Abstract
Objective
We examined 103 cases over the last five years and discussed diagnosis and treatment of alpha-fetoprotein (AFP)-negative small hepatic lesions.
Background: Small hepatic lesions (less than 2 cm in diameter) usually have no typical imaging characteristics and therefore are difficult to diagnose, especially when AFP tests provide a negative result.
Methods
A total of 103 patients with AFP-negative small hepatic lesions from January 2003 to December 2008 were retrospectively reviewed. Differential diagnosis was performed by digital subtraction angiography (DSA), dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI), contrast-enhanced ultrasound (CEUS), or positron emission tomography-computed tomography (PET-CT) based on the multiplicity of lesions. Ninety-four patients with suspected cancers underwent partial hepatectomy. Clinical data were collected from hospital records and follow-up questionnaires.
Results
Hepatocellular carcinoma (HCC) diagnostic sensitivity of DSA, DCE-MRI, CEUS and PET-CT was 88.2%, 93.9%, 88.9% and 88.9%, respectively. The surgery-related complication rate was 6.4%. Prognosis was good, with 1- and 3-year survival rates of 98.8% and 76.1%, respectively.
Conclusions
DSA, DCE-MRI, CEUS and PET-CT are valuable for diagnosis of small hepatic lesions. Partial hepatectomy is a preferred surgical procedure. Surgery for small liver cancers usually has little risk and good prognosis, therefore it can be actively applied in suspected HCC cases.
Key Words: Alpha-fetoprotein (AFP)-negative small hepatic lesions, contrast-enhanced ultrasound (CEUS), dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI), hepatectomy
Introduction
The diameter of malignant tumors of the liver is a key parameter in the staging and prognosis system. Therefore the clinical diagnosis and treatment of small liver carcinoma has become increasingly important. The practice guideline for hepatic cell carcinoma (HCC) of the American Association for the Study of Liver Diseases (AASLD) defined tumor diameter’s threshold at 2 cm (1). In this study, liver solid lesions with diameter less than 2 cm were referred as small hepatic lesions. Patients with small hepatic lesions usually are clinically asymptomatic, and most lesions are detected by ultrasound or computed tomography (CT) during routine physical examinations. These lesions usually have no typical imaging characteristics of liver tumors due to their small size, and therefore are difficult to diagnose, especially when alpha-fetoprotein (AFP) test shows negative result. As a result, physicians often miss the best opportunities for optimal diagnosis and treatment.
Materials and methods
General data
Between January 2003 and December 2008, 195 patients with small hepatic lesions were treated in our hospital. The lesions were mostly detected by ultrasound or CT during routine physical examinations or occasionally during examinations for epigastric discomfort. Since most patients were positive in hepatitis B surface antigen (HBsAg) testing or had a history of hepatitis B in this series (182/195; 93.3%), they were suspected to have hepatic malignancies (also based on the imaging results). Laboratory tests including routine blood tests, hepatic/renal function, clotting mechanism, AFP determination, and tests for hepatitis B/C-related antigens/antibodies were performed for all patients. The serum AFP level was more than 20 ng/mL in 92 patients [AFP ≥400 ng/mL in 52 patients, accounting for 30.4% (52/171) of surgically confirmed patients with HCC]. The remaining 103 patients with serum AFP level less than 20 ng/mL were regarded as AFP-negative cases.
Differential diagnosis
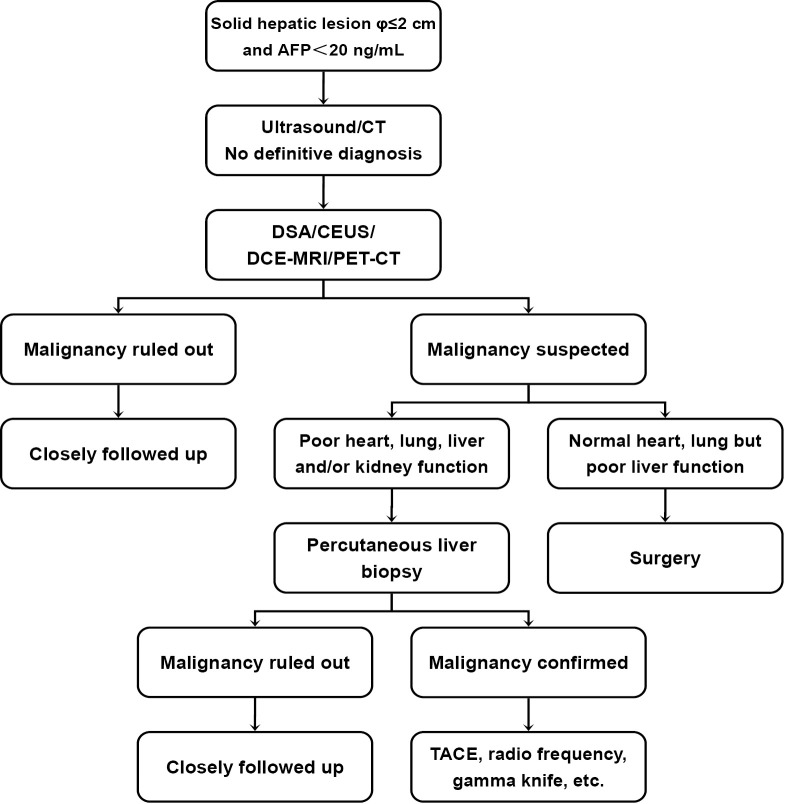
For the 103 patients (91 men and 12 women; age range, 35-79 years old) with AFP-negative small hepatic lesions whose characteristics could not be defined by ultrasound or contrast-enhanced CT (CECT), the differential diagnosis methods were mainly based on the multiplicity of hepatic tumors. Multiple hepatic lesions were mainly examined with digital subtraction angiography (DSA) or dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI), and solitary lesions with contrast-enhanced ultrasound (CEUS). With the advances in positron emission tomography-computed tomography (PET-CT) after 2006, we also applied it in some cases. When any imaging method suggested the possibility of malignancy, surgical treatment was recommended if the patient’s general health status was good and Child-Pugh less than 8 points in liver function evaluation. Patients whose possibility of malignancy was ruled out or who refused to undergo surgery were closely followed. For patients whose general health status was poor or whose functions of heart, lung, liver and/or kidney could not tolerate surgery, CT-guided liver biopsy was performed. Patients with confirmed malignancies in liver biopsy were treated with transarterial chemoembolization (TACE), radiofrequency ablation (RFA) or gamma knife treatment. Patients with pathologically confirmed benign diseases were closely followed. Figure 1 shows the clinical pathway of the diagnosis and treatment of AFP-negative small hepatic lesions.
Figure 1.
Clinical pathway of the diagnosis and treatment of AFP-negative small hepatic lesions
Results
Pathological findings
Of the 19 AFP-negative patients who underwent DSA, 17 received surgery; among these individuals the definitive diagnoses included 15 HCC and two focal nodular hyperplasia (FNH). Of the 37 patients who underwent DCE-MRI, 33 received surgery; Their definitive diagnoses included 29 HCC, one each of mixed-type liver malignant tumor, neuroendocrine tumor, nodular regenerative hyperplasia of the liver (NRHL) and solitary necrotic nodule (SNN). Of the 51 patients who underwent CEUS, 45 received surgery; their definitive diagnoses included 39 HCC, three FNH and one each of intrahepatic cholangio-carcinoma (IHCC), NRHL and inflammatory granuloma. Of the 12 patients who underwent PET-CT, nine received surgery; Their definitive diagnoses included eight HCC and one FNH. In clinical practice, 14 patients were examined with two or more imaging modalities, and the accuracy of surgical/pathological diagnosis was separately calculated. As a result, the data mentioned above may overlap. As confirmed by surgical-pathological findings, the diagnostic sensitivity of DSA, DCE-MRI, CEUS and PET-CT for hepatic malignancies was 88.2%, 93.9%, 88.9% and 88.9%, respectively (Figures 2,3). Detailed information of patients who received surgery is displayed in Table 1. Of the nine patients who did not undergo surgery, liver biopsy was performed in three patients, and two of these individuals were confirmed to have HCC; five patients have been followed for more than 2 years and no hepatic malignancy was found.
Figure 2.
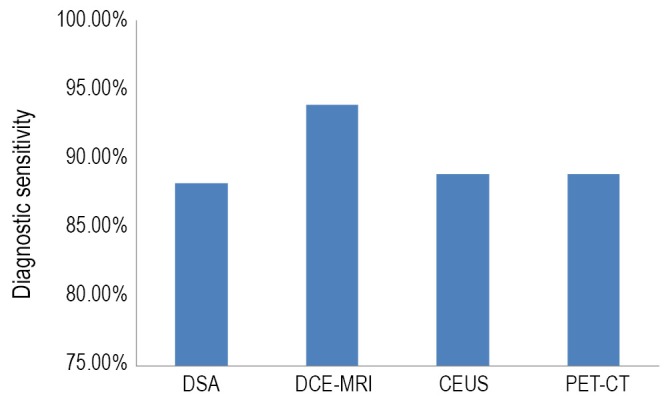
The diagnostic sensitivity of DSA, DCE-MRI, CEUS and PET-CT for hepatic malignancies
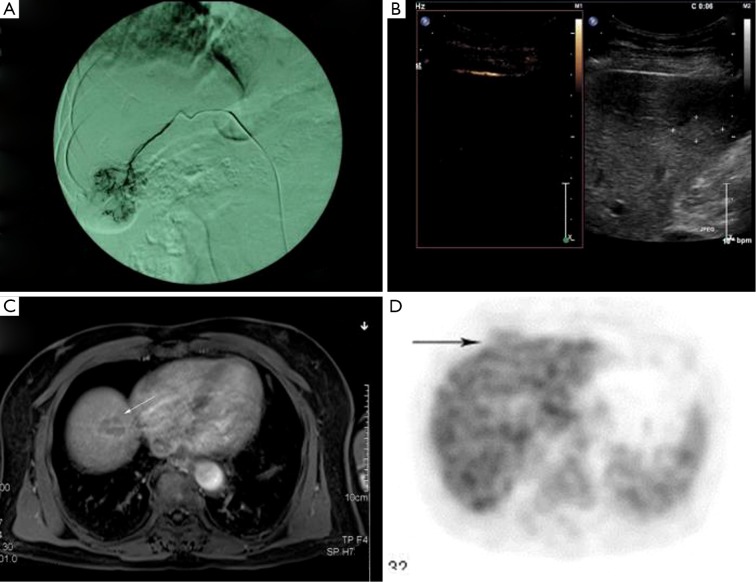
Figure 3.
DSA, DCE-MRI, CEUS and PET-CT imaging of hepatic malignancies. A. DSA; B. CEUS; C. DCE-MRI; D. PET-CT
Table 1. Clinical data of 94 patients with AFP-negative small hepatic lesions who underwent surgery.
| Benign cases (n=7) | Malignant cases (n=87) | ||
|---|---|---|---|
| Age range (years) | 35-71 | 38-79 | |
| Sex (male/female) | 3/4 | 84/3 | |
| HbsAg (+/-) | 5/2 | 84/3 | |
| Anti-HCV antibody (+/-) | 0/7 | 6/81 | |
| Child-Pugh grade (grade A/B) | 7/0 | 85/2 | |
| Cirrhosis (yes/no) | 6/1 | 82/5 | |
| Imaging modality | DSA | 2 | 15 |
| DCE-MRI | 2 | 31 | |
| CEUS | 5 | 40 | |
| PET-CT | 1 | 8 | |
| Tumor multiplicity* (single/multiple) | 7/0 | 75/12 | |
Benign or malignant nature of tumors was pathologically confirmed. The benign lesions included focal nodular hyperplasia (FNH; n=4), nodular regenerative hyperplasia of the liver (NRHL; n=1), solitary necrotic nodule (SNN; n=1), and inflammatory granuloma (n=1). The malignant lesions included hepatocellular carcinoma (HCC; n=84), mixed-type HCC (n=1), neuroendocrine tumor (n=1), and intrahepatic cholangio-carcinoma (IHCC; n=1). *Multiple hepatic lesions detected by preoperative imaging examinations and confirmed by postoperative pathology; If the multiple resected lesions had both benign and malignant lesions, the cases were recorded as “malignant”. In clinical practice, ten patients were examined with two or more imaging modalities, and the sensitivities of surgical and pathological diagnoses were calculated separately. As a result, the data may overlap
Follow-up
Ninety-four patients of the 103 AFP-negative cases received partial hepatectomy; Surgery-related complication rate was 6.4% (6/94) and no perioperative death was noted. The main complication was transient hepatic dysfunction, which was featured by medium-sized quantity of ascites and slightly increased total bilirubin.
Of the 87 patients with pathologically confirmed hepatic malignancies, 85 received postoperative follow-up for more than 1 year, and the 1-year survival rate was 98.8% (84/85). One patient died due to massive hemorrhage of the upper alimentary tract and liver failure at 6 months after surgery. The 1-year tumor-free survival rate was 90.6% (77/85).
Seventy-one patients were followed for more than 3 years, with a 3-year survival rate of 76.1% (54/71). The causes of death included metastasis and multiple organ failure (n=12), hemorrhage of the upper alimentary tract and liver failure (n=2), and other unrelated diseases (n=2); the remaining one patient died during perioperative stage of liver transplantation for tumor relapse. The 3-year tumor-free survival rate was 70.4% (50/71).
Discussion
Liver cancer, predominantly HCC, is one of the most common malignant tumors in China. Most HCCs with diameter more than 3 cm have typical characteristics on ultrasound or CT with satisfactory sensitivity. However, it is usually difficult to differentiate benign or malignant hepatic lesions when the size of hepatic lesion is small (less than 2 cm in diameter) for the lack of typical features of malignant tumors in imaging examinations (1,2).
The predictive value of HCC in remarked increase of serum AFP is significant when conditions such as pregnancy and genital cancers have been ruled out. According to official data released by the Chinese Society of Liver Cancer (CSLC), over 60% of patients with HCC showed serum AFP more than 400 ng/mL (3). Although quite a few physicians still use AFP as an important indicator to confirm the HCC diagnosis, AFP cannot meet the needs of clinical practice due to its controversial threshold value. When AFP value of 20 ng/mL is used as diagnostic cutoff value, it has good sensitivity but poor specificity; In other words, many false-positive cases will be included. However, when a threshold value of 400 ng/mL is taken as cutoff value, it is more specific but less sensitive with less than 65% accuracy in the literature (4), especially among Caucasian populations (5). As shown in our previous retrospective analysis of HCC patients who were treated in our hospital from 2002 to 2008, when serum AFP level of 200 ng/mL and of 400 ng/mL were used as cutoff value, the global sensitivities were 45.8% and only 39.2%, retrospectively. Therefore negative AFP test result is not enough to rule out hepatic malignancy.
Considering the observation that the majority of Chinese HCC patients also have complication of hepatitis B and cirrhosis, physicians should pay special attention to patients with history of hepatitis B or suspected cirrhosis (as shown by imaging examinations). Actually, hepatitis B and cirrhosis may have higher clinical significance than serum AFP level. According to the Guidelines for the Diagnosis and Treatment of Liver Cancers [2009] jointly developed and issued by CSLC, Chinese Society of Clinical Oncology (CSCO), and Liver Cancer Study Group, Chinese Society of Hepatology, Chinese Medical Association, physicians should pay adequate attention to background liver diseases to achieve early diagnosis of liver cancer (3).
DSA remains a sensitive imaging modality for the diagnosis of HCC (6), especially after ultra fluid lipiodol has been injected into the hepatic artery during hepatography; HCC-specific uptake of lipiodol can be observed by CT 3-4 weeks later, which further increases accuracy of DSA. However, DSA is a complicated and invasive procedure with certain risks. CEUS can be applied to observe the distribution of blood supply inside a tumor in real-time manner and thereby determine the nature of a tumor. CEUS is a simple and minimally invasive procedure. In recent years, DCE-MRI has increasingly been applied in clinical practice, especially for differential diagnosis of liver tumors. Both DCE-MRI and CEUS have shown higher accuracies in detecting liver lesions than conventional color ultrasound and contrast-enhanced CT (7).
In addition to history taking, differential diagnosis of AFP-negative small hepatic lesions must be based on more sensitive and specific examinations. In this series DSA, DCE-MRI, and CEUS showed satisfactory results in displaying AFP-negative small hepatic lesions, with diagnostic sensitivity about 90%. Therefore most patients can choose one of these modalities, and combination of two or three modalities may be appropriate for more complicated cases. For difficult-to-diagnose small hepatic lesions, the combination of CEUS with multi-dimensional CT or DCE-MRI is the optimal cost-effective and noninvasive method for differential diagnosis. In a study guided by AASLD criteria, for nodules less than 2 cm detected during conventional ultrasound surveillance, the combination of CEUS with DCE-MRI provided 100% specificity (8).
Only one lesion can be characterized after each contrast agent injection during CEUS; Therefore CEUS is the preferred modality for solitary liver tumor. For multiple liver lesions, DSA or DCE-MRI is recommended. For patients with poor general health status and those who refuse surgery, DSA is recommended. TACE may be applied concurrently when HCC is confirmed during DSA.
PET-CT is an imaging modality that characterizes tumors based on the metabolic level; however, its commonly used contrast agent [2-fluoro-2-deoxy-D-glucose (FDG)] is not sensitive for HCCs, especially relatively well-differentiated ones. 11C-acetate, a novel contrast agent synthesized by the Department of Nuclear Medicine of our hospital, has shown good efficacy in characterizing liver tumors when combined with FDG (9).
Many authors have argued whether percutaneous liver biopsy should be performed to establish pathological diagnosis. However, biopsy for liver lesions less than 2 cm may not provide reliable information for the following reasons. First, it is difficult to localize the lesion and to ensure that the specimen be harvested from the lesion. Second, the boundary between abnormally developed hepatocytes and well-differentiated HCC remains controversial, especially for small-sized liver lesions. Third, fine-needle aspiration cannot characterize the frame structure of HCC, making it even more difficult to differentiate well-differentiated HCC from normal hepatocytes. And fourth, a negative result of biopsy does not rule out malignancy (10,11). Therefore in our series only a small proportion of patients whose general health condition and/or liver function did not allow surgery underwent biopsy, with an attempt to find evidence to support the establishment of new treatment plans.
A recent study found that GP73, a resident Golgi glycoprotein, may be helpful for the early diagnosis of HCC (12). Our research team also reported that GP73 had higher sensitivity than AFP in diagnosing HCC (13). In the later stages of this study, we applied GP73 as an important indicator for diagnosing liver cancers and monitoring relapses, and our preliminary results were encouraging: in 19 patients with surgically confirmed HCC, 12 (63.2%) had remarkably increased GP73 levels. More studies are needed to test the novel biomarkers for HCC early diagnosis with higher sensitivity and specificity (14,15).
In this study 95.7% (90/94) of patients had the distance from the lesion to liver capsule less than 2 cm. Therefore the lesions could be easily localized by intraoperative exploration or ultrasound examination. Partial hepatectomy was the preferred surgical procedure for these 90 patients. The tumors, together with a margin of 1-2 cm of normal liver tissue around the tumor, were resected completely. The patients recovered quickly for small surgical trauma. For the remaining four patients whose lesions were located inside the liver parenchyma deeply, two also received partial hepatectomy (guided by ultrasound) and two received anatomical hepatectomy. No perioperative death was noted, and the surgery-associated complication rate was extremely low. Follow-up showed that the 1- and 3-year survival rate and tumor-free survival rate were satisfactory.
According to Kojiro and Roskams (16), patients can benefit dramatically from early diagnosis and prompt treatment when the diameter of liver tumor is less than 2 cm and no typical imaging features of tumor vessels occur; When the diameter is more than 2 cm and the imaging findings become obvious, the rate of microvascular invasion and the occurrence of satellite lesions will be high. Since small hepatic lesions usually incur small surgical trauma and good long-term prognosis, surgery should be considered as early as possible for patients with suspected malignant lesions, especially when they are accompanied by history of hepatitis B and/or clinical presentation of cirrhosis. The preferred surgical procedure is partial hepatectomy; Enlarging the resection scope is not helpful to improve the prognosis of small hepatic lesions.
In recent years, minimally invasive treatment, particularly RFA, has been applied for the treatment of hepatic malignancies (17,18). However, it has been demonstrated that the advantage of hepatic resection was still greater for patients with singular small tumors and patients with Child-Pugh Grade A liver function than RFA. In contrast, RFA might be recommended for patients who could not tolerate surgery, with higher surgical difficulties or with multinodular tumors (19).
Hepatitis B is highly prevalent in China. Along with recent improvements in living conditions, health care, and education, there has been increased public awareness of the progression of hepatitis B to cirrhosis and liver cancer. An increasing number of hepatitis B virus carriers actively take routine physical examination, making it possible to detect liver cancers in early stage. More small hepatic lesion cases will be early detected in the near future. Physicians should respond to this new situation by adopting more appropriate and sensitive examination methods and performing active surgical treatment for suspected cases.
Acknowledgements
This work was supported by China Medical Board in New York (CMB, 11-045), National Natural Science Foundation of China (30970623 and 81201566), International Science and Technology Cooperation Projects (2010DFA31840 and 2010DFB33720), Program for New Century Excellent Talents in University (NCET-11-0288), and Beijing Natural Science Foundation (5112030).
Disclosure: The authors declare no conflict of interest.
References
- 1.Bruix J, Sherman M, Practice Guidelines Committee, et al Management of hepatocellular carcinoma. Hepatology 2005;42:1208-36 [DOI] [PubMed] [Google Scholar]
- 2.Li C, Li G, Miao R, et al. Primary liver cancer presenting as pyogenic liver abscess: characteristics, diagnosis, and management. J Surg Oncol 2012;105:687-91 [DOI] [PubMed] [Google Scholar]
- 3.Ye SL, Qin SK. Expert consensus on the diagnosis and treatment of primary liver cancers. J Surg Concepts Pract 2009;14:469-76 [Google Scholar]
- 4.Sherman M.Alphafetoprotein: an obituary. J Hepatol 2001;34:603-5 [DOI] [PubMed] [Google Scholar]
- 5.Trevisani F, D’Intino PE, Morselli-Labate AM, et al. Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. J Hepatol 2001;34:570-5 [DOI] [PubMed] [Google Scholar]
- 6.Wang JC, Hu DY, Zhou XM, et al. Sensitivity of the hepatic arteriography for recurrent liver cancer after surgery. J Med Imaging 2005;15:900-3 [Google Scholar]
- 7.Lencioni R, Piscaglia F, Bolondi L.Contrast-enhanced ultrasound in the diagnosis of hepatocellular carcinoma. J Hepatol 2008;48:848-57 [DOI] [PubMed] [Google Scholar]
- 8.Forner A, Vilana R, Ayuso C, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology 2008;47:97-104 [DOI] [PubMed] [Google Scholar]
- 9.Huo L, Zhou Q, Dang YH, et al. Role of combination of 11C-acetate and 18FDG positron emission tomography in diagnosis of liver neoplasm. Bull Chin Cancer 2007;16:184-6 [Google Scholar]
- 10.Kojiro M.Focus on dysplastic nodules and early hepatocellular carcinoma: an Eastern point of view. Liver Transpl 2004;10:S3-8 [DOI] [PubMed] [Google Scholar]
- 11.Caturelli E, Solmi L, Anti M, et al. Ultrasound guided fine needle biopsy of early hepatocellular carcinoma complicating liver cirrhosis: a multicentre study. Gut 2004;53:1356-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marrero JA, Romano PR, Nikolaeva O, et al. GP73, a resident Golgi glycoprotein, is a novel serum marker for hepatocellular carcinoma. J Hepatol 2005;43:1007-12 [DOI] [PubMed] [Google Scholar]
- 13.Mao Y, Yang H, Xu H, et al. Golgi protein 73 (GOLPH2) is a valuable serum marker for hepatocellular carcinoma. Gut 2010;59:1687-93 [DOI] [PubMed] [Google Scholar]
- 14.Zhao X, Yang Z, Li G, et al. The role and clinical implications of microRNAs in hepatocellular carcinoma. Sci China Life Sci 2012;55:906-19 [DOI] [PubMed] [Google Scholar]
- 15.Prieto PA, Cha CH. DKK1 as a serum biomarker for hepatocellular carcinoma. Hepatobiliary Surg Nutr 2013;2:127-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kojiro M, Roskams T.Early hepatocellular carcinoma and dysplastic nodules. Semin Liver Dis 2005;25:133-42 [DOI] [PubMed] [Google Scholar]
- 17.Tateishi R, Shiina S, Teratani T, et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer 2005;103:1201-9 [DOI] [PubMed] [Google Scholar]
- 18.Nishikawa H, Osaki Y.Comparison of high-intensity focused ultrasound therapy and radiofrequency ablat ion for recur rent hepatocel lular carcinoma. Hepatobiliary Surg Nutr 2013;2:168-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ueno S, Sakoda M, Kubo F, et al. Surgical resection versus radiofrequency ablation for small hepatocellular carcinomas within the Milan criteria. J Hepatobiliary Pancreat Surg 2009;16:359-66 [DOI] [PubMed] [Google Scholar]