Abstract
Objective
The aim of the present study is to evaluate the association of air pollution with the onset of atrial fibrillation (AF).
Background
Air pollution in general and more specifically particulate matter has been associated with cardiovascular events. Although ventricular arrhythmias are traditionally thought to convey the increased cardiovascular risk, AF may also contribute.
Methods
Patients with dual chamber implantable cardioverter defibrillators (ICDs) were enrolled and followed prospectively. The association of AF onset with air quality including ambient PM2.5, black carbon, sulfate, particle number, NO2, SO2, and O3 in the 24 hours prior to the arrhythmia was examined utilizing a case-crossover analysis. In sensitivity analyses, associations with air pollution between 2 and 48 hours prior to the AF were examined.
Results
Of 176 patients followed for an average of 1.9 years, 49 patients had 328 episodes of AF lasting ≥ 30 seconds. Positive but nonsignificant associations were found for PM2.5 in the prior 24 hours, but stronger associations were found with shorter exposure windows. The odds of AF increased by 26% (95% CI 8% to 47%) for each 6.0 µg/m3 increase in PM2.5 in the 2 hours prior to the event (p=0.004). The odds of AF was highest at the upper quartile of mean PM2.5.
Conclusion
Particulate matter was associated with increased odds of AF onset within hours following exposure in patients with known cardiac disease. Air pollution is an acute trigger of AF, likely contributing to the pollution-associated adverse cardiac outcomes observed in epidemiological studies.
Keywords: Air pollution, Atrial fibrillation, Particulate matter, Traffic
INTRODUCTION
Air pollution has been linked to overall mortality in epidemiological investigations (1–4) and most evidence points to increased cardiovascular disease as the primary driver.(5–8) Although air pollution is composed of multiple pollutants; in most studies fine particular matter (PM2.5) is consistently associated with these cardiac events which include sudden cardiac death, heart failure, and myocardial infarctions.(2,4–9) The relative risk of daily cardiovascular mortality has been shown to be increased approximately 0.4% to 1.0% with a 10 µg/m3 increase in mean 24 hour PM2.5.(10) In addition, stroke has been associated with air pollution in several studies, with increased risks ranging from 0.4% to 18% with a 10 µg/m3 increase in PM2.5 on the day of the event.(11–15) PM2.5 is produced by direct emissions from local and regional sources such as motor vehicles plus secondary particles from upwind fossil fuel burning.(4) Black carbon (BC), particle number count (PNC), and NO2 are also produced predominantly by motor vehicles, while SO4 and ozone are secondary pollutants produced in atmospheric reactions of emissions from upwind sources.
These associations between air pollution and health are generally observed in large population-based studies evaluating epidemiological evidence for death, or hospital admission data for myocardial infarctions, heart failure and stroke. This literature also includes patient based studies on ventricular arrhythmias, usually in susceptible populations with low left ventricular ejection fractions and implantable cardioverter defibrillators (ICD).(4,16–20) Based on these investigations, it is generally assumed that the majority of air pollution’s cardiac effects are on the ventricle, and the cardiovascular complications of air pollution are mediated through ventricular arrhythmias, sudden death and worsened heart failure. However, a previous retrospective study by our group pointed to a possible association between atrial fibrillation (AF) with rapid ventricular responses and air pollution. That study was limited by the large proportion of these patients with single chamber ICDs which will only detect AF episodes that caused a rapid ventricular response.(21) Other studies have hinted at an association with AF, in that air pollution has been associated with some electrophysiological predictors of AF,(22) and also with atrial premature beats.(23,24)
The morbidity and mortality associated with AF is substantial. AF is responsible for more hospitalizations and longer hospital stays than any other arrhythmia, and may also lead to stroke, congestive heart failure, myocardial infarction and death.(25–31) Thus, AF, even if asymptomatic, is a very important cause of morbidity and mortality.(29,32) Whether air pollution contributes to AF has been difficult to detect, in large part because many episodes of AF are asymptomatic or minimally symptomatic. Therefore, individuals do not present for emergency treatment, making this arrhythmia unsuitable for population based studies. Even in the case of clinically recognized AF, the specific time of onset of these events is often not well defined. Electrocardiac monitoring studies are generally limited to short term monitoring for atrial arrhythmias and thus a true association may be missed. In this study, we evaluate the role of acute air pollution exposures as triggers of AF in a sample of high risk patients with continuous monitoring of atrial arrhythmias over an extended period of follow-up. Associations with traffic related and other air pollutants in the 24-hours prior to an AF event are evaluated, as well as alternative air pollution exposure windows relative to AF onset.
METHODS
Patient population
Subjects were recruited between September 2006 and March 2010 from patients followed at the Tufts Medical Center Cardiac Arrhythmia Center. Inclusion criteria included prior implantation of a dual (atrial and ventricular) chamber ICD and residential zip codes within a 50-kilometer radius of the Harvard Supersite air quality monitoring station. Exclusions included age younger than 18 years, chronic AF, lack of follow-up at Tufts Medical Center, terminal illness, or inability to give informed consent. The study protocol and informed consent were approved by the Institutional Review Board at Tufts Medical Center and the Harvard School of Public Health.
At the initial visit all patients completed an interviewer-administered questionnaire, including socio-demographic characteristics, medical history, detailed medication, lifestyle, and smoking history. They were measured for height and weight. A comprehensive past and current medical history, based on the National Cardiovascular Disease Data ICD Registry form was filled out by study coordinators based on review of medical records.
Arrhythmias
Patients were followed with either a clinic visit or by telephone every three months from study enrollment until June 30, 2010. At these encounters, ICD data which included the arrhythmia logbook and electrograms were downloaded directly or with trans-telephonic transmission and printed. All ICDs were dual chamber and capable of recording the date, time and real time electrograms of atrial and ventricular events. In addition to documenting the arrhythmia, the ICD characterizes each event as atrial or ventricular and as sustained or nonsustained, and records the total time of each episode. Heart rate detection and treatment rates for ventricular arrhythmias are programmed by the physician according to the specific needs of the patient. In general, treatment for ventricular arrhythmia begins at heart rates above 160 beats per minute (bpm). In addition to documenting rapid ventricular episodes, these devices also document and store electrograms of rapid atrial arrhythmias, even if the ventricular rate remains low.
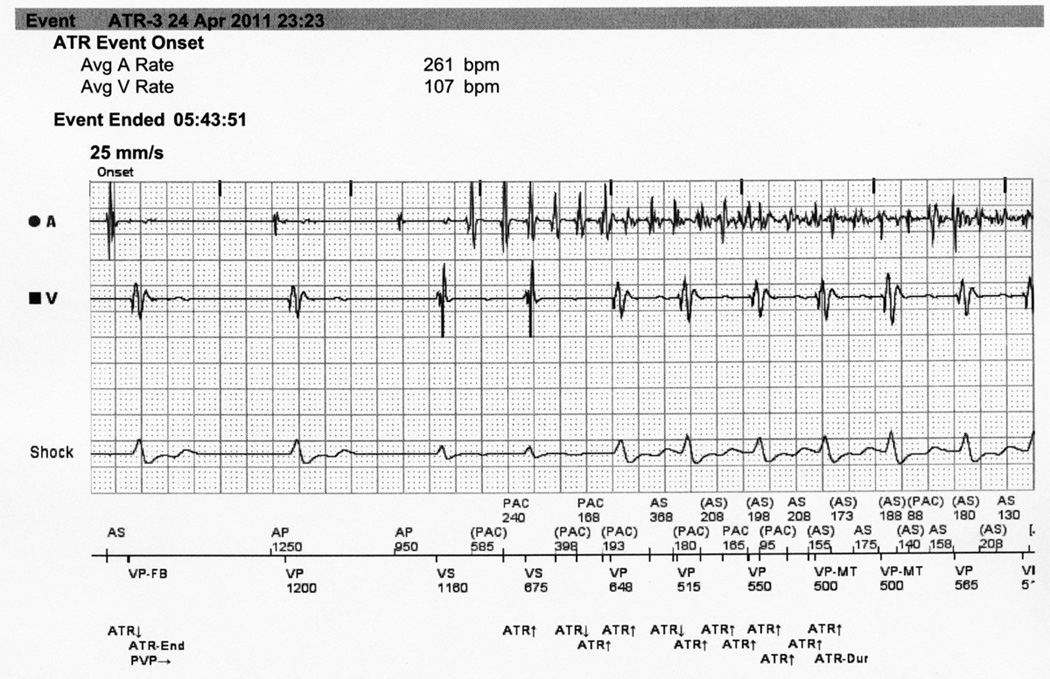
Arrhythmias documented by the ICD were later reviewed and interpreted by an electrophysiologist (MSL) blinded to air quality. These arrhythmias were characterized as ventricular, sinus tachycardia, AF, atrial arrhythmia other than AF, or not an arrhythmia. Sinus tachycardia is characterized by gradual onset and a 1 to 1 atrial to ventricular association. Acute onset atrial arrhythmias were subdivided into AF and atrial arrhythmias other than AF. These arrhythmias generally do not have 1 to 1 atrial to ventricular association; typically the atrial rate is much faster than the ventricular rate. Irregular rapid atrial electrograms were classified as AF (Figure 1).
Figure 1. Electrocardiogram of atrial fibrillation.
Intracardiac electrogram documenting the initiation of a disorganized atrial arrhythmia. Note the defibrillator documented time and date of arrhythmia onset as well as the intracardiac electrogram of both the atrium and the ventricle. The rapid and irregular atrial rate is typical of atrial fibrillation.
We restricted our analyses to clinically relevant AF defined as those lasting 30 seconds or longer as recommended by the American Heart Association, the American College of Cardiology and the Heart Rhythm Society. (27,33,34) To minimize clustering of events and overrepresentation of clustered events, if time periods between two successive AF episodes were shorter than 60 minutes, only the first episode of the arrhythmia was included in the analysis. Because of the possibility of pro-arrhythmia from the implantation of the ICD, all events occurring within 6 weeks of implant of the ICD device were excluded. Patients with less than 90 days of follow-up were excluded from analysis.
Air quality and weather data
Air quality was measured hourly at a Harvard Supersite monitor located on the roof of Countway Library 5 Km from Tufts Medical Center. Fine particle mass (PM2.5, particulate matter ≤ 2.5 microns in aerodynamic diameter) was measured by Tapered Element Oscillating Microbalance (TEOM, Rupprecht and Patashnick, East Greenbush, NY), black carbon (BC) by aethalometer (McGee Scientific, Berkeley, CA), particle number count (PNC, number of particles per cm3) by condensation particle counter (TSI Inc, Shoreview, MN), and sulfate (SO4) by sulfate particulate analyzer (Thermo Fisher Scientific, Waltham, MA). Gaseous air pollutants in the Boston metropolitan area also were measured hourly and averaged over three sites for sulfur dioxide (SO2), three sites for nitrogen dioxide (NO2), and two sites for ozone (O3), all operated by the Massachusetts Department of Environmental Protection.(35) Meteorological data (daily mean temperature, dew point, and barometric pressure) was obtained from the hourly surface observations of the National Weather Service at Logan Airport (East Boston).
Statistical Analysis
A case-crossover design allowed for investigation of the acute effects of exposure to air pollution.(36) The subject’s exposures before the time of the event (case period) is compared with the distribution of exposure estimated from separate control periods and the matched sets are analyzed using conditional logistic regression, thus each patient serves as his or her own control.(9,37–40) For these case-crossover analyses, only individuals who had AF in follow-up could be included for analysis.
Case periods were defined by the time prior to onset of each confirmed arrhythmic event, rounded to the nearest hour. Control periods (3–4 per case) were selected using a bidirectional time-stratified approach by matching on weekday and hour of the day within the same calendar month.(41) Hourly pollution concentrations and weather conditions were then matched to the case and control time periods for analysis. With this approach, the odds ratio from the logistic regression model is a consistent estimator of the incidence rate ratio. Average air pollution for the 24-hours prior to the AF, modeled as a continuous variable and in quartiles, was evaluated. Odds of AF associated with air pollution were estimated with conditional logistic regression, adjusted for temperature and dew point, and reported as percent increased odds (odds ratios minus one times 100) for an interquartile range (IQR) increase in exposure. The IQR reflects the range of exposures that the individual study subjects are likely to experience. Exposure–response relationships were examined based on estimated odds of arrhythmias versus quartiles of air pollution. In sensitivity analyses, associations with mean air pollution for the prior 2, 6, 12, and 48 hours were examined. Associations restricted to those patients less than the median distance (26 km) from the air pollution monitoring site, and associations restricted to patients with 20 or fewer AF events were also explored. Data management and all statistical analyses were conducted using SAS software version 9.1.3 (SAS Institute, Cary, NC, 2008).
RESULTS
Patient Population
Of the 1143 subjects screened, 843 were excluded, some for more than a single reason. The majority of the exclusions were for single chamber ICDs (n=502), living outside the 50-kilometer radius of the Harvard Supersite air quality monitoring station (n=345) and/or chronic AF (n=84). Of the 300 eligible patients, 200 were enrolled (66%) and 176 were followed for at least 90 days. The median distance of patients’ residence to the air pollution monitor was 20 km. Patients were 70% male, 93% Caucasian, had a mean age of 65 years (range 26 to 89 years) and a mean BMI of 29 kg/m2 (range 16 to 57) (Table 1). The mean left ventricular ejection fraction was 31.4% (range 10% to 70%). Indications for implantation of an ICD were primary prophylaxis in 111 subjects (63%), secondary prophylaxis in 33 subjects (19%), and syncope which clinically appeared arrhythmic, but without documentation of an arrhythmia in 32 subjects (18%). Twenty-nine percent of the population had a prior history of paroxysmal AF. Most patients were on standard pharmacologic therapy for cardiomyopathies which included beta blocking agents (93%), angiotension converting enzyme inhibitors or angiotension receptor blockers (84%), anti-platelet agents (68%) and statins (72%).
Table 1.
Patient Population (176 subjects followed for at least 90 days).
| All subjects N |
Subjects with AF>30 sec N |
|||
|---|---|---|---|---|
| Age (years) | 176 | 65.4 (26–89) | 49 | 67.9 (35–88) |
| Gender (male) | 123 (70%) | 33 (67%) | ||
| Race | ||||
| White | 164 (93%) | 47 (96%) | ||
| Black | 8 (5%) | 2 (4%) | ||
| Other | 4 (2%) | 0 (0%) | ||
| BMI (kg/m2) | 158 | 29.0 (16–57) | 40 | 27.8 (19–45) |
| Structural heart disease | 176 | 49 | ||
| Ischemic | 91 (52%) | 30 (61%) | ||
| Nonischemic | 52 (30%) | 10 (20%) | ||
| Other | 33 (19%) | 9 (18%) | ||
| Left ventricular ejection fraction (%) | 174 | 31.4 (10–70) | 49 | 31.4 (10–70) |
| History of revascularization | ||||
| CABG | 175 | 45 (26%) | 49 | 15 (31%) |
| PCI | 176 | 29 (17%) | 49 | 13 (27%)* |
| History of congestive heart failure | 176 | 96 (55%) | 49 | 31 (63%) |
| CHF class I | 19 (11%) | 8 (16%) | ||
| II | 31 (18%) | 10 (20%) | ||
| III | 46 (26%) | 13 (27%) | ||
| IV | 0 | 0 | ||
| Indications for implant | 176 | 49 | ||
| Primary prophylaxis | 111 (63%) | 31 (63%) | ||
| Secondary (cardiac arrest or sustained VT) | 33 (19%) | 7 (14%) | ||
| Syncope apparently arrhythmic | 32 (18%) | 11 (23%) | ||
| Co-morbidities | ||||
| Pulmonary Disease | 176 | 31 (18%) | 49 | 13 (27%) |
| Diabetes | 174 | 47 (27%) | 49 | 13 (27%) |
| Hypertension | 172 | 98 (57%) | 48 | 27 (56%) |
| History of AF | 175 | 51 (29%) | 49 | 24 (49%)* |
| Medications | 170 | 47 | ||
| ACE-I or ARB | 142 (84%) | 41 (87%) | ||
| Beta blocker | 158 (93%) | 43 (91%) | ||
| Antiarrhythmic agents: (Amiodarone, sotalol, or others not including beta blockers) | 28 (17%) | 10 (21%) | ||
| Platelet Aggregation Inhibitors | 115 (68%) | 31 (66%) | ||
| Statin | 123 (72%) | 38 (81%) | ||
| Smoking | ||||
| Current | 175 | 24 (14%) | 49 | 5 (11%) |
| Former | 175 | 85 (49%) | 49 | 25 (51%) |
| Never | 175 | 66 (38%) | 49 | 19 (39%) |
| Lived with Smoker | 175 | 111 (64%) | 49 | 32 (65%) |
| History of sleep apnea | 169 | 25 (15%) | 45 | 7 (16%) |
P< 0.05 between those with and without atrial fibrillation in follow-up.
For the 176 patients with at least 90 days of follow-up, the mean follow-up time was 1.9 years. In the course of the study 9 individuals (of the 200 enrolled) died, including 7 individuals followed for at least 90 days. Two of these subjects had AF during follow-up. The total time under observation was 340 person-years.
Arrhythmias
During the study period 328 incident AF with a duration of ≥ 30 seconds were observed among 49 patients (Table 1). The mean number of AF episodes among these 49 patients was 6.7 (median 4, range 1 to 65, and IQR 7). Patients with AF were more likely to have a past medical history of AF or percutaneous coronary intervention for revascularization but were similar in other respects to those without AF. Patients with AF were more likely to have a past medical history of AF or percutaneous coronary intervention for revascularization but were similar in other respects to those without AF.
Air quality
Daily mean air pollution concentrations during the study period were modest (Table 2). Mean PM2.5 was 8.4 µg/m3 with only one day above the EPA standard of 35 µg/m3 (maximum 52.4 µg/m3). The traffic related air pollutants, i.e. PM2.5, BC, and NO2, were highly correlated (Pearson correlation > 0.5), although the strongest correlation (r=0.82) was between PM2.5 and SO4, an indicator of secondary, transported fine particles (Table 2). PNC was negatively correlated with PM2.5 and SO4, but positively correlated with SO2 and NO2 (indicators of primary emissions).
Table 2.
Summary statistics and Pearson’s correlation coefficient of daily mean air pollutant concentrations and meteorological variables in Boston, USA, during the study period from September 1, 2006 until June 30, 2010.
| Summary statistics | Pearson correlation coefficient |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| # Days | Mean | 25th | 50th | 75th | PM2.5 | SO42− | BC | PNC | |
| Particles | |||||||||
| PM2.5 (µg/m3) | 1213 | 8.4 | 5.3 | 7.2 | 10.2 | 1 | 0.82 | 0.64 | −0.17 |
| SO42− (µg/m3) | 964 | 2.2 | 1.0 | 1.6 | 2.6 | 1 | 0.51 | −0.30 | |
| BC (µg/m3) | 1315 | 0.59 | 0.36 | 0.52 | 0.76 | 1 | 0.04 | ||
| PNC (1,000/cm3) | 1318 | 14.9 | 10.1 | 14.6 | 18.6 | 1 | |||
| Gases | |||||||||
| SO2 (ppb) | 1399 | 3.2 | 1.8 | 2.7 | 4.0 | 0.22 | 0.13 | 0.23 | 0.58 |
| NO2 (ppb) | 1399 | 16.1 | 11.9 | 15.2 | 19.2 | 0.37 | 0.24 | 0.59 | 0.47 |
| O3 (ppb) | 1399 | 24.8 | 17.5 | 24.3 | 31.5 | 0.18 | 0.22 | −0.21 | −0.39 |
| Weather | |||||||||
| Temperature (C) | 1398 | 10.6 | 3.4 | 11.0 | 18.3 | 0.31 | 0.37 | 0.24 | −0.74 |
| Dew point temperature (C) | 1398 | 3.5 | −3.7 | 4.1 | 11.9 | 0.34 | 0.41 | 0.35 | −0.72 |
Air Quality and AF
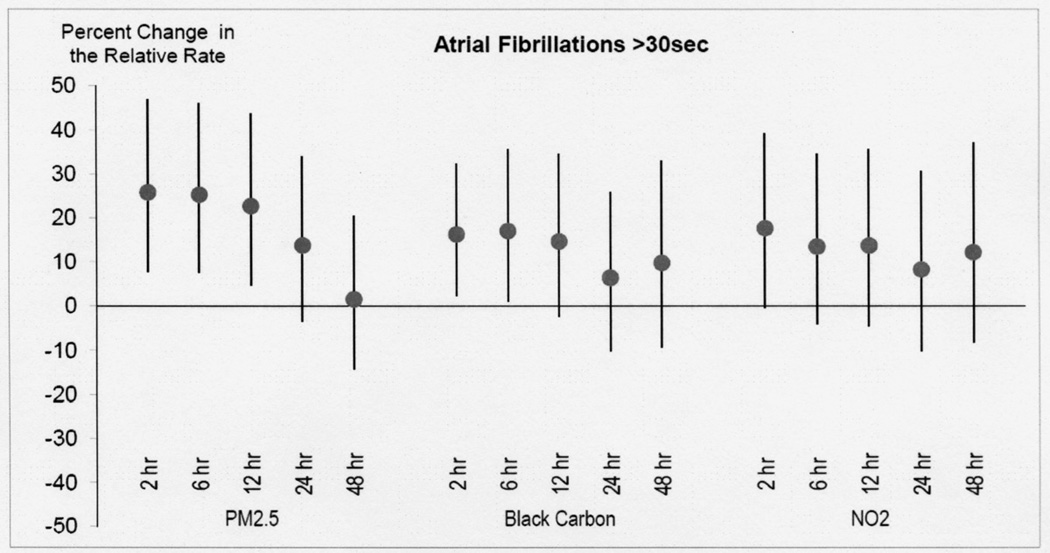
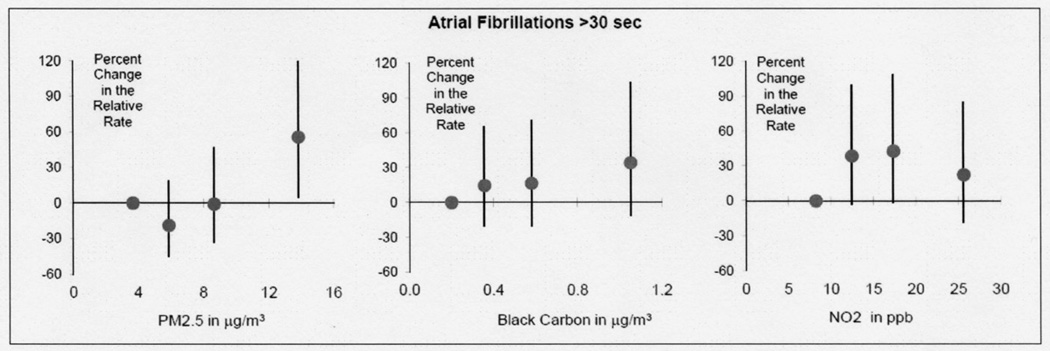
Increased odds were found between mean PM2.5 in the prior 24 hours and AF (Table 3). The strongest increased odds was a 14% increase (95% Confidence Interval (CI) −3% to 34%) for each 5.0 µg/m3 increase in 24-hour mean PM2.5. Stronger increased odds were found with shorter exposure periods before the AF event (Figure 2) with the strongest odds in the 2 hour moving average (26%; 95% CI 8% to 47%; Table 3) and effectively no association with mean over the previous 48 hours (Figure 2). A similar pattern was observed for BC, NO2 and PNC in that increased relative odds were also found for 2-hour moving averages compared to the 24 hour moving average. In quartile analysis there was an apparent exposure response effect in that the highest risk of AF was in the highest quartile of PM2.5 (Figure 3).
Table 3.
Percent change in risk of ICD detected arrhythmias associated with each interquartile range (IQR) increase in mean air pollution in the 2 and 24 hours prior to arrhythmic event, adjusted for temperature and dew point.
| Hours Prior | IQR | % change | (95% CI) | p-value | |
|---|---|---|---|---|---|
|
24 Hour Moving Average | |||||
| Particulates | |||||
| PM2.5 (µg/m3) | 24 | 5.0 | 14% | ( −3% , 34% ) | 0.12 |
| BC (µg/m3) | 24 | 0.39 | 6% | ( −10% , 26% ) | 0.47 |
| PNC (1000/cm3) | 24 | 8.4 | 12% | ( −19% , 56% ) | 0.49 |
| SO4 (µg/m3) | 24 | 1.6 | 0% | ( −14% , 15% ) | 0.96 |
| Gases | |||||
| NO2 (ppb) | 24 | 7.3 | 8% | ( −10% , 31% ) | 0.41 |
| SO2 (ppb) | 24 | 2.1 | 6% | ( −13% , 29% ) | 0.54 |
| O3 (ppb) | 24 | 14.0 | 18% | ( −12% , 56% ) | 0.27 |
|
2 Hour Moving Average | |||||
| Particulates | |||||
| PM2.5 (µg/m3) | 2 | 6.0 | 26% | ( 8% , 47% ) | 0.004 ** |
| BC (µg/m3) | 2 | 0.49 | 16% | ( 2% , 32% ) | 0.021 * |
| PNC (1000/cm3) | 2 | 10.9 | 24% | ( −4% , 61% ) | 0.099 |
| SO4 (µg/m3) | 2 | 1.7 | 2% | ( −10% , 16% ) | 0.71 |
| Gases | |||||
| NO2 (ppb) | 2 | 10.0 | 18% | ( −1% , 39% ) | 0.056 |
| SO2 (ppb) | 2 | 2.2 | 4% | ( −10% , 20% ) | 0.59 |
| O3 (ppb) | 2 | 17.0 | 3% | ( −20% , 31% ) | 0.83 |
Figure 2. Risk of atrial fibrillation with air pollution.
Percent increase in the occurrence (odds) of AF episodes associated with increase in air pollution (PM2.5, black carbon, and NO2) as a function of moving average in hours prior to event. Stronger increased odds were found with shorter exposure periods before the AF event with the strongest odds in the 2 hour moving average.
Figure 3. Risks of atrial fibrillation relative to quartile of pollution in the 2 hours prior to the arrhythmia.
This figure models the predictors as quartiles and plots each quartile at the median concentration for that quartile. Percent increased occurrence (odds) of AF for 2-hour moving averages of air pollution (PM2.5, black carbon and NO2) versus quartile medians. There was an apparent exposure response effect in that the highest risk of AF was in the highest quartile of PM2.5.
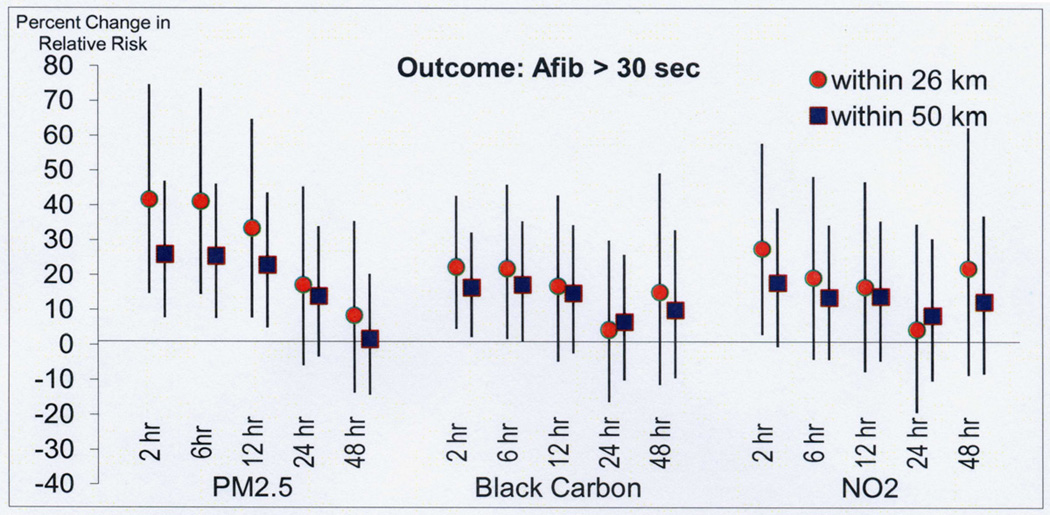
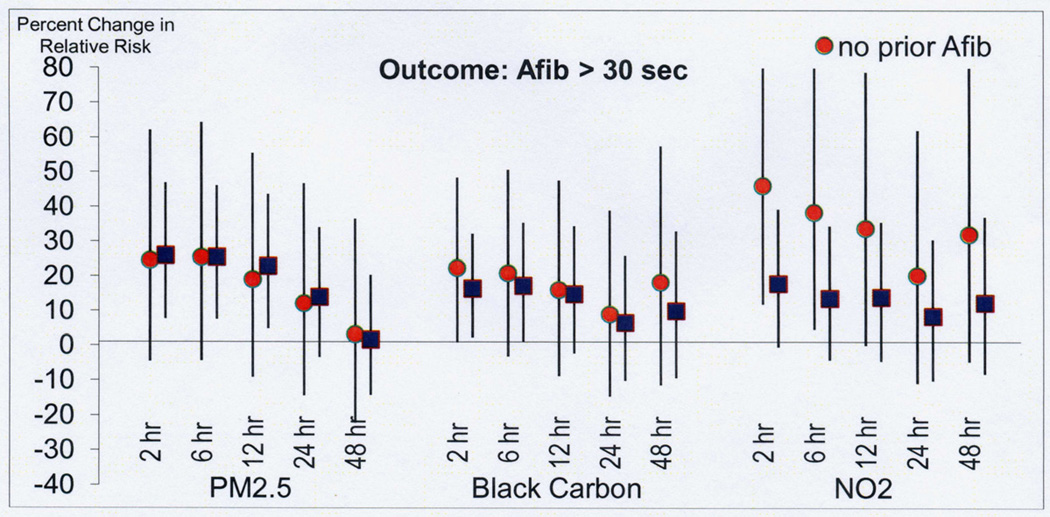
In sensitivity analyses restricted to the 25 patients (174 AF events) living closer than 26 km from the air pollution monitoring site, a stronger association was found with air pollution in the prior 2 hours for PM2.5 (42% increased odds compared to 26% in the whole population), BC (22% increased odds compared to 16% in the whole population), NO2 (28% increased odds compared to 18% in the whole population) (Figure 4). In analysis restricted to the 25 subjects with no history of AF prior to study enrollment (124 episodes of AF) the associations with PM2.5 and BC were similar and stronger for NO2 (Figure 5).
Figure 4. Risk of atrial fibrillation with air pollution in patients living within 26 km of the air pollution monitoring site.
In analyses restricted to the 25 patients (174 AF events) living closer than 26 km from the air pollution monitoring site, a stronger association was found with air pollution in the prior 2 hours for PM2.5 (42% increased odds compared to 26% in the whole population), BC (22% increased odds compared to 16% in the whole population), NO2 (28% increased odds compared to 18% in the whole population).
Figure 5. Risk of atrial fibrillation with air pollution in patients without a prior history of atrial fibrillation.
In analysis restricted to the 25 subjects with no history of AF prior to study enrollment (124 episodes of AF) the associations with PM2.5 and BC were similar and stronger for NO2 compared to all patients.
To ensure that patients with many events were not driving the positive association, the analysis was repeated with the exclusion of those individuals with > 20 AF episodes (3 individuals). Among these 46 patients (204 AF events), the increased odds of air pollution were either similar or of greater magnitude for PM2.5 (28%; 95% CI 4% to 59%), BC (26%; 95% CI 7% to 49%), NO2 (48%; 95% CI 19% to 85%), and PNC (44%; 95% CI 1% to 105%). There was still no association with SO4, SO2, or O3.
DISCUSSION
Fine particulate matter was an acute trigger of AF in this prospective study. This study is unique for its ability to capture all (including asymptomatic) atrial arrhythmias in a population at high risk for subclinical and clinical cardiac events. In addition, this study is able to specify the precise time of AF onset, and match time of onset with hourly ambient air pollution measurements. In many epidemiological studies the time window for cardiovascular effects of pollution is over days to years. However, we observed evidence of a shorter time window of increased exposure of 2 hours which was associated with an acutely increased risk. This short time window may be due to the nature of AF mechanism compared to other cardiovascular endpoints such as myocardial infarction or congestive heart failure or to the more precise timing of the events as is present in current ICD data collection.
Atrial fibrillation may be triggered by changes in autonomic tone,(42–45) inflammation and oxidative stress,(46–48) atrial ischemia,(49) and atrial pressures changes.(27,49,50) Acute alterations in sympathetic and parasympathetic tone and reduced heart rate variability is well documented in air pollution studies in humans,(51–53) and animals.(54,55) Increases in particulate air pollution have also been linked to inflammation.(56–58) Cardiac ischemia is worsened by air pollution.(59,60) And finally increases in right ventricular and thus likely right atrial pressures are seen with increases in particulate matter.(61) Thus, it biologically plausible that particulate air pollution can acutely trigger AF.
In addition to the strongest odds found with PM2.5, BC was associated with AF in the two hour moving average prior to AF. PM2.5 incorporates pollution from a multiple sources, both direct emissions from motor vehicles and other local and regional sources and secondary particles from upwind fossil fuel combustion.(51,53,62) BC is mainly a marker of local and regional vehicular emissions. Other pollutants associated with vehicular emissions, PNC and NO2 were also associated with AF, but did not reach statistical significance. Pollutants not associated with motor vehicular pollution (SO4, SO2, O3) were not associated with AF.
A strength of this study was the ability to precisely define the time of onset of AF. Because AF may be asymptomatic or of short-duration, it is important to accurately and precisely define the time of onset of these events. Misalignment of air pollution exposures with acute cardiac events has been shown to produce an underestimate of the association and can mask true associations.(63) Another strength of this study is the hourly pollution measurements obtained in the Boston area, allowing for more accurate determination of association of air quality and arrhythmias. These precise hourly measurements allowed for examination of acute triggering (as little as 2 hour moving averages) of AF, in contrast to most previous studies which have relied on integrated calendar day mean air pollution data.
AF is responsible for more hospitalizations and longer stays than any other arrhythmia, and may lead to stroke, congestive heart failure and myocardial infarction.(25–28) AF is the most common arrhythmia in the United States and most other countries.(27,64) The morbidity and mortality of AF is largely based on the risk of embolic events, the induction of heart failure and myocardial ischemia, and not on whether or not it causes arrhythmic symptoms. Given the known associations of AF and adverse cardiac outcomes,(25–29) and the results of this study it is likely that at least a portion of air pollution’s adverse cardiovascular effects are mediated by AF. Therefore, it is quite likely that AF contributes to the morbidity and mortality that has been associated with air pollution.
Most concerning is that the higher odds of AF were observed at air quality levels well under ambient air quality standards. The Environmental Protection Agency (EPA) 24 hour standard for PM2.5 is 35 µg/m3.(65) In the current study the maximum PM2.5 was 52.4 µg/m3 and the 75th percentile was10.2 µg/m3. In fact, in only one day did PM2.5 exceed 35 µg/m3 in Boston during the study time-frame. The average PM2.5 during the study period was less than 60% of the current EPA annual average standard of 15 µg/m3. The exposure-response shown in this study suggests that in cities with higher levels of pollution, the risk of AF may be even greater. Thus, these results raise important public health implications in that even low levels of air pollution such as that seen in Boston are associated with an increased occurrence of AF episodes.
Prior data linking air pollution to atrial arrhythmias are limited by a short duration of monitoring or incomplete monitoring of atrial arrhythmias. Incomplete arrhythmia monitoring is demonstrated by a sub-analysis of an earlier study of Boston ICD patients which reported associations of air pollution with atrial arrhythmias.(21) In this study, most patients had a single chamber ICD (ventricular) and thus only rapid AF such as would activate the ventricular arrhythmia zone were recorded. Consequently, these episodes were a highly selected subset of AF and likely were a minority of the atrial arrhythmia episodes.
Other studies on the association of atrial arrhythmias and air pollution utilize short and intermittent cardiac monitoring. For example, in a 24 week-30 minute/week Holter study of 32 adults in Ohio, 5-day moving averages of PM2.5, sulfate and ozone were associated with an increased odds of supraventricular ectopy.(23) In a 4 week-24 hour/week Holter study of 57 German men with coronary artery disease, elevated concentrations of particulate matter and NO2 in the prior 24-hours were associated with increased risk for atrial runs.(24) In another study monitoring PR prolongation and P wave complexity, possible predictors of AF, were associated with PM2.5 in the prior 24 hours.(22) No prior study to our knowledge has utilized such an extended time period of follow-up with complete monitoring for all AF as was done in the current study.
Limitations
As in many air pollution studies there are limitations to single site air quality monitoring. Single site ambient monitoring may lead to exposure misclassification. Exposure misclassification is less likely with PM2.5 because of the regional spatial homogeneity of this pollutant. Other pollutants with less spatial homogeneity may have weaker associations because of exposure misclassification. To overcome this issue we also analyzed only patients residing within 26 km of the monitoring station. In this analysis the effect of PM2.5, BC and NO2 were strong but with wider confidence intervals. The association with sulfate, PNC, SO2 and O3 remained weak and far from statistical significance.
Given the number of statistical tests performed (7 pollutants with 5 different lag times) it is possible that some of our results may be due to chance. However, the strongest associations were seen for the set of air pollutants indicative of motor vehicle exhaust. These associations were strongest for the 2 hour window prior to the AF and decreased with increasing lag times. There was also a dose dependent association with PM2.5 in that higher pollutants levels had an increased odds of AF. For these reasons we believe these results present a coherent, biologically plausible evidence of a true association which is not due to chance.
A significant proportion of the study population (29%) had episodic AF prior to their enrollment. The prior history of AF is surely one of the strongest risk factors for AF; thus these individuals are at particularly high risk for AF. Yet, it appears that air pollution may initiate a specific episode in these individuals. In analysis restricted to those patients without a prior history of AF the results were unchanged compared to the entire group (Figure 5). Patients in this study were at higher risk of AF because of their underlying heart disease and reduced left ventricular ejection fraction. However, AF is the most common arrhythmia in the United States and age is one of the strongest risk factors, yet it is unknown whether these results are relevant to other populations also at risk for AF.
This study evaluated the acute onset of AF, not the duration or total time in AF. The total duration of AF may be more important than the number of episodes of AF. This study also focused on AF and not on ventricular arrhythmias.
CONCLUSIONS
In this high risk population, ambient air pollution increased the occurrence of AF episodes. Importantly, the air pollution-associated increase in AF demonstrated an exposure response risk of AF with worsening air quality, even in a city with air quality which is within the EPA mandated levels. Since AF is associated with stroke and cardiovascular disease, it is likely that AF contributes to the adverse outcomes of air pollution seen in epidemiologic studies.
Acknowledgements
Funding: NIEHS Grants PO1 ES009825 and P30 ES000002 and the EPA-Harvard Clean Air Research Center (R83479801).
The National Institute of Environmental Health Sciences and the Environmental Protection Agency provides grant support to the Harvard School of Public Health, Department of Environmental Health. These grants partially supported Heike Luttmann-Gibson, Joel Schwartz, Murray A. Mittleman, Diane R. Gold, Douglas W. Dockery, and Francine Laden.
The authors would like to thank Michaela Owen, Melanie E. Marshall and Brandon Udelhofen for their work on this project. They were instrumental in the enrollment, follow-up and data collection of patients.
Abbreviations
- AF
Atrial fibrillation
- BC
Black Carbon
- bpm
Beats per minute
- ICD
Implantable Cardioverter Defibrillator
- NO2
Nitrogen Dioxide
- O3
Ozone
- PM2.5
Particulate Matter less than 2.5 µm aerodynamic diameter
- PNC
Particle Number Count
- SO2
Sulfur Dioxide
- SO42−
Sulfate
- µg/m3
micrograms per cubic meter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: There are no other conflicts of interests.
REFERENCES
- 1.Dockery DW. Epidemiologic evidence of cardiovascular effects of particulate air pollution. Environmental health perspectives. 2001;109(Suppl 4):483–486. doi: 10.1289/ehp.01109s4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brook RD, Rajagopalan S, Pope CA, 3rd, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 3.Brook RD, Franklin B, Cascio W, et al. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 4.Link MS, Dockery DW. Air pollution and the triggering of cardiac arrhythmias. Curr Opin Cardiol. 2010;25:16–22. doi: 10.1097/HCO.0b013e32833358cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zanobetti A, Schwartz J. The effect of particulate air pollution on emergency admissions for myocardial infarction: a multicity case-crossover analysis. Environmental health perspectives. 2005;113:978–982. doi: 10.1289/ehp.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peters A, Dockery DW, Muller JE, Mittleman MA. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103:2810–2815. doi: 10.1161/01.cir.103.23.2810. [DOI] [PubMed] [Google Scholar]
- 7.Forastiere F, Stafoggia M, Picciotto S, et al. A case-crossover analysis of out-of-hospital coronary deaths and air pollution in Rome, Italy. American journal of respiratory and critical care medicine. 2005;172:1549–1555. doi: 10.1164/rccm.200412-1726OC. [DOI] [PubMed] [Google Scholar]
- 8.Rosenthal FS, Carney JP, Olinger ML. Out-of-hospital cardiac arrest and airborne fine particulate matter: a case-crossover analysis of emergency medical services data in Indianapolis, Indiana. Environmental health perspectives. 2008;116:631–636. doi: 10.1289/ehp.10757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levy D, Sheppard L, Checkoway H, et al. A case-crossover analysis of particulate matter air pollution and out-of-hospital primary cardiac arrest. Epidemiology (Cambridge, Mass. 2001;12:193–199. [PubMed] [Google Scholar]
- 10.Pope CA, 3rd, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc. 2006;56:709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- 11.Wellenius GA, Schwartz J, Mittleman MA. Air pollution and hospital admissions for ischemic and hemorrhagic stroke among medicare beneficiaries. Stroke. 2005;36:2549–2553. doi: 10.1161/01.STR.0000189687.78760.47. [DOI] [PubMed] [Google Scholar]
- 12.O'Donnell MJ, Fang J, Mittleman MA, Kapral MK, Wellenius GA. Fine Particulate Air Pollution (PM2.5) and the Risk of Acute Ischemic Stroke. Epidemiology (Cambridge, Mass. 2011;22:422–431. doi: 10.1097/EDE.0b013e3182126580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller KA, Siscovick DS, Sheppard L, et al. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356:447–458. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- 14.Pope CA, 3rd, Burnett RT, Thurston GD, et al. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- 15.Wellenius GA, Burger MR, Coull BA, et al. Ambient air pollution and the risk of acute ischemic stroke. Arch Intern Med. 2012;172:229–234. doi: 10.1001/archinternmed.2011.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rich DQ, Kim MH, Turner JR, et al. Association of ventricular arrhythmias detected by implantable cardioverter defibrillator and ambient air pollutants in the St Louis, Missouri metropolitan area. Occupational and environmental medicine. 2006;63:591–596. doi: 10.1136/oem.2005.023457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dockery DW, Luttmann-Gibson H, Rich DQ, et al. Association of air pollution with increased incidence of ventricular tachyarrhythmias recorded by implanted cardioverter defibrillators. Environmental health perspectives. 2005;113:670–674. doi: 10.1289/ehp.7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson HR, Armstrong B, Hajat S, et al. Air pollution and activation of implantable cardioverter defibrillators in London. Epidemiology (Cambridge, Mass. 2010;21:405–413. doi: 10.1097/EDE.0b013e3181d61600. [DOI] [PubMed] [Google Scholar]
- 19.Ljungman PL, Berglind N, Holmgren C, et al. Rapid effects of air pollution on ventricular arrhythmias. Eur Heart J. 2008;29:2894–2901. doi: 10.1093/eurheartj/ehn463. [DOI] [PubMed] [Google Scholar]
- 20.Metzger KB, Klein M, Flanders WD, et al. Ambient air pollution and cardiac arrhythmias in patients with implantable defibrillators. Epidemiology (Cambridge, Mass. 2007;18:585–592. doi: 10.1097/EDE.0b013e318124ff0e. [DOI] [PubMed] [Google Scholar]
- 21.Rich DQ, Mittleman MA, Link MS, et al. Increased risk of paroxysmal atrial fibrillation episodes associated with acute increases in ambient air pollution. Environmental health perspectives. 2006;114:120–123. doi: 10.1289/ehp.8371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao D, Shaffer ML, He F, et al. Fine particulate air pollution is associated with higher vulnerability to atrial fibrillation--the APACR study. Journal of toxicology and environmental health. 2011;74:693–705. doi: 10.1080/15287394.2011.556056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarnat SE, Suh HH, Coull BA, Schwartz J, Stone PH, Gold DR. Ambient particulate air pollution and cardiac arrhythmia in a panel of older adults in Steubenville, Ohio. Occupational and environmental medicine. 2006;63:700–706. doi: 10.1136/oem.2006.027292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berger A, Zareba W, Schneider A, et al. Runs of ventricular and supraventricular tachycardia triggered by air pollution in patients with coronary heart disease. Journal of occupational and environmental medicine / American College of Occupational and Environmental Medicine. 2006;48:1149–1158. doi: 10.1097/01.jom.0000245921.15916.03. [DOI] [PubMed] [Google Scholar]
- 25.Roy D, Talajic M, Nattel S, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–2677. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 26.Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154:1449–1457. [PubMed] [Google Scholar]
- 27.Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 Guidelines for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257–e354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 28.Roy D, Talajic M, Dubuc M, et al. Atrial fibrillation and congestive heart failure. Curr Opin Cardiol. 2009;24:29–34. doi: 10.1097/hco.0b013e32831c8c58. [DOI] [PubMed] [Google Scholar]
- 29.Santini M, Gasparini M, Landolina M, et al. Device-detected atrial tachyarrhythmias predict adverse outcome in real-world patients with implantable biventricular defibrillators. J Am Coll Cardiol. 2011;57:167–172. doi: 10.1016/j.jacc.2010.08.624. [DOI] [PubMed] [Google Scholar]
- 30.Conen D, Chae CU, Glynn RJ, et al. Risk of death and cardiovascular events in initially healthy women with new-onset atrial fibrillation. JAMA. 2011;305:2080–2087. doi: 10.1001/jama.2011.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McManus DD, Rienstra M, Benjamin EJ. An update on the prognosis of patients with atrial fibrillation. Circulation. 2012;126:e143–e146. doi: 10.1161/CIRCULATIONAHA.112.129759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Healey JS, Connolly SJ, Gold MR, et al. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120–129. doi: 10.1056/NEJMoa1105575. [DOI] [PubMed] [Google Scholar]
- 33.Calkins H, Brugada J, Packer DL, et al. HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel policy procedures and follow-up A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2007;4:816–861. doi: 10.1016/j.hrthm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Estes NA, 3rd, Halperin JL, Calkins H, et al. ACC/AHA/Physician Consortium 2008 clinical performance measures for adults with nonvalvular atrial fibrillation or atrial flutter: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and the Physician Consortium for Performance Improvement (Writing Committee to Develop Clinical Performance Measures for Atrial Fibrillation): developed in collaboration with the Heart Rhythm Society. Circulation. 2008;117:1101–1120. doi: 10.1161/CIRCULATIONAHA.107.187192. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz J. The distributed lag between air pollution and daily deaths. Epidemiology (Cambridge, Mass. 2000;11:320–326. doi: 10.1097/00001648-200005000-00016. [DOI] [PubMed] [Google Scholar]
- 36.Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. American journal of epidemiology. 1991;133:144–153. doi: 10.1093/oxfordjournals.aje.a115853. [DOI] [PubMed] [Google Scholar]
- 37.Neas LM, Schwartz J, Dockery D. A case-crossover analysis of air pollution and mortality in Philadelphia. Environmental health perspectives. 1999;107:629–631. doi: 10.1289/ehp.99107629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sunyer J, Schwartz J, Tobias A, Macfarlane D, Garcia J, Anto JM. Patients with chronic obstructive pulmonary disease are at increased risk of death associated with urban particle air pollution: a case-crossover analysis. American journal of epidemiology. 2000;151:50–56. doi: 10.1093/oxfordjournals.aje.a010121. [DOI] [PubMed] [Google Scholar]
- 39.Lee JT, Schwartz J. Reanalysis of the effects of air pollution on daily mortality in Seoul, Korea: A case-crossover design. Environmental health perspectives. 1999;107:633–636. doi: 10.1289/ehp.99107633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mittleman MA, Maclure M, Robins JM. Control sampling strategies for case-crossover studies: an assessment of relative efficiency. American journal of epidemiology. 1995;142:91–98. doi: 10.1093/oxfordjournals.aje.a117550. [DOI] [PubMed] [Google Scholar]
- 41.Levy D, Lumley T, Sheppard L, Kaufman J, Checkoway H. Referent selection in case-crossover analyses of acute health effects of air pollution. Epidemiology (Cambridge, Mass. 2001;12:186–192. doi: 10.1097/00001648-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 42.Bettoni M, Zimmermann M. Autonomic tone variations before the onset of paroxysmal atrial fibrillation. Circulation. 2002;105:2753–2759. doi: 10.1161/01.cir.0000018443.44005.d8. [DOI] [PubMed] [Google Scholar]
- 43.Zimmermann M, Kalusche D. Fluctuation in autonomic tone is a major determinant of sustained atrial arrhythmias in patients with focal ectopy originating from the pulmonary veins. J Cardiovasc Electrophysiol. 2001;12:285–291. doi: 10.1046/j.1540-8167.2001.00285.x. [DOI] [PubMed] [Google Scholar]
- 44.Schauerte P, Scherlag BJ, Patterson E, et al. Focal atrial fibrillation: experimental evidence for a pathophysiologic role of the autonomic nervous system. J Cardiovasc Electrophysiol. 2001;12:592–599. doi: 10.1046/j.1540-8167.2001.00592.x. [DOI] [PubMed] [Google Scholar]
- 45.Lombardi F, Tarricone D, Tundo F, Colombo F, Belletti S, Fiorentini C. Autonomic nervous system and paroxysmal atrial fibrillation: a study based on the analysis of RR interval changes before, during and after paroxysmal atrial fibrillation. Eur Heart J. 2004;25:1242–1248. doi: 10.1016/j.ehj.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 46.Sata N, Hamada N, Horinouchi T, et al. C-reactive protein and atrial fibrillation. Is inflammation a consequence or a cause of atrial fibrillation? Jpn Heart J. 2004;45:441–445. doi: 10.1536/jhj.45.441. [DOI] [PubMed] [Google Scholar]
- 47.Aviles RJ, Martin DO, Apperson-Hansen C, et al. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006–3010. doi: 10.1161/01.CIR.0000103131.70301.4F. [DOI] [PubMed] [Google Scholar]
- 48.Chung MK, Martin DO, Sprecher D, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886–2891. doi: 10.1161/hc4901.101760. [DOI] [PubMed] [Google Scholar]
- 49.White CW, Kerber RE, Weiss HR, Marcus ML. The effects of atrial fibrillation on atrial pressure-volume and flow relationships. Circ Res. 1982;51:205–215. doi: 10.1161/01.res.51.2.205. [DOI] [PubMed] [Google Scholar]
- 50.Kamkin A, Kiseleva I, Wagner KD, Lozinsky I, Gunther J, Scholz H. Mechanically induced potentials in atrial fibroblasts from rat hearts are sensitive to hypoxia/reoxygenation. Pflugers Arch. 2003;446:169–174. doi: 10.1007/s00424-003-1032-0. [DOI] [PubMed] [Google Scholar]
- 51.Zanobetti A, Gold DR, Stone PH, et al. Reduction in heart rate variability with traffic and air pollution in patients with coronary artery disease. Environmental health perspectives. 2010;118:324–330. doi: 10.1289/ehp.0901003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gold DR, Litonjua A, Schwartz J, et al. Ambient pollution and heart rate variability. Circulation. 2000;101:1267–1273. doi: 10.1161/01.cir.101.11.1267. [DOI] [PubMed] [Google Scholar]
- 53.Schwartz J, Litonjua A, Suh H, et al. Traffic related pollution and heart rate variability in a panel of elderly subjects. Thorax. 2005;60:455–461. doi: 10.1136/thx.2004.024836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen LC, Hwang JS. Effects of subchronic exposures to concentrated ambient particles (CAPs) in mice. IV Characterization of acute and chronic effects of ambient air fine particulate matter exposures on heart-rate variability. Inhalation toxicology. 2005;17:209–216. doi: 10.1080/08958370590912789. [DOI] [PubMed] [Google Scholar]
- 55.Corey LM, Baker C, Luchtel DL. Heart-rate variability in the apolipoprotein E knockout transgenic mouse following exposure to Seattle particulate matter. Journal of toxicology and environmental health. 2006;69:953–965. doi: 10.1080/15287390500362105. [DOI] [PubMed] [Google Scholar]
- 56.Peters A, Frohlich M, Doring A, et al. Particulate air pollution is associated with an acute phase response in men; results from the MONICA-Augsburg Study. Eur Heart J. 2001;22:1198–1204. doi: 10.1053/euhj.2000.2483. [DOI] [PubMed] [Google Scholar]
- 57.Pekkanen J, Brunner EJ, Anderson HR, Tiittanen P, Atkinson RW. Daily concentrations of air pollution and plasma fibrinogen in London. Occupational and environmental medicine. 2000;57:818–822. doi: 10.1136/oem.57.12.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwartz J. Air pollution and blood markers of cardiovascular risk. Environmental health perspectives. 2001;109(Suppl 3):405–409. doi: 10.1289/ehp.01109s3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pekkanen J, Peters A, Hoek G, et al. Particulate air pollution and risk of ST-segment depression during repeated submaximal exercise tests among subjects with coronary heart disease: the Exposure and Risk Assessment for Fine and Ultrafine Particles in Ambient Air (ULTRA) study. Circulation. 2002;106:933–938. doi: 10.1161/01.cir.0000027561.41736.3c. [DOI] [PubMed] [Google Scholar]
- 60.Delfino RJ, Gillen DL, Tjoa T, et al. Electrocardiographic ST-segment depression and exposure to traffic-related aerosols in elderly subjects with coronary artery disease. Environmental health perspectives. 2011;119:196–202. doi: 10.1289/ehp.1002372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rich DQ, Freudenberger RS, Ohman-Strickland P, Cho Y, Kipen HM. Right heart pressure increases after acute increases in ambient particulate concentration. Environmental health perspectives. 2008;116:1167–1171. doi: 10.1289/ehp.11230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zanobetti A, Stone PH, Speizer FE, et al. T-wave alternans, air pollution and traffic in high-risk subjects. Am J Cardiol. 2009;104:665–670. doi: 10.1016/j.amjcard.2009.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lokken RP, Wellenius GA, Coull BA, et al. Air pollution and risk of stroke: underestimation of effect due to misclassification of time of event onset. Epidemiology (Cambridge, Mass. 2009;20:137–142. doi: 10.1097/ede.0b013e31818ef34a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 65.Environment Protection Agency. National Ambient Air Quality Standards for Particulate Matter. 2006:61144–61233. [Google Scholar]