Abstract
Background and Purpose
A novel quantitative susceptibility mapping (QSM) processing technology has been developed to map tissue susceptibility property without blooming artifacts. We hypothesize that hematoma volume measurement on QSM is independent of imaging parameters, eliminating its echo time (TE) dependence on gradient echo MRI.
Methods
Gradient echo MRI of 16 patients with intracerebral hemorrhage was processed with susceptibility weighted imaging (SWI), R2* (=1/T2*) mapping, and QSM at various TEs. Hematoma volumes were measured from these images.
Results
Linear regression of hematoma volume vs TE showed substantial slopes for gradient echo magnitude (0.45±0.31 L/s), SWI (0.52±0.46) and R2* (0.39±0.30) but nearly zero slope for QSM (0.01±0.05). At TE=20 msec, hematoma volume on QSM was 0.80x that on gradient echo magnitude image (R2=0.99).
Conclusions
QSM can provide reliable measurement of hematoma volume, which can be performed rapidly and accurately using a semi-automated segmentation tool.
Keywords: Intracerebral hemorrhage (ICH), quantitative susceptibility mapping (QSM)
Introduction
Gradient echo (GRE) MRI has been demonstrated to be more sensitive than CT in detecting intracerebral hemorrhage (ICH), which is characterized as hypointensity on T2* weighted GRE magnitude images1, 2. Hemoglobin in red blood cells becomes paramagnetic as it degenerates in hemorrhages3. GRE is sensitive to local magnetic inhomogeneities. However, T2* hypointensity is dependent on imaging parameters, including echo time (TE), field strength, and voxel size, making it difficult to reliably estimate the hematoma volume4, a vital clinical predictor in managing hemorrhagic patients5, 6. Recently, a novel quantitative susceptibility mapping (QSM) technology for post processing GRE MRI data through deconvolution to reveal intrinsic tissue magnetism and remove imaging parameter dependence7–9 has been applied successfully as a universal measurement of the burden of cerebral microbleeds, eliminating the T2* dependence on imaging parameters10. We hypothesize that QSM provides TE-independent measurement of hematoma volume in GRE MRI.
Methods
Patients
16 acute ICH patients (59 ± 11years, 13 males, 3 females) in stable condition within 6 hours after symptom-onset from August 7, 2009 to August 10, 2011 were recruited for MRI on a 3T scanner using a T2*-weighted three-dimensional multi-echo spoiled GRE sequence 10: 8–11 echoes with first TE =5msec, echo spacing=4.5–5msec, and TR=45–59msec. The GRE magnitude and phase data at each TE were processed to generate susceptibility weighted imaging (SWI), R2*(=1/T2*) and QSM images. Hematoma volumes (HVs) on magnitude, SWI, R2* and QSM at each TE were measured by a semi-automated region growth segmentation method and were normalizing to the volume measured at the reference echo (TEr≈20msec).
Statistical Analysis
Linear regression was performed on HVs measured at TEs in a given image type (magnitude, SWI, R2*, QSM) versus TE to assess volume dependence on TE, and among HVs measured from all image types at TEr for correlations.
Results
The normalized HVs at the last echo (TE>40msec) were 1.31±0.185 for magnitude, 1.35±0.37 for SWI, 1.24±0.14 for R2*, and 1.01±0.01 for QSM. The slope of HV-TE averaged over subjects was 0.44±0.31L/s for magnitude, 0.52±0.47L/s for SWI, 0.39±0.31L/s for R2* and 0.01±0.01L/s for QSM. Fig. 1 illustrates an example of the HV measurement, where the slopes of HV-TE were 0.2229 L/s (R2=0.88, p=0.06) for magnitude, 0.2688 L/s (R2=0.90, p=0.05) for SWI, 0.1684L/s (R2=0.95, p=0.03) for R2*, and 0.0003 L/s (R2=0.05, p=0.77) for QSM.
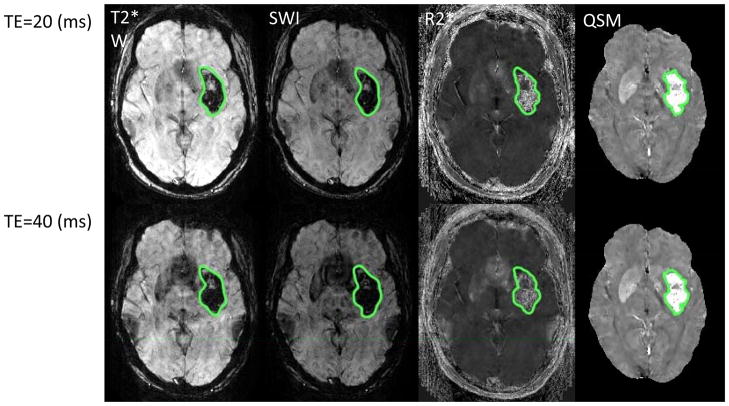
Figure 1.
Hematoma volume measurements by semi-automated segmentation on magnitude (Mag), SWI, R2*, QSM images. a) Axial section showing hemorrhage segmentation (red transparent overlays) at TE = 24 and 39 msec. b) Corresponding hematoma volume measured at TE=24, 29, 34, 39 msec. Hematoma volume increased by ~ 20% on Mag, SWI, R2* image, but ~ 0% on QSM.
The HV of QSM at TEr=20msec was linearly related to that of the magnitude image (magnitude volume/QSM volume = 1.24; R2=0.98, p<10−3), SWI (SWI volume/SWI volume = 1.43, R2=0.90, p<10−3), and R2* (R2* volume/QSM volume = 1.15, R2=0.92, p<10−3), shown in Fig. 2.
Figure 2.
Hematoma volume measured on magnitude, R2*, SWI, QSM at reference echo.
Hemorrhage volume measured on QSM ranged from 7.0 to 63.4mL, with a median of 17.65mL and interquartile from 11.7 to 24.2mL.
Discussion
Our results demonstrate that QSM reduced the TE dependence of GRE MRI hematoma volume measurements from magnitude, SWI and R2* images, providing a hematoma volume measurement independent of imaging parameters in GRE MRI.
This result is consistent with the report that QSM can provide a reliable measurement of the burden of cerebral microbleeds and is understood from physics underlying the GRE MRI data acquisition. Micro- and macrohemorrhages contain paramagnetic components (hemosiderins, methohemoglobins, etc.) that generate magnetic fields. Fields extending beyond their source locations cause blooming artifacts in GRE magnitude images. Blooming artifacts depend on phase accumulation, which is proportional to TE and local fields. Consequently, the SWI and R2* images are dependent on TE, resulting in TE-dependent overestimation in hematoma volume measurements from GRE MRI. Quantitatively, the magnetic field at a point in space, measurable from GRE phase images, is determined by convolving paramagnetic sources with the dipole kernel. To eliminate blooming artifacts dependent on GRE imaging parameters, the dipole kernel deconvolution must be performed to reveal tissue magnetic properties, which is QSM technology.
This technical study is limited by the number of patients and lack of CT correlation and may be followed by a future study on a large cohort of patients with CT correlation. Many stroke centers obtain a CT then a follow-up MRI, because of MRI’s unparalleled rich tissue contrast in imaging brain tissue. Therefore, estimating a precise hematoma volume by MRI in comparison to CT could be important in assessing hematoma expansion using different modalities. Hematoma volume from CT = 0.8* hematoma volume from the GRE magnitude image at TE=15–20 msec (~TEr here) was reported 11. We observed QSM volume/magnitude volume = 1/1.24=0.8 (inverse of the slope in Fig. 2). The suggestion that the hematoma volume measured by QSM may be approximately the hematoma volume measured by CT needs to be confirmed in future study.
Efforts exist to develop efficacious treatment of ICH, which has caused increasing hospital admissions with persistently high mortality rates 12. While hematoma volume serves as a measure for the effects of potential interventions in clinical trials, like INTERACT 13, in vivo MRI characterization of brain tissue and vasculature may allow better understanding and management of hematoma expansion 14, 15 and would be important in developing and applying ICH therapy. QSM of tissue magnetic property may become an essential part of a hemorrhage MRI protocol.
Acknowledgments
Funding Sources
This work was supported in part by grants from US NIH (R01EB013443 and R01NS07237), China National NSF (81171095), and Zhejiang Provincial NSF (LR12H09001).
Footnotes
Disclosures
Drs. Liu and Wang are listed as inventors on patent applications related to the QSM technique. .
References
- 1.Kidwell CS, Chalela JA, Saver JL, Starkman S, Hill MD, Demchuk AM, et al. Comparison of mri and ct for detection of acute intracerebral hemorrhage. JAMA. 2004;292:1823–1830. doi: 10.1001/jama.292.15.1823. [DOI] [PubMed] [Google Scholar]
- 2.Fiebach JB, Schellinger PD, Gass A, Kucinski T, Siebler M, Villringer, et al. Stroke magnetic resonance imaging is accurate in hyperacute intracerebral hemorrhage: A multicenter study on the validity of stroke imaging. Stroke. 2004;35:502–506. doi: 10.1161/01.STR.0000114203.75678.88. [DOI] [PubMed] [Google Scholar]
- 3.Bradley WG., Jr Mr appearance of hemorrhage in the brain. Radiology. 1993;189:15–26. doi: 10.1148/radiology.189.1.8372185. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Chang S, Liu T, Wang Q, Cui D, Chen X, Jin M, Wang B, Pei M, Wisnieff C, Spincemaille P, Zhang M, Wang Y. Reducing the object orientation dependence of susceptibility effects in gradient echo mri through quantitative susceptibility mapping. Magn Reson Med. 2012;65:1563–9. doi: 10.1002/mrm.24135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24:987–993. doi: 10.1161/01.str.24.7.987. [DOI] [PubMed] [Google Scholar]
- 6.Davis SM, Broderick J, Hennerici M, Brun NC, Diringer MN, Mayer SA, et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. 2006;66:1175–1181. doi: 10.1212/01.wnl.0000208408.98482.99. [DOI] [PubMed] [Google Scholar]
- 7.de Rochefort L, Liu T, Kressler B, Liu J, Spincemaille P, Lebon V, et al. Quantitative susceptibility map reconstruction from mr phase data using bayesian regularization: Validation and application to brain imaging. Magn Reson Med. 2010;63:194–206. doi: 10.1002/mrm.22187. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Liu T, de Rochefort L, Ledoux J, Khalidov I, Chen W, et al. Morphology-enabled dipole inversion for quantitative susceptibility mapping using structural consistency between the magnitude image and the susceptibility map. Neuroimage. 2012;59:2560–2568. doi: 10.1016/j.neuroimage.2011.08.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu T, Wisnieff C, Lou M, Chen W, Spincemaille P, Wang Y. Nonlinear formulation of the magnetic field to source relationship for robust quantitative susceptibility mapping. Magn Reson Med. 2012;69:467–476. doi: 10.1002/mrm.24272. [DOI] [PubMed] [Google Scholar]
- 10.Liu T, Surapaneni K, Lou M, Cheng L, Spincemaille P, Wang Y. Cerebral microbleeds: Burden assessment by using quantitative susceptibility mapping. Radiology. 2012;262:269–278. doi: 10.1148/radiol.11110251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burgess RE, Warach S, Schaewe TJ, Copenhaver BR, Alger JR, Vespa P, et al. Development and validation of a simple conversion model for comparison of intracerebral hemorrhage volumes measured on ct and gradient recalled echo mri. Stroke. 2008;39:2017–2020. doi: 10.1161/STROKEAHA.107.505719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayer SA, Brun NC, Begtrup K, Broderick J, Davis S, Diringer MN, et al. Efficacy and safety of recombinant activated factor vii for acute intracerebral hemorrhage. New England J Med. 2008;358:2127–2137. doi: 10.1056/NEJMoa0707534. [DOI] [PubMed] [Google Scholar]
- 13.Delcourt C, Huang Y, Arima H, Chalmers J, Davis SM, Heeley EL, et al. Hematoma growth and outcomes in intracerebral hemorrhage: The interact1 study. Neurology. 2012;79:314–319. doi: 10.1212/WNL.0b013e318260cbba. [DOI] [PubMed] [Google Scholar]
- 14.Demchuk AM, Dowlatshahi D, Rodriguez-Luna D, Molina CA, Blas YS, Dzialowski I, et al. Prediction of haematoma growth and outcome in patients with intracerebral haemorrhage using the ct-angiography spot sign (predict): A prospective observational study. Lancet neurology. 2012;11:307–314. doi: 10.1016/S1474-4422(12)70038-8. [DOI] [PubMed] [Google Scholar]
- 15.Jakubovic R, Aviv RI. Intracerebral hemorrhage: Toward physiological imaging of hemorrhage risk in acute and chronic bleeding. Front Neurol. 2012;3:86. doi: 10.3389/fneur.2012.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]