Abstract
The biological functions of the NRG1 (Neuregulin-1) and ERBB4 genes have received much recent attention due to several studies showing associations between these genes and schizophrenia. Moreover, reduced forebrain dendritic spine density is a consistent feature of schizophrenia. It is thus important to understand the mechanisms whereby NRG1 and erbB4 modulate spine morphogenesis. Here we show that long-term incubation with NRG1 increases both spine size and density in cortical pyramidal neurons. NRG1 also enhances the content of AMPA receptors in spines. Knockdown of ERBB4 expression prevented the effects of NRG1 on spine size, but not on spine density. The effects of NRG1 and erbB4 on spines were mediated by the RacGEF kalirin, a well-characterized regulator of dendritic spines. Finally, we show that environmental enrichment, known to promote spine growth, robustly enhances the levels of erbB4 protein in the forebrain. These findings provide a mechanistic link between NRG1 signaling and spine morphogenesis.
Introduction
Neuregulin 1 (NRG1) is a trophic factor that can be released presynaptically in a soluble form, and the postsynaptic erbB4 receptor tyrosine kinase is thought to be the predominant receptor for NRG1. NRG1 binds directly to erbB4, and this binding stimulates the intrinsic tyrosine kinase activity of the erbB4 receptor (Mei & Xiong 2008; Corfas et al. 2004). The biological functions of the NRG1 and ERBB4 genes have received much recent attention owing to several studies showing associations between these genes and schizophrenia (Harrison & Law 2006; Buonanno 2010). Nevertheless, the biological functions of NRG1 and erbB4 are incompletely understood.
Almost all NRG1 isoforms are initially trans-membrane-associated proteins termed pro-NRG1s (Mei & Xiong 2008). Proteolytic cleavage of pro-NRG1s causes shedding of the extracellular ecto-domain segment of NRG1 (Wang et al. 2001). On the extracellar side of pro-NRG1s lays the EGF-like domain proximal to the membrane, and this EGF-like domain is necessary and sufficient for erbB receptor binding and activation (Mei & Xiong 2008; Buonanno 2010). NRG1 proteins also contain other discrete domains such as an immunoglobulin domain, which in most isoforms lies between the EGF-like domain and the extreme N-terminus (Mei & Xiong 2008).
The target of cleaved, soluble NRG1s are the erbB receptor tyrosine kinase receptors. There are four erbB receptors expressed in the brain, erbB1 (epidermal growth factor receptor, EGFR), and erbB2–4. Erbb4 is the only receptor isoform that can both directly bind NRG1 and is catalytically active. Given this autonomous function of erbB4 as well as the association of erbB4 with schizophrenia, this receptor isoform has been the most extensively studied (Buonanno 2010). ErbB4 can homodimerize or can form a heterodimer with erbB2; however, unlike erbB4, erbB2 does not directly bind NRG1 (Tzahar et al. 1996; Mei & Xiong 2008).
Historically, the role for acute NRG1 and erbB4 activity in regulating neuronal function has received much attention, and studies have shown that NRG1/erbB4 impede synaptic plasticity in pyramidal neurons. NRG1 can reverse long-term potentiation (LTP) at CA1 hippocampal synapses when applied 20-minutes after theta burst stimulation (Kwon et al. 2005). In addition to reversing LTP, NRG1 suppresses LTP induction at the Schaffer collateral-CA1 synapse (SC-CA1) (Chen et al. 2010). NRG1 has been shown to inhibit spontaneous firing rates in prefrontal cortex (PFC) neurons, and also decreases the number of action potentials resulting from a 300 millisecond (ms) current injection (Wen et al. 2010). Most of the effects of NRG1 on regulating neuronal function are erbB4-dependent (Woo et al. 2007; Wen et al. 2010; Chen et al. 2010).
Long-term NRG1 activity, on the other hand, promotes plasticity, particularly the morphogenesis of dendritic spines on pyramidal neurons, the sites of most excitatory synapses in the brain. Notably, multi-day NRG1 treatment increases spine density and size in cultured forebrain neurons (Barros et al. 2009), and mice lacking NRG1 type III show a reduction in pyramidal neuron spine density (Chen et al. 2008). ErbB4 also has an established role in promoting spine morphogenesis as mice lacking erbB2/B4 show a reduction in spine density in the CA1 hippocampal field and in the prefrontal cortex (Barros et al. 2009). Knocking down erbB4 with a viral RNAi in the CA1 hippocampal field reduces spine density and area, while the overexpression of erbB4 in pyramidal neurons increases spine size (Li et al. 2007).
Given the links of NRG1 and erbB4 to schizophrenia, and because schizophrenia is characterized by alterations in forebrain spine density, a better understanding of the precise roles for these molecules in regulating spine morphogenesis remains an important question and could shed light on the contribution of these molecules to schizophrenia pathogenesis. Here we examine the role for NRG1 in regulating spines and determine the mechanisms important for these effects. Major regulators of spine morphogenesis are Rac1 guanine nucleotide exchange factors (GEFs). The kalirin-7 GEF plays a key role in regulating structural and functional plasticity at excitatory synapses (Penzes & Jones 2008), and kalirin has been functionally and genetically implicated in the pathogenesis of schizophrenia, including altered expression levels as well as genetic associations (Kushima et al. 2012; Rubio et al. 2012; Deo et al. 2012; Hill et al. 2006). Kalirin-7 interacts with erbB4, and is a critical regulator of NRG1-mediated interneuronal dendritic growth (Cahill et al. 2012). Here we wanted to determine the contribution of kalirin to NRG1’s effects on dendritic spines, and we show that NRG1 promotes spine morphogenesis in cortical pyramidal neurons and that kalirin is necessary for these effects. NRG1 also enhanced the expression of AMPA receptors in spines. Our data further suggest that erbB4 expression in pyramidal neurons is dispensable for NRG1’s effects on spine density, but surprisingly, not for spine area. Finally, because little is known about the means through which erbB4 expression is regulated, we examined how environmental enrichment, which is known to regulate spine morphogenesis, affects erbB4 expression. Overall, these findings help clarify the relationship between NRG1 activity and spine morphogenesis.
Methods
Antibodies and Reagents
The following antibodies were used: monoclonal and polyclonal GluA1 (Millipore), polyclonal GFP (Richard Huganir, Johns Hopkins University), monoclonal Rac1 (Millipore), polyclonal GFP (Richard Huganir, Johns Hopkins University). Antibody against GluN1 was a gift from Dr. Richard Huganir (John Hopkins University). The HFR erbB4 antibody clone was purchased from Santa Cruz Biotechnology. Polyclonal antibody against kalirin-7 was previously described (Ma et al. 2001; Penzes et al. 2000). Recombinant neuregulin-1 beta 1 (NRG1β) was purchased from R&D systems. The erbB4 plasmid was a gift from Dr. Gabriel Corfas (Harvard University). The kalirin RNAi used in this study, which uses plasmid pGsuper, thereby expressing siRNA and GFP simultaneously, has been previously described (Xie et al. 2007). ErbB4 shRNA and a scrambled control shRNA were obtained from GeneCopoeia (RSH050227-3-CH1).
Neuronal cultures
Medium and high density cortical neuron cultures were prepared from Sprague-Dawley rat E18 embryos as described previously (Xie et al. 2005). Neurons were plated onto coverslips or 60 mm dishes, coated with poly-D-lysine (0.2 mg/ml, Sigma), in plating media (feeding media plus 5% fetal calf serum). After 1 hr, the media was changed to feeding media (Neurobasal media supplemented with B27 (Invitrogen) and 0.5 mM glutamine). 200 µM D, Lamino-phosphonovalerate (D,L-APV, Ascent Scientific) was added to the media 4 days later. Dissociated postnatal DIV27–30 cortical cultures were assessed. Mouse cultures from WT or KALRN KO mice have been previously described (Xie et al. 2010), and were obtained using identical procedures as used for rat cultures, except they were generated from P1 pups. All mice used were c57/bl6. Kalirin knockout mice were generated as described previously (Cahill et al., 2009 PNAS) in which exons 27–28, which contain the active site of the GEF1 domain, were replaced with the neo cassette. Kalirin heterozygous x heterozygous matings were used to generate wildtype and full KO mice. The WT and KO mice obtained from these matings were used for WT x WT and KO x KO crosses. All mouse matings were done at the Northwestern University Center for Comparative Medicine (Chicago, IL). Rat cultures were derived from Sprague-Dawley rat E18 embryos obtained from Harlan.
Microscopy and Imaging
Confocal images of single- and double-stained neurons were obtained with a Ziess LSM5 Pascal confocal microscope. Images of neurons were taken using the 63x oil-immersion objective as a z-series of three to eight images, averaged four times, taken at 0.37 µm intervals, 1024 × 1024 pixel resolution at a scan speed of 8 seconds per section. Two-dimensional maximum projection reconstructions of images was done using MetaMorph software (Universal Imaging). The microscope images for all experiments were acquired and quantified by an experimenter blind to treatment conditions and data was obtained from a minimum of 2 experiments.
Quantitative immunofluorescence
To assess the spine expression level of GluA1, images were acquired as described above using a Ziess LSM5 Pascal confocal microscope. When imaging the GluA1 channel, detection parameters (e.g., gain) were held constant for all cells. The background corresponding to areas without cells were subtracted to generate a “background-subtracted” image. To measure the intensity of GluA1 clusters localized within spines, regions defining spines were generated based on GFP fluorescence in one channel. These regions were then transferred to the GluA1 fluorescence channel, and only the GluA1 fluorescence intensities within the corresponding spine regions were measured using MetaMorph (Universal Imaging); the GluA1 images were thresholded equally. Quantification was completed by an experimenter blind to treatment conditions.
Plasmid Transfections
Neurons were transfected with the appropriate cDNAs for 4 hours in Neurobasal media in the presence of Lipofectamine 2000 according to the manufacturer’s protocol. Following transfection, neurons were supplanted in Neurobasal media. Neurons were fixed for 10-minutes in 3.7% formaldehyde followed by 10-minutes of methanol pre-chilled to −20°C. Cells were then washed in phosphate buffer saline (PBS), blocked for 1-hour with PBS containing 2% normal goat serum (NGS) and 0.1% Triton. Primary and secondary antibodies were added to cells in PBS containing 2% NGS. hEK293 cells were transfected overnight with erbB4 cDNA in DMEM media without antibiotic using Lipofectamine 2000. The next morning, DMEM containing 10% fetal bovine serum (FBS) and pen/strep antibiotic was fed to cells. hEK293 cells were transfected when they reached ~40% confluency and were ~95% confluent at the time of harvesting.
Environmental enrichment rearing conditions
The control cage was a standard mouse cage used in the Center for Comparative Medicine at Northwestern University. C57BL/6 mice in the control cage were housed in groups of 4–5 mice per cage and had a single enrichment object throughout the housing period. For environmental enrichment, mice were housed in a cage 5x the volume of the control cage. Mice in the enriched cage were housed with 15 mice in the cage. The enriched cage contained several toys that were alternated 3x per week to drive novel exploration. Mice were also exposed to a running wheel periodically. Mice in both the standard and enriched cage had access to food and water ad lib. Mice were assigned to a housing conditioning after weaning from the parental cage at 3-weeks of age. Mice were then sacrificed at 18-weeks of age.
Biochemistry
For biochemical analysis, the frontal cortex and hippocampus of mice were dissected and sonicated in 15x by tissue weight radioimmunoprecipitation assay lysis buffer (RIPA) containing 150mM NaCl, 10mM Tris-HCl, pH 7.2, 5mM EDTA, 0.1% SDS, 1% Triton X-100, 1% deoxycholate, plus protease and phosphatase inhibitors. After sonication, tissue was centrifuged at 14,000xg for 10-minutes at 4°C to remove insoluble material. Laemmli sample buffer was added to the lysate and samples boiled for 5-minutes at 95°C. Samples were stored at −80°C until they were resolved using SDS-PAGE. Active Rac1 levels were assessed using a commercially available kit (Millipore) as described previously (Xie et al. 2007).
BS3 crosslinking
For BS3 crosslinking, Neurobasal media was removed from cultured cortical neurons, and the neurons were washed two times by brief immersion in ACSF. Cells were then immersed in ACSF containing Bis(sulfosuccinimidyl)suberate (BS3) for 20-minutes at 4°C. The reaction was then quenched with Tris-HCL buffer for 10-minutes at room temperature. Tris-HCL buffer was then removed, and RIPA buffer containing protease and phosphatase inhibitors was added to the cells. Non-crosslinked cells were used as a control. Cells were then sonicated, centrifuged at 14,000xg for 10-minutes at 4°C to remove insoluble material. Laemmli sample buffer was added to the lysate and samples boiled for 5-minutes at 95°C.
Golgi analysis
Golgi staining was performed using a modified Golgi-Cox impregnation method. Brains were processed and stained with a FD Rapid GolgiStain kit (FD NeuroTechnologies, Ellicott City, MD) following the manufacturer’s protocol. For spine density quantification, neurons were imaged using a Zeiss Axioplan 2 microscope. The number of spines was counted along equivalent lengths of secondary or tertiary dendritic segments of pyramidal neurons, and spine density quantified using Metamorph (Universal Imaging) by an experimenter blind to conditions.
Statistical analysis
All statistical analyses were done using GraphPad Prism software (La Jolla, CA). Analysis between 2 groups was done using students unpaired t-tests. Analysis between 3 or more groups was done using a one-way ANOVA with post-hoc tests to determine differences between groups.
Results
NRG1 promotes spine growth in cortical pyramidal neurons
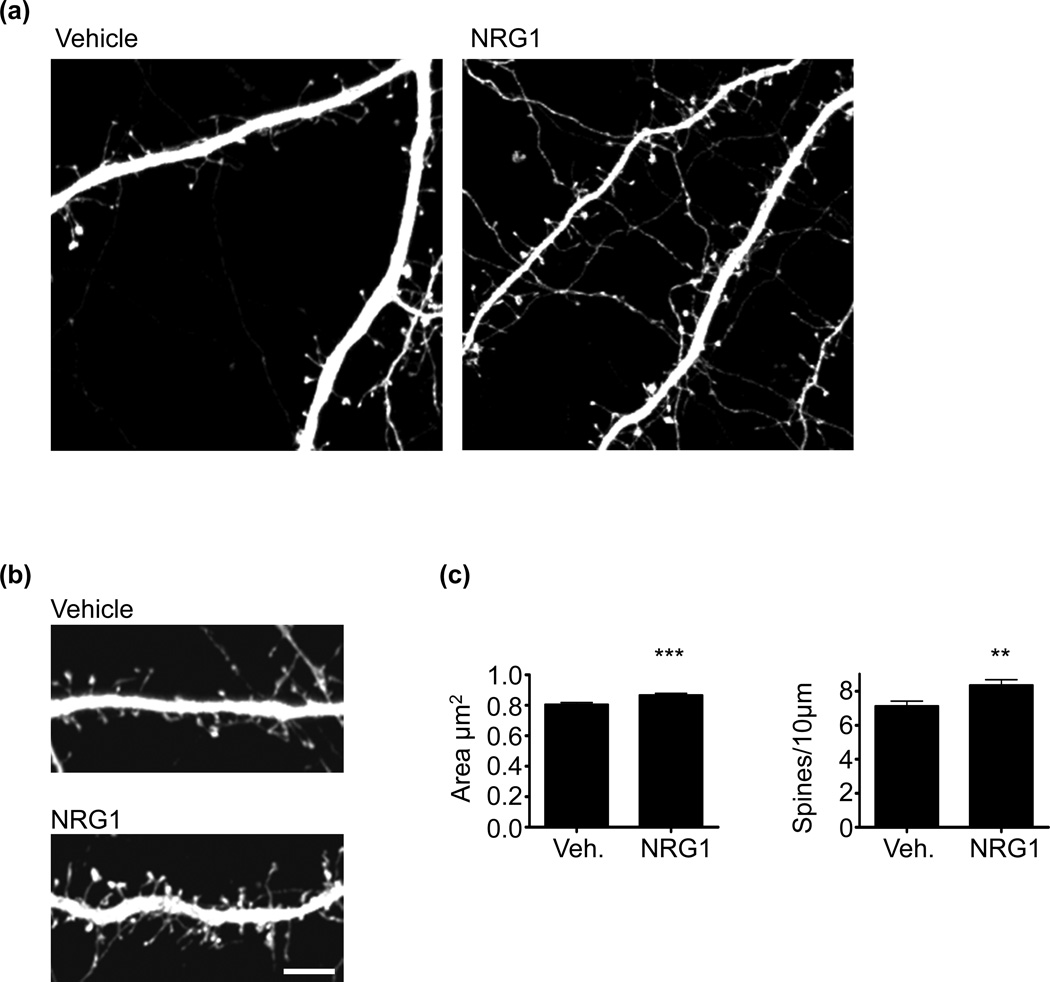
NRG1 has been show to promote spine growth in forebrain pyramidal neurons (Barros et al. 2009). Studies examining the role of NRG1 on spine morphogenesis have used NRG1 peptides solely containing the EGF-like domain. Although the EGF-like domain is necessary and sufficient for erbB4 activation (Buonanno 2010), this peptide lacks the other extracellular domains of soluble NRG1 that occur in vivo, and it is thus not clear if full-length NRG1 alters spine morphogenesis. To this end, we treated mature cultured cortical neurons with the entire NRG1 extracellular domain for 2 days. Prior to NRG1 treatment, neurons were transfected with GFP to allow for the visualization of spines. We found that cultured cortical neurons showed an increase in both spine size and density following treatment with NRG1 (Fig. 1a–c). This indicates that the full-length NRG1 ectodomain, identical to the presynaptically shed NRG1 that occurs in vivo, facilitates spine morphogenesis. NRG1 treatment also induced spine growth in hippocampal neurons (Figure S1a and b).
Fig. 1. NRG1 regulates spine morphogenesis in cortical neurons.
(a) DIV28 cultured cortical neurons expressing GFP were treated with vehicle or with NRG1β (5nM) for 2 days. Neurons were fixed and immunostained for GFP.
(b) High magnification images of dendrites for control and NRG1-treated neurons. Scale bar=10µm.
(c) Quantification of the effects of NRG1 on spine morphology. NRG1 increases spine area and density. N=19 cells, 1300 spines for vehicle; 17 cell, 1416 spines for NRG1. Data are the mean±SEM. **p<0.01, ***p<0.001.
NRG1 regulates GluA1 surface expression and spine content
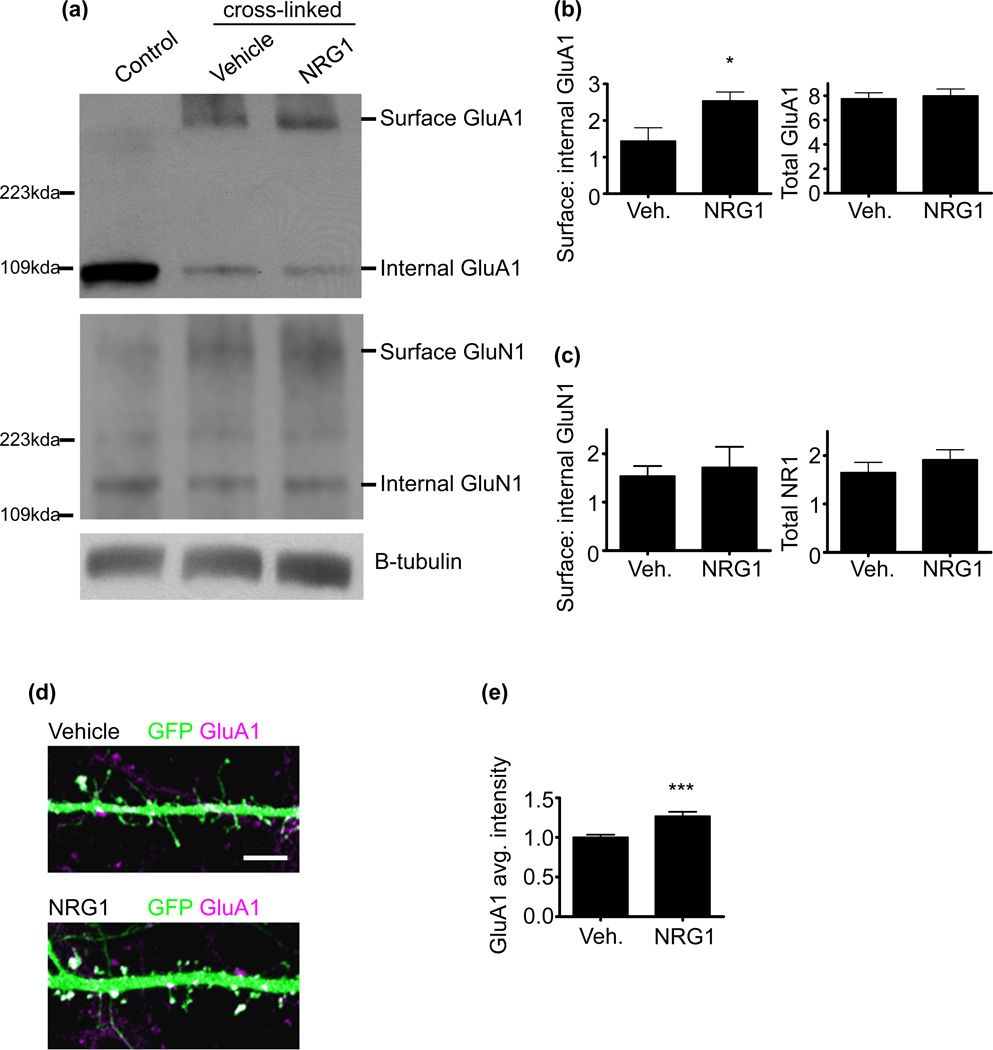
ErbB4 has been shown to regulate spine AMPAR content, and the forced stabilization of surface AMPARs occludes the effects of erbB4 RNAi on synaptic transmission and on spine morphology (Li et al. 2007). However, the effects of NRG1 on AMPAR surface expression and spine content have not been directly examined. We first assessed if treatment of mature cultured cortical neurons with the extracellular domain of NRG1 alters the surface expression of the AMPA subunit, GluA1. To this end, we used Bis(sulfosuccinimidyl) suberate (BS3) crosslinking to delineate surface from internal receptor stores. BS3 is a non-cleavable, membrane impermeable crosslinking agent that forms stable bonds with amine residues, and upon protein electrophoresis, BS3 crosslinked proteins migrate more slowly into the gel, while non-crosslinked proteins will show normal migration. This allows for the analysis of surface and internal receptor levels in the same SDS-PAGE lane. This method has been used previously to examine surface to internal glutamate receptor expression (Boudreau & Wolf 2005; Hall & Soderling 1997; Conrad et al. 2008). We found that NRG1 increased the ratio of surface to internal GluA1 levels (Fig. 2a and b). To determine the specificity of the effect of NRG1 on GluA1, we also probed for GluN1 and found no alterations in surface to internal levels (Fig. 2a and c). Collectively, these data indicate that NRG1 increases surface GluA1 levels, consistent with a role for NRG1 in regulating synaptic function.
Fig. 2. NRG1 enhances AMPA receptor surface expression and delivery to spines.
(a) DIV28 cultured cortical neurons were treated with vehicle or with NRG1β (5nM) for 3 days. BS3 crosslinking was used to determine surface and internal receptor levels. Non-crosslinked cells were used as a control (far left lane). Proteins were resolved using SDS-PAGE and probed with GluA1 and GluN1 antibodies.
(b) Quantification of the effects of NRG1 on surface to internal GluA1. NRG1 significantly increased surface to internal GluA1 levels. N=2 experiments. Data are the mean±SEM. *p<0.05.
(c) Quantification of the effects of NRG1 on surface to internal GluN1 levels. NRG1 did not alter surface to internal GluN1 levels. N=2 experiments. Data are the mean±SEM.
(d) DIV28 cultured cortical neurons expressing GFP were treated with vehicle or with NRG1β (5nM) for 2-days. Neurons were immunostained for GFP and endogenous GluA1. Scale bar=5µm.
(e) Quantification of the effects of NRG1 on spine GluA1 average intensity. NRG1 increased average GluA1 intensities in spines. Data are relative to the vehicle condition. N=9 cells, 716 spines for vehicle; 6 cells, 511 spines for NRG1. Data are the mean±SEM. ***p<0.001.
Next, we wanted to determine if pyramidal neuronal spines show an increase in GluA1 expression following NRG1 treatment. To this end, we treated mature cortical cultured neurons expressing GFP with NRG1 for 2-days and examined spine GluA1 average intensities. With NRG1 treatment we found a significant increase in spine GluA1 average intensity relative to vehicle-treated neurons (Fig. 2d and e). This indicates that NRG1 has distinct effects in regulating the spine GluA1 content of pyramidal neurons.
ErbB4 knockdown cell autonomously affects spine morphogenesis
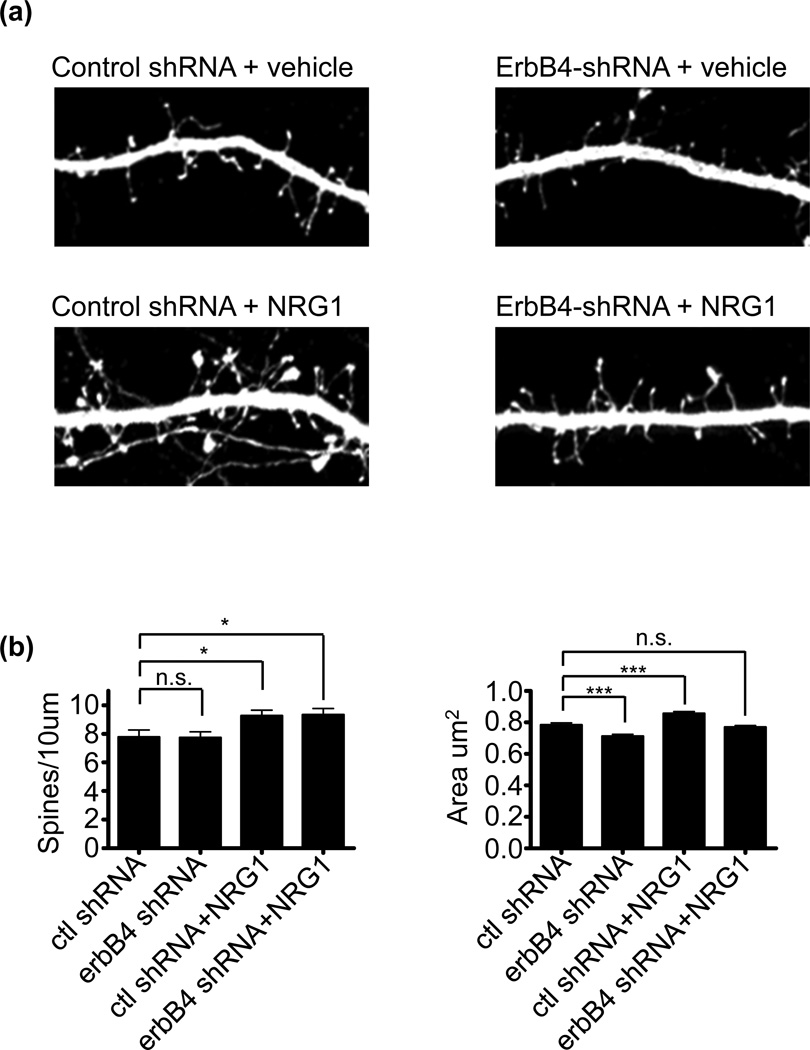
Viral-mediated knockdown of erbB4 reduces forebrain spine morphogenesis (Li et al. 2007), but it has been argued that owing to the widespread knockdown of erbB4 in the majority of cells, including interneurons, the effect of erbB4 knockdown on spines is indirect and caused by alterations in interneuronal output onto pyramidal neurons (Vullhorst et al. 2009). At present, it is unclear how the cell-autonomous knockdown of erbB4 affects spine morphogenesis. To test this, we overexpressed erbB4-shRNA or a scrambled control shRNA in cultured neurons for 6-days. The shRNA plasmids also express GFP allowing for the specific visualization of cells expressing the shRNA; the efficacy of the erbB4 shRNA in knocking down erbB4 protein levels is shown in Figure S1c . Three days post-transfection, neurons were treated with NRG1 or vehicle (Fig. 3a). Consistent with the idea that erbB4 expression in pyramidal neurons is not the direct mediator of NRG1-mediated increases in spine density, erbB4 knockdown affected neither basal spine numbers nor occluded NRG1’s effects in increasing spine density. Surprisingly, loss of erbB4 did reduce basal spine area and blocked NRG1’s effects on spine area (Fig. 3b). Taken together, these findings indicate that effects of NRG1 on spine density, but not spine area, occur irrespective of erbB4 expression in pyramidal neurons. Because the transfection method used results in only a small percentage of neurons expressing the GFP shRNA (less than 1%), it is highly unlikely that the effects on pyramidal neurons were indirect.
Fig. 3. ErbB4 knockdown prevents NRG1-dependent spine enlargement, but not new spine formation.
(a) Mature cultured cortical neurons were transfected with either GFP-expressing ERBB4-shRNA or a GFP-expressing control shRNA for 6-days. 3-days post transfection, neurons were treated with 5nm NRG1 or vehicle for 3-days.
(b) Quantification of cells in a. ErbB4 knockdown did not affect basal spine density, and did not affect NRG1-mediated increases in spine density. Cells expresssing erbB4 shRNA showed a reduction in both basal spine area and in NRG1-mediated increases in spine area. N=15 cells, 1181 spines for control shRNA; 14 cells, 1111 spines for erbB4 shRNA; 22 cells, 2042 spines for control shRNA+NRG1; 17 cells, 1606 spines for erbB4 shRNA+NRG1. Data are the mean±SEM. *p<0.05, ***p<0.001.
NRG1 and erbB4-induced spine growth require kalirin
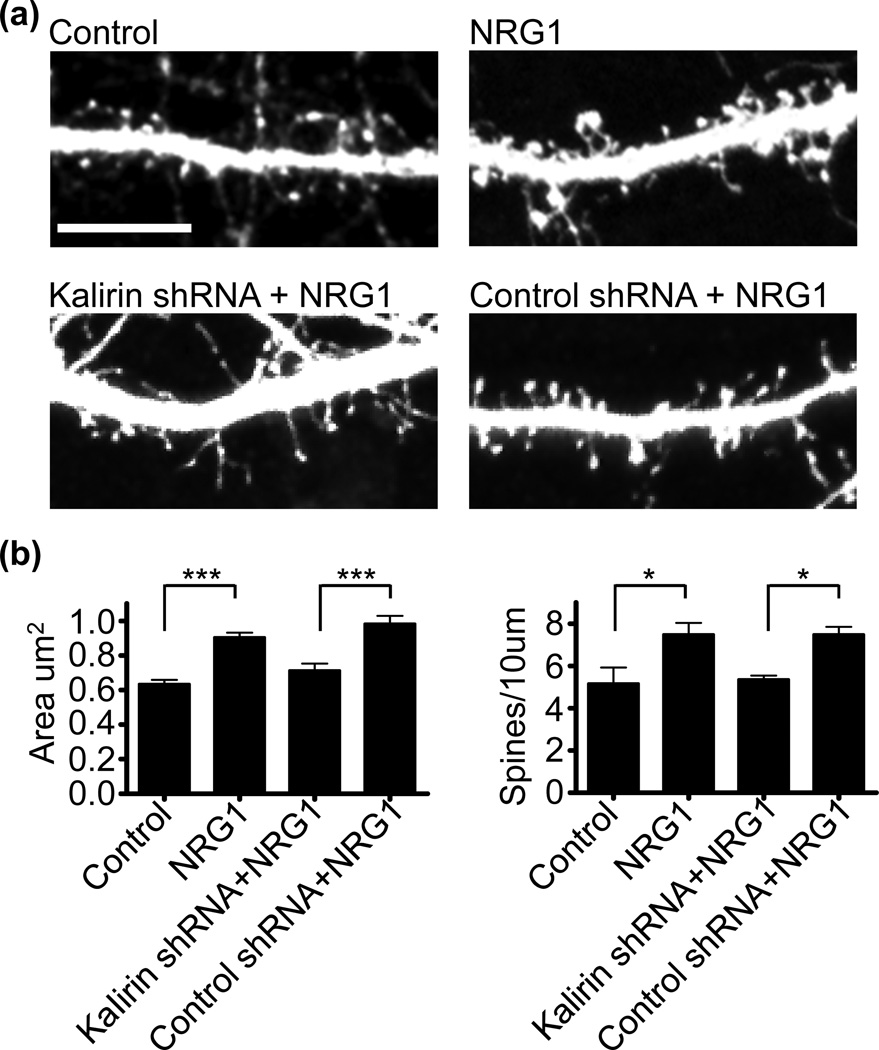
Kalirin is a critical regulator of forebrain spine morphogenesis as its loss reduces both spine density and area (Xie et al. 2007), and NRG1 regulates interneuron growth through kalirin (Cahill et al. 2012). We found that NRG1 increases the activity of kalirin-7’s downstream effector, Rac1 (Figure S1d), and we thus wanted to determine if kalirin is important for NRG1’s effects on spine morphogenesis. To determine if the acute loss of kalirin is sufficient to block NRG1-mediated spine morphogenesis, we overexpressed kalirin-shRNA or a control scrambled shRNA in cortical neurons for 3 days in combination with NRG1 treatment (Fig. 4a and Figure S1e). The specificity of the kalirin-shRNA has been previously validated (Xie et al. 2007). First, we found that NRG1 treatment of GFP-expressing cortical cultures increased spine area and density, similar to our previous results. We found that following NRG1 treatment, neurons expressing the control RNAi show a greater increase in spine area and density relative to neurons expressing kalirin RNAi, indicating that partial knockdown of kalirin blocked NRG1-dependent increases in spine area and density (Fig. 4b). To extend these findings, we treated mature cultured cortical neurons from WT or KALRN KO mice with NRG1 for 3 days and examined spine morphology in GFP expressing neurons (Figure S2a). In WT neurons, NRG1 treatment caused an enlargement of cortical spine area and de novo spine formation (Figure S2b). Conversely, NRG1 treatment did not affect spine area or density in kalirin KO cortical neurons. Collectively, these data indicate that kalirin is important for NRG1-mediated spine growth.
Fig. 4. Kalirin is required for NRG1-dependent spine morphogenesis.
(a) Mature cultured cortical neurons were transfected with either GFP, GFP-expressing kalirin-shRNA, or a GFP-expressing control shRNA for 3-days, with or without 3-day NRG1 treatment. Scale bar=10µm.
(b) Quantification of cells treated as in a. NRG1 increased spine area and spine density in GFP-expressing neurons. With NRG1 treatment, cells expressing the control RNAi show a greater spine area and density relative to cells expressing kalirin RNAi. N=6 cells per condition, 162 spines for control, 366 spines for NRG1, 110 spines for kalirin shRNA+NRG1, 140 spines for control shRNA+NRG1. Data are the mean±SEM. *p<0.05, ***p<0.001.
We next assessed the cell-autonomous effects of overexpressing erbB4 in cultured cortical neurons, and the dependence of these effects on kalirin. ErbB4 overexpression increased spine area but not spine density (Fig. 5a and b), consistent with our previous finding of cell autonomous erbB4 knockdown in reducing spine size but not density, and with the reported effects of erbB4 overexpression in hippocampal slices (Li et al. 2007). On the contrary, kalirin KO neurons failed to show alterations in spine size following erbB4 overexpression (Fig. 5a and b), suggesting that kalirin is necessary for the spine enlargement promoting effects of erbB4.
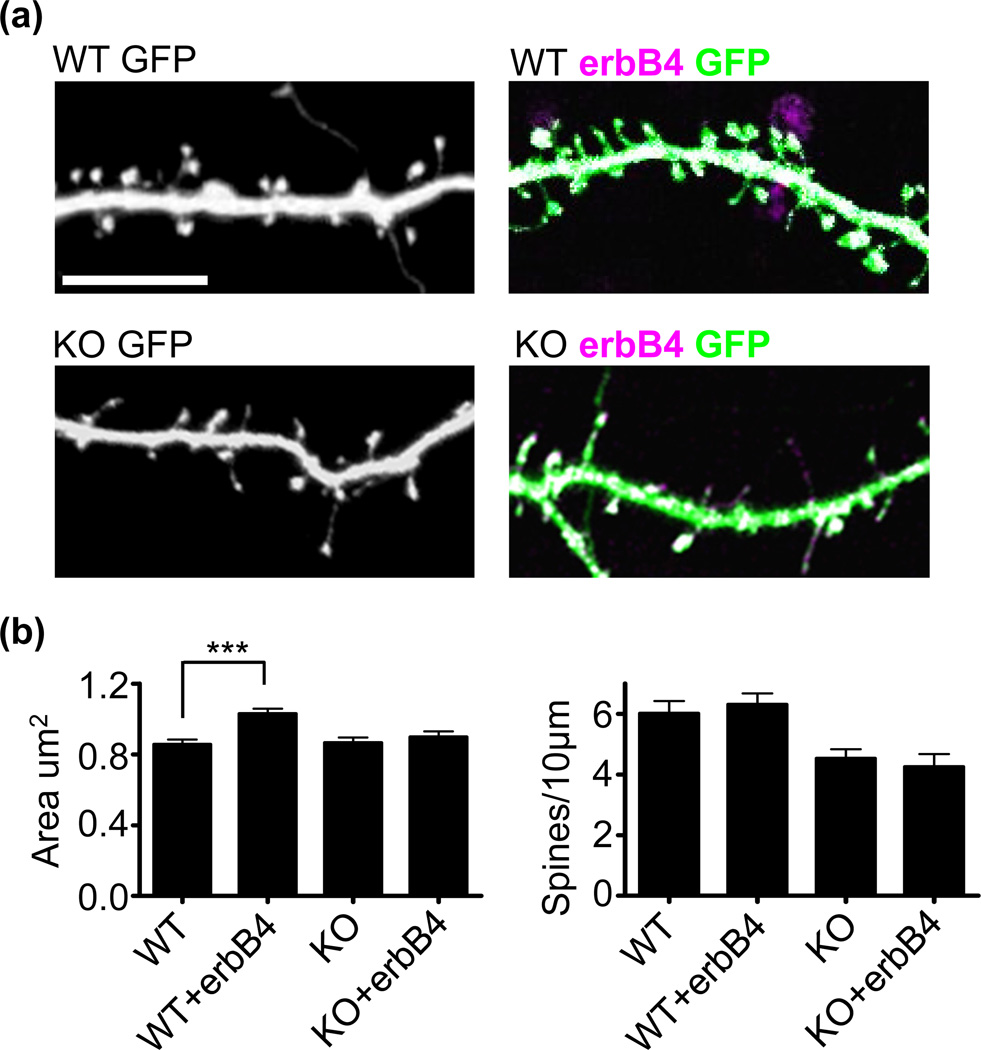
Fig. 5. Kalirin is required for erbB4-dependent spine morphogenesis.
(a) Cultured cortical neurons generated from WT or KALRN KO mice were transfected with either GFP alone or GFP plus a plasmid expressing erbB4 for 3-days. Scale bar=10µm.
(b) Quantification of cells in a. ErbB4 overexpression increased spine area in WT, but not KO neurons, without affecting spine density. N=8 cells, 423 spines for WT, 8 cells, 454 spines for WT+erbB4; 8 cells, 344 spines for KO; 8 cells, 336 spines for KO+erbB4. Data are the mean±SEM. ***p<0.001.
Environmental enrichment upregulates erbB4 expression
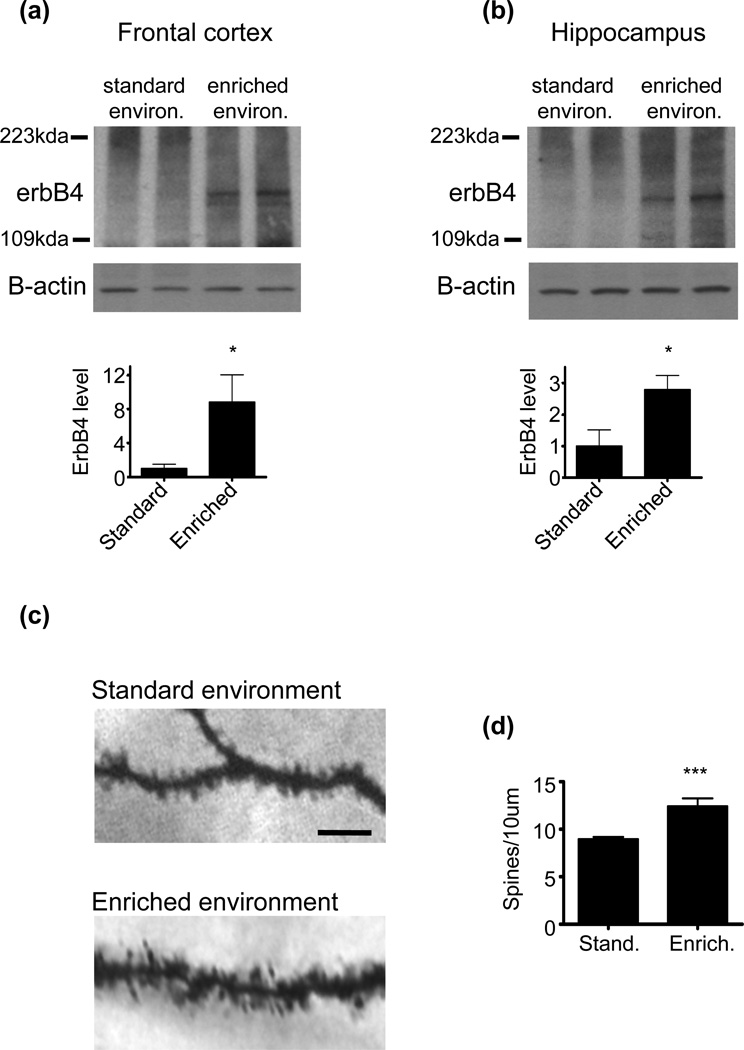
The expression level of erbB4 in the forebrain is generally low (Eilam et al. 1998), but a number of environmental manipulations, such as pharmacological treatments, have been shown to increase erbB4 forebrain expression (Wang et al. 2008). Here we wanted to determine how an environmental manipulation known to drive spine morphogenesis affects erbB4 forebrain expression. To this end, we studied how rearing mice for a prolonged period in an enriched environment affects erbB4 expression relative to mice reared in standard environments. We found that mice reared in enriched conditions show greater levels of erbB4 expression in frontal cortical and hippocampal lysates as compared to mice raised in standard environments (Fig. 6a and b). The erbB4 antibody used in this experiment detected exogenously expressed erbB4 in hEK293 cells, and did not cross-react with other endogenous protein (Figure S3). An increase in hippocampal spine density is a defining feature of environmental enrichment, as enrichment has consistently been shown to drive hippocampal spine morphogenesis (Nithianantharajah & Hannan 2006). We assessed the validity of our enrichment protocol by examining spine density in the CA1 region, and found that enriched mice showed increased spine numbers (Fig. 6c and d).
Fig. 6. Environmental enrichment increases erbB4 protein levels.
(a) Frontal cortical lysate from mice reared in either a standard or enriched enivronment was resolved using SDS-PAGE and probed with an erbB4 antibody. Enrichment resulted in a significant increase in frontal cortical erbB4 expression. Graph shows quantification of erbB4 with normalization to the corresponding amount of β-actin, relative to the standard environment condition. N=6 mice per rearing condition. Data are the mean±SEM. *p<0.05.
(b) Hippocampal lysate from mice reared in either the standard or enriched enivronment was resolved using SDS-PAGE and probed with an erbB4 antibody. Enrichment resulted in a significant increase in hippocampal erbB4 expression. Graph shows quantification of erbB4 with normalization to the corresponding amount of β-actin, relative to the standard environment condition. N=6 mice per rearing condition. Data are the mean±SEM. *p<0.05.
(c) CA1 spines were visualized in hippocampal slices from mice reared in standard and enriched environments using Golgi staining. Scale bar=10µm.
(d) Quantification of spine linear densities as in c shows that enrichment increased spine density. N=16 cells for standard environment; 12 cells for enriched environment. Data are the mean±SEM. ***p<0.001.
Discussion
Here we show that NRG1 promotes spine morphogenesis in cortical pyramidal neurons and our findings indicate that kalirin is responsible for these effects. Our findings are further supportive of the idea that long-term chronic NRG1 stimuation has trophic effects on pyramidal neuron spines (Barros et al. 2009). Consistent with this, the long-term loss of NRG1 as seen in NRG1 mutant mice results in a reduction in forebrain spine density (Chen et al. 2008). NRG1 signaling seems to have differential effects on excitatory synapses depending on the length of stimulation. Notably, acute NRG1 treatment (around 30-minutes) reduces pyramidal neuronal synaptic plasticity (Kwon et al. 2005). Many of these effects are mediated through interneurons as acute NRG1 treatment can stimulate GABA release from interneurons thereby inhibiting pyramidal neuron activity (Wen et al. 2009). That acute NRG1 treatment inhibits pyramidal neuronal activity, while long-term NRG1 promotes plasticity, could indicate that under different circumstances excessive acute NRG1 signaling and/or deficient chronic NRG1 signaling might both impede synaptic plasticity and be relevant to schizophrenia pathogenesis.
Within the forebrain, erbB4 expression is highest in interneurons, and despite evidence that NRG1 and erbB4 have trophic effects on spine morphogenesis, the expression profile of erbB4 in pyramidal neurons is a matter of ongoing debate. Previous studies have detected erbB4 protein expression in the dendritic spines of mature pyramidal neurons using immuno-electron microscopy (immuno-EM) (Mechawar et al. 2007), but the specificity of the antibody used in these EM studies has been questioned, and antibodies against different erbB4 epitopes have turned up negative results (Neddens & Buonanno 2009; Vullhorst et al. 2009). However, several studies using antibodies from different sources against various epitopes of the erbB4 protein have detected protein expression in mature pyramidal neurons, albeit at significantly lower levels than in interneurons (Bernstein et al. 2006; Okada & Corfas 2004). Furthermore, several in situ studies have detected ERBB4 mRNA expression in mature pyramidal neurons (Fox & Kornblum 2005; Gerecke et al. 2001). Complicating matters, single cell RT-PCR experiments have failed to detect ERBB4 in hippocampal pyramidal neurons (Vullhorst et al. 2009), making it uncertain if erbB4 is truly expressed in forebrain pyramidal neurons.
Interestingly, our results indicate that the effect of NRG1 on spine numbers is likely independent of erbB4 expression, as hypothesized by other groups (Vullhorst et al. 2009), suggesting distinct role for erbB4 in mediating NRG1’s effects on spine area and density (Figure S4). Moreover, erbB4 overexpression is capable of inducing spine enlargement, but not new spines, in a cell autonomous fashion. This suggests that erbB4 may not completely underlie NRG1-induced spine growth. Several explanations for the effects of long-term NRG1 activity on facilitating plasticity in pyramidal neurons have been offered. Namely, that the trophic effects are due to the direct activation of low-levels of erbB4 receptors on pyramidal neurons (Li et al. 2007), or conversly, that these effects are compensatory mechanisms to preserve excitatability in the face of increased GABAergic input to pyramdial neurons resulting from NRG1 (Buonanno 2010). Similar to other studies (Li et al. 2007), we found that erbB4 overexpression increases spine size. Additionally, we found that the knockdown of erbB4 cell autonomously blocked NRG1-mediated increases in spine size, and a previous study found that erbB4 knockdown in pyramidal neurons reduced AMPAR-mediated transmission in a cell autonomous manner (Li et al. 2007). This leaves open the possibility that low levels of pyramidal erbB4 expression mediate some of NRG1’s effects on plasticity. It is also possible that NRG1’s effects are mediated by receptors other than erbB4. In this later case, overexpression of erbB4 might engage their downstream signaling without actually being expressed in these cells. Another possibility is that environmental and therapeutic treatments enhance erbB4 expression, which is than able to further enhance the spine growth promoting effects of NRG1. In this case, upregulation of erbB4 might be a mediator of some therapeutic approaches.
The cleavage of NRG1 is critical for the feed-forward signaling of most isoforms. NRG1 processing occurs after delivery of NRG1 to the cell surface (Loeb et al. 1998), and numerous lines of evidence indicate that synaptic activity increases levels of NRG1 (Eilam et al. 1998; Loeb et al. 1998; Ozaki et al. 2004). It is thus likely that neurons are exposed to constitutive levels of NRG1, making it important to determine the effects on long-term NRG1 activity on neurons. Our findings, in combination with previous studies (Li et al. 2007), indicate that chronic NRG1 activity increases glutamate receptor levels in spines, which would cause increased synaptic transmission onto pyramidal neurons. This increased synaptic transmission would be expected to further amplify NRG1 release contributing to long-term and sustainable plasticity (Mei & Xiong 2008). These trophic effects of NRG1 on synaptic plasticity could contribute to the neuroprotective effects of chronic NRG1 in animal models of neurodegeneration (Woo et al. 2012; Shyu et al. 2004; Guo et al. 2006).
We also found that that kalirin-7, a key regulator of spine remodeling, is important for NRG1-dependent increases in spine morphogenesis. The importance of investigating interactions between kalirin-7 and schizophrenia-associated genes is underscored by a recent study showing that the disrupted-in-schizophrenia protein (DISC1) regulates spine morphogenesis through a signaling complex involving kalirin-7. Other studies have found a reduction in kalirin mRNA expression in the prefrontal cortex of schizophrenia patients (Hill et al. 2006; Narayan et al. 2008), and the protein expression of kalirin-7 is reduced in the schizophrenia dorsolateral prefrontal cortex and anterior cingulate cortex (Rubio et al. 2012). Furthermore, kalirin loss correlates strongly with prefrontal cortical spine loss in schizophrenia patients irrespective of antipsychotic treatment (Hill et al. 2006). Interestingly, a reduction in cortical spine density has been detected in NRG1, erbB4, and kalirin mutant mice, and mice hypomorphic or with mutations in these genes show similar behavioral phenotypes, including locomotor hyperactivity and deficits in pre-pulse inhibition, spatial working memory, and social behavior (Stefansson et al. 2002; Gerlai et al. 2000; Cahill et al. 2009; Clapcote et al. 2007; Pletnikov et al. 2008; Barros et al. 2009; Golub et al. 2004).
We found that environmental enrichment robustly upregulates the expression of erbB4 protein in both the cortex and hippocampus. Schizophrenia is thought to result from a complex interaction between genes and the environment (Le Strat et al. 2009), and understanding how environmental manipulations affect the expression of schizophrenia susceptibility molecules could provide a more complete understanding of potential disease processes. The effect of therapeutic approaches on the expression profile of erbB4 has received recent attention. Notably, treatment with the antipsychotic clozapine increases forebrain erbB4 expression in rodents (Wang et al. 2008). Overall, this suggests that altered erbB4 levels might be related to certain treatment strategies. Enrichment has been shown to alleviate deficits in neuronal ultrastructure, including deficits in spine density, in numerous animal models of neuropsychiatric disorders (Nithianantharajah & Hannan 2006). In humans, early life participation in enrichment programs has been shown to preemptively reduce schizotypal personality behavior, including reduced cognitive disorganization (Raine et al. 2003). The expression profile of erbB4 in schizophrenia remains complex, as some studies suggest that overexpression of the receptor occurs in schizophrenia (Law et al. 2007), while other findings indicate that erbB4 activity is decreased in the disease (Walsh et al. 2008). Nevertheless, our findings would support enrichment as a potentially useful model for studying how alterations in rodent erbB4 expression levels impact brain structure and function, with potential disease implications.
Supplementary Material
Acknowledgements
This work was supported by grants from NIH-NIMH (R01MH071316, R01MH097216), National Alliance for Research on Schizophrenia and Depression (NARSAD) to P.P., Ruth L. Kirschstein National Research Service Awards 1F31AG031621-01A2 to M.E.C and 1F31MH085362 to K.A.J , and research grants from NIH (R01 MH 071533, R01 AG027224) and from the VHA (I01 BX000452) to RAS. All experiments involving animals were done according to the Institutional Animal Care and Use Committee of Northwestern University. M.E.C, C.R, K.A.J., Z.X. performed research and analyzed results. M.E.C., C.R., and P.P. wrote the paper, and P.P. and R.A.S. contributed resources necessary to complete this work.
Abbreviations used
- NRG1
(neuregulin 1)
- PFC
(prefrontal cortex)
- BS3
Bis(sulfosuccinimidyl)suberate
- RNAi
(RNA interference)
Footnotes
Conflict of interest
None of the authors have completing financial interests in relation to the work described.
References
- Barros CS, Calabrese B, Chamero P, et al. Impaired maturation of dendritic spines without disorganization of cortical cell layers in mice lacking NRG1/ErbB signaling in the central nervous system. Proc Natl Acad Sci U S A. 2009;106:4507–4512. doi: 10.1073/pnas.0900355106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein HG, Lendeckel U, Bertram I, et al. Localization of neuregulin-1alpha (heregulin-alpha) and one of its receptors, ErbB-4 tyrosine kinase, in developing and adult human brain. Brain Res Bull. 2006;69:546–559. doi: 10.1016/j.brainresbull.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonanno A. The neuregulin signaling pathway and schizophrenia: from genes to synapses and neural circuits. Brain Res Bull. 2010;83:122–131. doi: 10.1016/j.brainresbull.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill ME, Jones KA, Rafalovich I, Xie Z, Barros CS, Muller U, Penzes P. Control of interneuron dendritic growth through NRG1/erbB4-mediated kalirin-7 disinhibition. Mol Psychiatry. 2012;17(1):99–107. doi: 10.1038/mp.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill ME, Xie Z, Day M, et al. Kalirin regulates cortical spine morphogenesis and disease-related behavioral phenotypes. Proc Natl Acad Sci U S A. 2009;106:13058–13063. doi: 10.1073/pnas.0904636106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YJ, Johnson MA, Lieberman MD, et al. Type III neuregulin-1 is required for normal sensorimotor gating, memory-related behaviors, and corticostriatal circuit components. J Neurosci. 2008;28:6872–6883. doi: 10.1523/JNEUROSCI.1815-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YJ, Zhang M, Yin DM, et al. ErbB4 in parvalbumin-positive interneurons is critical for neuregulin 1 regulation of long-term potentiation. Proc Natl Acad Sci U S A. 2010;107:21818–21823. doi: 10.1073/pnas.1010669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapcote SJ, Lipina TV, Millar JK, et al. Behavioral phenotypes of Disc1 missense mutations in mice. Neuron. 2007;54:387–402. doi: 10.1016/j.neuron.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corfas G, Roy K, Buxbaum JD. Neuregulin 1-erbB signaling and the molecular/cellular basis of schizophrenia. Nat Neurosci. 2004;7:575–580. doi: 10.1038/nn1258. [DOI] [PubMed] [Google Scholar]
- Deo AJ, Cahill ME, Li S, et al. Increased expression of Kalirin-9 in the auditory cortex of schizophrenia subjects: its role in dendritic pathology. Neurobiol Dis. 2012;45:796–803. doi: 10.1016/j.nbd.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilam R, Pinkas-Kramarski R, Ratzkin BJ, Segal M, Yarden Y. Activity-dependent regulation of Neu differentiation factor/neuregulin expression in rat brain. Proc Natl Acad Sci U S A. 1998;95:1888–1893. doi: 10.1073/pnas.95.4.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox IJ, Kornblum HI. Developmental profile of ErbB receptors in murine central nervous system: implications for functional interactions. J Neurosci Res. 2005;79:584–597. doi: 10.1002/jnr.20381. [DOI] [PubMed] [Google Scholar]
- Gerecke KM, Wyss JM, Karavanova I, Buonanno A, Carroll SL. ErbB transmembrane tyrosine kinase receptors are differentially expressed throughout the adult rat central nervous system. J Comp Neurol. 2001;433:86–100. doi: 10.1002/cne.1127. [DOI] [PubMed] [Google Scholar]
- Gerlai R, Pisacane P, Erickson S. Heregulin, but not ErbB2 or ErbB3, heterozygous mutant mice exhibit hyperactivity in multiple behavioral tasks. Behav Brain Res. 2000;109:219–227. doi: 10.1016/s0166-4328(99)00175-8. [DOI] [PubMed] [Google Scholar]
- Golub MS, Germann SL, Lloyd KC. Behavioral characteristics of a nervous system-specific erbB4 knock-out mouse. Behav Brain Res. 2004;153:159–170. doi: 10.1016/j.bbr.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Guo WP, Wang J, Li RX, Peng YW. Neuroprotective effects of neuregulin-1 in rat models of focal cerebral ischemia. Brain Res. 2006;1087:180–185. doi: 10.1016/j.brainres.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Hall RA, Soderling TR. Differential surface expression and phosphorylation of the N-methyl-D-aspartate receptor subunits NR1 and NR2 in cultured hippocampal neurons. J Biol Chem. 1997;272:4135–4140. doi: 10.1074/jbc.272.7.4135. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Law AJ. Neuregulin 1 and schizophrenia: genetics, gene expression, and neurobiology. Biol Psychiatry. 2006;60:132–140. doi: 10.1016/j.biopsych.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Hill JJ, Hashimoto T, Lewis DA. Molecular mechanisms contributing to dendritic spine alterations in the prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2006;11:557–566. doi: 10.1038/sj.mp.4001792. [DOI] [PubMed] [Google Scholar]
- Kushima I, Nakamura Y, Aleksic B, et al. Resequencing and association analysis of the KALRN and EPHB1 genes and their contribution to schizophrenia susceptibility. Schizophr Bull. 2012;38:552–560. doi: 10.1093/schbul/sbq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon OB, Longart M, Vullhorst D, Hoffman DA, Buonanno A. Neuregulin-1 reverses long-term potentiation at CA1 hippocampal synapses. J Neurosci. 2005;25:9378–9383. doi: 10.1523/JNEUROSCI.2100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law AJ, Kleinman JE, Weinberger DR, Weickert CS. Disease-associated intronic variants in the ErbB4 gene are related to altered ErbB4 splice-variant expression in the brain in schizophrenia. Hum Mol Genet. 2007;16:129–141. doi: 10.1093/hmg/ddl449. [DOI] [PubMed] [Google Scholar]
- Le Strat Y, Ramoz N, Gorwood P. The role of genes involved in neuroplasticity and neurogenesis in the observation of a gene-environment interaction (GxE) in schizophrenia. Curr Mol Med. 2009;9:506–518. doi: 10.2174/156652409788167104. [DOI] [PubMed] [Google Scholar]
- Li B, Woo RS, Mei L, Malinow R. The neuregulin-1 receptor erbB4 controls glutamatergic synapse maturation and plasticity. Neuron. 2007;54:583–597. doi: 10.1016/j.neuron.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb JA, Susanto ET, Fischbach GD. The neuregulin precursor proARIA is processed to ARIA after expression on the cell surface by a protein kinase C-enhanced mechanism. Mol Cell Neurosci. 1998;11:77–91. doi: 10.1006/mcne.1998.0676. [DOI] [PubMed] [Google Scholar]
- Ma XM, Johnson RC, Mains RE, Eipper BA. Expression of kalirin, a neuronal GDP/GTP exchange factor of the trio family, in the central nervous system of the adult rat. J Comp Neurol. 2001;429:388–402. doi: 10.1002/1096-9861(20010115)429:3<388::aid-cne3>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Mechawar N, Lacoste B, Yu WF, Srivastava LK, Quirion R. Developmental profile of neuregulin receptor ErbB4 in postnatal rat cerebral cortex and hippocampus. Neuroscience. 2007;148:126–139. doi: 10.1016/j.neuroscience.2007.04.066. [DOI] [PubMed] [Google Scholar]
- Mei L, Xiong WC. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat Rev Neurosci. 2008;9:437–452. doi: 10.1038/nrn2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan S, Tang B, Head SR, Gilmartin TJ, Sutcliffe JG, Dean B, Thomas EA. Molecular profiles of schizophrenia in the CNS at different stages of illness. Brain Res. 2008 doi: 10.1016/j.brainres.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neddens J, Buonanno A. Selective populations of hippocampal interneurons express ErbB4 and their number and distribution is altered in ErbB4 knockout mice. Hippocampus. 2009 doi: 10.1002/hipo.20675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006;7:697–709. doi: 10.1038/nrn1970. [DOI] [PubMed] [Google Scholar]
- Okada M, Corfas G. Neuregulin1 downregulates postsynaptic GABAA receptors at the hippocampal inhibitory synapse. Hippocampus. 2004;14:337–344. doi: 10.1002/hipo.10185. [DOI] [PubMed] [Google Scholar]
- Ozaki M, Itoh K, Miyakawa Y, Kishida H, Hashikawa T. Protein processing and releases of neuregulin-1 are regulated in an activity-dependent manner. J Neurochem. 2004;91:176–188. doi: 10.1111/j.1471-4159.2004.02719.x. [DOI] [PubMed] [Google Scholar]
- Penzes P, Johnson RC, Alam MR, Kambampati V, Mains RE, Eipper BA. An isoform of kalirin, a brain-specific GDP/GTP exchange factor, is enriched in the postsynaptic density fraction. The Journal of biological chemistry. 2000;275:6395–6403. doi: 10.1074/jbc.275.9.6395. [DOI] [PubMed] [Google Scholar]
- Penzes P, Jones KA. Dendritic spine dynamics--a key role for kalirin-7. Trends Neurosci. 2008;31:419–427. doi: 10.1016/j.tins.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletnikov MV, Ayhan Y, Nikolskaia O, Xu Y, Ovanesov MV, Huang H, Mori S, Moran TH, Ross CA. Inducible expression of mutant human DISC1 in mice is associated with brain and behavioral abnormalities reminiscent of schizophrenia. Mol Psychiatry. 2008;13:173–186. doi: 10.1038/sj.mp.4002079. 115. [DOI] [PubMed] [Google Scholar]
- Raine A, Mellingen K, Liu J, Venables P, Mednick SA. Effects of environmental enrichment at ages 3–5 years on schizotypal personality and antisocial behavior at ages 17 and 23 years. Am J Psychiatry. 2003;160:1627–1635. doi: 10.1176/appi.ajp.160.9.1627. [DOI] [PubMed] [Google Scholar]
- Rubio MD, Haroutunian V, Meador-Woodruff JH. Abnormalities of the Duo/Ras-related C3 botulinum toxin substrate 1/p21-activated kinase 1 pathway drive myosin light chain phosphorylation in frontal cortex in schizophrenia. Biol Psychiatry. 2012;71:906–914. doi: 10.1016/j.biopsych.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu WC, Lin SZ, Chiang MF, Yang HI, Thajeb P, Li H. Neuregulin-1 reduces ischemia-induced brain damage in rats. Neurobiol Aging. 2004;25:935–944. doi: 10.1016/j.neurobiolaging.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Stefansson H, Sigurdsson E, Steinthorsdottir V, et al. Neuregulin 1 and susceptibility to schizophrenia. Am J Hum Genet. 2002;71:877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzahar E, Waterman H, Chen X, Levkowitz G, Karunagaran D, Lavi S, Ratzkin BJ, Yarden Y. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol Cell Biol. 1996;16:5276–5287. doi: 10.1128/mcb.16.10.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vullhorst D, Neddens J, Karavanova I, Tricoire L, Petralia RS, McBain CJ, Buonanno A. Selective expression of ErbB4 in interneurons, but not pyramidal cells, of the rodent hippocampus. J Neurosci. 2009;29:12255–12264. doi: 10.1523/JNEUROSCI.2454-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh T, McClellan JM, McCarthy SE, et al. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320:539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- Wang JY, Miller SJ, Falls DL. The N-terminal region of neuregulin isoforms determines the accumulation of cell surface and released neuregulin ectodomain. J Biol Chem. 2001;276:2841–2851. doi: 10.1074/jbc.M005700200. [DOI] [PubMed] [Google Scholar]
- Wang XD, Su YA, Guo CM, Yang Y, Si TM. Chronic antipsychotic drug administration alters the expression of neuregulin 1beta, ErbB2, ErbB3, and ErbB4 in the rat prefrontal cortex and hippocampus. Int J Neuropsychopharmacol. 2008;11:553–561. doi: 10.1017/S1461145707008371. [DOI] [PubMed] [Google Scholar]
- Wen L, Lu YS, Zhu XH, et al. Neuregulin 1 regulates pyramidal neuron activity via ErbB4 in parvalbumin-positive interneurons. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0910302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L, Lu YS, Zhu XH, et al. Neuregulin 1 regulates pyramidal neuron activity via ErbB4 in parvalbumin-positive interneurons. Proc Natl Acad Sci U S A. 2010;107:1211–1216. doi: 10.1073/pnas.0910302107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo RS, Lee JH, Kim HS, Baek CH, Song DY, Suh YH, Baik TK. Neuregulin-1 protects against neurotoxicities induced by Swedish amyloid precursor protein via the ErbB4 receptor. Neuroscience. 2012;202:413–423. doi: 10.1016/j.neuroscience.2011.11.026. [DOI] [PubMed] [Google Scholar]
- Woo RS, Li XM, Tao Y, et al. Neuregulin-1 enhances depolarization-induced GABA release. Neuron. 2007;54:599–610. doi: 10.1016/j.neuron.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Xie Z, Cahill ME, Penzes P. Kalirin loss results in cortical morphological alterations. Mol Cell Neurosci. 2010;43:81–89. doi: 10.1016/j.mcn.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Huganir RL, Penzes P. Activity-dependent dendritic spine structural plasticity is regulated by small GTPase Rap1 and its target AF-6. Neuron. 2005;48:605–618. doi: 10.1016/j.neuron.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Xie Z, Srivastava DP, Photowala H, Kai L, Cahill ME, Woolfrey KM, Shum CY, Surmeier DJ, Penzes P. Kalirin-7 controls activity-dependent structural and functional plasticity of dendritic spines. Neuron. 2007;56:640–656. doi: 10.1016/j.neuron.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.