Abstract
Preterm birth is a leading cause of perinatal morbidity and mortality that is often associated with ascending infections from the lower genital tract. Recent studies with animal models have suggested that developmental exposure to the environmental toxin 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) can increase the risk of preterm birth in the offspring. How TCDD may modify placental immunity to ascending infections is unclear. Therefore, we studied the effects of TCDD treatment on basal and Escherichia coli-stimulated cytokine production by placental explants. Cultures of second-trimester placentas were treated with up to 40 nM TCDD for 72 h and then stimulated with 107 CFU/ml E. coli for an additional 24 h. Concentrations of cytokines and PGE2 were measured in conditioned medium by immunoassay. TCDD exposure increased mRNA levels of IL-1β by unstimulated cultures, but no effects on protein levels of this cytokine were detected. TNF-α production was unaffected by TCDD for unstimulated cultures, but pre-treatment with 40 nM TCDD significantly increased E. coli-stimulated TNF-α production. Both basal and bacteria-stimulated PGE2 and COX-2 gene expression were enhanced by TCDD pretreatment. In contrast, production of the anti-inflammatory cytokine, IL-10, was reduced by TCDD pretreatment for both unstimulated and E. coli-stimulated cultures. No effect of TCDD on the viability of the cultures was detected. These results suggest that TCDD exposure may shift immunity to enhance a proinflammatory phenotype at the maternal–fetal interface that could increase the risk of infection-mediated preterm birth.
Keywords: TCDD, Placenta, Preterm birth, Interleukin-10, Cytokine
1. Introduction
Persistent organic pollutants are a world-wide health concern owing to their endocrine and immunological disrupting and pro-oncogenic effects (Grassman et al., 1998). Among them, 2,3,7,8-tetrachlorodibenzo-p-dioxin(TCDD) is one of the most toxic. Subcutaneous injection of 75 µg/kg/week killed 93% of the rats exposed within 16 weeks (Chahoud et al., 1989). Exposure to lower amounts of TCDD has profound effects on animal growth (Håkansson et al., 1987), sexual development (Gray and Ostby, 1995; Gray et al., 1995; Gray, 1998), metabolism (Lakshman et al., 1991), and immune function (Thigpen et al., 1975).
Exposure to TCDD may also be associated with adverse pregnancy outcomes. In Chapayevsk, Russia, a town contaminated with dioxin from a chemical plant, rates of preterm deliveries and abortion were significantly higher compared with surrounding areas (Revich et al., 2001). Although results did not reach statistical significance, women exposed to large amounts of TCDD after an explosion at a trichlorophenol plant near Seveso, Italy, had a 20–50% increased risk of preterm birth (Eskenazi et al., 2003).
TCDD may increase the risk of preterm birth through its immunomodulatory properties. Successful pregnancy requires tight regulation of immunity at the maternal–fetal interface in order to ensure survival of the fetal allograft (Medawar, 1956). Pro-inflammatory mediators such as IL-1β and TNF-α in the placenta are tightly regulated in part by the anti-inflammatory cytokine IL-10 (Pomini et al., 1999; Hanna et al., 2000). Disruption of this delicate balance of cytokines by bacteria is a leading cause of preterm birth (Hillier et al., 1988, 1991; Gibbs et al., 1992; Hillier et al., 1995). Bacteria stimulate the placenta to increase production of IL-1β and TNF-α (Silver et al., 1997, 1994). These cytokines increase the expression of cyclooygenase- 2 (COX-2) that makes prostaglandins (PG) such as PGE2 and PGF2α (Griesinger et al., 2001; Sato et al., 2003; Lappas et al., 2006). These prostaglandins can stimulate cervical ripening and uterine contractions that can lead to preterm birth. Previous studies have demonstrated that TCDD enhances production of proinflammatory proteins such as COX-2 in hepatic (Puga et al., 1997), spleen (Lawrence and Kerkvliet, 1998), and fibroblastic (Wölfle et al., 2000) cells. This may be due to TCDD enhancement of TNF-α and IL-1β expression. TCDD increased expression of TNF-α by primate PBML (Rier et al., 2001) and a human monocytic cell line (Sciullo et al., 2009). IL-1β production was enhanced by TCDD exposure for thymus (Lai et al., 1997) and lung cells (Wong et al., 2010). Conversely, production of the antiinflammatory cytokine, IL-10 was suppressed by TCDD for mouse splenocytes (Shepherd et al., 2000) and CD11c–derived dendritic cells (Lee et al., 2007). Whether TCDD has these immunotoxic properties with regard to the placenta is unclear. Therefore, we used a well-established placental explant culture system to test the hypothesis that TCDD will enhance basal and bacteria-stimulated production of IL-1β, TNF-α, and PGE2, but inhibit IL-10 production, changes that could enhance the risk of preterm birth.
2. Materials and methods
The methods for this study are standard for our laboratory and presented in detail elsewhere (Peltier et al., 2011, 2012). All procedures were conducted under Institutional Review Board-approved protocols.
2.1. Materials
Escherichia coli strain J5 was purchased from ATCC (# 33908 ATCC, Manassas, VA, USA) and prepared for this study by cultivating the organism in nutrient broth to late log-phase. Bacteria were then harvested by centrifugation at 10,000 × g and resuspended in DMEM. Quantitative cultures of the resuspended bacteria were then prepared to estimate CFU/ml of the bacterial stock, which was then heat-killed by incubation at 85°C for one hour. Frozen aliquots of this stock were stored at −80°C until use in the cell culture studies as described below.
2.2. Placental explant cultures
Reproductive tissues were harvested from women having elective terminations of apparently healthy pregnancies (no known complications) at 16–22 weeks’ gestation (n = 10).
Tissues were transported to the laboratory where the villous placenta was isolated by sharp dissection, washed extensively with PBS and blood clots were removed by gentle blotting with sterile gauze. Segments (1–2cm3) of tissues were then chopped for three passes on a +McIllwian Tissue Chopper (Stoelting Co., Wood Dale, IL, USA) set to cut at 0.1-µm increments and washed with DMEM. Tissues (60 mg wet weight) were then cultured in 3.0 ml DMEM + 5% fetal bovine serum + 100 U/ml penicillin/100µg streptomycin overnight in a humidified incubator in 60-mm culture dishes. Cultures were then randomly assigned to different treatment groups so that any variation due to weighing would bias results toward the null. TCDD was then added to final concentrations of 0 nM, 4 nM or 40 nM. These concentrations were chosen based on previous studies that used an environmentally relevant concentration range of 0–100 nM (Karman et al., 2012). Cultures were incubated for three days at 37°C and then stimulated with E. coli at a final concentration of 107 CFU/ml or an equivalent volume of sterile DMEM for a final overnight incubation. Previous experiments demonstrated that three days’ exposure to TCDD is required to detect changes in placental steroidogenesis (Augustowska et al., 2003b). We administered E. coli after exposing the tissues to the toxin to mimic the order in which exposure would likely occur in utero where the placenta would be exposed to TCDD from the beginning of pregnancy through maternal dietary habits and then bacteria would at some point ascend from the lower genital tract. Conditioned medium was harvested by centrifugation and stored at −80°C until assay.
2.3. Immunoassays
Concentrations of IL-1β, TNF-α, IL-10 in the conditioned medium were determined using the Bioplex™ system from Bio-Rad (Hercules, CA, USA). Samples were diluted with assay buffer (PBS + 1% bovine serum albumin) as needed to fit onto the standard curve. All assays were performed in duplicate and samples that fell below the sensitivity of the assay were set equal to the sensitivity of the assay for analysis. PGE2 concentrations were determined using a Luminex™-based assay (Cayman Chemical, Ann Arbor, MI, USA) according to the manufacturer’s instructions. Assays for progesterone (P4) were performed using an enzyme immunoassay kit purchased from Calbiotech (Spring City, CA, USA).
2.4. Real-time RT-PCR
Total RNA was extracted from tissues after cultures performed as described above using kits purchased from Qiagen according to the manufacturer’s instructions. Quantity and quality of the isolated RNA was estimated using UV spectroscopy and 1µg total RNA was reverse transcribed using kits purchased from Applied Biosystems (Carlsbad, CA, USA). cDNA ( µl) was then amplified using primer sets (purchased from Applied Biosystems) for IL-1β, TNF-α, IL-10, COX-2, aryl hydrocarbon receptor (AhR), ayrl hydrocarbon receptor nuclear translocator (AhRNT), and cytochrome P450, family 1, subfamily A, polypeptide 1 (CYP1A1), a gene activated by the AhR–AhRNT complex, on a Roche II Lightcycler. Cycles to amplification (Ct) time were quantified using the instrument’s software.
2.5. Viability assays
Placental explants were cultured as described above and after the conditioned medium was harvested, tetrazolium salt (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide, MTT; Sigma, St. Louis, MO, USA) was added to a final concentration of 5 mg/ml. Cultures were incubated at 37°C for 30 min. Tissues and crystals were then pelleted by centrifugation and extracted with isopropanol. The absorbance of the A570–690 of the extract was quantified on a computer-controlled, microplate spectrophotometer.
2.6. Statistical analyses
Data were evaluated using linear mixed effects models with the lme4 package of R (www.r-project.org). The mathematical model included effects of TCDD, E. coli stimulation, and their interaction as fixed effects and the patient as a random effect. Separate models were fitted and compared using likelihood ratio tests to determine if the random effect of the patient was best fitted with a separate intercept (to adjust for patient-to-patient variation in basal secretion) or a separate intercept and slope (to normalizing responsiveness to bacterial stimulation as well as background levels of the immunomodulators). Residuals of the fitted models were analyzed for compliance with parametric statistical techniques (normality, equivalence, and independence or errors) and log-transformed, as needed, for hypothesis testing (estimating P-values). Potential differences between individual treatments were evaluated using contrast procedures (using parametric bootstrapping or the esticon function of the doBy package of R) for pre-planned comparisons. Results are presented as least-squares means ± SEM and contrasts ± 95% confidence intervals. Data from real-time PCR analyses was normalized to GAPDH expression for analysis and results transformed to 2−ΔΔCtwith unstimulated, TCDD-untreated cultures as the reference for clarity of presentation. Statistical significance was declared when P ≤ 0.05.
3. Results
3.1. Effect of TCDD exposure on placental cytokine gene expression and secretion
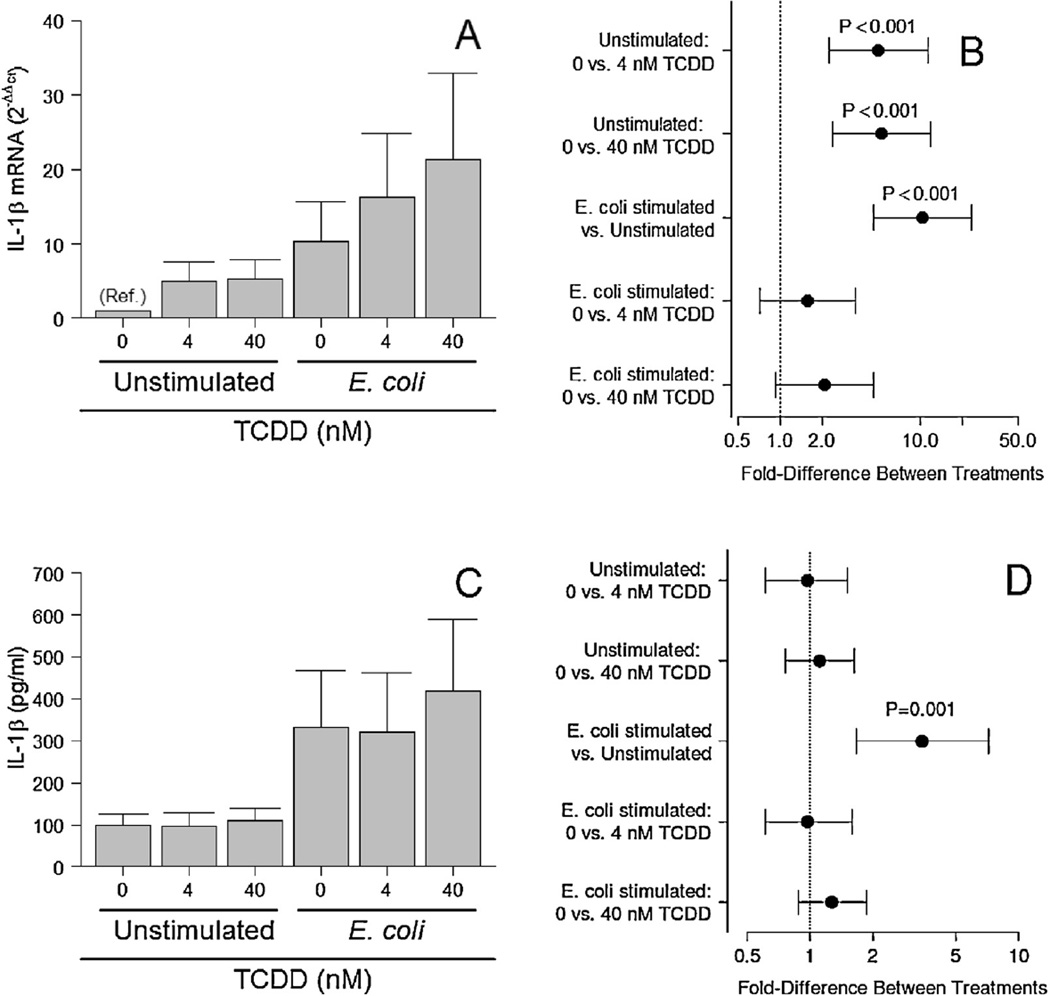
As expected, E. coli significantly increased IL-1β mRNA expression (P < 0.001; Fig. 1A and B) and IL-1β secretion by placental explants (P = 0.001; Fig. 1C and D). Analysis of the tissue explants using real-time PCR methods indicated that basal, but not E. coli-stimulated IL-1β gene expression was significantly increased by both doses of TCDD (P < 0.001; Fig. 1A and B). No effect of pretreatment with 4 or 40 nM TCDD on basal or E. coli-stimulated IL-1β protein levels was detected (Fig. 1C and D), however.
Fig. 1.
Effect of three days’ pretreatment of TCDD on IL-1β mRNA levels (A and B)and secretion (C and D) by unstimulated and E. coli-stimulated placental explant cultures. Least-squares means ± SEM (A and C) and contrasts ± 95% CI (B and D) for experiments performed on tissues from eight (mRNA) or ten (protein) different women are shown. Contrasts that have error bars crossing 1 (indicated by a vertical dotted line) are not statistically significant.
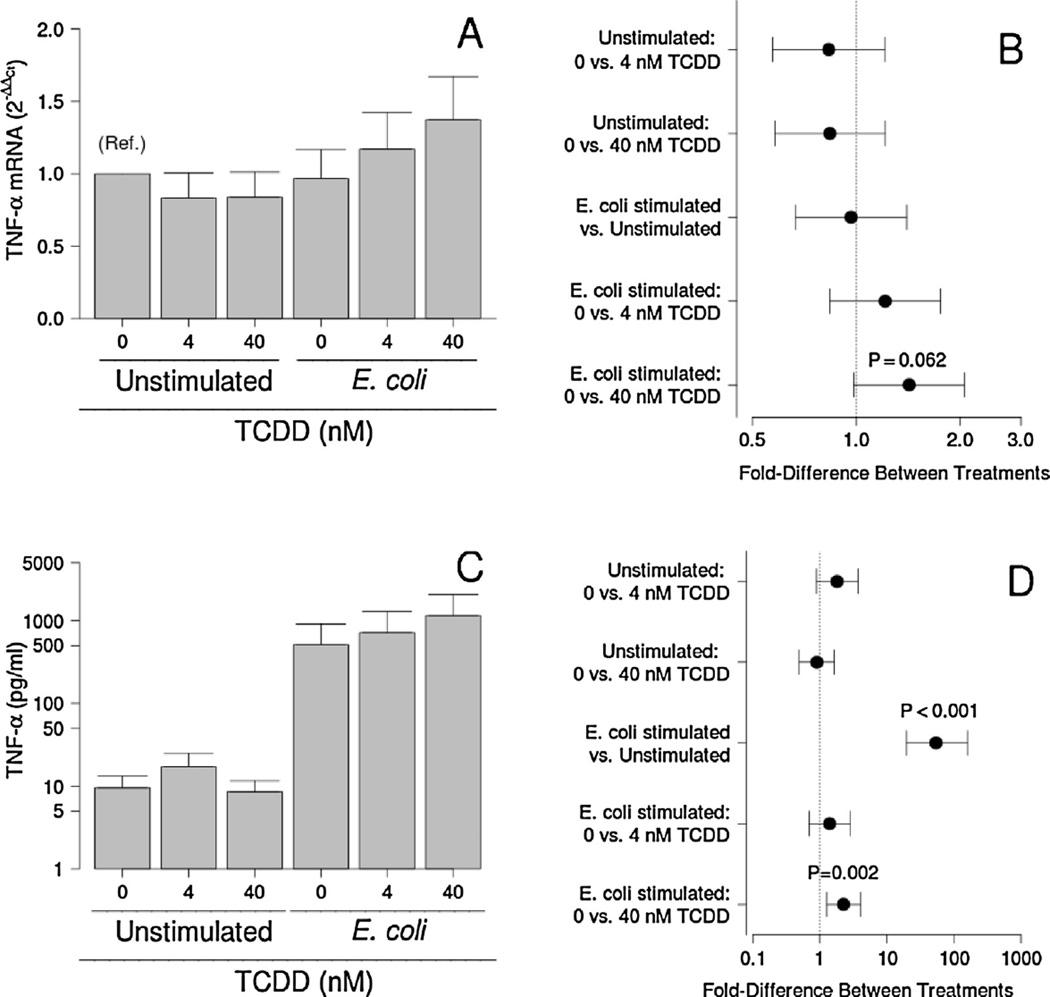
Although no significant effect of bacteria exposure or TCDD was observed for TNF-α gene expression, 40 nM TCDD tended to increase mRNA levels in the placenta at the time of tissue harvest (P = 0.062; Fig. 2A and B). Placental explant cultures responded to E. coli with about a 56-fold increase in TNF-α secretion (P < 0.001) that was further enhanced by pre-exposure to 40, but not 4nM, TCDD (P = 0.002; Fig. 2C and D). No significant effects of TCDD on TNF-α secretion by unstimulated cultures were detected, however.
Fig. 2.
Effect of TCDD on TNF-α mRNA levels (A and B) and secretion (C and D) by unstimulated and E. coli-stimulated placental explant cultures. Least-squares means ±SEM (A and C) and contrasts ±95% CI (Band D) for experiments performed on tissues from eight (mRNA) or ten (protein) different women are shown. Contrasts that have error bars crossing 1 (indicated by a vertical dotted line) are not statistically significant.
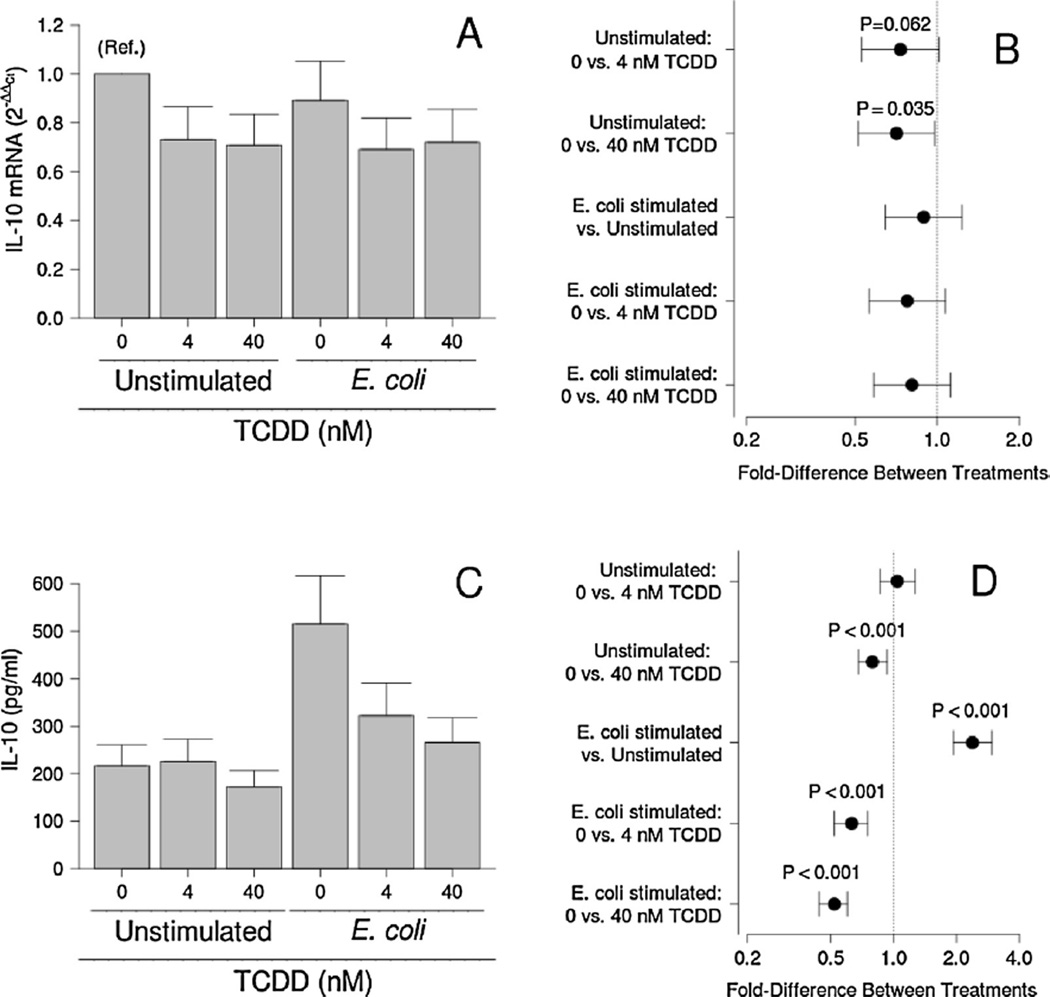
Although no effect of E. coli exposure was detected, TCDD tended to reduce IL-10 mRNA levels in the tissue explants (P ≤ 0.062; Fig. 3A and B). Over all bacterial treatments, however, TCDD significantly reduced IL-10 mRNA levels (P = 0.012). Concentrations of this antiinflammatory cytokine in conditioned medium were increased in response to bacterial stimulation, however (P < 0.001; Fig. 3C and D). For unstimulated cultures, 40 nM, but not 4 nM TCDD, significantly reduced IL-10 production (P < 0.001; Fig. 3C and D). Three days of TCDD pretreatment at 4 and 40 nM also attenuated bacteria-stimulated IL-10 production (P < 0.001; Fig. 3C and D).
Fig. 3.
Effect of TCDD on IL-10 mRNA levels (A and B) and secretion (C and D) by unstimulated and E. coli-stimulated placental explant cultures. Least-squares means ± SEM (A and C) and contrasts ± 95% CI (B and D) for experiments performed on tissues from eight (mRNA) or ten (protein) different women are shown. Contrasts that have error bars crossing 1 (indicated by a vertical dotted line) are not statistically significant.
3.2. Effect of TCDD exposure on PGE2 and P4 secretion
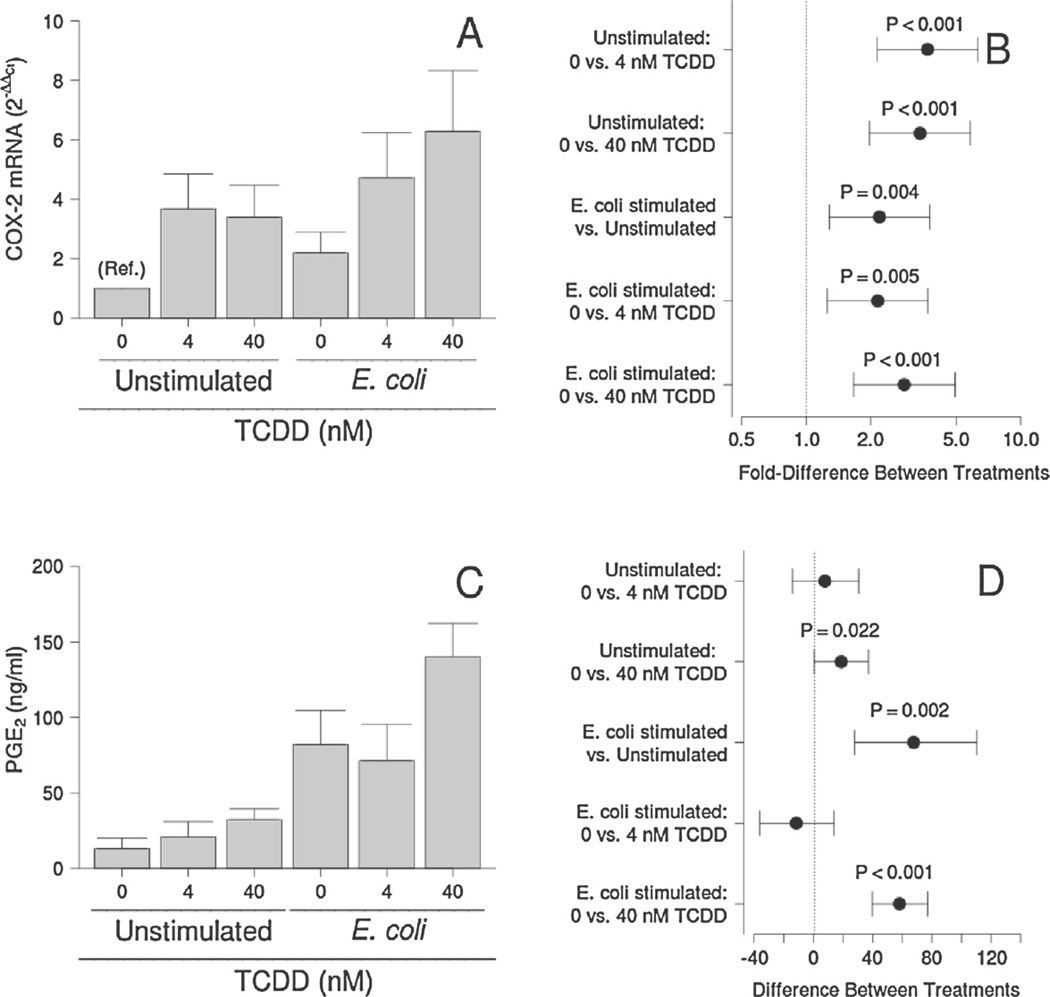
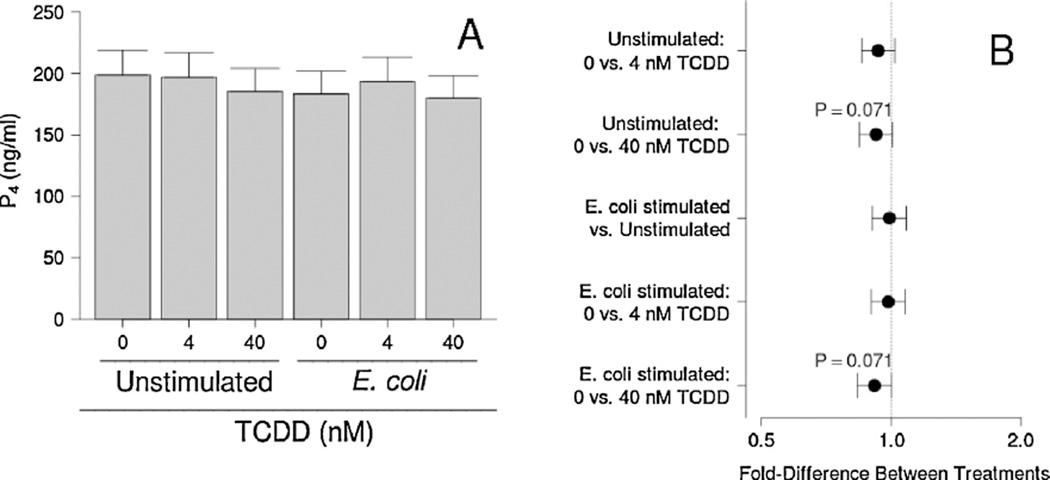
COX-2 mRNA expression was enhanced by both E. coli and TCDD (P < 0.001; Fig. 4A and B). Concentrations of PGE2 in conditioned medium followed a similar trend, except that no effect of 4 nM TCDD was detected for basal or E. coli-stimulated cultures (Fig. 4C and D). Bacterial stimulation had no detectable effect, but TCDD tended to reduce P4 levels in the conditioned medium in the presence and absence of bacteria (P = 0.071; Fig. 5). This effect was statistically significant when averaged over all bacterial treatments (P = 0.039).
Fig. 4.
Effect of TCDD on COX-2 mRNA levels (A and B) and PGE2 secretion (C and D) by unstimulated and E. coli-stimulated placental explant cultures. Least-squares means ±SEM (A and C) and contrasts ±95% CI (B and D) for experiments performed on tissues from eight (COX-2) or ten (PGE2) different women are shown. Contrasts that have error bars crossing 1 (COX-2) or 0 (PGE2; indicated by a vertical dotted line) are not statistically significant.
Fig. 5.
Effect of TCDD on P4 levels by unstimulated and E. coli-stimulated placental explant cultures. Least-squares means±SEM (A) and contrasts ± 95% CI (B) for experiments performed on tissues from nine different women are shown. Contrasts that have error bars crossing 1 (indicated by a vertical dotted line) are not statistically significant.
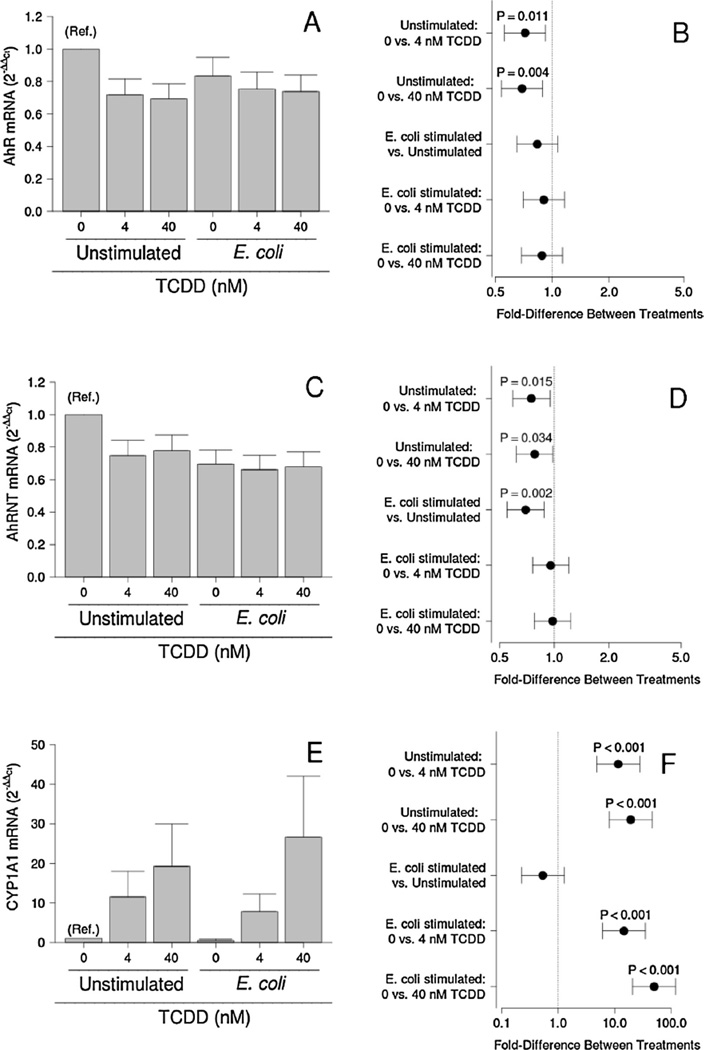
3.3. Effect of TCDD exposure on AhR gene expression
To explore how bacteria and TCDD may interact with the AhR system, we studied the expression of AhR, AhRNT, and CYP1A1-a gene regulated by the AhR in our model system. E. coli treatment and no detectable effect on AhR mRNA expression (Fig. 6A and B). TCDD significantly reduced AhR mRNA expression by unstimulated cultures (P = 0.011 and P = 0.004 for cultures treated with 4 and 40 nM TCDD), but had no effect on cultures treated with E. coli (Fig. 6A and B). Although no effect of TCDD was detected on AhRNT mRNA expression by bacteria-treated cultures, AhRNT expression was significantly reduced by 4 or 40 nM TCDD (P = 0.015 and P = 0.034 respectively; Fig. 6C and D). E. coli treatment also significantly reduced AhRNT mRNA expression by the placental explant cultures (P = 0.002; Fig. 6C and D).
Fig. 6.
Effect of TCDD on mRNA expression for AhR (A and B), AhRNT (C and D), and CYP1A1 (E and F) by unstimulated and E. coli-stimulated placental explant cultures. Least-squares means ± SEM (A, C and E) and contrasts ± 95% CI (B, D and F) for experiments performed on tissues from eight different women are shown. Contrasts that have error bars crossing 1 (indicated by a vertical dotted line) are not statistically significant.
CYP1A1 mRNA levels, which are regulated by the AhR, were not affected by E. coli treatment (Fig. 6E and F). TCDD, an AhR agonist, however, significantly increased mRNA levels of this detoxifying enzyme in a dose-dependent manner.
3.4. Effect of TCDD exposure on placental explant viability
To exclude the possibility that TCDD exposure affects cytokine production through changes in viability of the cultures, we monitored mitochondrial activity of TCDD-treated cultures using a variant of the MTT assay. As shown in Table 1, no effect of TCDD was detected at concentrations up to 40 nM for up to 72 h, the maximum levels of exposure used in this study.
Table 1.
Effect of TCDD exposure on mitochondrial dehydrogenase activity of placental explant cultures using a variant of the MTT assay. Shown are least-squares means ± SEM for experiments performed with tissues isolated from 3 different women.
| Treatment | A570–690 | P-value |
|---|---|---|
| Vehiclea | 0.650 ± 0.019 | Reference |
| 4 nM TCDD | 0.637 ± 0.019 | 0.513 |
| 40 nM TCDD | 0.618 ± 0.028 | 0.739 |
0.1% DMSO (v/v) in culture medium (DMEM + 20% fetal bovine serum + 100U/ml penicillin +100 µg/ml streptomycin).
4. Discussion
Most of the general population are exposed to low levels of TCDD throughout their lifetimes. The major route of human exposure to dioxins (>90%) is through consumption of fish, meat, eggs, and dairy products (Huisman et al., 1995). Of the 50 foods that are most contaminated with TCDD, 16 are consumed in greater portions by pregnant women (Houlihan et al., 2000), placing this population at increased risk of exposure. Animal and cell culture studies demonstrate that TCDD is rapidly taken up into the placenta (Abbott et al., 1996; Augustowska et al., 2003b). Furthermore, concentrations of TCDD in the placenta are at significantly greater levels than blood, suggesting that TCDD may bioaccumulate at the maternal–fetal interface (Wang et al., 2004). The immunomodulatory properties of TCDD are well-described and since pregnancy reflects a delicate immunological situation, we hypothesized that exposure to TCDD might lead to changes in cytokine production to favor preterm birth.
We found that TCDD significantly reduced IL-10 production, but increased TNF-α and PGE2 production by placental explants. Although no effects on IL-1β levels were detected, analysis of the explant cultures by realtime PCR, a more sensitive technique, revealed increased IL-1β mRNA-levels by unstimulated cultures. COX-2 gene expression was also enhanced by exposure to TCDD. These changes are consistent with our hypothesis that TCDD might shift cytokine production to favor a proinflammatory environment.
Our finding of reduced IL-10 production by TCDD is similar to those of previous studies that demonstrated that TCDD reduced IL-10 production by murine splenocytes (Shepherd et al., 2000) and dendritic cells (Lee et al., 2007). The enhanced bacteria-stimulated TNF-α production by E. coli-stimulated explant cultures is consistent with a previous study that reported increased TNF-α production by mitogen-stimulated lymphocytes from TCDD-exposed rhesus monkeys (Rier et al., 2001).
It is notable that the effects of TCDD on TNF-α and IL-10 levels in conditioned medium were not reflected by mRNA levels at the time the tissue was harvested. It is likely that the cytokine gene expression and secretion change during the culture period. Analyses of tissue mRNA content only reflect the mRNA levels at the time tissues were harvested.
In contrast, cytokine levels in the conditioned medium reflect the total amount of cytokine produced since the treatments were added as the cytokines can accumulate in the medium during the culture period.
Recent studies have demonstrated that exposure of mice to TCDD increases their sensitivity to LPS-mediated preterm birth and is associated with increased placental inflammation (Ding et al., 2011). Low levels of bacteria in the upper genital tract are common, even in pregnancies with normal outcomes (Andrews et al., 2005). Placental IL-10 may help to contain the inflammatory response to these pathogens in order to continue the pregnancy. Therefore, reductions in IL-10 production by TCDD may increase the risk of preterm birth by inhibiting this regulatory response to ascending infections. In our study, TCDD tended to increase E. coli-stimulated IL-1β and TNF-α production.
Enhanced production of PGE2 by the placenta after TCDD exposure is consistent with previous findings where TCDD exposure to mice caused a three-fold increase in peritoneal PGE2 during an immune response to allogeneic cells (Lawrence and Kerkvliet, 1998).
PGE2 promotes the ripening and shortening of the cervix. Reduced cervical length at midgestation is a well-established risk factor for infection-mediated preterm birth (Grimes-Dennis and Berghella, 2007, Berghella, 2009). Increased PGE2 production by the placenta could promote premature cervical ripening, increasing the likelihood of ascending infections from the lower genital tract. To our knowledge, no association between TCDD exposure and cervical length has been reported in women, however.
The mechanisms by which TCDD might enhance inflammation are unclear (Stejskalova et al., 2011). Previous studies on the effects of TCDD on the placental function have largely been limited to evaluating its endocrine functions, which may also control the cytokine milieu at the maternal–fetal interface. Progesterone (P4) has well-described immunosuppressive properties (Stites and Siiteri, 1983; Hansen, 1998; Arroyo and Montor, 2011). In ovariectomized animals it prolongs the survival of allografts in the uterus (Hansen, 1998) and removal of progesterone by RU486 (Garfield et al., 1987; Dudley et al., 1996) or ovariectomy (Hirsch and Muhle, 2002; Garfield et al., 1982; Csapo et al., 1982) induces preterm birth in rodent models. Furthermore, supplementation of progesterone in women with a short cervix (Romero et al., 2012; Hassan et al., 2011) or a history of preterm delivery (Meis et al., 2003; da Fonseca et al., 2003) reduced their risk of preterm birth. Some of the actions of P4 may be mediated through cytokines, as P4 and/or proteins induced by it have been shown to favor IL-10 production and inhibit IL-1β and TNF-α production (Marks et al., 2010; Hudić et al., 2011; Raghupathy et al., 2009). Our finding that TCDD significantly inhibited P4 production by placental explant cultures is in agreement with previous studies that used choriocarcinoma cells (Augustowska et al., 2007), isolated trophoblast (Augustowska et al., 2003a), and luteal cells (Gregoraszczuk et al., 2000, 2001). TCDD-mediated reduction of P4 production by luteal cells was reversed by α-naphthoflavone (Gregoraszczuk et al., 2000), an antagonist to the aryl hydrocarbon receptor (AhR) that mediates the majority of known TCDD effects. We also found significant levels of mRNA for the AhR and AhRNT in our placental explant cultures. Increased expression of CYP1A1, which is regulated by the AhR, after TCDD treatment suggests that the AhR system might be functional in our explants culture model. Other studies have also demonstrated that the placenta and placenta-like cells express functional AhR (Stejskalova and Pavek, 2011; Stejskalova et al., 2011). Further experiments with α-naphtophlavone are needed, however, to determine if the observed effects of TCDD on cytokine production are mediated through reductions in P4 secretion and the AhR-regulated genes.
This study has several strengths. First, we used tissues harvested from elective terminations of apparently healthy pregnancies in the second trimester. This is the time point when the effects leading to preterm birth are thought to occur. By applying each of the treatments to the placental explants prepared from the same patient, our statistical models are able to account and make adjustments for patient-to-patient variability in background levels of cytokine and their responsiveness to E. coli. Use of placental explant cultures in lieu of isolated trophoblasts maintains some of the three-dimensional structure of the tissues. This allows us to model the response of the many different types of cells in the placenta to experimental treatments and avoids processing artifacts such as exposure to enzymes and plating of cells.
Our study is not without limitations, however. We are using tissues from women having a controversial obstetrical procedure to end an apparently healthy pregnancy. This requires strict protocols to protect patient privacy. As a result of this, we are unable to obtain any information from the patients aside from gestational age at the time of the procedure, although we designed the experiment to control for patient-to-patient differences in cytokine production and responsiveness to bacteria. Women who have elective terminations of pregnancy in the second trimester may differ, as a population, from those having preterm birth or from the general obstetrical population.
This study is also limited to studying local immune responses to bacteria. In pregnant women, immunity to ascending infection may involve recruitment of additional leukocytes subsets that contribute to the cytokine milieu at the maternal–fetal interface. We are also limited to studying the effects of a single TCDD exposure over a period of a few days.
It is unclear what effects exposure to lower levels of TCDD over a longer period of time would have on the host response to bacterial infections. These limitations, however, are characteristic of tissue culture experiments and none would bias results toward finding an effect.
In summary, we found that TCDD pretreatment increased TNF-α and PGE2 and decreased IL-10 production by placental explants. Furthermore, TCDD increased the expression of mRNA for IL-1β and COX-2. These changes may push the balance of cytokines at the maternal–fetal interface toward a proinflammatory phenotype increasing the risk of preterm birth. Further studies using patient samples and pregnancy outcome data are needed to determine if there is a correlation between TCDD exposure and risk of infection-mediated preterm birth and to better understand the significance of TCDD exposure in the general population.
Acknowledgment
This study was funded in part by grants from the National Institutes of Health (5R21ES017320-02).
References
- Abbott BD, Birnbaum LS, Diliberto JJ. Rapid distribution of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) to embryonic tissues in C57BL/6N mice and correlation with palatal uptake in vitro. Toxicol. Appl. Pharmacol. 1996;141:256–263. doi: 10.1006/taap.1996.0282. [DOI] [PubMed] [Google Scholar]
- Andrews WW, Goldenberg RL, Hauth JC, Cliver SP, Conner M, Goepfert AR. Endometrial microbial colonization and plasma cell endometritis after spontaneous or indicated preterm versus term delivery. Am. J. Obstet. Gynecol. 2005;193:739–745. doi: 10.1016/j.ajog.2005.02.128. [DOI] [PubMed] [Google Scholar]
- Arroyo IC, Montor JM. Non-reproductive effects of sex steroids: their immunoregulatory role. Curr. Top. Med. Chem. 2011;11:1661–1670. doi: 10.2174/156802611796117603. [DOI] [PubMed] [Google Scholar]
- Augustowska K, Gregoraszczuk EL, Milewicz T, Krzysiek J, Grochowal-ski A, Chrzaszcz R. Effects of dioxin (2,3,7,8-TCDD) and PCDDS/PCDFS congeners mixture on steroidogenesis in human placenta tissue culture. Endocr. Regul. 2003a;37:11–19. [PubMed] [Google Scholar]
- Augustowska K, Łucja Gregoraszczuk E, Grochowalski E, Milewicz A, Mika T, Krzysiek M, Chrzaszcz JR. Comparison of accumulation and altered steroid secretion by placental tissue treated with TCDD and natural mixture of PCDDS-PCDFS. Reproduction. 2003b;126:681–687. doi: 10.1530/rep.0.1260681. [DOI] [PubMed] [Google Scholar]
- Augustowska K, Magnowska Z, Kapiszewska M, Gregoraszczuk EL. Is the natural PCDD/PCDF mixture toxic for human placental JEG-3 cell line? The action of the toxicants on hormonal profile, CYP1A1 activity, DNA damage and cell apoptosis. Hum. Exp. Toxicol. 2007;26:407–417. doi: 10.1177/0960327107073119. [DOI] [PubMed] [Google Scholar]
- Berghella V. Novel developments on cervical length screening and progesterone for preventing preterm birth. BJOG. 2009;116:182–187. doi: 10.1111/j.1471-0528.2008.02008.x. [DOI] [PubMed] [Google Scholar]
- Chahoud I, Krowke R, Schimmel A, Merker HJ, Neubert D. Reproductive toxicity and pharmacokinetics of 2,3,7,8-tetrachlorodibenzo-p-dioxin. 1. Effects of high doses on the fertility of male rats. Arch. Toxicol. 1989;63:432–439. doi: 10.1007/BF00316444. [DOI] [PubMed] [Google Scholar]
- Csapo AI, Puri CP, Tarro S, Henzl MR. Deactivation of the uterus during normal and premature labor by the calcium antagonist nicardipine. Am. J. Obstet. Gynecol. 1982;142:483–491. doi: 10.1016/0002-9378(82)90749-9. [DOI] [PubMed] [Google Scholar]
- da Fonseca EB, Bittar RE, Carvalho MHB, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am. J. Obstet. Gynecol. 2003;188:419–424. doi: 10.1067/mob.2003.41. [DOI] [PubMed] [Google Scholar]
- Ding T, McConaha M, Boyd KL, Osteen KG, Bruner-Tran KL. Developmental dioxin exposure of either parent is associated with an increased risk of preterm birth in adult mice. Reprod. Toxicol. 2011;31:351–358. doi: 10.1016/j.reprotox.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley DJ, Branch DW, Edwin SS, Mitchell MD. Induction of preterm birth in mice by RU486. Biol. Reprod. 1996;55:992–995. doi: 10.1095/biolreprod55.5.992. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Mocarelli P, Warner M, Chee WY, Gerthoux PM, Samuels S, Needham LL, Patterson DG. Maternal serum dioxin levels and birth outcomes in women of Seveso, Italy. Environ. Health Perspect. 2003;111:947–953. doi: 10.1289/ehp.6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfield RE, Puri CP, Csapo AI. Endocrine, structural, and functional changes in the uterus during premature labor. Am. J. Obstet. Gynecol. 1982;142:21–27. doi: 10.1016/s0002-9378(16)32279-7. [DOI] [PubMed] [Google Scholar]
- Garfield RE, Gasc JM, Baulieu EE. Effects of the antiprogesterone RU486on preterm birth in the rat. Am. J. Obstet. Gynecol. 1987;157:1281–1290. doi: 10.1016/s0002-9378(87)80315-0. [DOI] [PubMed] [Google Scholar]
- Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. Am. J. Obstet. Gynecol. 1992;166:1515–1520. doi: 10.1016/0002-9378(92)91628-n. [DOI] [PubMed] [Google Scholar]
- Grassman JA, Masten SA, Walker NJ, Lucier GW. Animal models of human response to dioxins. Environ. Health Perspect. 1998;106(Suppl. 2):761–775. doi: 10.1289/ehp.98106761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray LE. Xenoendocrine disrupters: laboratory studies on male reproductive effects. Toxicol. Lett. 1998;102–103:331–335. doi: 10.1016/s0378-4274(98)00327-0. [DOI] [PubMed] [Google Scholar]
- Gray LE, Ostby JS. In utero 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) alters reproductive morphology and function in female rat offspring. Toxicol. Appl. Pharmacol. 1995;133:285–294. doi: 10.1006/taap.1995.1153. [DOI] [PubMed] [Google Scholar]
- Gray LE, Kelce WR, Monosson E, Ostby JS, Birnbaum LS. Exposure to TCDD during development permanently alters reproductive function in male Long Evans rats and hamsters: reduced ejaculated and epididymal sperm numbers and sex accessory gland weights in offspring with normal androgenic status. Toxicol. Appl. Pharmacol. 1995;131:108–118. doi: 10.1006/taap.1995.1052. [DOI] [PubMed] [Google Scholar]
- Gregoraszczuk EL, Wójtowicz AK, Zabielny E, Grochowalski A. Dose-and-time dependent effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on progesterone secretion by porcine luteal cells cultured in vitro. J. Physiol. Pharmacol. 2000;51:127–135. [PubMed] [Google Scholar]
- Gregoraszczuk EL, Zabielny E, Ochwat D. Aryl hydrocarbon receptor (AhR) linked inhibition of luteal cell progesterone secretion in 2,3,7,8-tetrachlorodibenzo-p-dioxin treated cells. J. Physiol. Pharmacol. 2001;52:303–311. [PubMed] [Google Scholar]
- Griesinger G, Saleh L, Bauer S, Husslein P, Knöfler M. Production of pro- and antiinflammatory cytokines of human placental trophoblasts in response to pathogenic bacteria. J. Soc. Gynecol. Investig. 2001;8:334–340. [Google Scholar]
- Grimes-Dennis J, Berghella V. Cervical length and prediction of preterm delivery. Curr. Opin. Obstet. Gynecol. 2007;19:191–195. doi: 10.1097/GCO.0b013e3280895dd3. [DOI] [PubMed] [Google Scholar]
- Håkansson H, Waern F, Ahlborg UG. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in the lactating rat on maternal and neonatal vitamin a status. J. Nutr. 1987;117:580–586. doi: 10.1093/jn/117.3.580. [DOI] [PubMed] [Google Scholar]
- Hanna N, Hanna I, Hleb M, Wagner E, Dougherty J, Balkundi D, Padbury J, Sharma S. Gestational age-dependent expression of IL-10andits receptor in human placental tissues and isolated cytotrophoblasts. J. Immunol. 2000;164:5721–5730. doi: 10.4049/jimmunol.164.11.5721. [DOI] [PubMed] [Google Scholar]
- Hansen PJ. Regulation of uterine immune function by progesterone-lessons from the sheep. J. Reprod. Immunol. 1998;40:63–79. doi: 10.1016/s0165-0378(98)00035-7. [DOI] [PubMed] [Google Scholar]
- Hassan SS, Romero R, Vidyadhari D, Fusey S, Baxter JK, Khandelwal M, Vijayaraghavan J, Trivedi Y, Soma-Pillay P, Sambarey P, Dayal A, Potapov V, O’Brien J, Astakhov V, Yuzko O, Kinzler W, Dattel B, Sehdev H, Mazheika L, Manchu-lenko D, Gervasi MT, Sullivan L, Conde-Agudelo A, Phillips JA, Creasy GW PREGNANT Trial. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet. Gynecol. 2011;38:18–31. doi: 10.1002/uog.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillier SL, Martius J, Krohn M, Kiviat N, Holmes KK, Eschenbach DA. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N. Engl. J. Med. 1988;319:972–978. doi: 10.1056/NEJM198810133191503. [DOI] [PubMed] [Google Scholar]
- Hillier SL, Krohn MA, Kiviat NB, Watts DH, Eschenbach DA. Microbiologic causes and neonatal outcomes associated with chorioamnion infection. Am. J. Obstet. Gynecol. 1991;165:955–961. doi: 10.1016/0002-9378(91)90447-y. [DOI] [PubMed] [Google Scholar]
- Hillier SL, Nugent RP, Eschenbach DA, Krohn MA, Gibbs RS, Martin DH, Cotch MF, Edelman R, Pastorek JG, Rao AV. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The vaginal infections and prematurity study group. N. Engl. J. Med. 1995;333:1737–1740. doi: 10.1056/NEJM199512283332604. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Muhle R. Intrauterine bacterial inoculation induces labor in the mouse by mechanisms other than progesterone withdrawal. Biol. Reprod. 2002;67:1337–1340. doi: 10.1095/biolreprod67.4.1337. [DOI] [PubMed] [Google Scholar]
- Houlihan J, Campbell C, Wiles R Environmental Working Group. Moms and pops. Persistent organic pollutants in the diets of pregnant and nursing women. 2000 www.ewr.org.
- Hudić I, Szekeres-Bartho J, Fatušić Z, Stray-Pedersen B, Dizdarević-Hudić L, Latifagić A, Hotić N, Kamerić L, Mandžić A. Dydrogesterone supplementation in women with threatened preterm delivery–the impact on cytokine profile, hormone profile, and progesterone-induced blocking factor. J. Reprod. Immunol. 2011;92:103–107. doi: 10.1016/j.jri.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Huisman M, Eerenstein SE, Koopman-Esseboom C, Brouwer M, Fidler V, Muskiet FA, Sauer PJ, Boersma ER. Perinatal exposure to polychlorinated biphenyls and dioxins through dietary intake. Chemosphere. 1995;31:4273–4280. doi: 10.1016/0045-6535(95)00296-k. [DOI] [PubMed] [Google Scholar]
- Karman BN, Basavarajappa MS, Craig ZR, Flaws JA. 2,3,7,8-Tetrachlorodibenzo-p-dioxin activates the aryl hydrocarbon receptor and alters sex steroid hormone secretion without affecting growth of mouse antral follicles in vitro. Toxicol. Appl. Pharmacol. 2012;261:88–96. doi: 10.1016/j.taap.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai ZW, Hundeiker C, Gleichmann E, Esser C. Cytokine gene expression during ontogeny in murine thymus on activation of the aryl hydrocarbon receptor by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Mol. Pharmacol. 1997;52:30–37. doi: 10.1124/mol.52.1.30. [DOI] [PubMed] [Google Scholar]
- Lakshman MR, Ghosh P, Chirtel SJ. Mechanism of action of 2,3,7,8-tetrachlorodibenzo-p-dioxin on intermediary metabolism in the rat. J. Pharmacol. Exp. Ther. 1991;258:317–319. [PubMed] [Google Scholar]
- Lappas M, Yee K, Permezel M, Rice GE. Lipopolysaccharide and TNF-alpha activate the nuclear factor kappa B pathway in the human placental JEG-3 cells. Placenta. 2006;27:568–575. doi: 10.1016/j.placenta.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Lawrence BP, Kerkvliet NI. Role of altered arachidonic acid metabolism in 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced immune suppression in c57bl/6 mice. Toxicol. Sci. 1998;42:13–22. doi: 10.1006/toxs.1997.2418. [DOI] [PubMed] [Google Scholar]
- Lee JA, Hwang JA, Sung HN, Jeon CH, Gill BC, Youn HJ, Park JH. 2,3,7,8-Tetrachlorodibenzo-p-dioxin modulates functional differentiation of mouse bone marrow-derived dendritic cells down-regulation of RelB by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Lett. 2007;173:31–40. doi: 10.1016/j.toxlet.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Marks MA, Gravitt PE, Burk RD, Studentsov Y, Farzadegan H, Klein SL. Progesterone and 17beta-estradiol enhance regulatory responses to human papillomavirus type 16 virus-like particles in peripheral blood mononuclear cells from healthy women. Clin. Vaccine Immunol. 2010;17:609–617. doi: 10.1128/CVI.00441-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medawar PB. The immunology of transplantation. Harvey Lect. 1956:144–176. [PubMed] [Google Scholar]
- Meis PJ, Klebanoff M, Thom E, Dombrowski MP, Sibai B, Moawad AH, Spong CY, Hauth JC, Miodovnik M, Varner MW, Leveno KJ, Caritis SN, Iams JD, Wapner RJ, Conway D, O’Sullivan MJ, Carpenter M, Mercer B, Ramin SM, Thorp JM, Peaceman AM, Gabbe S National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N. Engl. J. Med. 2003;348:2379–2380. doi: 10.1056/NEJMoa035140. [DOI] [PubMed] [Google Scholar]
- Peltier MR, Gurzenda EM, Murthy A, Chawala K, Lerner V, Kharode I, Arita Y, Rhodes A, Maari N, Moawad A, Hanna N. Can oxygen tension contribute to an abnormal placental cytokine milieu? Am. J. Reprod. Immunol. 2011;66:279–285. doi: 10.1111/j.1600-0897.2011.00998.x. [DOI] [PubMed] [Google Scholar]
- Peltier MR, Klimova NG, Arita Y, Gurzenda EM, Murthy A, Chawala K, Lerner V, Richardson J, Hanna N. Polybrominateddiphenyl ethers enhance the production of proinflammatory cytokines by the placenta. Placenta. 2012;33:745–749. doi: 10.1016/j.placenta.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomini F, Caruso A, Challis JR. Interleukin-10 modifies the effects of interleukin-1beta and tumor necrosis factor-alpha on the activity and expression of prostaglandin Hsynthase-2 and the NAD+-dependent 15-hydroxyprostaglandin dehydrogenase in cultured term human villous trophoblast and chorion trophoblast cells. J. Clin. Endocrinol. Metab. 1999;84:4645–4650. doi: 10.1210/jcem.84.12.6188. [DOI] [PubMed] [Google Scholar]
- Puga A, Hoffer A, Zhou S, Bohm JM, Leikauf GD, Shertzer HG. Sustained increase in intracellular free calcium and activation of cyclooxygenase-2 expression in mouse hepatoma cells treated with dioxin. Biochem. Pharmacol. 1997;54:1287–1290. doi: 10.1016/s0006-2952(97)00417-6. [DOI] [PubMed] [Google Scholar]
- Raghupathy R, Al-Mutawa E, Al-Azemi M, Makhseed M, Azizieh F, Szekeres-Bartho J. Progesterone-induced blocking factor (PIBF) modulates cytokine production by lymphocytes from women with recurrent miscarriage or preterm delivery. J. Reprod. Immunol. 2009;80:91–99. doi: 10.1016/j.jri.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Revich B, Aksel E, Ushakova T, Ivanova I, Zhuchenko N, Klyuev N, Brodsky B, Sotskov Y. Dioxin exposure and public health in Chapaevsk, Russia. Chemosphere. 2001;43:951–966. doi: 10.1016/s0045-6535(00)00456-2. [DOI] [PubMed] [Google Scholar]
- Rier SE, Coe CL, Lemieux AM, Martin DC, Morris R, Lucier GW, Clark GC. Increased tumor necrosis factor-alpha production by peripheral blood leukocytes from TCDD-exposed rhesus monkeys. Toxicol. Sci. 2001;60:327–337. doi: 10.1093/toxsci/60.2.327. [DOI] [PubMed] [Google Scholar]
- Romero R, Nicolaides K, Conde-Agudelo A, Tabor A, O’Brien JM, Cetingoz E, Fonseca ED, Creasy GW, Klein K, Rode L, Soma-Pillay P, Fusey S, Cam C, Alfirevic Z, Hassan SS. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: a systematic review and metaanalysis of individual patient data. Am. J. Obstet. Gynecol. 2012;206:124e1–1250e. doi: 10.1016/j.ajog.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato TA, Keelan JA, Mitchell MD. Critical paracrine interactions between TNF-alpha and IL-10 regulate lipopolysaccharide-stimulated human choriodecidual cytokine and prostaglandin e2 production. J. Immunol. 2003;170:158–166. doi: 10.4049/jimmunol.170.1.158. [DOI] [PubMed] [Google Scholar]
- Sciullo EM, Dong B, Vogel CFA, Matsumura F. Characterization of the pattern of the nongenomic signaling pathway through which TCDD-induces early inflammatory responses in U937 human macrophages. Chemosphere. 2009;74:1531–1540. doi: 10.1016/j.chemosphere.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd DM, Dearstyne EA, Kerkvliet NI. The effects of TCDD on the activation of ovalbumin (OVA)-specific DO11.10 transgenic CD4(+) T cells in adoptively transferred mice. Toxicol. Sci. 2000;56:340–350. doi: 10.1093/toxsci/56.2.340. [DOI] [PubMed] [Google Scholar]
- Silver RM, Lohner WS, Daynes RA, Mitchell MD, Branch DW. Lipopolysaccharide-induced fetal death: the role of tumor-necrosis factor alpha. Biol. Reprod. 1994;50:1108–1110. doi: 10.1095/biolreprod50.5.1108. [DOI] [PubMed] [Google Scholar]
- Silver RM, Edwin SS, Umar F, Dudley DJ, Branch DW, Mitchell MD. Bacterial lipopolysaccharide-mediated murine fetal death: the role of interleukin-1. Am. J. Obstet. Gynecol. 1997;176:544–549. doi: 10.1016/s0002-9378(97)70545-3. [DOI] [PubMed] [Google Scholar]
- Stejskalova L, Pavek P. The function of cytochrome P450 1A1 enzyme (CYP1A1) and aryl hydrocarbon receptor (AhR) in the placenta. Curr. Pharm. Biotechnol. 2011;12:715–730. doi: 10.2174/138920111795470994. [DOI] [PubMed] [Google Scholar]
- Stejskalova L, Vecerova L, Peréz LM, Vrzal R, Dvorak Z, Nachtigal P, Pavek P. Aryl hydrocarbon receptor and aryl hydrocarbon nuclear translocator expression in human and rat placentas and transcription activity in human trophoblast cultures. Toxicol. Sci. 2011;123:26–36. doi: 10.1093/toxsci/kfr150. [DOI] [PubMed] [Google Scholar]
- Stites DP, Siiteri PK. Steroids as immunosuppressants in pregnancy. Immunol. Rev. 1983;75:117–138. doi: 10.1111/j.1600-065x.1983.tb01093.x. [DOI] [PubMed] [Google Scholar]
- Thigpen JE, Faith RE, McConnell EE, Moore JA. Increased susceptibility to bacterial infection as a sequela of exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Infect. Immun. 1975;12:1319–1320. doi: 10.1128/iai.12.6.1319-1324.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SL, Lin CY, Guo YL, Lin LY, Chou WL, Chang LW. Infant exposure to polychlorinated dibenzo-p-dioxins, dibenzofurans and biphenyls (PCDD/FS, PCBS) – correlation between prenatal and postnatal exposure. Chemosphere. 2004;54:1459–1460. doi: 10.1016/j.chemosphere.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Wölfle D, Marotzki S, Dartsch D, Schäfer W, Marquardt H. Induction of cyclooxygenase expression and enhancement of malignant cell transformation by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Carcinogenesis. 2000;21:15–21. doi: 10.1093/carcin/21.1.15. [DOI] [PubMed] [Google Scholar]
- Wong PS, Vogel CF, Kokosinski K, Matsumura F. Arylhydrocar-bon receptor activation in NCI-H441 cells and C57BL/6 mice: possible mechanisms for lung dysfunction. Am. J. Respir. Cell Mol. Biol. 2010;42:210–217. doi: 10.1165/rcmb.2008-0228OC. [DOI] [PMC free article] [PubMed] [Google Scholar]