Abstract
Xanomeline is thought to be a M1/M4 functionally selective agonist at muscarinic receptors. We have previously demonstrated that it binds in a unique manner at the M1 receptor. In the current study, we examined the ability of xanomeline to bind to the M3 receptor and determined the long-term consequences of this mode of binding in Chinese hamster ovary cells expressing M3 receptors. Xanomeline binds in a reversible and wash-resistant manner at the M3 receptor and elicits a functional response under both conditions. Long-term exposure to xanomeline resulted in changes in the binding profile of [3H]NMS and a decrease in cell-surface receptor density. Additionally, pretreatment with xanomeline was associated with antagonism of the functional response to subsequent stimulation by conventional agonists. Our results indicate that xanomeline binds to and activates the M3 muscarinic receptor in a wash-resistant manner, and that this type of binding results in time-dependent receptor regulation.
Keywords: Muscarinic receptors, Xanomeline, CHO cells, Receptor binding and inositol phosphates
Introduction
There are five subtypes of muscarinic acetylcholine receptors (mAChR), denoted M1–M5, which are part of the G-protein-coupled receptor superfamily [1]. The orthosteric binding site is well conserved across subtypes making it difficult to develop an agonist that specifically activates a particular subtype [2]. However, allosteric binding sites on the receptors are less conserved across subtypes, which may aid in the development of drugs that are subtype selective [3]. The M1, M3 and M5 receptors are functionally coupled to the Gq/11 G-proteins that lead to the activation of phospholipase C [4]. The M1 receptor is involved in learning and memory whereas the M3 receptor is known to be involved in salivation, food intake and gastrointestinal tract motility and secretion [3].
One characteristic of Alzheimer’s disease (AD) is loss of cholinergic neurons in the basal forebrain that project to the hippocampus [5]. There is a decrease in the levels of choline acetyl transferase and presynaptic M2 receptors [5]; however, the postsynaptic M1 receptors seem to remain at normal levels [3]. One current avenue that is being pursued for the treatment of Alzheimer’s disease is the use of cholinergic agonists to replace the deficiency in endogenous acetylcholine. Such agonists would be bene-ficial because they do not rely on presynaptic cholinergic projections to be present [6]. However, it is necessary to find an agonist that selectively activates the M1 receptor to avoid unwanted side effects.
In the literature, xanomeline has been purported to be a potent agonist that is functionally selective for M1 and M4 receptors [6, 7]. This is in spite of its binding with equal potency to all subtypes of muscarinic receptors [6, 7]. Current research has primarily focused on the interaction of xanomeline at the M1 receptor. The binding of xanomeline to this receptor is thought to occur through a mechanism that is distinct from other agonists, with binding occurring both at the primary orthosteric site (reversible) and at a secondary allosteric site (wash-resistant) [8]. Binding of xanomeline in a wash-resistant manner at the M1 receptor occurs rapidly and takes place even when the primary binding site is blocked [9]. In contrast, its wash-resistant activation of the M1 receptor appears to involve the orthosteric receptor domain. It appears that xanomeline also has unique binding properties at the M2 [10] and M5 [11] receptors. However, it is unclear whether the molecular mechanism of xanomeline binding occurs in a similar manner at all the receptor subtypes. Previously, xanome-line was tested in clinical trials for treatment of Alzheimer’s disease. Although there were positive effects on cognitive measures, thought to occur through activation of the M1 receptor [6], the adverse side effects due to activation of other muscarinic receptors eventually resulted in the cessation of further clinical trials [12]. However, recent research has explored the possibility of xanomeline treatment of schizophrenia. Current research has demonstrated that treatment with xanomeline results in a decrease in symptoms of schizophrenia and an improvement in cognitive function [13]. In this case the side effects of xanomeline treatment were more well tolerated [13]. It is currently unknown if these beneficial actions of xanome-line are a result of activation of M1 or M4 receptors or a combination of both.
The current study extended this work to examine the ability of xanomeline to bind to the M3 receptor and to determine the long-term consequences that wash-resistant xanomeline exerts on receptor binding and functional response. Elucidation of the mode of interaction of xanomeline with various muscarinic receptor subtypes and the underlying mechanisms may lead to the development of both muscarinic agonists and antagonists with long duration of action and perhaps better pharmacological selectivity.
Experimental Procedures
Materials
[3H]N-Methylscopolamine (NMS) (81 Ci/mmol) was purchased from DuPont (Wilmington, DE); myo-[3H]inositol (71 Ci/mmol) was purchased from GE Healthcare (Little Chalfont, Buckinghamshire, UK); [14C]inositol-1-phosphate (300 mCi/mmol) was purchased from American Radiolabeled Chemicals (St. Louis, MO); Dulbecco’s modified Eagle’s medium was purchased from Invitrogen (Carlsbad, CA); geneticin was obtained from Calbiochem (San Diego, CA); and bovine calf serum was supplied by Hyclone Laboratories (Logan, UT). Xanomeline tartrate was a generous gift from Eli Lilly & Co. (Indianapolis, IN); all other reagents were purchased from Sigma-Aldrich (St. Louis, MO).
Cell Culture
Chinese hamster ovary (CHO) cells stably transfected with the human M3 muscarinic acetylcholine receptor (hM3) (provided by Dr. M. Brann, University of Vermont Medical School) were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% bovine calf serum and 50 μg/ml geneticin. Cells were grown to confluency in 24-well plates at 37°C in a humidified atmosphere consisting of 5% CO2/95% air.
Pretreatment With Xanomeline
CHO cells expressing the hM3 receptor were incubated with xanomeline in culture media at 37°C. After incubation for 1 min or 1 h, the cells were washed three times with HEPES buffer (110 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 1 mM MgSO4, 25 mM glucose, 20 mM HEPES, 58 mM sucrose; pH 7.4 ± 0.02; 340 ± 5 mOsm) to remove any unbound drug. Cells were used immediately or allowed to incubate for 23–24 h in ligand-free media. Additional cells were incubated with xanomeline in media for 24 h before washing three times with HEPES buffer. All pretreatments and radioligand assays were performed on cells in monolayer unless otherwise noted.
Time Course
These experiments were conducted to determine the time course of development of xanomeline wash-resistant binding. CHO hM3 cells were incubated for various times (<15 s to 30 min) with a single concentration of xanomeline (10 μM) and then washed three times with HEPES buffer. In the binding assay, cells were incubated with the muscarinic receptor ligand [3H]N-methylscopolamine ([3H]NMS) (0.2 nM) for 1 h at 37°C. In all instances, non-specific binding was determined using 10 μM atropine. Incubations were terminated by washing away free radioligand and then cells with bound radioligand were dissolved in 1 M NaOH. Bound radioactivity (disintegrations per minute (dpm)) was quantitated by liquid scintillation spectrometry.
Inhibition of [3H]NMS Binding by Xanomeline
This protocol was used to assess the potency of xanomeline binding to the hM3 receptor. CHO hM3 cells were incubated for 1 h concurrently with a fixed concentration of the muscarinic receptor ligand [3H]NMS (0.2 nM) and increasing concentrations of xanomeline (1 nM–100 μM). In order to assess persistent binding of xanomeline, cells were pretreated for 1 min or 1 h with increasing concentrations of xanomeline (1 pM-100 μM), washed three times with HEPES buffer and then used immediately or after 23–24 h incubation in control media. In order to assess the effects of prolonged xanomeline treatment, cells were pretreated for 24 h with increasing concentrations of xanomeline before washing three times with HEPES buffer. Subsequently, cells were incubated with [3H]NMS (0.2 nM) for 1 h. In all instances, pretreatments and radi-oligand binding were performed in monolayer at 37°C. Non-specific binding was determined using 10 μM atropine. Incubations were terminated as described above. Protein determinations were performed according to the method of Bradford [14]. Bound radioactivity (dpm) was quantitated by liquid scintillation spectrometry.
[3H]NMS Saturation Binding
A saturation binding paradigm was used to determine the total cell-surface receptor density and radioligand–receptor equilibrium dissociation constant. CHO hM3 cells were incubated in monolayer for 1 h at 37°C in the absence or presence of xanomeline (10 μM) followed by three washes with HEPES buffer and used immediately or after 23 h incubation in control media. An additional group of cells was treated with xanomeline for 24 h before washing three times with HEPES buffer. Subsequently, cells were incubated in monolayer with increasing concentrations of [3H]NMS (0.01–6.5 nM) for 1 h at 37°C. Non-specific binding was determined using 10 μM atropine. Reactions were terminated and bound radioactivity was quantitated as described above.
Assay of Phosphoinositide (PI) Hydrolysis
CHO hM3 cells were loaded with myo-[3H]inositol (1 μCi/ ml) at 37°C 24 h prior to being subjected to the following several different treatment protocols with agonists and determination of inositol phosphate production. (1) Labeled untreated cells were incubated in the presence of increasing concentrations of xanomeline, pilocarpine, or carbachol in the presence of 10 mM LiCl for 1 h at 37°C. (2) Cells were pretreated with increasing concentrations of xanomeline (1 nM–100 μM) for 1 min or 1 h, at which time cells were washed three times with HEPES buffer. Cells were then incubated for 1 h at 37°C in the presence of 10 mM LiCl but in the absence of further agonist stimulation. (3) Cells were pretreated for 1 min or 1 h with a single concentration of xanomeline (10 μM), washed three times with HEPES buffer and then used immediately or incubated in control media for 23 or 24 h, respectively. (4) Cells were pretreated with xanomeline (10 μM) for 24 h, washed three times with HEPES buffer and used immediately in the functional assay. For treatments three and four, cells were then incubated with increasing concentrations of carbachol (10 nM–10 mM), pilocarpine (10 nM–1 mM) or xanomeline (1 nM–100 μM) for 1 h in the presence of 10 mM LiCl. (5) The time course of xanomeline-induced persistent activation and antagonism of carbachol-elicited PI hydrolysis was established. CHO hM3 cells were exposed to xanomeline (10 μM) for increasing amounts of time (30 min–24 h) followed by washing and immediate use in the functional assay. Alternatively, cells were treated with xanomeline (10 μM) for 1 h followed by extensive washing and incubation in control media for increasing lengths of time (1–23 h) as indicated in Results. In both cases, cells were incubated for 1 h in a LiCl-containing buffer in the absence of further agonist stimulation or in the presence of a single high concentration of carbachol (10 μM).
In all cases, reactions were stopped with 0.3 M HClO4 and neutralized with 0.15 M K2CO3. The samples were centrifuged (1,500×g; 15 min) and total inositol phosphates in the supernatant were separated by ion exchange chromatography (AG1-X8 resin). In all cases, [14C]inositol-1-phosphate was used as an internal recovery standard. Radioactivity (dpm) was quantitated by liquid scintillation spectrometry.
Data Analysis
All analyses were conducted using Prism 4.0 (GraphPad Software Inc., San Diego, CA). Inhibition curves of [3H]NMS binding were analyzed via non-linear regression to derive estimates of IC50 (midpoint location or potency parameter). Data were refitted according to both one- and two-site mass-action binding models, and the better model was determined by an extra sum-of-squares test. The non-reversible nature of xanomeline binding did not permit transforming IC50 values to inhibition constants because such transformation assumes reversible competitive interaction. Saturation binding curves were analyzed by nonlinear regression to derive individual estimates of Bmax (total cell-surface receptor density) and Kd (radioligand–receptor equilibrium dissociation constant). PI hydrolysis concentration–response curves were analyzed via non-linear regression. Data shown are the means ± standard error of the mean. Comparisons between mean values were performed by one-way analysis of variance (ANOVA). A probability (P) value<0.05 was taken to indicate statistical significance.
Results
Time Course of Xanomeline Wash-Resistant Binding
To determine the time course of the formation of xanom-eline wash-resistant binding at the M3 receptor, cells were exposed to 10 μM xanomeline for various lengths of time (<15 s to 30 min) and extensively washed. The formation of wash-resistant xanomeline binding occurred rapidly at the M3 receptor (Fig. 1). There was an almost instantaneous decrease of 41% in [3H]NMS binding following very brief xanomeline exposure (<15 s). This was succeeded by a slower phase of inhibition of radioligand binding that plateaued after 10 min of xanomeline exposure at a decrease in [3H]NMS binding of 83%.
Fig. 1.
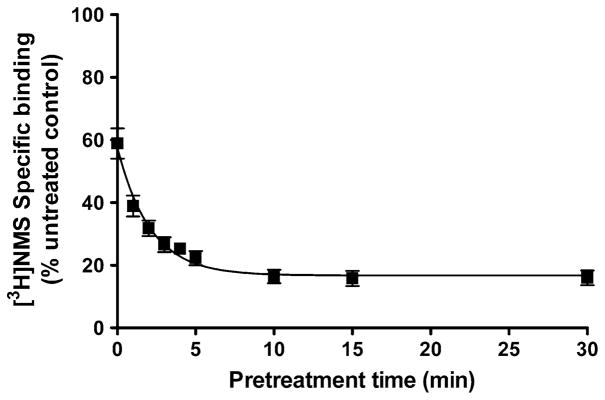
Time course of the development of xanomeline wash-resistant binding in CHO cells stably expressing human M3 muscarinic receptors. Cells were incubated with xanomeline for increasing lengths of time followed by extensive washing and determination of 0.2 nM [3H]NMS specific binding. Values represent the means ± SE of three experiments conducted in triplicate. Binding in the absence of xanomeline pretreatment is denoted by 100%. Individual experiments were normalized to control binding in the absence of xanomeline treatment, which had a mean of 5,800 dpm/well
Effects of Brief and Long-Term Exposure of Cells to Xanomeline on [3H]NMS Binding
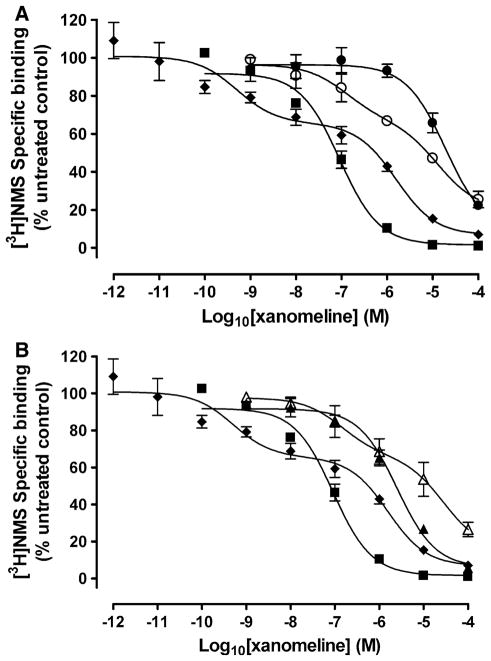
Experiments were conducted to determine the concentration dependence of both reversible and wash-resistant xanomeline binding in its ability to inhibit 0.2 nM [3H]NMS binding to the M3 receptor. In untreated cells, xanomeline caused complete concentration-dependent inhibition of [3H]NMS binding when it was included in the binding assay buffer (Fig. 2a, 2b). Brief pretreatment of cells with xanomeline followed by washing and immediate use in the binding assay resulted in wash-resistant binding of xanomeline with a potency that was 2.4 and 1.5 orders of magnitude lower than that obtained when xanomeline was continually present in the binding assay in case of prein-cubation for 1 min (Fig. 2a) or 1 h (Fig. 2b), respectively. In all cases non-linear regression analysis indicated the data were adequately fit by a one-site binding model.
Fig. 2.
Inhibition of specific [3H]NMS binding following treatment with increasing concentrations of xanomeline in CHO cells expressing the human M3 receptor. The binding of 0.2 nM [3H]NMS was determined in the continuous presence of increasing concentrations of xanomeline (closed squares). Cells were treated with xanomeline for 1 min (closed circles, a) or 1 h (closed triangles, b) followed by washing and immediate application in the binding assay or followed by washing and waiting for 24 h (open circles, A) or 23 h (open triangles, b), respectively. An additional group of cells was treated with xanomeline for 24 h followed by washing and use in binding assay (closed diamonds). Following all pretreatments cells were incubated with 0.2 nM [3H]NMS for 1 h at 37°C. Individual experiments were normalized to control binding in the absence of xanomeline that averaged 21,300 dpm/well. Values represent the means ± SE. of 3–8 experiments conducted in triplicate
To determine the long-term effects of wash-resistant xanomeline on the binding of 0.2 nM [3H]NMS, cells were incubated with increasing concentrations of xanomeline for 1 min or 1 h, washed extensively and then incubated further in control media for 24 or 23 h, respectively. An additional group of cells was treated with xanomeline for 24 h before washing. Incubation with xanomeline for 1 min (Fig. 2a) or 1 h (Fig. 2b) followed by washing and waiting resulted in the appearance of a biphasic binding curve. The wash-resistant inhibition of [3H]NMS binding was incomplete under these conditions (Fig. 2a, b; Table 1). The high-potency site observed following washing and waiting was 3 and 1.2 orders of magnitude higher than the single site obtained prior to waiting in the case of 1 min or 1 h pre-treatments, respectively (Table 1). Interestingly, the low-potency site observed 24 h after 1 min pretreatment was indistinguishable from the single-potency curve obtained immediately following 1 min pretreatment (Table 1).
Table 1.
Acute and delayed effects of xanomeline pretreatments on inhibition of [3H]NMS binding in CHO hM3 cells
| Xanomeline pretreatment | pIC50a | pIC50 highb | pIC50 lowc | Imaxd | ne |
|---|---|---|---|---|---|
| Control (untreated)f | 7.1 ± 0.10 | 99 ± 0.4 | 8 | ||
| 1-min pretreatment | |||||
| No wait | 4.7 ± 0.08# | 93 ± 3.0 | 3 | ||
| 24-h wait | 7.7 ± 0.75† (40 ± 4.3%) | 4.8 ± 0.43 | 82 ± 9.6‡ | 3 | |
| 1-h pretreatment | |||||
| No wait | 5.6 ± 0.07# | 95 ± 0.9 | 3 | ||
| 23-h wait | 6.8 ± 0.39 (32 ± 9.9%) | 4.3 ± 0.48 | 89 ± 5.6 | 3 | |
| 24-h pretreatment | |||||
| No wait | 8.9 ± 0.39*† (38 ± 4.1%) | 5.7 ± 0.05 | 94 ± 0.6 | 6 | |
Cells were treated with increasing concentrations of xanomeline for 1 min or 1 h followed by washing and immediate use in the binding assay or subsequent to incubation for 24/23 h in the absence of free xanomeline. An additional group of cells was treated with xanomeline for 24 h prior to washing. Cells were then incubated with 0.2 nM [3H]NMS at 37°C for 1 h. Parameters (±SEM) were derived from computer-assisted nonlinear regression analysis
Negative logarithm of the IC50 for binding to a single-potency site
Negative logarithm of the IC50 for the high-potency agonist binding site; percent of total binding sites shown in parentheses
Negative logarithm of the IC50 for the low-potency agonist binding site
Maximal percent inhibition of [3H]NMS binding
Number of experiments
Naive cells were incubated simultaneously with xanomeline and radioligand
Unpaired t-test detected a significant difference (P <0.05) in pIC50 between either the 1 min/wash/no wait or 1 h/wash/no wait group compared to control
ANOVA followed by Dunnett’s post-test comparison detected a significant difference (P <0.05) in pIC50 where indicated between 1 min/ wash/24 h wait or 24 h/wash/no wait pretreated groups compared to xanomeline 1 min/wash/no wait treatment
ANOVA followed by Dunnett’s post-test comparison detected a significant difference (P <0.05) in pIC50 where indicated between 1 h/wash/ 23 h wait or 24 h/wash/no wait pretreated groups compared to xanomeline 1 h/wash/no wait treatment
Significantly different (P <0.05) from 100 as determined by ANOVA followed by Dunnett’s post-test comparison
However, when pretreatment times were increased to 1 h before washing, the low-potency site observed following 23 h wait was 1.3 orders of magnitude lower than that seen when cells were used immediately following washing (Table 1). A similar biphasic binding curve was obtained when cells were continuously incubated with xanomeline for 24 h prior to washing, albeit with markedly higher potencies at both sites (Fig. 2a, b; Table 1).
Due to the fact that xanomeline was dissolved in dimethyl sulfoxide (DMSO), control experiments were designed to ensure that DMSO did not contribute to the observed effects of xanomeline. Cells were treated with DMSO concentrations corresponding to those in contact with cells during the various treatment modalities with xanomeline. DMSO did not result in a decrease in radio-ligand binding at the highest concentrations used to prepare xanomeline dilutions (1%; data not shown).
It has previously been reported that long-term pretreatment with xanomeline at the M1 receptor results in decreased protein content [15]. Therefore, additional experiments were conducted using the method of Bradford [14] to determine if there were changes in protein levels following the various xanomeline pretreatment protocols at the M3 receptor. There was no change in protein content compared to control for any of the aforementioned treatment groups (data not shown).
Effects of Xanomeline Treatments on [3H]NMS Saturation Binding
Increasing concentrations of [3H]NMS were used to determine the effects of 10 μM xanomeline pretreatment on cell-surface receptor density and radioligand affinity in CHO cells expressing the M3 receptor. As shown in Fig. 3, treatment with xanomeline for 1 h followed by washing and immediate use led to a significant (P <0.05) reduction in radioligand affinity, but no change in maximal binding compared to untreated cells. In contrast, pretreatment with xanomeline for 1 h followed by washing and waiting for 23 h resulted in a significant (P <0.05) decrease in cell-surface receptor number without a change in radioligand affinity (Fig. 3; Table 2). Interestingly, cells that were incubated with xanomeline for 24 h prior to washing displayed a significant (P <0.05) decrease in both the radioligand affinity and the number of cell-surface receptors (Fig. 3; Table 2).
Fig. 3.
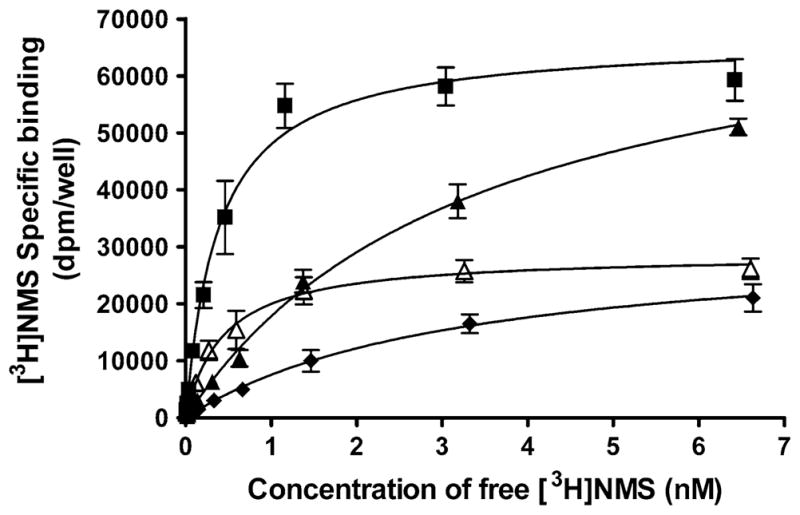
Effects of xanomeline pretreatment on [3H]NMS saturation binding in CHO cells expressing human M3 receptors. Cells were pretreated with 10 μM xanomeline for 1 h followed by washing and immediate application in the binding assay (closed triangles) or after 23 h incubation in the absence of free xanomeline (open triangles). An additional group of cells was treated with xanomeline for 24 h followed by washing and addition to the binding assay mixture (closed diamonds). Untreated control cells (closed squares) and xanomeline-pretreated and washed cells were subsequently incubated with increasing concentrations of [3H]NMS (0.01–6.5 nM) for 1 h at 37°C. Non-specific binding was determined in the presence of 10 μM atropine. Values represent the means ± SE of four experiments conducted in triplicate
Table 2.
Effects of xanomeline pretreatment and waiting periods on [3H]NMS saturation binding in CHO hM3 cells
| Xanomeline pretreatment group | Kda (nM) | Bmaxb | nc |
|---|---|---|---|
| Control (untreated) | 0.42 ± 0.06 | 68,000 ± 3,200 | 4 |
| 1-h/washout/no wait | 3.52 ± 0.52* | 79,000 ± 1,600 | 4 |
| 1-h/washout/23 h wait | 0.59 ± 0.21 | 30,000 ± 2,000* | 4 |
| 24-h/washout/no wait | 4.19 ± 0.93* | 37,000 ± 4,600* | 4 |
Cells were treated with 10 μM xanomeline for 1 h followed by washing and immediate use in the binding assay or subsequent to incubation for 23 h in the absence of free xanomeline. An additional group of cells was treated with 10 μM xanomeline for 24 h prior to washing. Cells were then incubated with increasing concentrations of [3H]NMS (0.01–6.5 nM) at 37°C for 1 h. Parameters (±SEM) were derived from computer-assisted non-linear regression analysis
Equilibrium dissociation constant for [3H]NMS binding
Cell-surface receptor density (dpm/well)
Number of experiments
ANOVA followed by Dunnetts post-test comparison detected a significant difference (P <0.05) in Kd or Bmax between the pretreated groups compared with control
Effects of Xanomeline Pretreatment on M3 Receptor-Mediated Stimulation of Inositol Phosphate Production
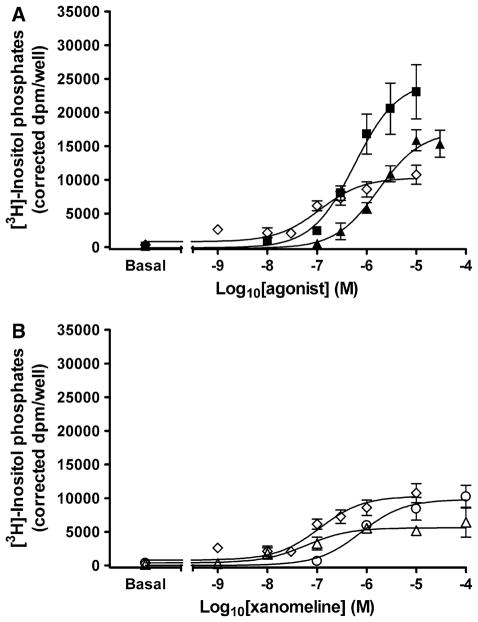
Initial experiments were designed to compare the functional response elicited by xanomeline to that of the M3 full agonist carbachol and the partial agonist pilocarpine [16] in untreated cells. The maximal response stimulated by xanomeline was slightly lower than pilocarpine and approximately half that of carbachol, demonstrating that xanomeline displays properties of a partial agonist at the M3 receptor under our specific experimental conditions (Fig. 4a). However, xanomeline exhibited a significantly (P <0.05) higher potency than either carbachol or pilocarpine (Table 3).
Fig. 4.
M3 receptor activation by xanomeline reversible and wash-resistant binding. a Cells were incubated at 37°C for 1 h with increasing concentrations of carbachol (closed squares), pilocarpine (closed triangles) or xanomeline (open diamonds) in the presence of 10 mM lithium chloride and inositol phosphate production was determined; b cells were pretreated with increasing concentrations of xanomeline for 1 min (open circles) or 1 h (open diamonds) followed by washing and determination of the accumulation of inositol phosphates in the absence of free xanomeline. In the third group (diamonds) xanomeline was only present during determination of inositol phosphates accumulation. Values represent the means ± SE. of 3–5 experiments conducted in triplicate
Table 3.
Effects of agonist treatment on inositol phosphate production in CHO hM3 cells
| PEC50a | Emaxb | nc | |
|---|---|---|---|
| Agonist stimulation | |||
| Carbachol | 6.3 ± 0.04 | 23,300 ± 3,900 | 4 |
| Pilocarpine | 5.8 ± 0.08 | 16,400 ± 2,100 | 4 |
| Xanomeline | 6.9 ± 0.07† | 11,100 ± 1,300* | 5 |
| Xanomeline pretreatments | |||
| 1-min/washout/no wait | 6.1 ± 0.10 | 9,800 ± 2,000 | 3 |
| 1-h/washout/no wait | 7.1 ± 0.52 | 6,200 ± 1,300 | 4 |
Cells were incubated at 37°C for 1 h with increasing concentrations of carbachol, pilocarpine or xanomeline and the levels of inositol phosphate production were determined in the presence of 10 mM LiCl. Additional groups of cells were treated with xanomeline for 1 min or 1 h followed by washing and use in the functional assay in the absence of further agonists stimulation. Parameters (±SEM) were derived from computer-assisted non-linear regression analysis
Negative logarithm of the midpoint (potency) parameter
Maximal response. Values are expressed as dpm/well after correcting for standard recovery
Number of experiments
ANOVA followed by Tukey’s post-test comparison detected a significant difference (P <0.05) in pEC50 between xanomeline compared with carbachol or pilocarpine
ANOVA followed by Dunnetts post-test comparison detected a significant difference (P <0.05) in Emax between xanomeline compared with carbachol
No statistical differences in pEC50 or Emax were found between the xanomeline-pretreated and washed groups and xanomeline continuous presence during the receptor function assay
Wash-resistant xanomeline binding resulted in persistent activation of the M3 receptor (Fig. 4b). Exposure of cells to xanomeline for 1 min followed by washing led to a concentration-dependent increase in the hydrolysis of inositol phosphates, with a maximal response similar to that observed when it was continually present with untreated cells, but with a lower potency (Table 3). Interestingly, increasing the preincubation time with xanomeline to 1 h prior to washing reduced the maximal response without a change in potency (Table 3). In order to demonstrate that the observed effects of xanomeline are not due to depletion of the pool of labeled polyphosphoinositides, cells were stimulated with exogenous phospholipase C following the various pretreatments with xanomeline. No differences in the production of inositol phosphates were observed in any of the experimental groups (data not shown).
Effects of Acute Xanomeline Exposure on the Ability of Various Muscarinic Agonists to Stimulate the Production of Inositol Phosphates
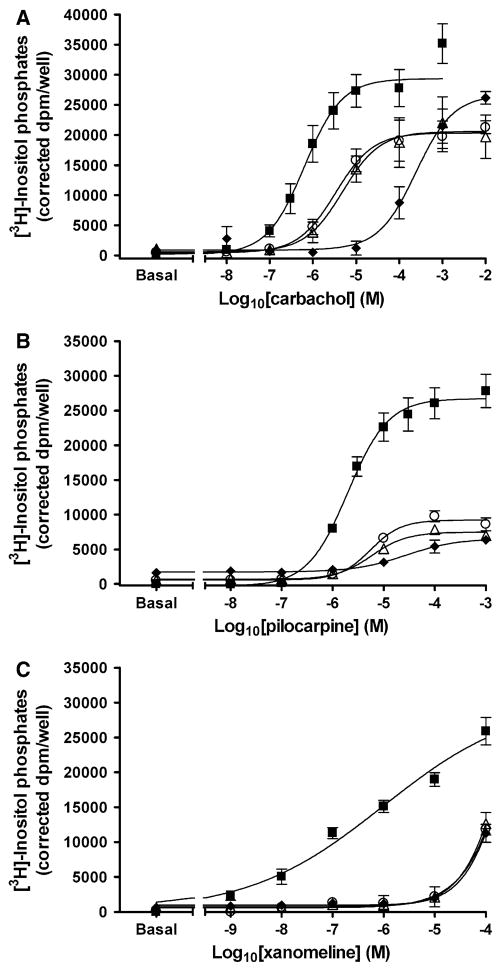
Brief pretreatment with 10 μM xanomeline followed by washing resulted in significant decreases in carbachol potency (P <0.05), with a more marked effect in the case of 1 h pretreatment (Fig. 5a; Table 4). No changes were observed in the maximal response elicited by carbachol following 1 min or 1 h pretreatment with xanomeline and washing (Fig. 5a). The concurrent presence of xanomeline and carbachol during the functional assay resulted in a larger reduction in carbachol potency compared to that obtained following either pretreatment condition (Fig. 5a; Table 4).
Fig. 5.
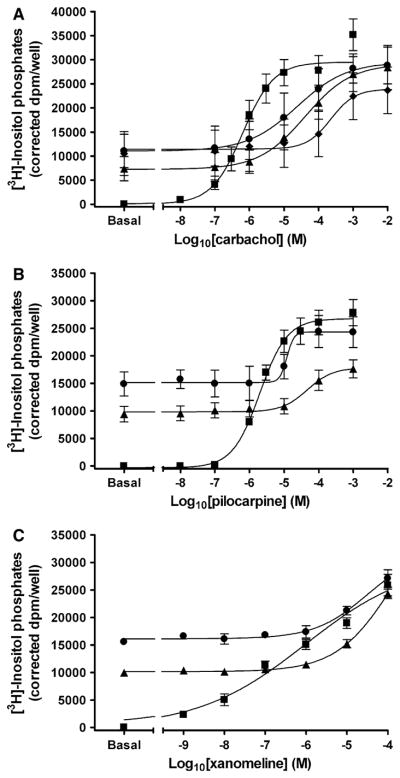
Effects of acute xanomeline exposure on the ability of muscarinic agonists to stimulate the production of inositol phosphates. Cells were pretreated with 10 μM xanomeline for 1 min (closed circles) or 1 h (closed triangles) followed by washing and immediate use. Subsequently, cells were treated with increasing concentrations of a carbachol; b pilocarpine; or c xanomeline and the accumulation of inositol phosphates was determined over a 1 h incubation period at 37°C in the presence of 10 mM lithium chloride for untreated cells (closed squares) and those pretreated with xanomeline. a An additional group of cells was simulated simultaneously with increasing concentrations of carbachol and 10 μM xanomeline (closed diamonds). Values represent the means ± SE. of 3–7 experiments conducted in triplicate
Table 4.
Effects of carbachol, pilocarpine or xanomeline stimulation following short- and long-term 10 μM xanomeline pretreatment and washout on inositol phosphates production in CHO hM3 cells
| Xanomeline pretreatment group | Carbachol stimulation
|
Pilocarpine stimulation
|
Xanomeline stimulation
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| PEC50a | Emaxb | nc | pEC50 | Emax | n | pEC50 | Emax | n | |
| Control (untreated) | 6.2 ± 0.09 | 28,300 ± 3,100 | 7 | 5.7 ± 0.004 | 26,800 ± 2,300 | 3 | 6.2 ± 0.14 | 28,100 ± 3,000 5 | 5 |
| 1-min/washout/no wait | 4.7 ± 0.19* | 30,700 ± 4,100 | 4 | 4.9 ± 0.05 | 24,500 ± 2,800 | 3 | 4.5 ± 0.10 | 32,200 ± 2,800 3 | 3 |
| 1-h/washout/no wait | 4.2 ± 0.06* | 28,900 ± 5,100 | 3 | 4.3 ± 0.05 | 17,900 ± 1,500 | 3 | 4.4 ± 0.15 | 32,200 ± 2,800 3 | 3 |
| Xanomeline and carbachol—co- addition | 3.7 ± 0.11* | 24, ± 4,800 | 3 | ||||||
| 1 min/washout/wait 24 h | 5.5 ± 0.14* | 20,± 2,000 | 3 | 5.3 ± 0.06 | 9,200 ± 880 | 3 | ND | ND | 3 |
| 1 h/washout/wait 23 h | 5.1 ± 0.10* | 25,600 ± 2,700 | 3 | 5.2 ± 0.05 | 7,000 ± 600 | 3 | ND | ND | 3 |
| 24 h/washout/no wait | 3.6 ± 0.20* | 26,800 ± 1,000 | 3 | 4.0 ± 0.04 | 6,600 ± 500 | 3 | ND | ND | 3 |
Cells were pretreated with 10 μM xanomeline for 1 min or 1 h followed by washing and immediate use in the functional assay or subsequent to incubation for 24 or 23 h, respectively, in the absence of free xanomeline. An additional group of cells was treated with 10 μM xanomeline for 24 h prior to washing. In the functional assay, cells were stimulated with increasing concentrations of carbachol, pilocarpine or xanomeline for 1 h at 37°C in the presence of 10 mM lithium chloride. Parameters (±SEM) were derived from computer-assisted non-linear regression analysis
Negative logarithm of the midpoint (potency) parameter
Maximal response. Values are expressed as dpm/well after correcting for standard recovery
Number of experiments
ND—parameter value was not determined
ANOVA followed by Dunnetts post-test comparison detected a significant difference (P <0.05) in pEC50 between xanomeline-pretreated groups with carbachol stimulation compared to carbachol stimulation in naïve cells
ANOVA followed by Dunnetts post-test comparison detected a significant difference (P <0.05) in pEC50 and Emax between xanomeline-pretreated groups with pilocarpine stimulation compared to pilocarpine stimulation in naïve cells
ANOVA followed by Dunnetts post-test comparison detected a significant difference (P <0.05) in pEC50 between xanomeline-pretreated groups with xanomeline stimulation compared to xanomeline stimulation in naïve cells
Further experiments were conducted to determine the effects of brief xanomeline treatment and washing on the response to partial agonists. Pretreatment with 10 μM xanomeline for 1 min or 1 h followed by washing led to a significant (P <0.05) decrease in pilocarpine potency (by ~1 order of magnitude) (Fig. 5b). This was accompanied by a significant (P <0.05) decrease in pilocarpine maximal response in the 1 h pretreatment group, but not the 1 min group (Fig. 5b; Table 4). Xanomeline wash-resistant binding also resulted in antagonism of the response to a second addition of xanomeline. This was evidenced by a decrease in xanomeline potency without an alteration in efficacy (Fig. 5c; Table 4).
Effects of Long-Term Xanomeline Exposure on the Ability of Various Muscarinic Agonists to Stimulate Inositol Phosphate Production
The full agonist carbachol was utilized to determine the long-term effects of xanomeline pretreatment on receptor response. Pretreatment with 10 μM xanomeline for 1 min or 1 h followed by washing and waiting for a day resulted in nearly identical concentration–response curves, displaying significant (P <0.05) decreases in the potency of carbachol without significant changes in maximal response (Fig. 6a; Table 4). A more marked decrease in carbachol potency (2.4 orders of magnitude) was observed when cells were pretreated with 10 μM xanomeline for 24 h before washing (Fig. 6a; Table 4). Again, there was no change in carbachol maximal response.
Fig. 6.
Effects of prolonged xanomeline exposure on the ability of muscarinic agonists to stimulate the production of inositol phosphates. Cells were pretreated with 10 μM xanomeline for 1 min (open circles) or 1 h (open triangles) followed by washing and waiting 24 or 23 h, respectively. An additional group of cells was treated with xanomeline for 24 h (closed diamonds) prior to washing. Subsequently, cells were treated with increasing concentrations of a carbachol; b pilocarpine; or c xanomeline and the accumulation of inositol phosphates was determined over a 1 h incubation period at 37°C in the presence of 10 mM lithium chloride for untreated cells (closed squares) or those pretreated with xanomeline. Values represent the means ± SE. of 3–7 experiments conducted in triplicate
Lack of demonstrable effects of xanomeline pretreatment on the maximal response elicited by the full agonist carbachol, in spite of the observed large decrease in the number of available cell-surface receptors (Fig. 3), might be due to the involvement of spare receptors. We therefore tested the effects of xanomeline on the receptor response to partial agonists, as such ligands require full receptor occupancy to elicit maximal receptor activation. Cells were pretreated with 10 μM xanomeline for 24 h prior to washing, or pre-incubated with 10 μM xanomeline for either 1 min or 1 h followed by washing and prolonged waiting in the absence of free xanomeline. In all cases, comparable significant (P <0.05) decreases in the maximal response to the partial agonist pilocarpine were observed (Fig. 6b). These effects were accompanied by a significant (P <0.05) reduction in pilocarpine potency, with a more pronounced effect in case of the 24 h pretreatment group (Fig. 6b; Table 4). Noteworthy, pretreatment with xanomeline resulted in more marked attenuation of its own stimulatory effects as compared to those of carbachol and pilocarpine (Fig. 6c). However, xanomeline maximal receptor activation and potency under these conditions could not be quantified since a full concentration–response curve could not be attained, due to the concentration limitations presented by using DMSO as a solvent.
Time Course of the Switch of Xanomeline Pharmacological Profile From an Agonist to an Antagonist
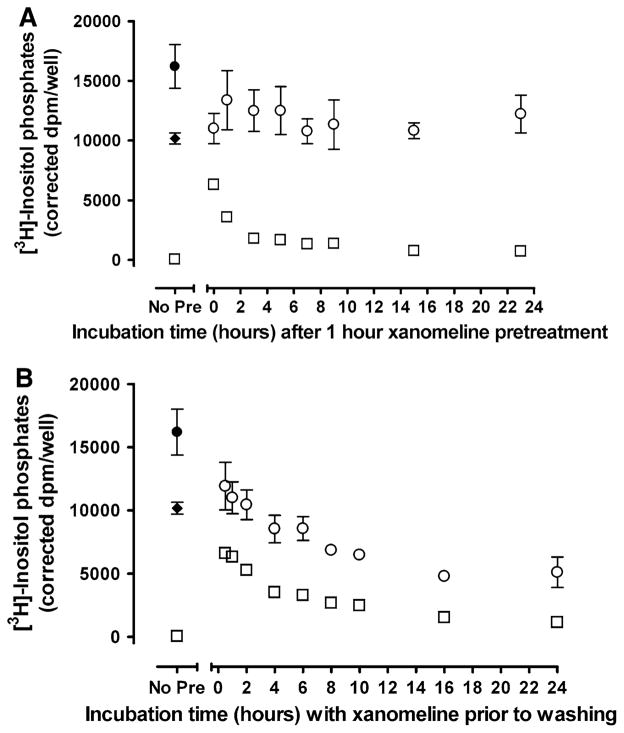
We have shown that the presence of acute wash-resistant xanomeline binding results in an increase in basal levels of inositol phosphate production (Fig. 5). However, inositol phosphate levels were no longer elevated following prolonged waiting in control media (Fig. 6). Similar results were obtained when cells were incubated with xanomeline for 24 h prior to washing. To determine the time course of the reversal of receptor stimulation by wash-resistant xanomeline, cells were treated with 10 μM xanomeline for 1 h followed by washing and waiting for different durations. The initial receptor activation following pretreatment for 1 h and washing decreased rapidly and subsided upon further incubation in control media, approaching control levels after 3 h (Fig. 7a). As can be seen in Fig. 7b, a similar response profile was observed when xanomeline was present for increasing durations of time prior to washing (30 min–24 h), although the time-dependent reversal of persistent receptor activation occurred at a slower rate.
Fig. 7.
Time course of switching of the pharmacological profile of xanomeline from an agonist to an antagonist. a Cells were pretreated with 10 μM xanomeline for 1 h followed by washing and waiting for increasing lengths of time (0–23 h); b Cells were pretreated with 10 μM xanomeline for increasing amounts of time (30 min–24 h) prior to washing. Subsequently, the accumulation of inositol phosphates was determined over a 1 h incubation period in the presence of 10 mM lithium chloride following no further stimulation (open squares) or stimulation with 10 μM carbachol (open circles). For comparison, untreated cells were stimulated with carbachol (closed circles) or xanomeline (closed diamonds). No pre: untreated cells that were not exposed to xanomeline. Values represent the means of two experiments conducted in triplicate
Xanomeline has been shown to antagonize the functional response to carbachol following washing and prolonged incubation in control media (Fig. 6). Further experiments were conducted to determine the time-dependence of development of xanomeline antagonistic activity. A reduction in the ability of carbachol to elicit a response was observed immediately following xanomeline treatment for 1 h. This effect was maintained at a steady state upon further incubation of pretreated and washed cells in control media for various time points up to 23 h (Fig. 7a). In contrast, a time-dependent reduction in the ability of carbachol to stimulate a response was observed when cells were treated with xanomeline for 30 min–24 h followed by washing (Fig. 7b).
Discussion
We have recently shown that brief interaction of xanome-line with the M1 muscarinic receptor results in wash-resistant binding and receptor activation that are associated with delayed consequences on receptor binding and activity. In clinical trials of Alzheimer’s disease treatment with xanomeline, patients experienced side effects such as excessive salivation and gastrointestinal tract problems. These effects are most likely due to activation of the M3 receptor [6, 17]. In the current study, we were interested in investigating the short- and long-term effects of xanome-line interaction with the M3 receptor on radioligand binding and receptor function.
Our results indicate rapid biphasic kinetics of development of wash-resistant xanomeline binding at the M3 receptor. A rapid phase of binding takes place within the first minute of exposure, followed by a slower phase that reaches equilibrium after ~10 min (Fig. 1). These kinetics are similar to those observed at the M1 receptor [9], but are in contrast to the slower formation of wash-resistant xanomeline binding at the M2 receptor [10].
Previous literature suggests that xanomeline binds equally well to all five muscarinic receptor subtypes [7]. In our current study, xanomeline was shown to bind with high-potency at the M3 receptor, similar to that obtained previously at the M1 [18] and M5 receptor [11] using similar expression systems. We have also shown that xanomeline binds to the M3 receptor in a wash-resistant manner similar to other muscarinic receptor subtypes [10, 18]. When cells expressing M3 muscarinic receptors were exposed to xanomeline for 1 min or 1 h followed by washing and immediate use in the binding assay, it resulted in concentration-dependent wash-resistant binding (Fig. 2). Interestingly, overnight incubation of briefly pretreated and washed cells in ligand-free media resulted in the development of a biphasic binding curve. Similar results were observed under the same conditions at the M1 receptor [15]. It is well-documented that prolonged incubation with an agonist leads to internalization/down-regulation of the receptor [19, 20]. Decreased radioligand binding associated with these phenomena might underlie the appearance of the new high-potency xanomeline-induced decrease in [3H]NMS binding. It is noteworthy that prolonged waiting following brief exposure of M3 receptors to xanomeline results in incomplete inhibition of [3H]NMS binding (Fig. 2), a hallmark of allosteric receptor modulation [21]. Thus, changes in the cooperative interactions between the receptor orthosteric and allosteric sites might also play a role in the observed delayed effects of xanomeline. However, incomplete inhibition of [3H]NMS binding may suggest that a portion of the receptor population is not susceptible to internalization/down-regulation [22]. Additionally, it is also possible that incomplete inhibition of [3H]NMS binding is a result of the inability to test higher concentrations of xanomeline due to solubility limitations. The complete inhibition of [3H]NMS binding following pretreatment with xanomeline for 24 h prior to washing suggests a predominant role of receptor internalization/down-regulation under these conditions.
Saturation binding experiments were performed to determine the effects of short- and long-term xanome-line binding on the number of cell-surface receptors and radioligand binding affinity (Fig. 3). Brief xanomeline pretreatment followed by washing did not affect maximal [3H]NMS binding. However, there was a significant decrease in its binding affinity. This may be due to allosteric modulation of the receptor orthosteric domain by xanomeline binding at its allosteric site [9, 23–25]. It is also possible that the reduction in radioligand affinity could be a result of incomplete washout of xanomeline. However, this is unlikely because exposure of untreated cells to the supernatant from the third or seventh washes did not affect on radioligand binding (data not shown). When cells were subjected to brief xanomeline pretreatment followed by a long waiting period, the effects of xanomeline were confined to a decrease in the number of receptors without a change in their affinity for the radioligand, indicative of internalization/down-regulation of the receptor [26, 27]. In contrast, incubation with xanomeline for 24 h prior to washing resulted in a reduction of both the number of receptors and the affinity of the radioligand. The change in radioligand affinity may suggest modulation of receptor conformation by xanomeline binding at the allosteric site in addition to receptor down-regulation.
The functional consequences of xanomeline treatment were studied by determining the accumulation of inositol phosphates (Fig. 4). Xanomeline was found to be a potent partial agonist at the M3 muscarinic receptor. This is in agreement with previous studies [6]. However, Wood et al. [28] showed that xanomeline acted as a full agonist at M3 receptors in their expression system. This discrepancy may be due to different receptor expression levels or variation in the functional assays employed to assess receptor sensitivity. The wash-resistant component of xanomeline binding established after exposure to the drug for 1 min was able to stimulate a concentration-dependent functional response at the M3 receptor. The maximal response was similar to that observed when xanomeline was present during the functional assay, albeit with a lower potency under the former protocol. These findings are similar to what was observed at the M1 receptor [8, 15]. However, pretreatment of cells with xanomeline for 1 h followed by washing resulted in a slightly lower efficacy compared to xanomeline pretreatment for 1 min. This time-dependent reduction in receptor sensitivity might be due to xanome-line-induced desensitization of the receptor. It has been demonstrated that an agonist can cause desensitization without changing receptor expression [29].
This notion was tested further by examining the effects of xanomeline treatment on the ability of other agonists to stimulate the production of inositol phosphates (Fig. 5). When xanomeline was present for 1 min or 1 h followed by washing and immediate use it decreased the potency of carbachol without reduction of its maximal response. These results are in agreement with saturation binding experiments in which there was marked reduction in the affinity of the radioligand for the receptor in the absence of change in receptor density. However, pretreatment with xanome-line for 1 h resulted in wash-resistant attenuation of not only the potency of the partial agonist pilocarpine, but also its maximal response. This might be due to the higher susceptibility of partial agonists to changes in receptor sensitivity [30]. Alternatively, these results could be explained by differential allosteric modulation by xanomeline of the interaction of different agonists with the receptor. Previous research has shown that compounds that are thought to act in allosteric manner result in a change in the response of cells to additional agonists [24, 25]. When cells were treated with xanomeline for 1 min or 1 h followed by washing and prolonged waiting there was also a decrease in the potency of both carbachol and pilocarpine, albeit the shift in potency was less pronounced than that observed following acute xanomeline pretreatments (Fig. 6). This corresponds well to the observed reversal of the effects of xanomeline on [3H]NMS affinity following prolonged incubation of pretreated and washed cells in control media. In contrast, acute wash-resistant xanomeline binding at the M3 receptor resulted in additional delayed reduction in the maximal functional response elicited by carbachol and pilocarpine that is likely due to reduction in the number of cell-surface receptors detected in radioligand binding experiments. Pretreatment with xanomeline for 24 h prior to washing resulted in a more pronounced effect on the potency of carbachol and pilocarpine compared to acute xanomeline pretreatment followed by washing and waiting. This might be due to the larger decrease in receptor affinity detected in radioligand binding studies under the latter treatment conditions.
Increases in activation of phosphoinositide hydrolysis were seen in cells briefly treated with xanomeline and then washed. However, when pretreated and washed cells were incubated for a long period in the absence of free xanomeline, this wash-resistant receptor activation was reversed to control unstimulated levels. The same phenomenon was observed in cells continuously treated with xanomeline for prolonged durations. It is possible that receptor uncoupling from the G-protein, internalization or down-regulation could account for these changes [27, 31]. We further explored the time-dependence of the development of this biphasic pharmacological profile. Elevated levels of inositol phosphates that occur during 1 h xanomeline treatment followed by washing rapidly decline within the first 3 h of incubation in control media. A similar decline in wash-resistant activation was observed when cells were treated with xanomeline for 30 min–24 h followed by washing just prior to performing the receptor functional assay.
Reversal of xanomeline wash-resistant activation of the receptor is accompanied by attenuation of receptor activation by carbachol. Noteworthy, while there was complete reversal of wash-resistant receptor activation by xanomeline following waiting after brief exposure, its antagonistic effects on carbachol reached a maximum of only ~25%. In contrast, the ability of carbachol to elicit a response following exposure of cells to xanomeline for 30 min–24 h followed by washing decreases as the time of xanomeline exposure increases, with a maximal decrease of approximately 67%. The higher magnitude of the antagonistic effects of the latter treatment protocol on the response to carbachol might be due to its demonstrated combined effect on receptor number and affinity.
In summary, these data demonstrate that wash-resistant xanomeline binding occurs rapidly at the M3 receptor and is able to stimulate a functional inositol phosphate response. Incubation with xanomeline for brief periods of time followed by prolonged waiting leads to marked delayed changes in cell-surface receptor density that are accompanied by development of antagonism of the functional response to agonists. Mechanistically, the long-term effects of xanomeline treatments may be due to internalization or down-regulation of the receptor as a result of wash-resistant receptor activation and/or allosteric modification of the receptor conformation. Further studies will be undertaken to elucidate the mechanisms of xanomeline-induced regulation of the M3 receptor.
Acknowledgments
This work was supported by National Institutes of Health Grant NS25743. The project described was also supported by Grant Number T32DE007288 from the National Institute of Dental & Craniofacial Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Dental & Craniofacial Research or the National Institutes of Health.
References
- 1.Caulfield MP, Birdsall NJ. International union of pharmacology Xvii. Classification of muscarinic acetylcholine receptors. Pharmacol Rev. 1998;50:279–290. [PubMed] [Google Scholar]
- 2.Wess J. Muscarinic acetylcholine receptor knockout mice: novel phenotypes and clinical implications. Annu Rev Pharmacol Toxicol. 2004;44:423–450. doi: 10.1146/annurev.pharmtox.44.101802.121622. 10.1146/annurev.pharmtox.44.101802. 121622. [DOI] [PubMed] [Google Scholar]
- 3.Eglen RM. Muscarinic receptor subtype pharmacology and physiology. Prog Med Chem. 2005;43:105–136. doi: 10.1016/S0079-6468(05)43004-0. [DOI] [PubMed] [Google Scholar]
- 4.Volpicelli LA, Levey AI. Muscarinic acetylcholine receptor subtypes in cerebral cortex and hippocampus. Prog Brain Res. 2004;145:59–66. doi: 10.1016/S0079-6123(03)45003-6. [DOI] [PubMed] [Google Scholar]
- 5.Francis PT, Palmer AM, Snape M, et al. The cholinergic hypothesis of alzheimer’s disease: a review of progress. J Neurol Neurosurg Psychiatry. 1999;66:137–147. doi: 10.1136/jnnp.66.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bymaster FP, Whitesitt CA, Shannon HE, et al. Xanomeline: a selective muscarinic agonist for the treatment of alzhiemer’s disease. Drug Dev Res. 1997;40:158–170. doi: 10.1002/(SICI)1098-2299(199702)40:2<158::AID-DDR6>3.0.CO;2-K. [DOI] [Google Scholar]
- 7.Shannon HE, Bymaster FP, Calligaro DO, et al. Xanomeline: a novel muscarinic receptor agonist with functional selectivity for m1 receptors. J Pharmacol Exp Ther. 1994;269:271–281. [PubMed] [Google Scholar]
- 8.Christopoulos A, Pierce TL, Sorman JL, et al. On the unique binding and activating properties of xanomeline at the m1 muscarinic acetylcholine receptor. Mol Pharmacol. 1998;53:1120–1130. [PubMed] [Google Scholar]
- 9.Jakubik J, Tucek S, El-Fakahany EE. Allosteric modulation by persistent binding of xanomeline of the interaction of competitive ligands with the m1 muscarinic acetylcholine receptor. J Pharmacol Exp Ther. 2002;301:1033–1041. doi: 10.1124/jpet.301.3.1033. 10.1124/ jpet.301.3.1033. [DOI] [PubMed] [Google Scholar]
- 10.Jakubik J, El-Fakahany EE, Dolezal V. Differences in kinetics of xanomeline binding and selectivity of activation of g proteins at m(1) and m(2) muscarinic acetylcholine receptors. Mol Pharmacol. 2006;70:656–666. doi: 10.1124/mol.106.023762. [DOI] [PubMed] [Google Scholar]
- 11.Grant MK, El-Fakahany EE. Persistent binding and functional antagonism by xanomeline at the muscarinic m5 receptor. J Pharmacol Exp Ther. 2005;315:313–319. doi: 10.1124/jpet.105.090134. [DOI] [PubMed] [Google Scholar]
- 12.Sramek JJ, Hurley DJ, Wardle TS, et al. The safety and tolerance of xanomeline tartrate in patients with alzheimer’s disease. J Clin Pharmacol. 1995;35:800–806. doi: 10.1002/j.1552-4604.1995.tb04123.x. [DOI] [PubMed] [Google Scholar]
- 13.Shekhar A, Potter WZ, Lightfoot J, et al. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am J Psychiatry. 2008;165:1033–1039. doi: 10.1176/appi.ajp.2008.06091591. [DOI] [PubMed] [Google Scholar]
- 14.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 15.De Lorme KC, Grant MK, Noetzel MJ, et al. Long-term changes in the muscarinic m1 receptor induced by instantaneous formation of wash-resistant xanomeline-receptor complex. J Pharmacol Exp Ther. 2007;323:868–876. doi: 10.1124/jpet.107.129940. [DOI] [PubMed] [Google Scholar]
- 16.Wang SZ, el-Fakahany EE. Application of transfected cell lines in studies of functional receptor subtype selectivity of muscarinic agonists. J Pharmacol Exp Ther. 1993;266:237–243. [PubMed] [Google Scholar]
- 17.Sramek JJ, Cutler NR, Hurley DJ, et al. The utility of salivary amylase as an evaluation of m3 muscarinic agonist activity in alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:85–91. doi: 10.1016/0278-5846(94)00107-S. [DOI] [PubMed] [Google Scholar]
- 18.Christopoulos A, El-Fakahany EE. Novel persistent activation of muscarinic m1 receptors by xanomeline. Eur J Pharmacol. 1997;334:R3–R4. doi: 10.1016/S0014-2999(97)01162-X. [DOI] [PubMed] [Google Scholar]
- 19.Dohlman HG, Thorner J, Caron MG, et al. Model systems for the study of seven-transmembrane-segment receptors. Annu Rev Biochem. 1991;60:653–688. doi: 10.1146/annurev.bi.60.070191.003253. 10.1146/annurev.bi.60.070191. 003253. [DOI] [PubMed] [Google Scholar]
- 20.el-Fakahany EE, Cioffi CL. Molecular mechanisms of regulation of neuronal muscarinic receptor sensitivity. Membr Biochem. 1990;9:9–27. doi: 10.3109/09687689009026820. [DOI] [PubMed] [Google Scholar]
- 21.Lee NH, el-Fakahany EE. Allosteric antagonists of the muscarinic acetylcholine receptor. Biochem Pharmacol. 1991;42:199–205. doi: 10.1016/0006-2952(91)90703-8. [DOI] [PubMed] [Google Scholar]
- 22.Feigenbaum P, El-Fakahany EE. Regulation of muscarinic cholinergic receptor density in neuroblastoma cells by brief exposure to agonist: possible involvement in desensitization of receptor function. J Pharmacol Exp Ther. 1985;233:134–140. [PubMed] [Google Scholar]
- 23.Christopoulos A. Allosteric binding sites on cell-surface receptors: novel targets for drug discovery. Nat Rev Drug Discov. 2002;1:198–210. doi: 10.1038/nrd746. [DOI] [PubMed] [Google Scholar]
- 24.May LT, Lin Y, Sexton PM, et al. Regulation of m2 muscarinic acetylcholine receptor expression and signaling by prolonged exposure to allosteric modulators. J Pharmacol Exp Ther. 2005;312:382–390. doi: 10.1124/jpet.104.073767. [DOI] [PubMed] [Google Scholar]
- 25.Lanzafame AA, Sexton PM, Christopoulos A. Interaction studies of multiple binding sites on m4 muscarinic acetylcholine receptors. Mol Pharmacol. 2006;70:736–746. doi: 10.1124/mol.106.024711. [DOI] [PubMed] [Google Scholar]
- 26.Wang SZ, Hu JR, Long RM, et al. Agonist-induced down-regulation of m1 muscarinic receptors and reduction of their mrna level in a transfected cell line. FEBS Lett. 1990;276:185–188. doi: 10.1016/0014-5793(90)80538-T. [DOI] [PubMed] [Google Scholar]
- 27.Cioffi CL, el-Fakahany EE. Differential sensitivity of phosphoinositide and cyclic gmp responses to short-term regulation by a muscarinic agonist in mouse neuroblastoma cells Correlation with down-regulation of cell surface receptors. Biochem Pharmacol. 1989;38:1827–1834. doi: 10.1016/0006-2952(89)90418-8. 10.1016/0006-2952(89)90 418-8. [DOI] [PubMed] [Google Scholar]
- 28.Wood MD, Murkitt KL, Ho M, et al. Functional comparison of muscarinic partial agonists at muscarinic receptor subtypes hm1, hm2, hm3, hm4 and hm5 using microphysiometry. Br J Pharmacol. 1999;126:1620–1624. doi: 10.1038/sj.bjp.0702463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maloteaux JM, Hermans E. Agonist-induced muscarinic cholinergic receptor internalization, recycling and degradation in cultured neuronal cells cellular mechanisms and role in desensitization. Biochem Pharmacol. 1994;47:77–88. doi: 10.1016/0006-2952(94)90439-1. [DOI] [PubMed] [Google Scholar]
- 30.Hu J, Wang SZ, el-Fakahany EE. Effects of agonist efficacy on desensitization of phosphoinositide hydrolysis mediated by m1 and m3 muscarinic receptors expressed in chinese hamster ovary cells. J Pharmacol Exp Ther. 1991;257:938–945. [PubMed] [Google Scholar]
- 31.van de Westerlo E, Yang J, Logsdon C, et al. Down-regulation of the g-proteins gq alpha and g11 alpha by transfected human m3 muscarinic acetylcholine receptors in chinese hamster ovary cells is independent of receptor down-regulation. Biochem J. 1995;310(Pt 2):559–563. [PMC free article] [PubMed] [Google Scholar]