Abstract
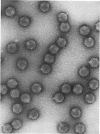
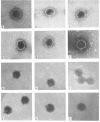
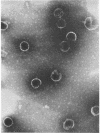
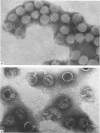
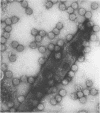
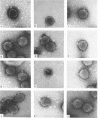
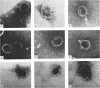
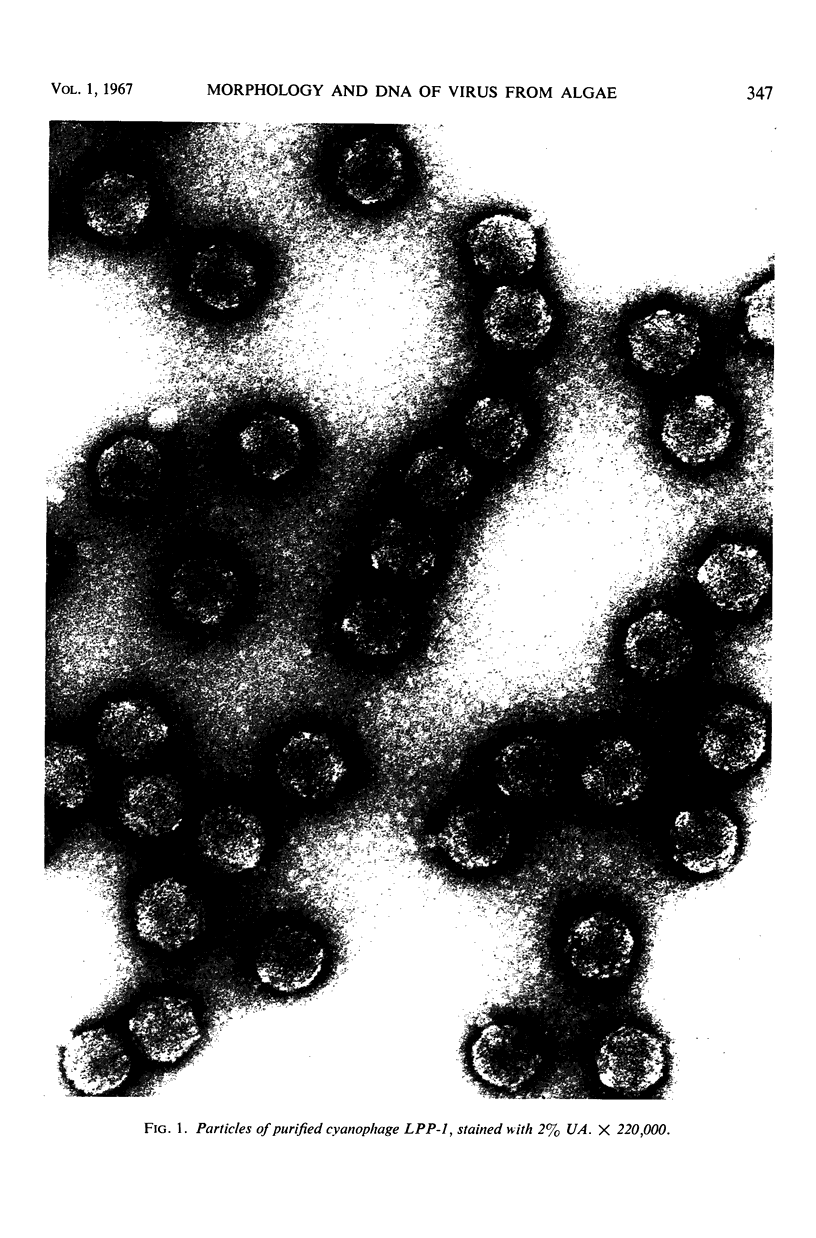
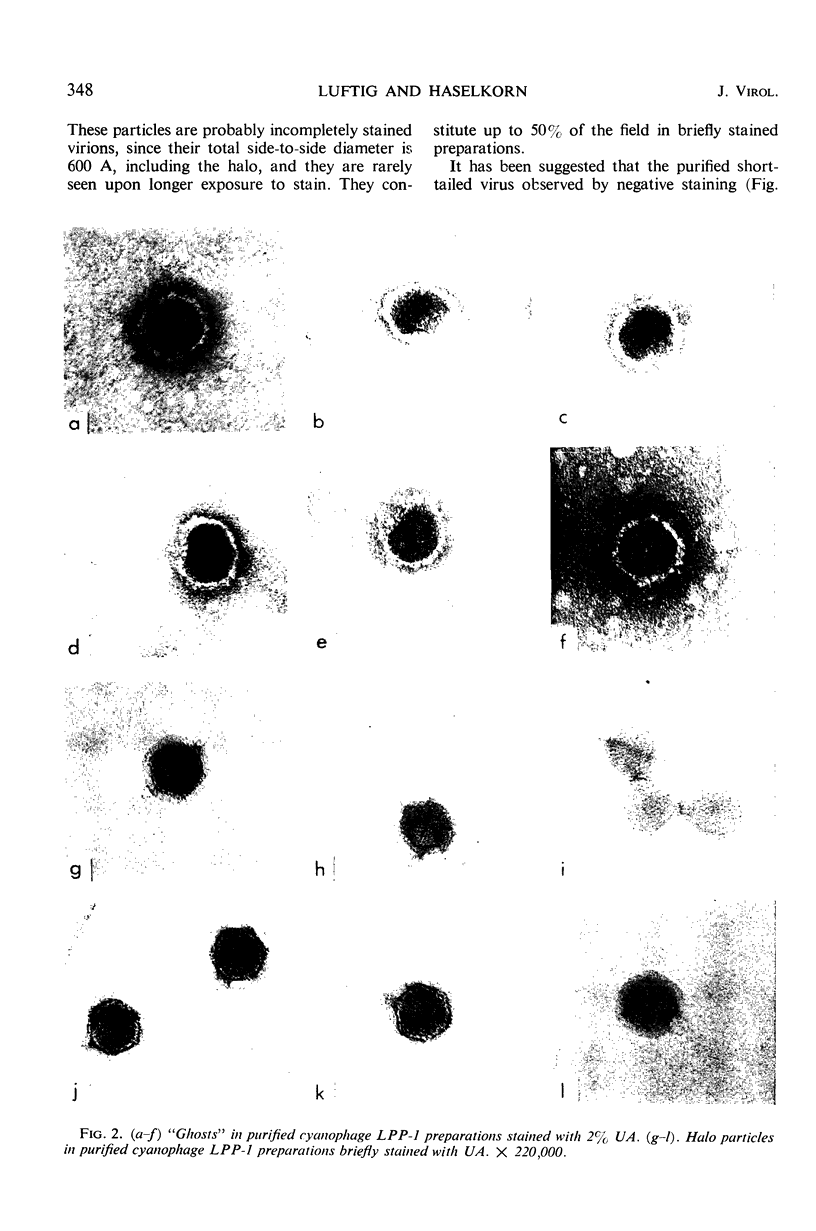
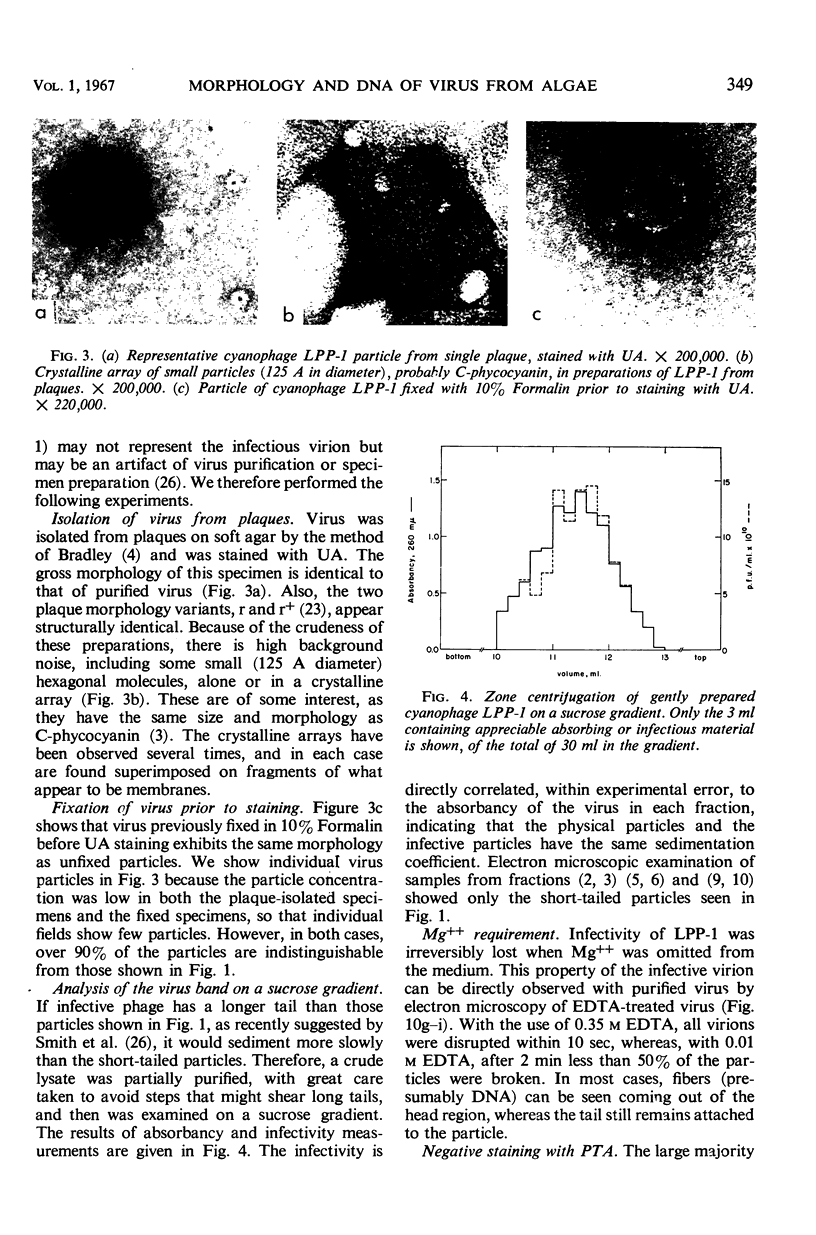
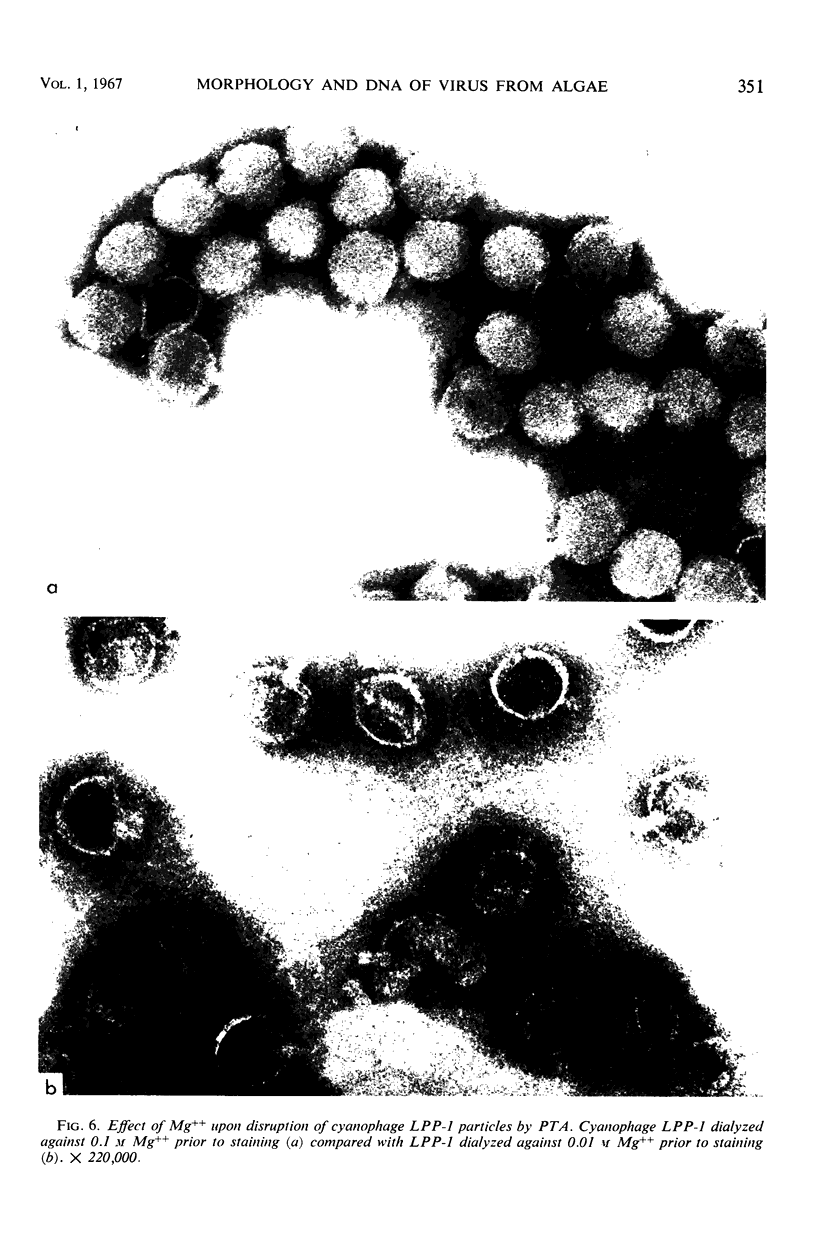
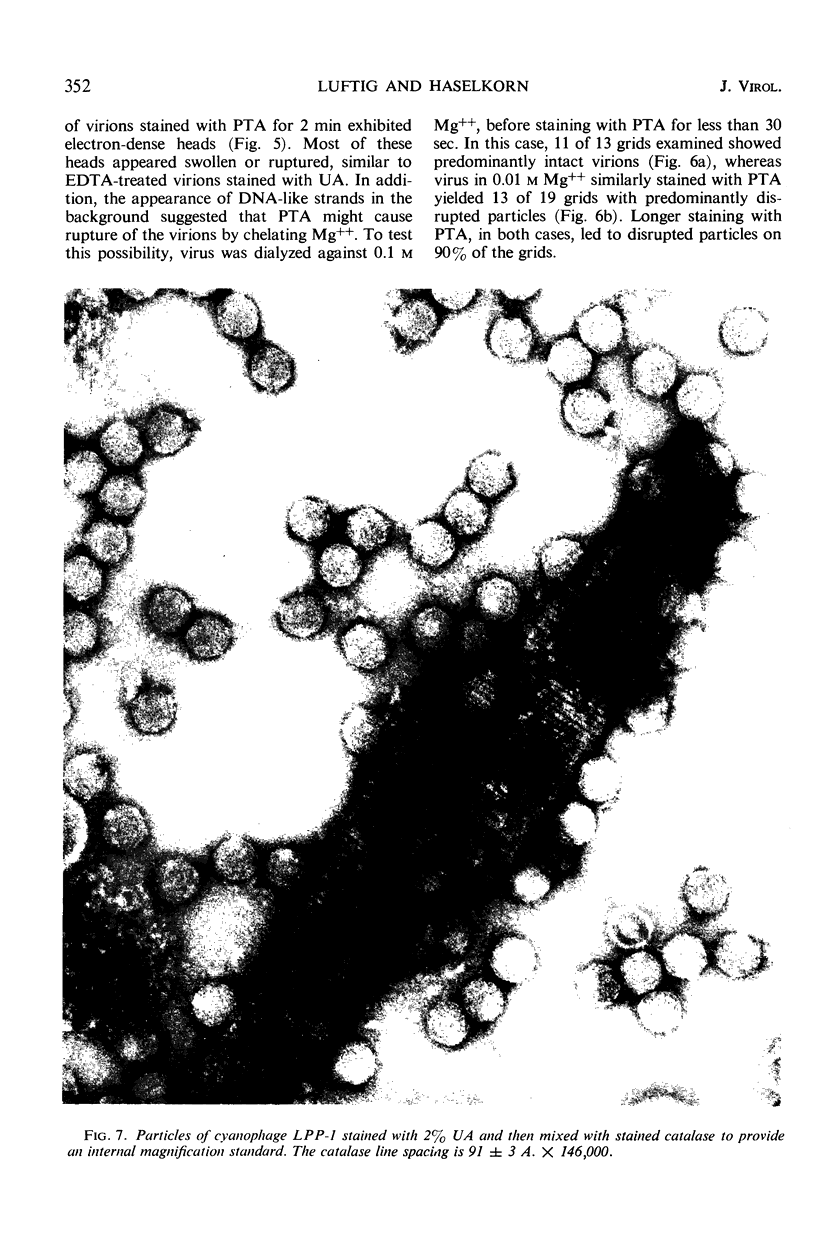
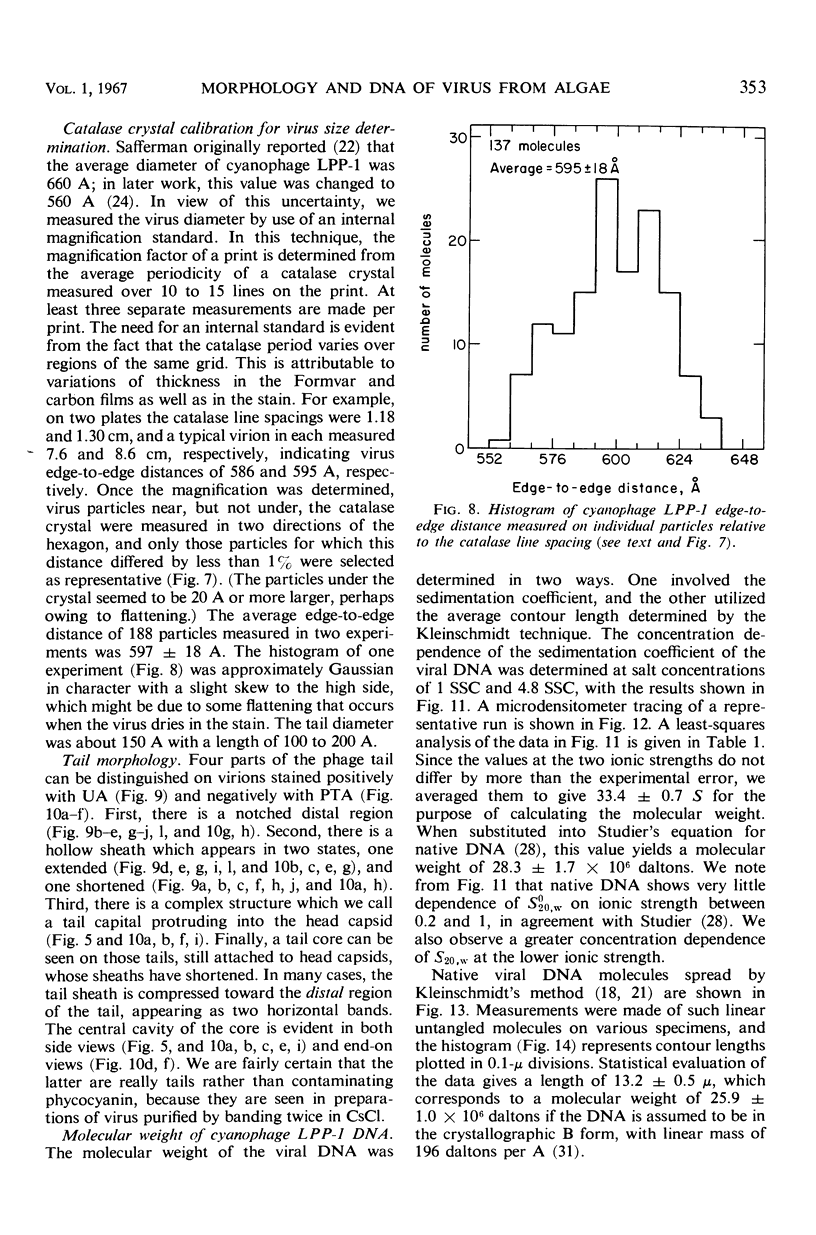
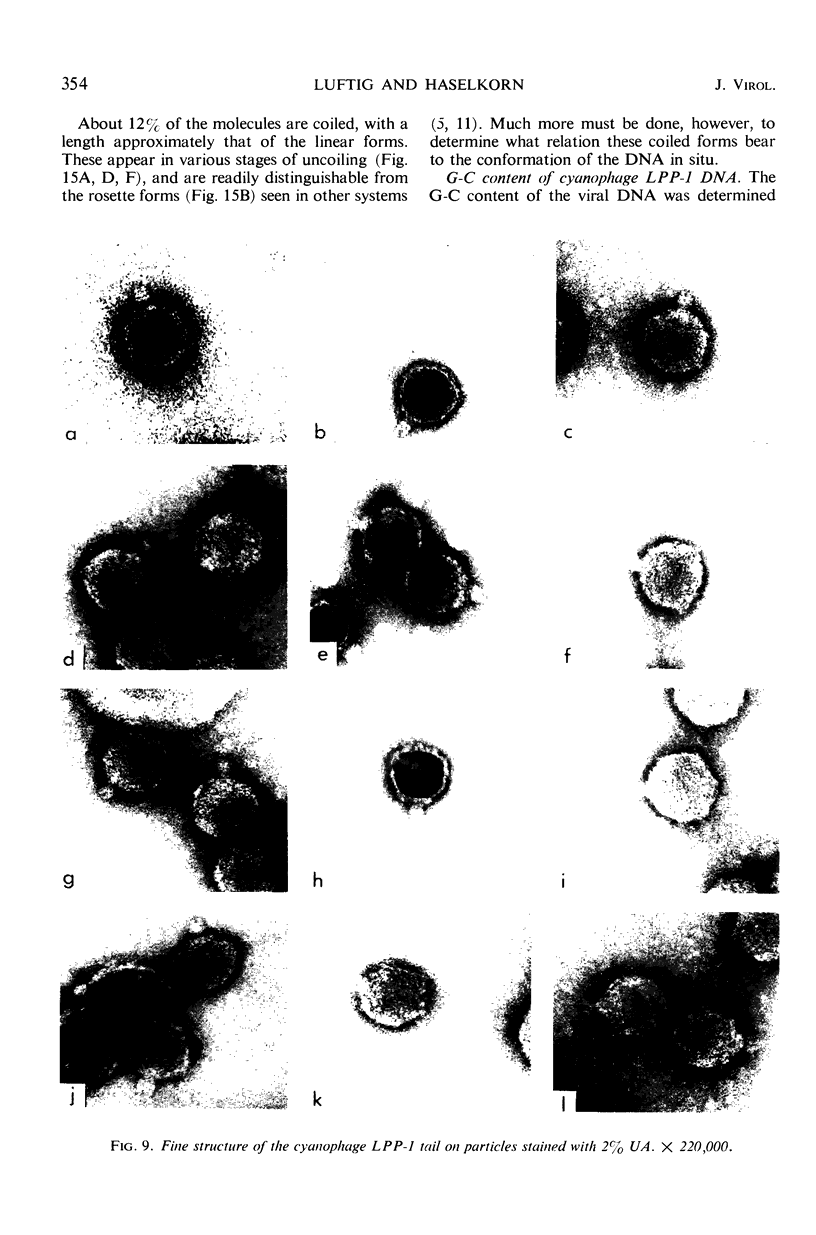
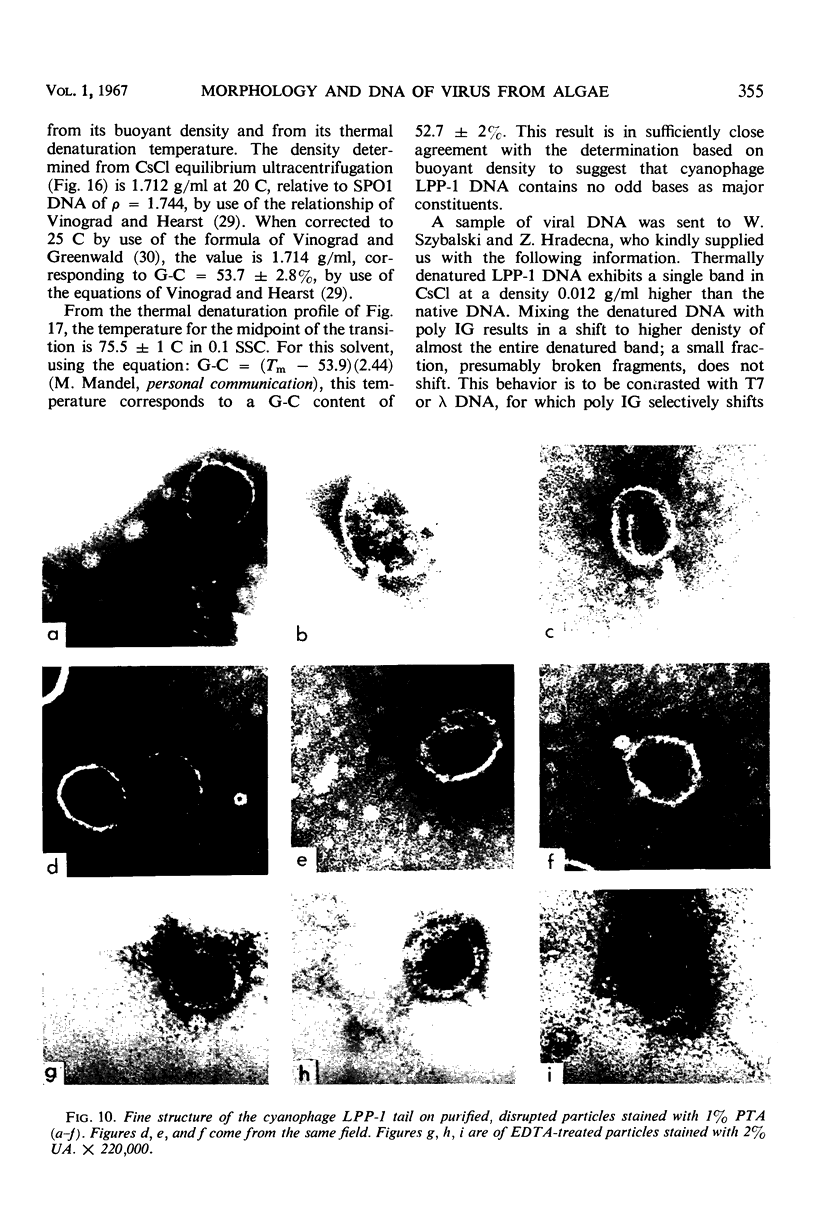
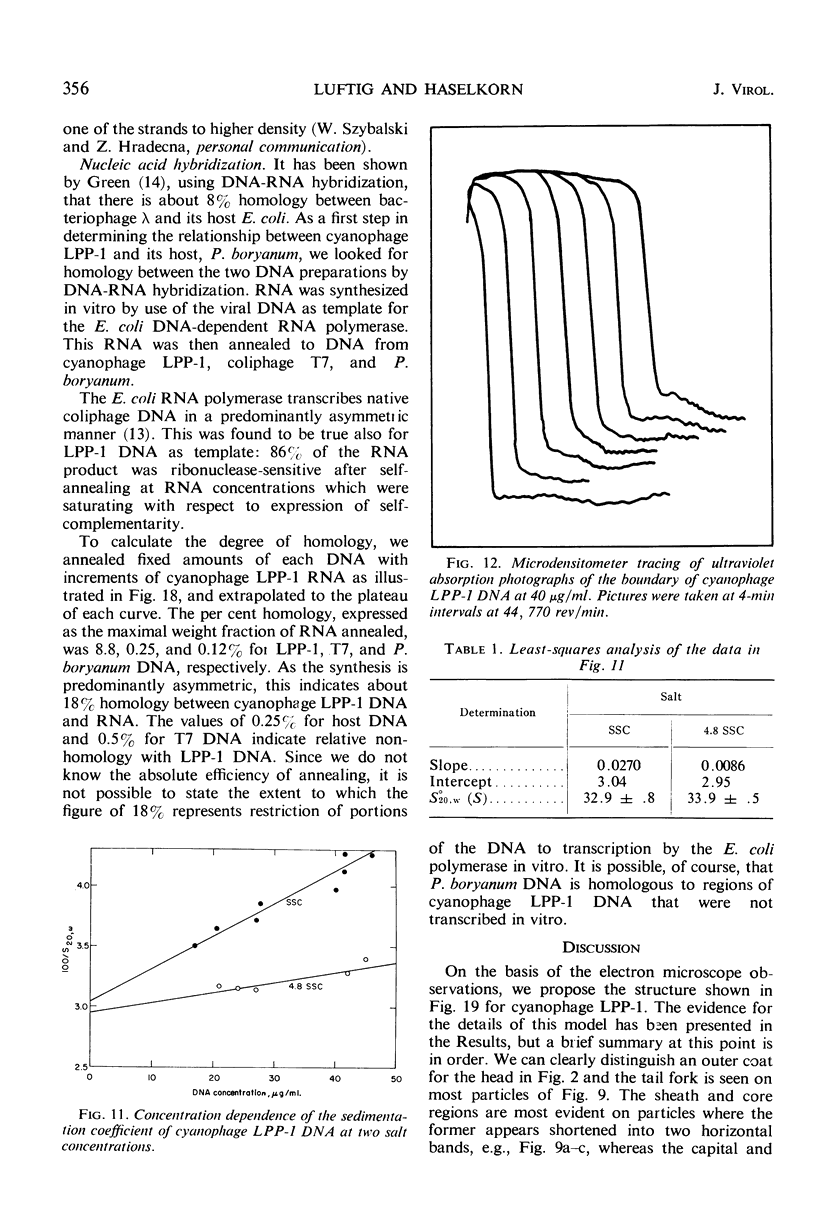
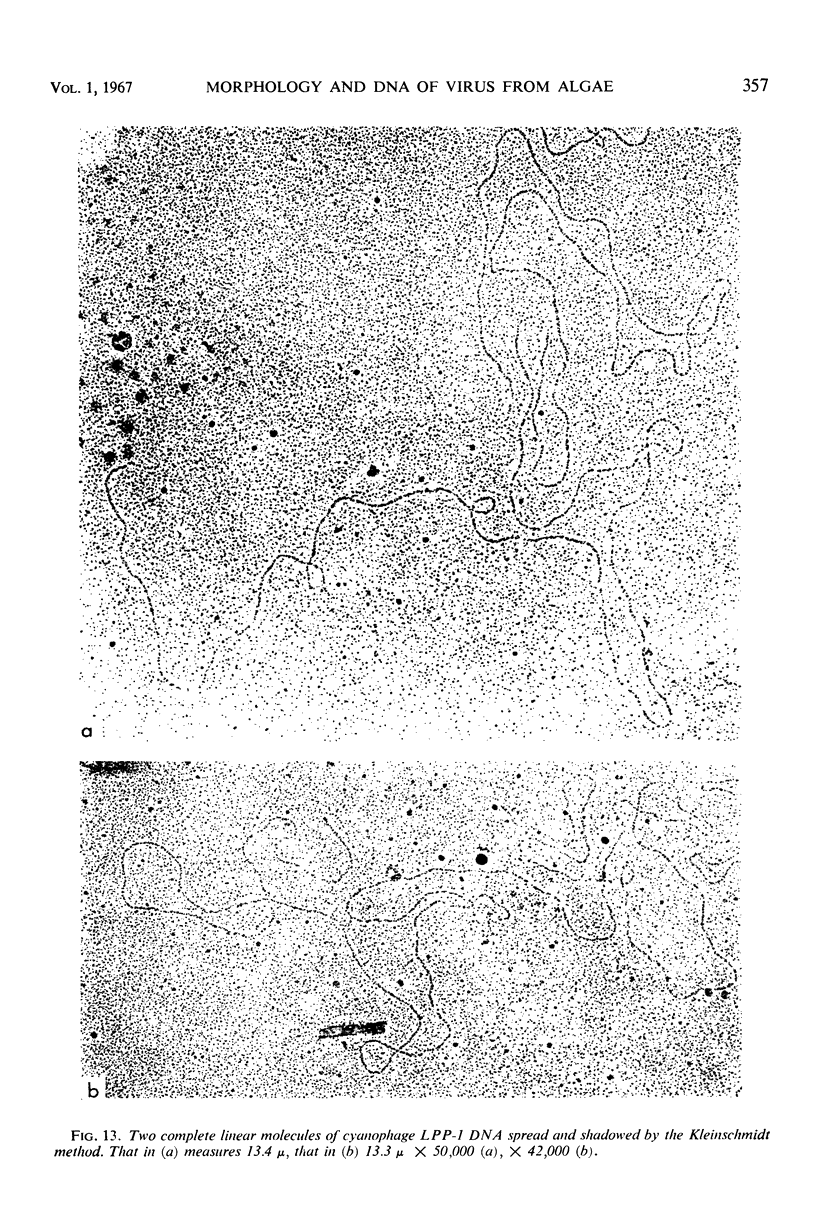
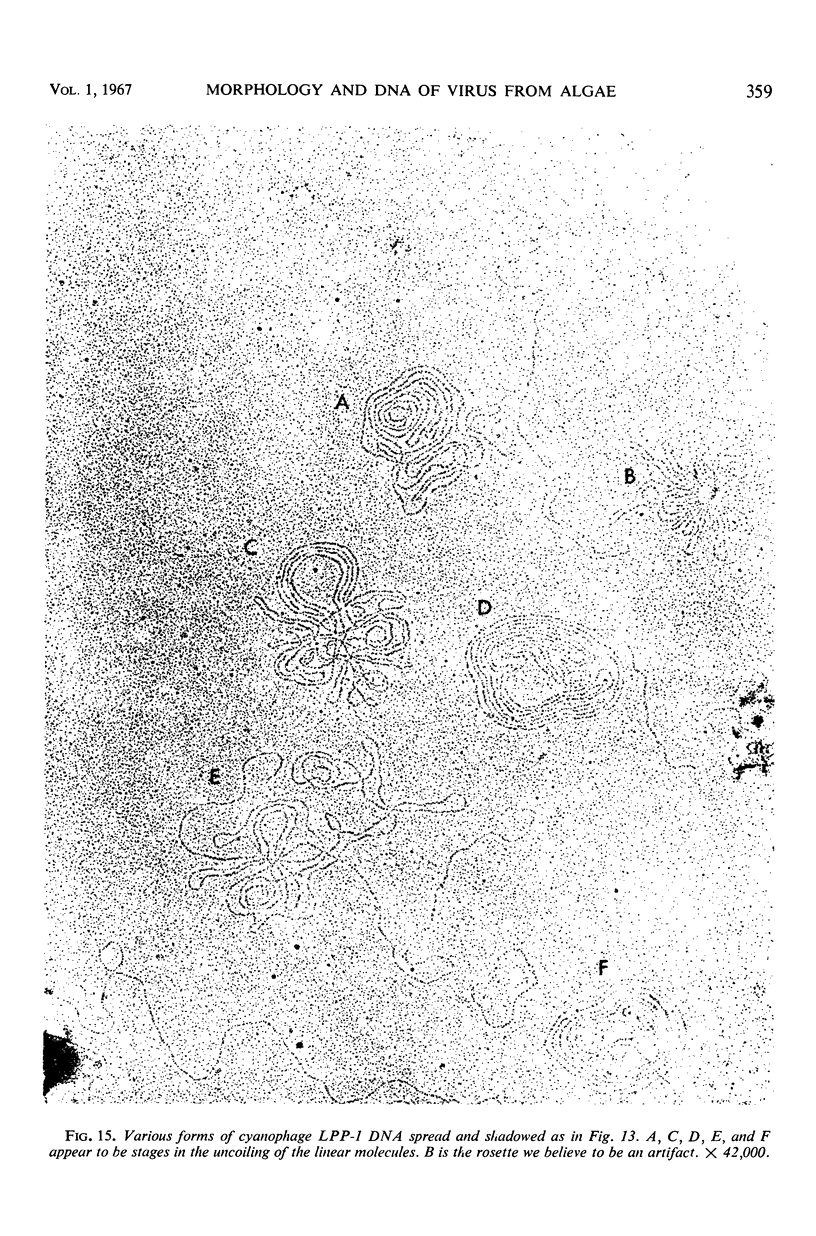
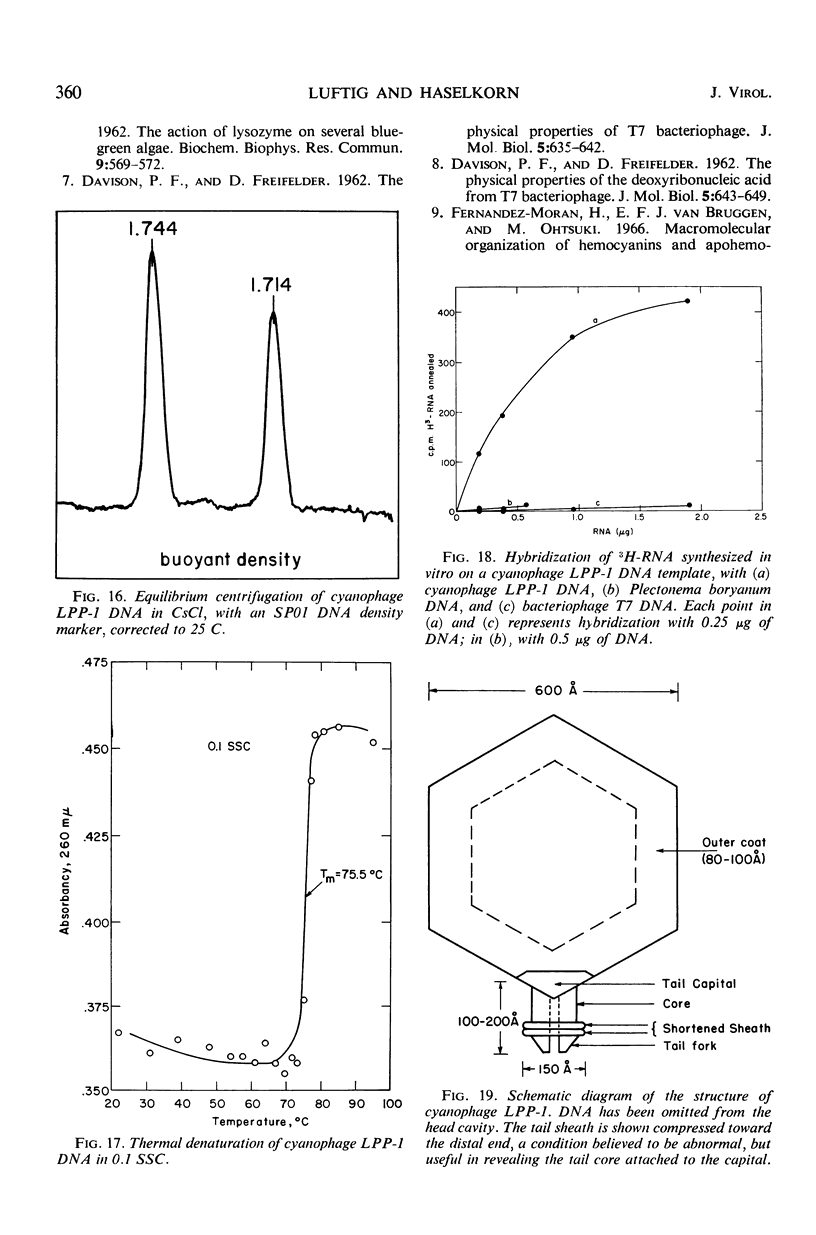
The morphology of Safferman's virus of blue-green algae (phycovirus LPP-1) has been studied by electron microscopy and physicochemical methods. The virion has a short (100 to 200 A long, 150 A in diameter) forked tail, with an outer sheath, an inner core, and a capital attached to one of the vertices of a polyhedral head. The head capsid edge-to-edge distance is 600 A, based upon internal calibration of the magnification in electron micrographs by use of the line-line spacing of catalase crystals. Measurements of absorbancy and infectivity, and electron microscopy across the band of virus after zone centrifugation on a sucrose gradient, indicated that infectivity was correlated with the short-tailed particles described. The viral deoxyribonucleic acid (DNA) is linear, with a contour length of 13.2 ± 0.5 μ, measured by the Kleinschmidt method. Its sedimentation coefficient, S020, w, is 33.4 ± 0.7 S. These values are consistent with a molecular weight of 27 × 106 for the viral DNA. Based upon buoyant density in CsCl and thermal denaturation, the guanine-cytosine content of the DNA is 53%. The viral DNA was used as template for in vitro ribonucleic acid (RNA) synthesis by Escherichia coli RNA polymerase. This RNA annealed to 18% of the sequences in the viral DNA, 0.5% of the sequences in bacteriophage T7 DNA, and 0.25% of the sequences in Plectonema boryanum DNA, at saturating levels of RNA in the Hall-Nygaard hybridization assay. The lack of homology with T7 DNA is of interest because the two viruses are very similar morphologically. The lack of homology with host DNA suggests that this algal virus is a poor candidate for transduction.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. L., Hickman D. D., Reilly B. E. Structure of Bacillus subtilis bacteriophage phi 29 and the length of phi 29 deoxyribonucleic acid. J Bacteriol. 1966 May;91(5):2081–2089. doi: 10.1128/jb.91.5.2081-2089.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns D. S., Edwards M. R. Electron micrographic investigations of C-phycocyanin. Arch Biochem Biophys. 1965 Jun;110(3):511–516. doi: 10.1016/0003-9861(65)90444-3. [DOI] [PubMed] [Google Scholar]
- Bradley D. E. The morphology and physiology of bacteriophages as revealed by the electron microscope. J R Microsc Soc. 1965 Sep;84(3):257–316. [PubMed] [Google Scholar]
- CARO L. G. THE MOLECULAR WEIGHT OF LAMBDA DNA. Virology. 1965 Feb;25:226–236. doi: 10.1016/0042-6822(65)90201-1. [DOI] [PubMed] [Google Scholar]
- DAVISON P. F., FREIFELDER D. The physical properties of the deoxyribonucleic acid from T7 bacteriophage. J Mol Biol. 1962 Dec;5:643–649. doi: 10.1016/s0022-2836(62)80092-8. [DOI] [PubMed] [Google Scholar]
- Freifelder D. A rapid technique for the preparation of purified bacteriophage DNA or RNA from crude lysates. Biochim Biophys Acta. 1965 Oct 11;108(2):318–319. doi: 10.1016/0005-2787(65)90020-1. [DOI] [PubMed] [Google Scholar]
- Freifelder D., Kleinschmidt A. K. Single-strand breaks in duplex DNA of coliphage T7 as demonstrated by electron microscopy. J Mol Biol. 1965 Nov;14(1):271–278. doi: 10.1016/s0022-2836(65)80246-7. [DOI] [PubMed] [Google Scholar]
- GEIDUSCHEK E. P. "Reversible" DNA. Proc Natl Acad Sci U S A. 1961 Jul 15;47:950–955. doi: 10.1073/pnas.47.7.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN M. H. COMPLEMENTARITY BETWEEN LAMBDA (LAMBDA) PHAGE AND ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1963 Dec;50:1177–1184. doi: 10.1073/pnas.50.6.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiduschek E. P. Some aspects of RNA synthesis on a DNA template. Bull Soc Chim Biol (Paris) 1965;47(8):1571–1577. [PubMed] [Google Scholar]
- HEARST J. E. The specific volume of various cationic forms of deoxyribonucleic acid. J Mol Biol. 1962 May;4:415–417. doi: 10.1016/s0022-2836(62)80024-2. [DOI] [PubMed] [Google Scholar]
- HUXLEY H. E., ZUBAY G. Preferential staining of nucleic acid-containing structures for electron microscopy. J Biophys Biochem Cytol. 1961 Nov;11:273–296. doi: 10.1083/jcb.11.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NYGAARD A. P., HALL B. D. A method for the detection of RNA-DNA complexes. Biochem Biophys Res Commun. 1963 Jul 18;12:98–104. doi: 10.1016/0006-291x(63)90242-0. [DOI] [PubMed] [Google Scholar]
- SAFFERMAN R. S., MORRIS M. E. Algal virus: isolation. Science. 1963 May 10;140(3567):679–680. doi: 10.1126/science.140.3567.679. [DOI] [PubMed] [Google Scholar]
- SAFFERMAN R. S., MORRIS M. E. GROWTH CHARACTERISTICS OF THE BLUE-GREEN ALGAL VIRUS LPP-1. J Bacteriol. 1964 Sep;88:771–775. doi: 10.1128/jb.88.3.771-775.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHNEIDER I. R., DIENER T. O., SAFFERMAN R. S. BLUE-GREEN ALGAL VIRUS LPP-1: PURIFICATION AND PARTIAL CHARACTERIZATION. Science. 1964 May 29;144(3622):1127–1130. doi: 10.1126/science.144.3622.1127. [DOI] [PubMed] [Google Scholar]
- STOECKENIUS W. Electron microscopy of DNA molecules "stained" with heavy metal salts. J Biophys Biochem Cytol. 1961 Nov;11:297–310. doi: 10.1083/jcb.11.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Smith K. M., Brown R. M., Jr, Goldstein D. A., Walne P. L. Culture methods for the blue-green alga Plectonema boryanum and its virus, with an electron microscope study of virus-infected cells. Virology. 1966 Apr;28(4):580–591. doi: 10.1016/0042-6822(66)90243-1. [DOI] [PubMed] [Google Scholar]
- Smith K. M., Brown R. M., Jr, Walne P. L., Goldstein D. A. Electron microscopy of the infection process of the blue-green alga virus. Virology. 1966 Oct;30(2):182–192. doi: 10.1016/0042-6822(66)90094-8. [DOI] [PubMed] [Google Scholar]
- VINOGRAD J., HEARST J. E. Equilibrium sedimentation of macromolecules and viruses in a density gradient. Fortschr Chem Org Naturst. 1962;20:373–422. [PubMed] [Google Scholar]
- WILKINS M. H. Molecular configuration of nucleic acids. Science. 1963 May 31;140(3570):941–950. doi: 10.1126/science.140.3570.941. [DOI] [PubMed] [Google Scholar]