Summary
Single-chain glycoprotein CD44 is a major cell surface receptor for hyaluronan and mediates epithelial cell adhesion by its involvement in cell-cell and cell-matrix interactions. Recently, CD44 has been identified as a biomarker of cancer stem cells in many malignancies, including ovarian carcinoma. However, its clinical significance in human ovarian carcinoma has been controversial until recently. The aim of our current study was to clarify the clinical role of CD44 expression in human ovarian carcinoma. Immunohistochemical staining of 483 primary ovarian carcinoma, and 27 paired primary and recurrent ovarian carcinoma samples for CD44 standard form (CD44s) was performed using tissue microarray. The associations between CD44s expression and clinical factors (histological types, tumor grade, International Federation of Gynecology and Obstetrics stage, and response to chemotherapy), and overall or disease-free survivals were analyzed. We observed CD44s expression in 38% of the ovarian carcinoma samples. Results of Fisher’s exact test suggested that CD44s expression was associated with high-grade carcinoma (P = .013); advanced International Federation of Gynecology and Obstetrics stage (III–IV, P < .001); age at diagnosis less than 60 years old (P = .011); and transitional cell carcinoma (P = .039). However, CD44s expression was not associated with overall survival (P = .529) or disease-free survival (P = .218) by the log-rank test. Moreover, there was no statistical difference in CD44s expression between the primary and recurrent ovarian carcinomas. Our results showed that CD44s expression is not a prognostic predictor in ovarian cancer.
Keywords: CD44, immunohistochemistry, ovarian carcinoma, prognosis
1. Introduction
The adhesion molecule CD44 is a major cell surface receptor for hyaluronan and mediates epithelial cell adhesion by its involvement in cell-cell and cell-matrix interactions [1]. It is encoded by a single gene on chromosome 11p13; this gene is composed of 19 exons, 10 of which (exons 1–5 and 15–19) are included in the standard form of CD44, termed CD44s. The remaining exons can be differentially inserted into the mature mRNA via alternative splicing and may in theory give rise to hundreds of protein variants [2]. These CD44 isoforms have been implicated in cell migration and tumor progression [3], and their expression levels reportedly have prognostic value in many solid malignant tumors, such as bladder [4], breast [5], and colorectal cancers [6].
Over the past decade, attention has focused on CD44 because it has been proposed to be a biomarker for cancer stem cells (CSCs) in many solid malignant tumors. These CSCs have been suggested to be present as a unique subpopulation of cells that may contribute to tumor development, progression, metastasis, and recurrence as well as exhibit chemo- or radio-resistant properties [7]. Separate studies have reported that CD44(+)/CD24(-/low) populations have the ability to form new tumors, undergo heterogeneous differentiation, and resist chemotherapy and/or radiation therapy in breast [8–10] and ovarian carcinomas [11]. CD44 (hi) or CD44 (+) populations have been identified as CSC-like cells in human colon carcinoma [12], squamous cell carcinoma of the head and neck, and prostate carcinoma [13].
Ovarian carcinoma is the deadliest gynecologic malignancy in women worldwide. Most patients receive a diagnosis when the cancer is at an advanced stage due to a lack of effective detection methods. Moreover, although most cancer patients initially respond to standard chemotherapeutic regimens, the majority of them ultimately have relapse with chemo-resistant disease.
Because CD44 expression is a common marker of CSCs in many malignant tumors, including ovarian carcinoma, it may be associated with the clinical outcome of patients. However, its clinical significance has been controversial until recently [14–16]. The purpose of our current study was to evaluate the association between CD44s expression and clinical factors including histopathological diagnosis, tumor grade, International Federation of Gynecology and Obstetrics (FIGO) stage, ascites status, serum cancer antigen 125 (CA125) level, and clinical response to chemotherapy, as well as overall survival (OS) and disease-free survival (DFS). Our study used 510 specimens: 483 human primary ovarian carcinoma specimens and 27 paired primary and recurrent ovarian carcinoma specimens. To our knowledge, this is the largest sample linking CD44s expression to human ovarian carcinoma.
2. Materials and methods
2.1. Patients and clinicopathologic data
We analyzed a total of 510 samples of human ovarian carcinoma obtained from patients who underwent surgery at The University of Texas MD Anderson Cancer Center between January 1, 1990, and February 22, 2007. Relevant clinical data were obtained via retrospective review of the patients' medical files. These data included demographic information, histopathological diagnosis, tumor grade, disease stage, ascites status, CA125 level, chemotherapy regimen, and response to clinical treatment or chemotherapy. The follow-up information of patients was updated through July 30, 2011, by reviewing their medical records and the U.S. Social Security Death Index. Use of tissue blocks and chart reviews was approved by the MD Anderson Institutional Review Board.
The patients’ pathologic diagnoses were based on World Health Organization criteria [17]. Nonserous carcinomas were graded using the Gynecologic Oncology Group criteria [18–20], and serous carcinomas were graded according to a two-tier system (low and high grade) [21]. Tumors from patients with ovarian carcinoma were staged using the FIGO system [22].
To analyze responses to chemotherapy, we categorized the patients as responders or nonresponders. Responders were patients who experienced complete clinical remission with a normal serum CA125 level (normal range, 0–35 U/ml) after chemotherapy for histologically diagnosed ovarian carcinoma and who had a treatment-free interval of at least 6 months [23]. Nonresponders were subdivided according to whether patients had progressive or recurrent disease. Progressive disease was defined as carcinoma that progressed without any observed remission after the initiation of treatment, whereas recurrent disease was defined as carcinoma detected after a period of clinically documented remission shorter than 6 months [24].
OS duration was defined as the time from the date of first biopsy to the date of death caused by ovarian carcinoma or to the last follow-up date. DFS duration was defined as the time from the date of first biopsy to the date of recurrence. Recurrence was indicated clinically by the appearance of new lesions or an increase in the serum CA125 level to more than twice the upper limit of the normal range [25].
2.2. Immunohistochemical analysis
Tissue microarray slides were stained according to the immunohistochemical protocol of the manufacturer (Biocare Medical, Concord, CA, USA). After the tissue microarray sections were deparaffinized and rehydrated, antigen retrieval was performed using a universal decloaker (UD1000M; Biocare Medical) to unmask the epitopes in an autoclave at 121°C for 5 minutes and blocked using Peroxidazed 1 endogenous peroxidase blocker (PX968; Biocare Medical) at room temperature for 10 minutes. The sections were then incubated with Avidin (AB972H-A, Biocare Medical) and Biotin solution (AB972H-B, Biocare Medical) for 10 minutes separately. Nonspecific binding was blocked using Background Sniper (BS966M; Biocare Medical) for 15 minutes at room temperature. The slides were incubated with a primary mouse monoclonal antibody against CD44s (156-3C11, 1:50; Cell Signaling Technology, Beverly, MA, USA) overnight at 4°C, with a biotin-labeled secondary antibody for 20 minutes (Universal Goat Link, GU600H; Biocare Medical), and finally with 4plus HRP 1000 Universal for 20 minutes (HP604; Biocare Medical). After being stained with 3,3'-diaminobenzidine chromogen (DB801L; Biocare Medical) for 2 minutes, the sections were counterstained with hematoxylin, dehydrated, and mounted. Negative controls were constructed by replacing the primary antibody with phosphate-buffered saline. All of the controls yielded satisfactory results.
The slides showing CD44s immunohistochemical staining were independently analyzed by two gynecologic pathologists (J.L. and J.Z.). A majority of ovarian carcinoma samples showed similar intensity (moderate or high positive intensity) for CD44s expression. There were no significant associations between the intensity scores (negative, weak, moderate, and high) and OS or DFS duration. Rather, we considered that only cellular membrane staining in tumor cells was regarded as an indicator of positivity for CD44s expression. The degree of staining was semi-quantified using a five-score grading system: 0, samples without CD44s-positive cells; 1, samples with less than 5% CD44s-positive cells; 2, samples with 5–25% CD44s-positive cells; 3, samples with 26–50% CD44s-positive cells; 4, samples with >51% CD44spositive cells. For statistical analysis, these cases were classified as negative (0% CD44s-positive cells) or positive (>0% CD44s-positive cells) for CD44s expression.
2.3. Statistical analysis
Fisher’s exact test and logistic regression analysis were used to evaluate the association of CD44s expression with clinical factors. One-way analysis of variance for multiple comparisons was performed to evaluate the association of CD44s expression with response to chemotherapy and the grade of serous or endometrioid adenocarcinoma. The Kaplan-Meier method and log-rank test were used to estimate the association of CD44s expression with OS and DFS rates. Unconditional logistic regression analysis using multiple covariates were used to evaluate the association of clinical factors (age at diagnosis, tumor grade, response to clinical treatment, pathologic diagnosis, and FIGO stage) with OS and DFS duration. P-values less than .05 were considered statistically significant. For statistical analyses, we used the SPSS (version 17.0; SPSS, Chicago, IL, USA) and Stata (version 8.0; StataCorp, College Station, TX, USA) software programs.
3. Results
3.1. Patient characteristics
The mean age of the patients was 59 years (range, 22–89 years). At the initiation of the current study, 76 patients were alive without ovarian cancer, 37 were alive with ovarian cancer, 264 had died of ovarian cancer, and 94 had died of causes other than ovarian cancer; 12 were lost to follow-up and were excluded from the OS analysis. Median OS duration was 4.4 years (95% confidence interval [CI], 3.8–5.1 years), and the OS rates were 63% (95% CI, 61–65%) at 3 years, 45% (95% CI, 43–47%) at 5 years, and 35% (95% CI, 32–38%) at 10 years. Among 483 patients, 63 did not have relapse, 203 did have relapse, and 180 had disease progression; 37 patients were lost to follow-up or had unknown serum CA125 levels. Only the patients with and without disease relapse were included in the DFS analysis. Median DFS duration was 1.4 years (95% CI, 1.2- 1.7 years), and the DFS rates were 35% (95% CI, 32–38%) at 3 years, 26% (95% CI, 23–29%) at 5 years, and 23% (95% CI, 20–26%) at 10 years.
3.2. CD44s expression and localization
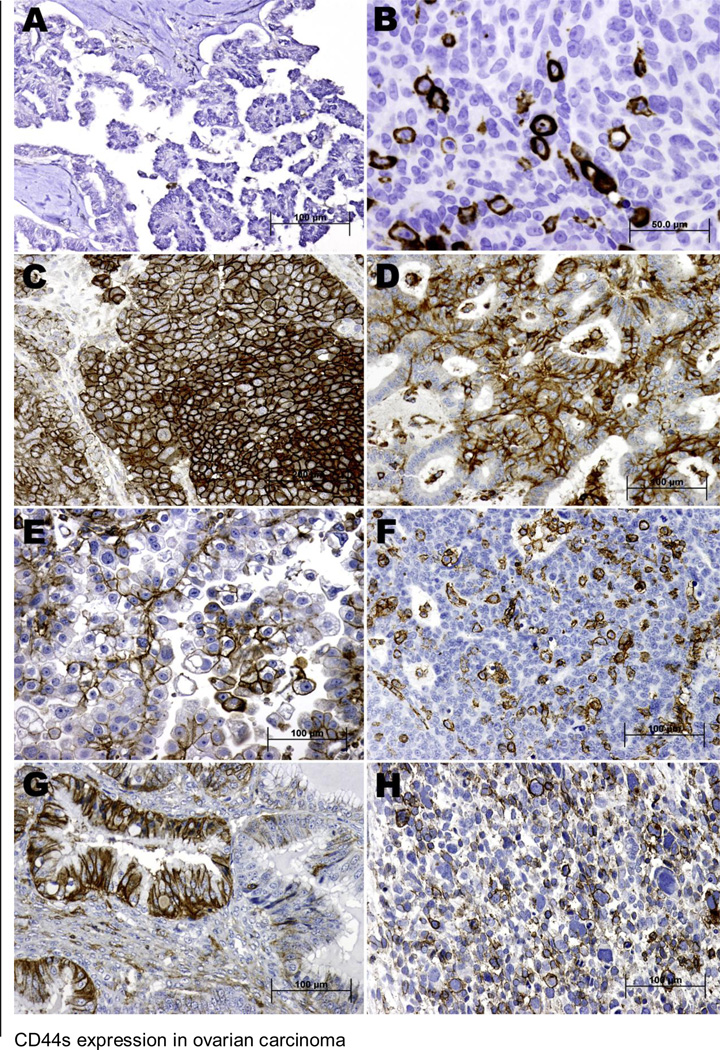
Representative samples of CD44s staining are shown in Figure 1. The positive staining of CD44s showed cellular membrane localization, and 184 (38%) of 483 ovarian carcinoma samples were positive for CD44s expression. Among these 184 cases, the immunohistochemical scores were 1 in 97 patients (53%), 2 in 34 patients (18%), 3 in 22 patients (12%), and 4 in 31 patients (17%). A majority of samples with an immunohistochemical score of 1 showed scattered positive cells. CD44s immunoreactivity was also found in some stromal fibroblasts and lymphocytes.
Figure 1.
Immunohistochemical staining for CD44s in human primary ovarian carcinoma samples. A, Tumor cells in a low-grade serous carcinoma sample exhibiting negative expression for CD44s. B and C, High-grade serous carcinoma samples with less than 10% (B) and more than 50% (C) of the tumor cells exhibiting CD44s expression. D, CD44s-positive expression in an endometrioid adenocarcinoma sample. E, A clear cell carcinoma sample showing positive CD44s expression. F, Transitional cell carcinoma cells with less than 10% of the tumor cells showing expression of CD44s. G, Mucinous adenocarcinoma exhibiting membrane staining for CD44s. H, Malignant mixed müllerian tumor cells with CD44s expression. (A, C-H) Original magnification X200; (B) Original magnification X400; see text for immunostaining procedure.
3.3. Association of CD44s expression with clinicopathologic variables
The results of CD44s immunostaining in the ovarian carcinoma microarrays, organized according to clinicopathologic characteristics of the patients, are shown in Table 1. In summary, CD44s-positive expression was associated with transitional cell carcinoma (P = .039), high-grade carcinoma (P = .013), advanced-stage tumors (FIGO III–IV, P < .001), and age at diagnosis of less than 60 years (P = .011). Considering different tumor grading systems, we analyzed the associations of CD44s expression with ovarian serous or endometrioid adenocarcinoma grades separately. CD44s expression was identified in only 12% (2/17) of low-grade serous carcinomas, compared with 39% (145/276) of high-grade serous carcinomas. There was a significant association between CD44s-positive expression and high-grade serous carcinoma (P = .037), but no significant associations between CD44s expression status and different tumor grades in endometrioid adenocarcinoma (P = .487) (data not shown).
Table 1.
Associations of CD44s expression with clinicopathologic factors
| Characteristic |
CD44s expression |
Pa | ||
|---|---|---|---|---|
|
Total no. of patients |
Negative (%) |
Positive (%) |
||
| Histologic type | .039 | |||
| High-grade serous carcinoma | 376 | 231 (61) | 145 (39) | |
| Low-grade serous carcinoma | 17 | 15 (88) | 2 (12) | |
| Endometrioid adenocarcinoma | 35 | 24 (69) | 11 (31) | |
| Clear cell carcinoma | 16 | 10 (63) | 6 (37) | |
| Transitional cell carcinoma | 11 | 3 (27) | 8 (73) | |
| Other b | 28 | 16 (57) | 12 (43) | |
| FIGO stage | < .001 | |||
| I–II | 51 | 45 (88) | 6 (12) | |
| III–IV | 432 | 254 (59) | 178 (41) | |
| Grade | .013 | |||
| Low | 26 | 22 (85) | 4 (15) | |
| High | 457 | 277 (61) | 180 (39) | |
| Ascites status | .097 | |||
| No | 54 | 39 (72) | 15 (28) | |
| Yes | 338 | 201 (60) | 137 (40) | |
| Unknown | 91 | 59 (65) | 32 (35) | |
| Response to clinical treatment | .059 | |||
| No | 59 | 30 (51) | 29 (49) | |
| Yes | 386 | 249 (65) | 137 (35) | |
| Unknown | 38 | 20 (53) | 18 (47) | |
| Age at diagnosis | .011 | |||
| <60 years | 242 | 136 (56) | 106 (44) | |
| ≥60 years | 241 | 163 (68) | 78 (32) | |
| Serum CA125 level | .378 | |||
| <500 U/ml | 138 | 86 (62) | 52 (38) | |
| ≥500 U/ml | 222 | 127 (57) | 95 (43) | |
| Unknown | 123 | 86 (70) | 37 (30) | |
Fisher’s exact test.
mucinous adenocarcinoma (five cases), malignant mixed müllerian tumor (ten cases), undifferentiated carcinoma (ten cases), and mixed type carcinoma (three cases).
The correlation of CD44s expression with response to chemotherapy is shown in Table 2. In total, 462 (96%) patients received chemotherapy. Among these patients, 346 patients (75%) received postsurgical cisplatin-based treatment either alone or with other drugs; 66 patients (14%) received presurgical cisplatin-based treatment; and eight patients (2%) received other forms of chemotherapy (tamoxifen, yttrium, paclitaxel [Taxol], melphalan, and paclitaxel plus cyclophosphamide [Cytoxan]). However, there was no significant association between chemotherapy response and CD44s expression.
Table 2.
Correlation of expression of CD44s with response to chemotherapy
| CD44s expression |
|||
|---|---|---|---|
| Response to chemotherapy | Total no. of patients |
Negative (%) |
Positive (%) |
| Responders a | |||
| Postsurgery cisplatin-based regimenb | 185 | 116 (63) | 69 (37) |
| Presurgery cisplatin-based regimenc | 20 | 13 (65) | 7 (35) |
| Other regimen | 3 | 1 (33) | 2 (67) |
| Unknown regimen | 2 | 0 (0) | 2 (100) |
| Nonresponders, progressive diseasea | |||
| Postsurgery cisplatin-based regimenb | 118 | 68 (58) | 50 (42) |
| Presurgery cisplatin-based regimenc | 45 | 30 (67) | 15 (33) |
| Other regimen | 3 | 2 (67) | 1 (33) |
| Unknown regimen | 1 | 1 (100) | 0 (0) |
| Nonresponders, recurrent diseasea | |||
| Postsurgery cisplatin-based regimenb | 43 | 30 (70) | 13 (30) |
| Presurgery cisplatin-based regimenc | 1 | 1 (100) | 0 (0) |
| Other regimen | 2 | 1 (50) | 1 (50) |
| No chemotherapy | 21 | 16 (76) | 5 (24) |
| Unknown response | 39 | 20 (51) | 19 (49) |
| Total | 483 | 299 (62) | 184 (38) |
Responders versus nonresponders progressive and recurrent disease (P = .528).
Responders versus nonresponders (postsurgery cisplatin-based regimen subgroup) progressive and recurrent disease (P = .205).
Responders versus nonresponders (presurgery cisplatin-based regimen subgroup) progressive and recurrent disease (P = .769).
P-values were calculated using one-way analysis of variance.
3.4. Expression of CD44s in 27 paired primary and recurrent ovarian carcinomas
To identify the association between CD44s expression and tumor recurrence, we used the paraffin-embedded blocks from 27 patients’ primary and recurrent ovarian carcinoma tissues. Two representative cores were taken from every primary and recurrent tumor block to make a tissue microarray containing a total of 108 cores. Of the 27 cases, 15 cases showed CD44s-negative expression and three cases showed CD44s-positive expression in both primary and recurrent tumor tissues. Three cases (11%) had CD44s-positive expression in the primary tumor tissues and negative expression in recurrent tumor tissues. Six cases had CD44s-positive expression in recurrent tumor tissues and negative expression in the primary tumor tissues (Table 3). Statistically, there was no association between CD44s expression in primary and recurrent tumors samples (P = .367).
Table 3.
CD44s expression in 27 paired primary and recurrent ovarian carcinoma
| CD44s expression status | No. of patients |
|---|---|
| Primary & recurrent tumor both 0% | 15 |
| Primary & recurrent tumor both >0% | 3 |
| Primary tumor >0%, recurrent tumor 0% | 3 |
| Primary tumor 0%, recurrent tumor >0% | 6 |
P = .367 (Fisher’s exact test); r = .189 (Two-tailed Person correlation)
3.5. Association of CD44s expression with OS or DFS
The association of CD44s expression with OS or DFS durations and their rates at 3, 5, and 10 years was shown in Table 4. There were no associations between CD44s expression and OS (P = .529) or DFS (P = .218). Furthermore, we observed no significant associations among the different immunohistochemical scores for CD44s expression (0, 1, 2, 3, or 4) and OS (P = .254) or DFS (P = .939). Multivariate Cox proportional hazards regression analysis indicated that FIGO stage was significantly associated with OS and DFS (data not shown).
Table 4.
Association of CD44s expression with overall or disease-free survival
| OS (n= 471) | DFS (n = 266) | |||
|---|---|---|---|---|
| Variable | CD44s- negative |
CD44s- positive |
CD44s- negative |
CD44s- positive |
| No. of patients | 295 | 176 | 172 | 94 |
| Median survival duration, years (95% CI) | 4.1 (3.4–4.9) | 4.4 (3.1–5.8) | 1.5 (1.0–2.0) | 1.3 (0.8–1.7) |
| Survival rate (95% CI) | ||||
| 3 years | 0.64 (0.61–0.67) | 0.59 (0.55–0.63) | 0.37 (0.33–0.41) | 0.31 (0.26–0.36) |
| 5 years | 0.44 (0.41–0.47) | 0.46 (0.42–0.50) | 0.29 (0.25–0.33) | 0.21 (0.17–0.25) |
| 10 years | 0.36 (0.33–0.39) | 0.21 (0.15–0.27) | 0.27 (0.23–0.31) | N/A (<<0.17) |
| Pa | .529 | .218 | ||
Log-rank test.
4. Discussion
Recently CD44 has been proposed to be a common biomarker of CSCs in many malignancies. Theoretically, its expression should be correlated with tumor progression, recurrence, and resistance to chemotherapy or radiotherapy [7]. Because of extensive alternative splicing and post-translational modification, CD44 is a polymorphic group of transmembrane glycoproteins [2]. In ovarian tumor tissues, there are different expression levels of CD44s and CD44-v3~10 expression [16, 26–28]. Of these proteins, CD44s is the major species and has been most extensively studied [29].
Although a few studies also have evaluated the prognostic significance of CD44s expression in patients with ovarian carcinoma, whether CD44s is a prognostic predictor remained a matter of controversy until recently. Some investigators have demonstrated an association between CD44s expression and poor prognosis or survival [30, 31], whereas others could not demonstrate such results [15, 28], or even reached the opposite conclusion [16, 26]. Our data showed that no statistical correlation was found between CD44s expression and response to chemotherapy in our study. Moreover, in the 27 paired primary and recurrent ovarian carcinoma samples, there was no association between CD44s expression and tumor recurrence. Considering the heterogeneous nature of ovarian carcinoma, we believe that the reliability of using a single CSC marker, CD44s, as the way to isolate ovarian CSCs will have low specificity.
Epithelial ovarian carcinoma spreads by implantation of tumor cells through the mesothelial lining of the peritoneal cavity. In vitro studies have suggested that CD44 on the surface of ovarian cancer cells binds to hyaluronan on mesothelial cells and may contribute to peritoneal metastasis [29, 32]. Monoclonal antibodies against CD44 significantly inhibit ovarian cancer cell adhesion to mesothelial cells and peritoneal implantation in mice [33, 34]. Our study, which found that CD44s expression was associated with high-grade, and advanced stage ovarian carcinoma, also revealed a possible association between CD44s expression and tumor progression.
In conclusion, our study found that CD44s expression is not a prognostic predictor but associated with high-grade and advanced stage ovarian carcinoma. The combined use of CD44s with the other markers to isolate CSCs in ovarian carcinoma may be more reliable than the use of CD44s alone.
Acknowledgements
The authors thank for MD Anderson Cancer Center's Department of Scientific Publication for insightful manuscript editing and revision. J. Liu was supported by an R01 grant (R01CA131183-01A2) and ovarian cancer Specialized Programs of Research Excellence (SPORE) grant (IP50CA83638) from the National Institutes of Health and the Ovarian Cancer Research Fund (OCRF). This work was also supported in part by the National Institutes of Health through the MD Anderson Cancer Center Support Grant (CA016672). J. Zhang and B. Chang were visiting scientists at MD Anderson Cancer Center. J. Zhang was supported by National Natural Science Foundation of China (NSFC 81070582) and B. Chang was supported by NSFC 81160316.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: the authors declare no conflicts of interest.
References
- 1.Nagano O, Murakami D, Hartmann D, et al. Cell-matrix interaction via CD44 is independently regulated by different metalloproteinases activated in response to extracellular Ca(2+) influx and PKC activation. J Cell Biol. 2004;165:893–902. doi: 10.1083/jcb.200310024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naor D, Sionov RV, Ish-Shalom D. CD44: structure, function, and association with the malignant process. Adv Cancer Res. 1997;71:241–319. doi: 10.1016/s0065-230x(08)60101-3. [DOI] [PubMed] [Google Scholar]
- 3.Marhaba R, Zoller M. CD44 in cancer progression: adhesion, migration and growth regulation. J Mol Histol. 2004;35:211–231. doi: 10.1023/b:hijo.0000032354.94213.69. [DOI] [PubMed] [Google Scholar]
- 4.Stavropoulos NE, Filliadis I, Ioachim E, et al. CD44 standard form expression as a predictor of progression in high risk superficial bladder tumors. Int Urol Nephrol. 2001;33:479–483. doi: 10.1023/a:1019589923706. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe O, Kinoshita J, Shimizu T, et al. Expression of a CD44 variant and VEGF-C and the implications for lymphatic metastasis and long-term prognosis of human breast cancer. J Exp Clin Cancer Res. 2005;24:75–82. [PubMed] [Google Scholar]
- 6.Lugli A, Iezzi G, Hostettler I, et al. Prognostic impact of the expression of putative cancer stem cell markers CD133, CD166, CD44s, EpCAM, and ALDH1 in colorectal cancer. Br J Cancer. 2010;103:382–390. doi: 10.1038/sj.bjc.6605762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curley MD, Garrett LA, Schorge JO, Foster R, Rueda BR. Evidence for cancer stem cells contributing to the pathogenesis of ovarian cancer. Front Biosci. 2011;16:368–392. doi: 10.2741/3693. [DOI] [PubMed] [Google Scholar]
- 8.Phillips TM, McBride WH, Pajonk F. The response of CD24(−/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98:1777–1785. doi: 10.1093/jnci/djj495. [DOI] [PubMed] [Google Scholar]
- 9.Ling LJ, Wang S, Liu XA, et al. A novel mouse model of human breast cancer stem-like cells with high CD44+CD24−/lower phenotype metastasis to human bone. Chin Med J (Engl) 2008;121:1980–1986. [PubMed] [Google Scholar]
- 10.Tanaka H, Nakamura M, Kameda C, et al. The Hedgehog signaling pathway plays an essential role in maintaining the CD44+CD24−/low subpopulation and the side population of breast cancer cells. Anticancer Res. 2009;29:2147–2157. [PubMed] [Google Scholar]
- 11.Shi MF, Jiao J, Lu WG, et al. Identification of cancer stem cell-like cells from human epithelial ovarian carcinoma cell line. Cell Mol Life Sci. 2010;67:3915–3925. doi: 10.1007/s00018-010-0420-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu P, Clanton DJ, Snipas TS, et al. Characterization of a subpopulation of colon cancer cells with stem cell-like properties. Int J Cancer. 2009;124:1312–1321. doi: 10.1002/ijc.24061. [DOI] [PubMed] [Google Scholar]
- 13.Patrawala L, Calhoun T, Schneider-Broussard R, et al. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25:1696–1708. doi: 10.1038/sj.onc.1209327. [DOI] [PubMed] [Google Scholar]
- 14.Sillanpaa S, Anttila MA, Voutilainen K, et al. CD44 expression indicates favorable prognosis in epithelial ovarian cancer. Clin Cancer Res. 2003;9:5318–5324. [PubMed] [Google Scholar]
- 15.Zagorianakou N, Stefanou D, Makrydimas G, et al. CD44s expression, in benign, borderline and malignant tumors of ovarian surface epithelium. Correlation with p53, steroid receptor status, proliferative indices (PCNA, MIB1) and survival. Anticancer Res. 2004;24:1665–1670. [PubMed] [Google Scholar]
- 16.Cho EY, Choi Y, Chae SW, Sohn JH, Ahn GH. Immunohistochemical study of the expression of adhesion molecules in ovarian serous neoplasms. Pathol Int. 2006;56:62–70. doi: 10.1111/j.1440-1827.2006.01925.x. [DOI] [PubMed] [Google Scholar]
- 17.Tavassoeli FA, Devilee P. Pathology and genetics of tumours of the breast and female genital organs. Lyon IARC press. 2003:117–145. [Google Scholar]
- 18.Russell P. The pathological assessment of ovarian neoplasms. I: Introduction to the common 'epithelial' tumours and analysis of benign 'epithelial' tumours. Pathology. 1979;11:5–26. doi: 10.3109/00313027909063533. [DOI] [PubMed] [Google Scholar]
- 19.Russell P. The pathological assessment of ovarian neoplasms. II: The proliferating 'epithelial' tumours. Pathology. 1979;11:251–282. doi: 10.3109/00313027909061951. [DOI] [PubMed] [Google Scholar]
- 20.Russell P. The pathological assessment of ovarian neoplasms. III: The malignant "epithelial" tumours. Pathology. 1979;11:493–532. doi: 10.3109/00313027909059027. [DOI] [PubMed] [Google Scholar]
- 21.Malpica A, Deavers MT, Lu K, et al. Grading ovarian serous carcinoma using a two-tier system. Am J Surg Pathol. 2004;28:496–504. doi: 10.1097/00000478-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Shepherd JH. Revised FIGO staging for gynaecological cancer. Br J Obstet Gynaecol. 1989;96:889–892. doi: 10.1111/j.1471-0528.1989.tb03341.x. [DOI] [PubMed] [Google Scholar]
- 23.Rustin GJ, Nelstrop AE, Crawford M, et al. Phase II trial of oral altretamine for relapsed ovarian carcinoma: evaluation of defining response by serum CA125. J Clin Oncol. 1997;15:172–176. doi: 10.1200/JCO.1997.15.1.172. [DOI] [PubMed] [Google Scholar]
- 24.Rustin GJ, Nelstrop AE, Tuxen MK, Lambert HE. Defining progression of ovarian carcinoma during follow-up according to CA 125: a North Thames Ovary Group Study. Ann Oncol. 1996;7:361–364. doi: 10.1093/oxfordjournals.annonc.a010602. [DOI] [PubMed] [Google Scholar]
- 25.Rosen DG, Yang G, Deavers MT, et al. Cyclin E expression is correlated with tumor progression and predicts a poor prognosis in patients with ovarian carcinoma. Cancer. 2006;106:1925–1932. doi: 10.1002/cncr.21767. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez-Rodriguez L, Sancho-Torres I, Mesonero C, Gibbon DG, Shih WJ, Zotalis G. The CD44 receptor is a molecular predictor of survival in ovarian cancer. Med Oncol. 2003;20:255–263. doi: 10.1385/MO:20:3:255. [DOI] [PubMed] [Google Scholar]
- 27.Afify AM, Ferguson AW, Davila RM, Werness BA. Expression of CD44S and CD44v5 is more common in stage III than in stage I serous ovarian carcinomas. Appl Immunohistochem Mol Morphol. 2001;9:309–314. doi: 10.1097/00129039-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Berner HS, Davidson B, Berner A, et al. Expression of CD44 in effusions of patients diagnosed with serous ovarian carcinoma--diagnostic and prognostic implications. Clin Exp Metastasis. 2000;18:197–202. doi: 10.1023/a:1006711320107. [DOI] [PubMed] [Google Scholar]
- 29.Catterall JB, Jones LM, Turner GA. Membrane protein glycosylation and CD44 content in the adhesion of human ovarian cancer cells to hyaluronan. Clin Exp Metastasis. 1999;17:583–591. doi: 10.1023/a:1006756518500. [DOI] [PubMed] [Google Scholar]
- 30.Teyssier JR, Couillin P, Benard J, Ravisse N, Ulrich E, Cornillet P. A multidrug-resistant ovarian carcinoma cell line with a malignant suppressed phenotype is a CD44 gene expression defective mutant. Cancer Genet Cytogenet. 1992;60:14–19. doi: 10.1016/0165-4608(92)90225-w. [DOI] [PubMed] [Google Scholar]
- 31.Ross JS, Sheehan CE, Williams SS, Malfetano JH, Szyfelbein WM, Kallakury BV. Decreased CD44 standard form expression correlates with prognostic variables in ovarian carcinomas. Am J Clin Pathol. 2001;116:122–128. doi: 10.1309/KUK0-1M3D-LGNE-THXR. [DOI] [PubMed] [Google Scholar]
- 32.Bourguignon LY, Gilad E, Rothman K, Peyrollier K. Hyaluronan-CD44 interaction with IQGAP1 promotes Cdc42 and ERK signaling, leading to actin binding, Elk-1/estrogen receptor transcriptional activation, and ovarian cancer progression. J Biol Chem. 2005;280:11961–11972. doi: 10.1074/jbc.M411985200. [DOI] [PubMed] [Google Scholar]
- 33.Strobel T, Swanson L, Cannistra SA. In vivo inhibition of CD44 limits intra-abdominal spread of a human ovarian cancer xenograft in nude mice: a novel role for CD44 in the process of peritoneal implantation. Cancer Res. 1997;57:1228–1232. [PubMed] [Google Scholar]
- 34.Lessan K, Aguiar DJ, Oegema T, Siebenson L, Skubitz AP. CD44 and beta1 integrin mediate ovarian carcinoma cell adhesion to peritoneal mesothelial cells. Am J Pathol. 1999;154:1525–1537. doi: 10.1016/s0002-9440(10)65406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]