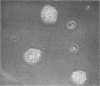
Abstract
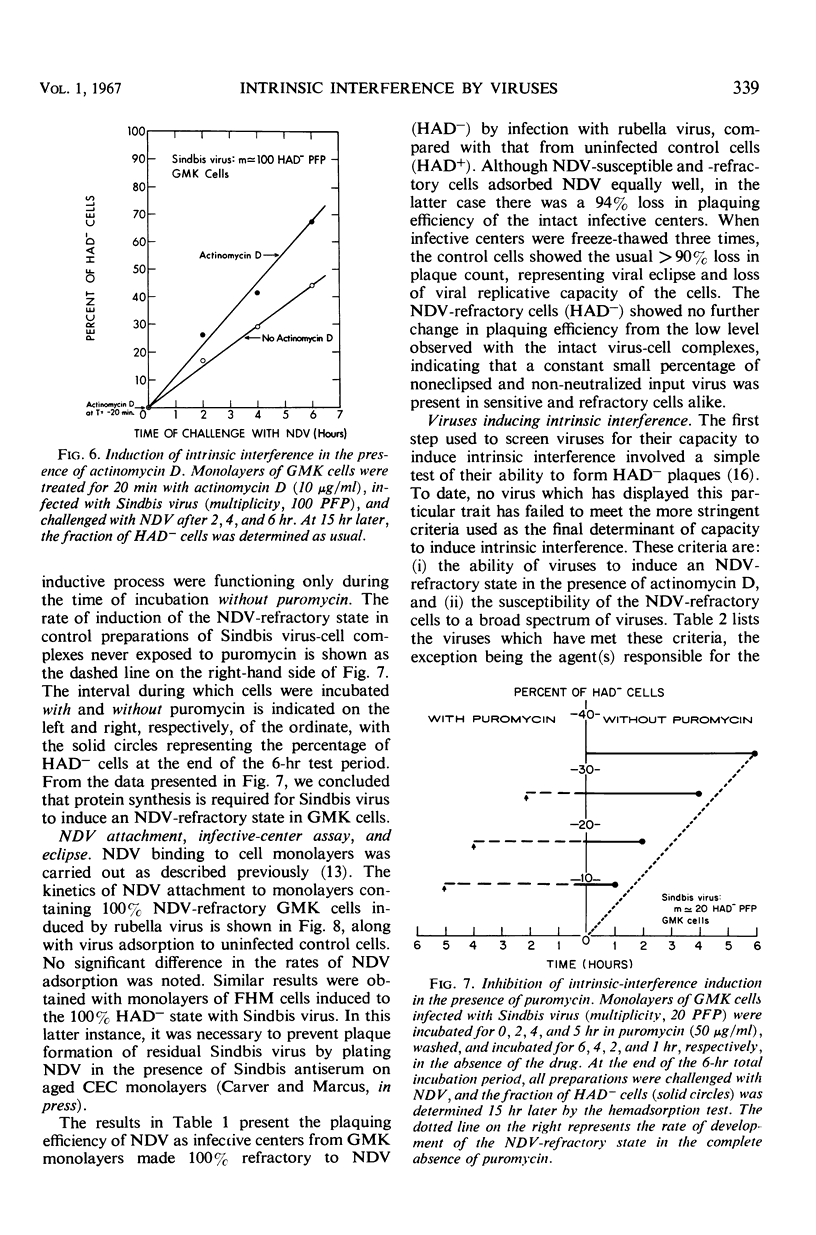
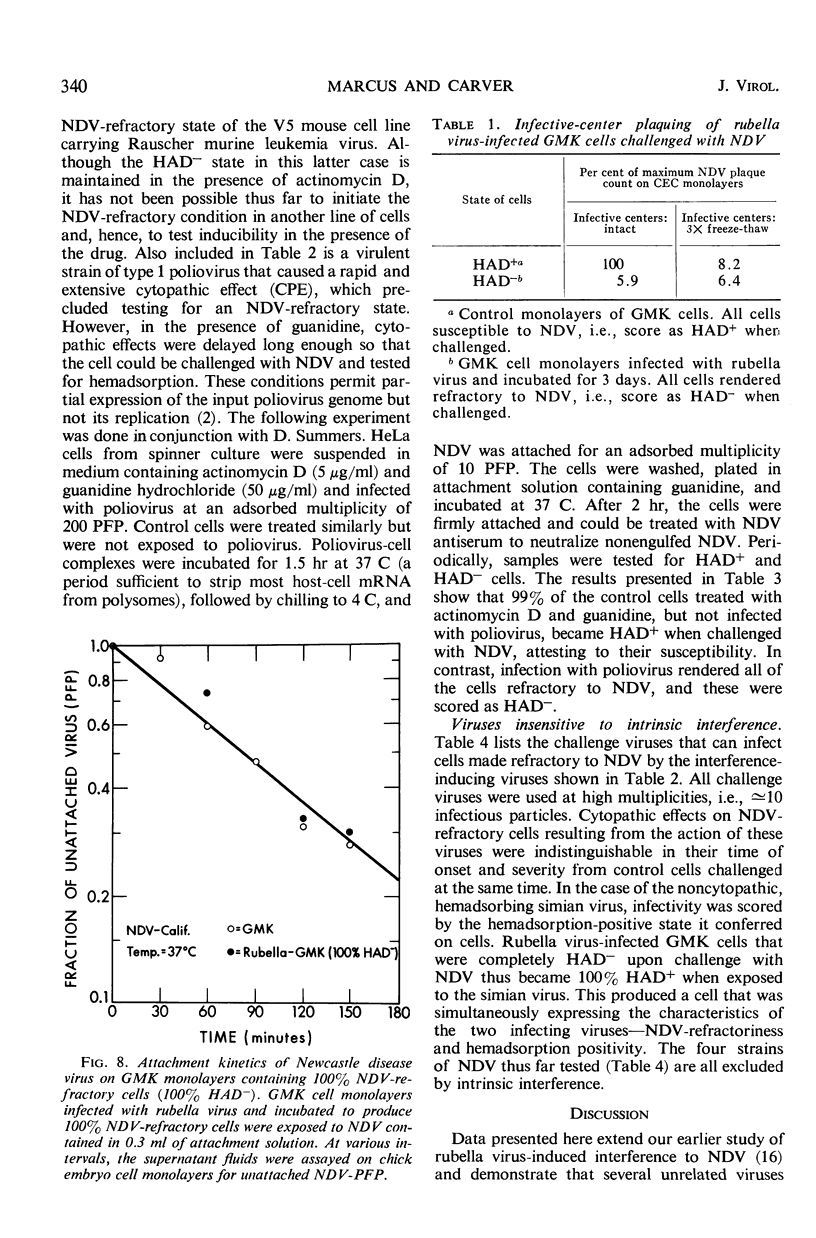
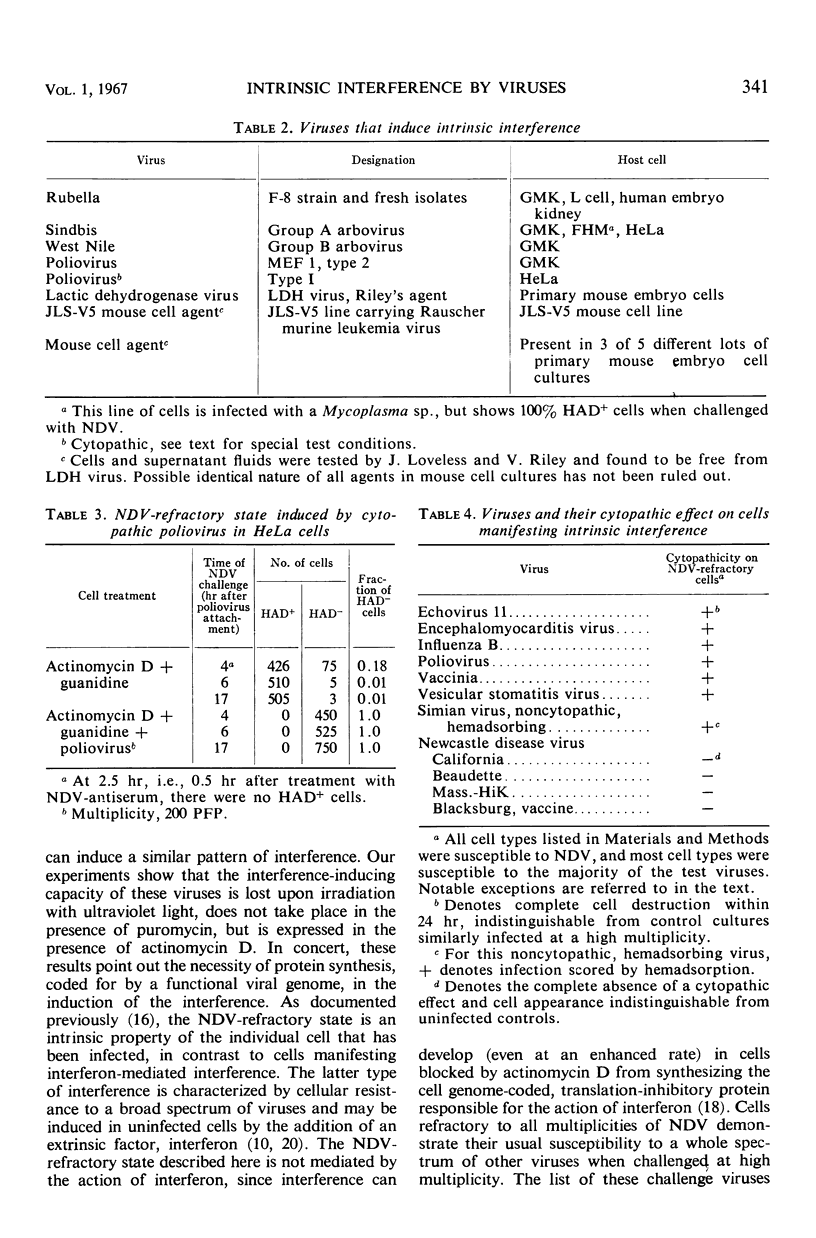
The hemadsorption-negative plaque test has revealed a new type of viral interference, termed intrinsic interference. Several unrelated types of noncytopathic viruses were shown to induce in infected host cells a state of interference unique in being directed solely against superinfection by Newcastle disease virus (NDV). The NDV-refractory state arises only in those individual cells of a population actually infected by the inducing virus, and presumably results from the action of a protein(s) coded for by the viral genome. Thus, intrinsic interference differs fundamentally from that mediated by an extrinsic protein detectable under conditions favoring resistance to a broad spectrum of viruses and characteristic of interference induced by interferon, the latter being coded for by the cell genome. Intrinsic interference is defined as a viral genome-induced cellular state of resistance to challenge by high multiplicities of NDV, coexistent with a state of susceptibility to a broad spectrum of other viruses, similarly tested at high multiplicities. The capacity to induce intrinsic interference was demonstrated with rubella virus, Sindbis virus (arbovirus, group A), West Nile virus (arbovirus, group B), poliovirus (MEF, type 2), the lactic dehydrogenase virus (Riley's agent), and an unidentified nonhemadsorbing, noncytopathic adventitious virus. A state of intrinsic interference was also observed in the V5 line of mouse cells carrying a murine leukemia virus, probably resulting from some heretofore unsuspected contaminating virus. The molecular basis for intrinsic interference is not known, but it appears to involve a step in the NDV growth cycle beyond that of viral attachment, entry, and eclipse.
Full text
PDF

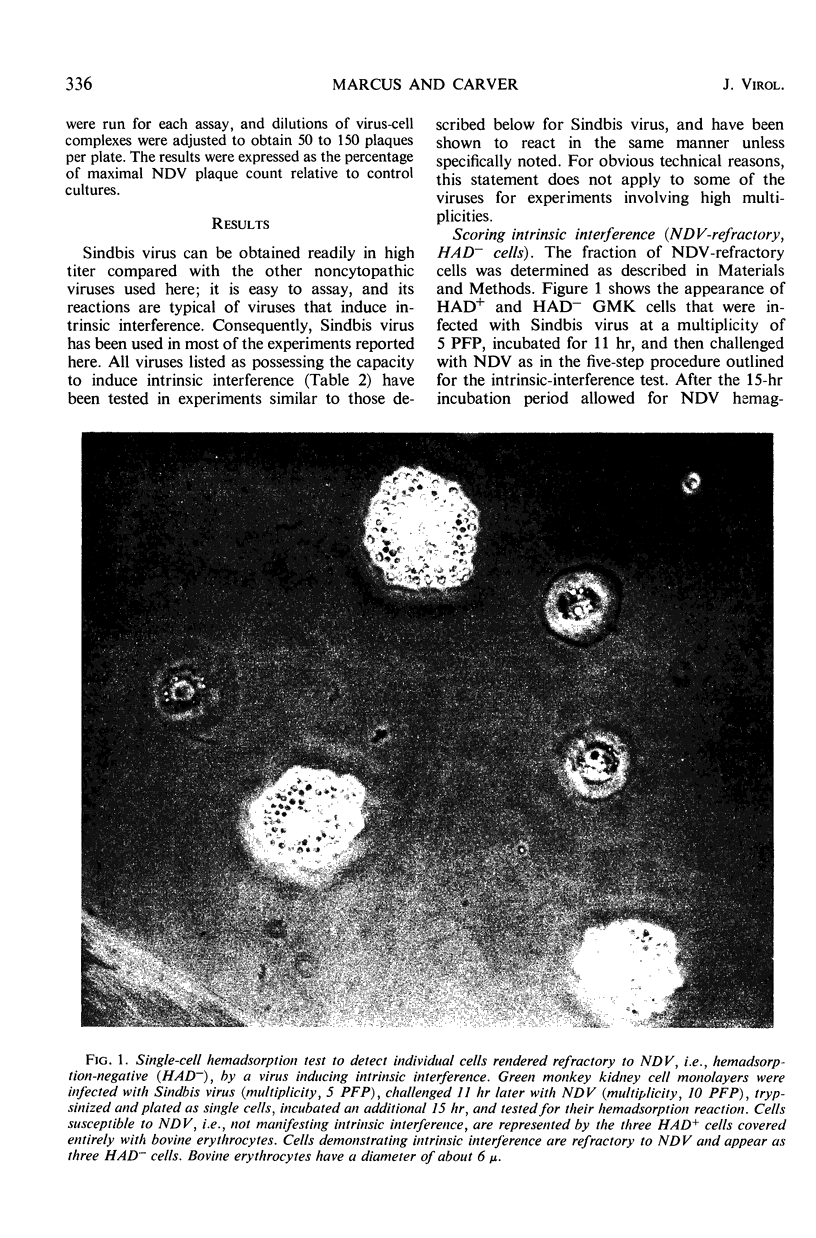
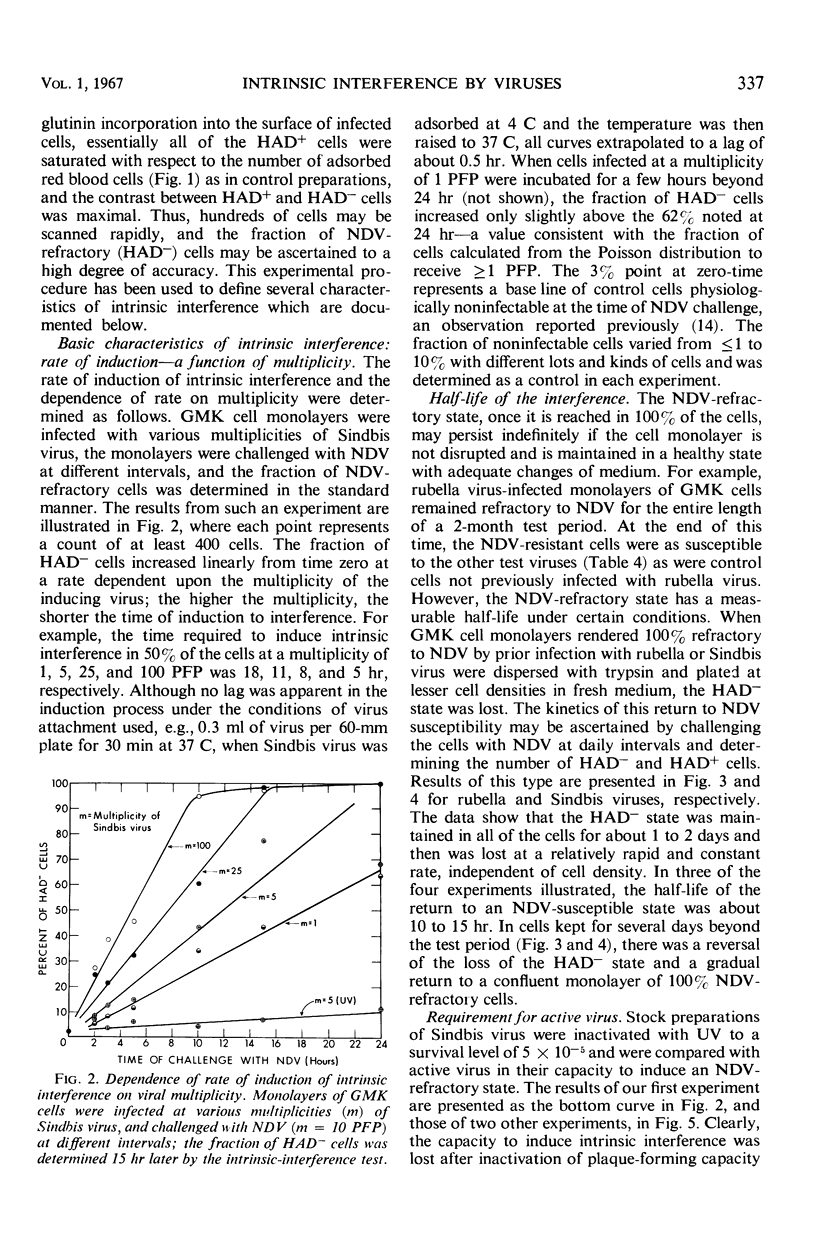
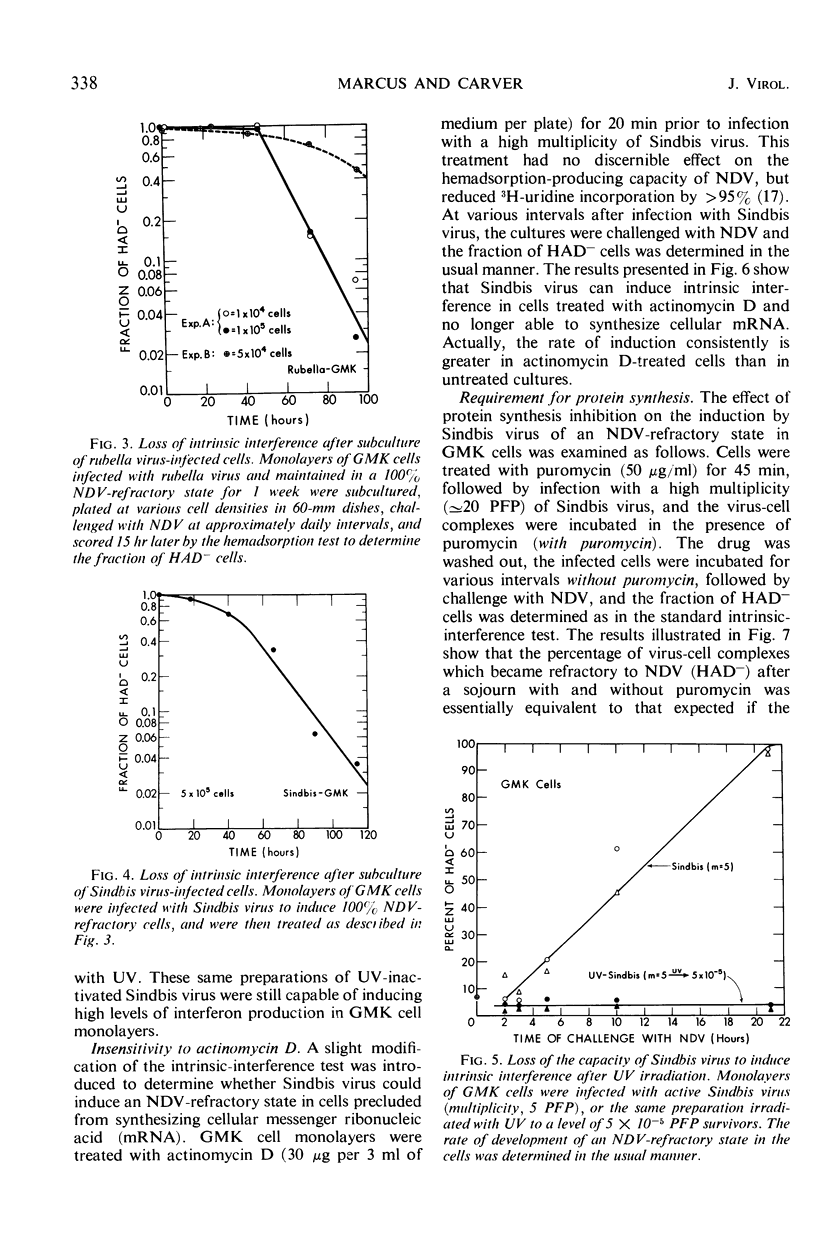


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BABLANIAN R., EGGERS H. J., TAMM I. STUDIES ON THE MECHANISM OF POLIOVIRUS-INDUCED CELL DAMAGE. I. THE RELATION BETWEEN POLIOVIRUS,-INDUCED METABOLIC AND MORPHOLOGICAL ALTERATIONS IN CULTURED CELLS. Virology. 1965 May;26:100–113. doi: 10.1016/0042-6822(65)90030-9. [DOI] [PubMed] [Google Scholar]
- BALTIMORE D., EGGERS H. J., FRANKLIN R. M., TAMM I. Poliovirus-induced RNA polymerase and the effects of virus-specific inhibitors on its production. Proc Natl Acad Sci U S A. 1963 Jun;49:843–849. doi: 10.1073/pnas.49.6.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALUDA M. A. Loss of viral receptors in homologous interference by ultraviolet-irradiated Newcastle disease virus. Virology. 1959 Mar;7(3):315–327. doi: 10.1016/0042-6822(59)90201-6. [DOI] [PubMed] [Google Scholar]
- CORDS C. E., HOLLAND J. J. INTERFERENCE BETWEEN ENTEROVIRUSES AND CONDITIONS EFFECTING ITS REVERSAL. Virology. 1964 Feb;22:226–234. doi: 10.1016/0042-6822(64)90007-8. [DOI] [PubMed] [Google Scholar]
- Gravell M., Malsberger R. G. A permanent cell line from the fathead minnow (Pimephales promelas). Ann N Y Acad Sci. 1965 Aug 10;126(1):555–565. doi: 10.1111/j.1749-6632.1965.tb14302.x. [DOI] [PubMed] [Google Scholar]
- HAM R. G. An improved nutrient solution for diploid Chinese hamster and human cell lines. Exp Cell Res. 1963 Feb;29:515–526. doi: 10.1016/s0014-4827(63)80014-2. [DOI] [PubMed] [Google Scholar]
- HERMODSSON S. Inhibition of interferon by an infection with parainfluenza virus type 3 (PIV-3). Virology. 1963 Jun;20:333–343. doi: 10.1016/0042-6822(63)90123-5. [DOI] [PubMed] [Google Scholar]
- HOTCHIN J. E., DEIBEL R., BENSON L. M. Location of noncytopathic myxovirus plaques by hemadsorption. Virology. 1960 Feb;10:275–280. doi: 10.1016/0042-6822(60)90047-7. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Wagner R. R. Defective T particles of vesicular stomatitis virus. II. Biologic role in homologous interference. Virology. 1966 Oct;30(2):173–181. doi: 10.1016/0042-6822(66)90093-6. [DOI] [PubMed] [Google Scholar]
- ISAACS A. INTERFERON. Adv Virus Res. 1963;10:1–38. [PubMed] [Google Scholar]
- KUMAGAI T., SHIMIZU T., IKEDA S., MATUMOTO M. A new in vitro method (END) for detection and measurement of hog cholera virus and its antibody by means of effect of HC virus on Newcastle disease virus in swine tissue culture. I. Establishment of standard procedure. J Immunol. 1961 Sep;87:245–256. [PubMed] [Google Scholar]
- MARCUS P. I. Dynamics of surface modification in myxovirus-infected cells. Cold Spring Harb Symp Quant Biol. 1962;27:351–365. doi: 10.1101/sqb.1962.027.001.033. [DOI] [PubMed] [Google Scholar]
- MARCUS P. I. Host-cell interaction of animal viruses. II. Cell-killing particle enumeration: survival curves at low multiplicities. Virology. 1959 Dec;9:546–563. doi: 10.1016/0042-6822(59)90148-5. [DOI] [PubMed] [Google Scholar]
- MARCUS P. I., ROBBINS E. VIRAL INHIBITION IN THE METAPHASE-ARREST CELL. Proc Natl Acad Sci U S A. 1963 Dec;50:1156–1164. doi: 10.1073/pnas.50.6.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCUS P. I. Symposium on the biology of cells modified by viruses or antigens. IV. Single-cell techniques in tracing virus-host interactions. Bacteriol Rev. 1959 Dec;23(4):232–249. doi: 10.1128/br.23.4.232-249.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeno K., Yoshii S., Nagata I., Matsumoto T. Growth of Newcastle disease virus in a HVJ carrier culture of HeLa cells. Virology. 1966 Jun;29(2):255–263. doi: 10.1016/0042-6822(66)90032-8. [DOI] [PubMed] [Google Scholar]
- Marcus P. I., Carver D. H. Hemadsorption-negative plaque test: new assay for rubella virus revealing a unique interference. Science. 1965 Aug 27;149(3687):983–986. doi: 10.1126/science.149.3687.983. [DOI] [PubMed] [Google Scholar]
- Marcus P. I., Salb J. M. Molecular basis of interferon action: inhibition of viral RNA translation. Virology. 1966 Nov;30(3):502–516. doi: 10.1016/0042-6822(66)90126-7. [DOI] [PubMed] [Google Scholar]
- Roizman B. Abortive infection of canine cells by herpes simplex virus. 3. The interference of conditional lethal virus with an extended host range mutant. Virology. 1965 Sep;27(1):113–117. doi: 10.1016/0042-6822(65)90148-0. [DOI] [PubMed] [Google Scholar]
- WAGNER R. R. INTERFERON. A REVIEW AND ANALYSIS OF RECENT OBSERVATIONS. Am J Med. 1965 May;38:726–737. doi: 10.1016/0002-9343(65)90193-2. [DOI] [PubMed] [Google Scholar]