BANKS I. S., SMITH C. W., HOGGAN M. D. Demonstration of Venezuelan equine encephalomyelitis in tissue culture by immunofluorescence. Proc Soc Exp Biol Med. 1961 Jan;106:212–214. doi: 10.3181/00379727-106-26289. [DOI] [PubMed] [Google Scholar]
- MUSSGAY M., WEIBEL J. Electron microscopic and biological studies on the growth of Venezuelan equine encephalitis virus in KB cells. Virology. 1962 Jan;16:52–62. doi: 10.1016/0042-6822(62)90201-5. [DOI] [PubMed] [Google Scholar]
- PAYNE F. E., KURTZ H., ACKERMANN W. W. Initial stages of the interaction of HeLa cells with poliovirus. Arch Gesamte Virusforsch. 1958;8(1):1–15. doi: 10.1007/BF01242308. [DOI] [PubMed] [Google Scholar]
- PHILIPSON L. THE EARLY INTERACTION OF ANIMAL VIRUSES AND CELLS. Prog Med Virol. 1963;5:43–78. [PubMed] [Google Scholar]
- POSTLETHWAITE R. A plaque technique for the titration of vaccinia virus in chick embryo cells and some features of vaccinial infection in this system. Virology. 1960 Apr;10:466–482. doi: 10.1016/0042-6822(60)90130-6. [DOI] [PubMed] [Google Scholar]
- PUCK T. T., GAREN A., CLINE J. The mechanism of virus attachment to host cells. I. The role of ions in the primary reaction. J Exp Med. 1951 Jan;93(1):65–88. doi: 10.1084/jem.93.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PUCK T. T., TOLMACH L. J. The mechanism of virus attachment to host cells. IV. Physicochemical studies on virus and cell surface groups. Arch Biochem Biophys. 1954 Jul;51(1):229–245. doi: 10.1016/0003-9861(54)90470-1. [DOI] [PubMed] [Google Scholar]
- Paranchych W. Stages in phage R17 infection: the role of divalent cations. Virology. 1966 Jan;28(1):90–99. doi: 10.1016/0042-6822(66)90309-6. [DOI] [PubMed] [Google Scholar]
- RIGGS J. L., SEIWALD R. J., BURCKHALTER J. H., DOWNS C. M., METCALF T. G. Isothiocyanate compounds as fluorescent labeling agents for immune serum. Am J Pathol. 1958 Nov-Dec;34(6):1081–1097. [PMC free article] [PubMed] [Google Scholar]
- RODRIGUEZ J., DEINHARDT F. Preparation of a semipermanent mounting medium for fluorescent antibody studies. Virology. 1960 Oct;12:316–317. doi: 10.1016/0042-6822(60)90205-1. [DOI] [PubMed] [Google Scholar]
- SCOTT T. F., CORIELL L. L., BLANK H., GRAY A. The growth curve of the virus of herpes simplex on the choriollantoic membrane of the embryonated hen's egg. J Immunol. 1953 Sep;71(3):134–144. [PubMed] [Google Scholar]
- SMITH K. O., SHARP D. G. Interaction of virus with cells in tissue cultures. I. Adsorption on and growth of vaccinia virus in L cells. Virology. 1960 Jul;11:519–532. doi: 10.1016/0042-6822(60)90097-0. [DOI] [PubMed] [Google Scholar]
- VALENTINE R. C., ALLISON A. C. Virus particle adsorption. I. Theory of adsorption and experiments on the attachment of particles to non-biological surfaces. Biochim Biophys Acta. 1959 Jul;34:10–23. doi: 10.1016/0006-3002(59)90228-8. [DOI] [PubMed] [Google Scholar]
- WATKINS J. F. The adsorption, penetration and eclipse of herpes simplex virus in chorio-allantoic membrane ectoderm. J Gen Microbiol. 1960 Feb;22:40–56. doi: 10.1099/00221287-22-1-40. [DOI] [PubMed] [Google Scholar]
- WHEELOCK E. F., TAMM I. Enumeration of cell-infecting particles of Newcastle disease virus by the fluorescent antibody technique. J Exp Med. 1961 Feb 1;113:301–316. doi: 10.1084/jem.113.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Roles of metallic ions in host-parasite interactions. Bacteriol Rev. 1966 Mar;30(1):136–151. doi: 10.1128/br.30.1.136-151.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]