Abstract
Background
Survival of patients on left ventricular assist devices (LVADs) has improved. We examined the differences in risk of adverse outcomes between LVAD-supported and medically managed candidates on the heart transplant waiting list.
Methods and Results
We analyzed mortality and morbidity in 33 073 heart transplant candidates registered on the United Network for Organ Sharing (UNOS) waiting list between 1999 and 2011. Five groups were selected: patients without LVADs in urgency status 1A, 1B, and 2; patients with pulsatile-flow LVADs; and patients with continuous-flow LVADs. Outcomes in patients requiring biventricular assist devices, total artificial heart, and temporary VADs were also analyzed. Two eras were defined on the basis of the approval date of the first continuous-flow LVAD for bridge to transplantation in the United States (2008). Mortality was lower in the current compared with the first era (2.1%/mo versus 2.9%/mo; P<0.0001). In the first era, mortality of pulsatile-flow LVAD patients was higher than in status 2 (hazard ratio [HR], 2.15; P<0.0001) and similar to that in status 1B patients (HR, 1.04; P=0.61). In the current era, patients with continuous-flow LVADs had mortality similar to that of status 2 (HR, 0.80; P=0.12) and lower mortality compared with status 1A and 1B patients (HR, 0.24 and 0.47; P<0.0001 for both comparisons). However, status upgrade for LVAD-related complications occurred frequently (28%) and increased the mortality risk (HR, 1.75; P=0.001). Mortality was highest in patients with biventricular assist devices (HR, 5.00; P<0.0001) and temporary VADs (HR, 7.72; P<0.0001).
Conclusions
Mortality and morbidity on the heart transplant waiting list have decreased. Candidates supported with contemporary continuous-flow LVADs have favorable waiting list outcomes; however, they worsen significantly once a serious LVAD-related complication occurs. Transplant candidates requiring temporary and biventricular support have the highest risk of adverse outcomes. These results may help to guide optimal allocation of donor hearts.
Keywords: heart-assist devices, mortality, outcome assessment, transplantation
Heart transplantation provides remarkable improvement of quality of life and survival in selected patients with advanced heart failure. However, the number of heart transplant procedures is limited by donor availability.1 The Organ Procurement and Transplantation Network (OPTN), through a contract with the United Network for Organ Sharing (UNOS), has proposed, implemented, and updated policies that direct allocation of donor organs in the United States. UNOS has strived to create allocation policies based on algorithms that prioritize patients with the highest mortality risk in the period preceding transplantation.2 Despite these policies and the multiple interventions aimed at increasing donor heart availability, mortality on the transplant waiting list remains considerable.3–7
Left ventricular assist devices (LVADs) are increasingly used to improve hemodynamic status in patients with advanced heart failure awaiting heart transplantation.8–11 The efficacy and risk profile of these devices have been further confirmed by postmarket approval data.12–14 However, first-generation pulsatile-flow (PF) LVADs were associated with significant device-related complications, including device failure and suboptimal long-term outcomes.8,9 Mortality risk was high during the first 3 weeks after PF-LVAD implantation (15%–30%),2 and based on these data, a 1999 UNOS thoracic organ allocation policy revision allowed listing of LVAD patients in the high-urgency 1A status if the device had been in place for <30 days, with indefinite intermediate-urgency 1B status listing thereafter. Additional modification in 2002 allowed physicians to use 30 days of 1A status time at their discretion at any point after LVAD implantation.2,15
Advances in mechanical circulatory support (MCS) technology, specifically the introduction of the new-generation, smaller, and more durable continuous-flow (CF) LVADs, have further reduced the morbidity and mortality of advanced heart failure patients in need of LVAD bridging to transplantation.11–13,16–18 These encouraging results, along with continued donor organ shortage and recent data suggesting that LVAD bridging is no longer associated with increased mortality after transplantation,19 resulted in a substantial increase in the use of LVADs for bridging to transplantation.1,7,14 In view of these results, it has been questioned whether the allocation advantage given to patients bridged with LVADs is still justified.15 In this study, we sought to quantify the differences in risk of mortality and mortality or delisting as a result of worsening clinical status between LVAD-supported and medically managed candidates listed in the various urgency statuses of the heart transplant waiting list.
Methods
Data Source and Study Population
UNOS/OPTN provided deidentified patient-level data from the Waiting List Registry (data source No. 412012-2). These data included all heart transplant candidates registered on the waiting list between September 1985 and December 2011. There is 1 record per waiting list registration, and each record includes the most recent follow-up information, including patient survival, inactivation, and delisting reported to OPTN. Data entry by all US transplant centers has been mandatory since the passage of the National Transplantation Act of 1984.
We included adult candidates (age ≥18 years) registered for single-organ, primary heart transplantation between January 1999 and December 2011. The study cohort was divided on the basis of patient UNOS status at the time of last follow-up, transplantation, death, or delisting and the need for MCS with a durable LVAD (regardless of UNOS status) at the time of listing. The following 5 groups of patients were the focus of our analysis: medically managed candidates in (1) high-urgency UNOS status 1A, (2) intermediate-urgency UNOS status 1B, and (3) low-urgency UNOS status 2 and candidates requiring circulatory support with (4) PF-LVADs and (5) CF-LVADs. Durable LVADs are the most common type of assist device used for bridge to transplantation; therefore, among candidates requiring MCS, these patients were the main focus of our analyses. For completeness, patients who required support with biventricular assist devices (BIVADs), total artificial heart (TAH), and temporary extracorporeal VADs were also included in this analysis. For patients whose status on the waiting list changed, the cumulative time spent throughout the listed period in the status that was reported last was used for the analysis. Patients requiring implantation of an LVAD while on the waiting list were also included in the study but were assigned to their respective medically managed UNOS status groups. Patients without an LVAD who were listed for transplantation in an inactive status and remained inactive; patients with an unknown type of assist device and those registered after December 2, 2011 (to allow a minimum follow-up of 3 months) were excluded.
Two eras were defined within the study period. The first era included candidates registered between January 20, 1999, and April 20, 2008, and the current era included candidates registered between April 21, 2008, and December 2, 2011. The era boundaries were based on the following 2 events: The first era started on the day when a 3-tier allocation system (status 1 patients were subdivided into status 1A and 1B) went into effect, and the current era started on the day when the first CF-LVAD (HeartMate II) was approved by the Food and Drug Administration for clinical use in the bridge-to-transplant indication in the United States.
The primary outcome of our study was all-cause mortality on the waiting list. Some transplant candidates are removed from the waiting list when their clinical condition worsens and transplantation is no longer believed to be a good therapeutic option. Many of these patients die or are transitioned to hospice care soon after delisting. To ascertain that our results captured this clinical scenario, we designated as the secondary outcome of our study a composite end point of all-cause mortality or delisting as a result of worsening clinical status.
Statistical Analysis
Continuous variables were summarized as mean±SD and compared by means of ANOVA. Testing of normality and equal-variance assumptions was not performed before ANOVA because this test is known to be robust to both of these assumptions.20,21 Categorical variables were summarized as frequencies and percentages and were compared by the Pearson χ2 test or, when <5 outcomes were expected per cell, by the Fisher exact test. The P values for pairwise group comparisons were adjusted for multiplicity by use of the Holm multiple-comparison procedure. Cumulative survival rates were estimated with Kaplan-Meier survival analysis and compared between groups by means of the log-rank test.22,23 The time to event was the amount of time spent in the listing status during which the patient was transplanted, delisted, or died. A death within 2 weeks of removal (resulting from any reason) from the waiting list, identified through Social Security Death Index data, was considered waiting list mortality and was included in the primary outcome. For the primary outcome of waiting list mortality, survival was censored at the time of delisting or transplant or at the last follow-up reported to UNOS for patients remaining on the waiting list. For the secondary outcome of death or delisting as a result of worsening clinical status, survival was censored at the time of transplantation or at the last follow-up reported to UNOS for patients remaining on the waiting list. Survival curves were constructed to illustrate multiple possible outcomes at any time after registration on the waiting list (competing outcomes) in the group of patients supported with durable LVADs: death, delist-ing as a result of worsening clinical status, delisting owing to clinical recovery, transplantation, and alive on waiting list.
The association of the different risk factors with hazard of the primary and secondary outcomes while on the waiting list was assessed separately by use of univariable Cox proportional hazards regression models.24 The proportional hazards assumption of the Cox regression models was assessed graphically with log-log curves and was found to be adequately met. The models examined the effect of the following candidate characteristics present at registration on the heart transplant waiting list: age, sex, body mass index, ABO blood type, heart failure etiology, history of diabetes mellitus, tobacco use, serum creatinine, pulmonary artery pressures, pulmonary capillary wedge pressure, use of inotropes, dialysis, mechanical ventilation, TAH, BIVADs, temporary VADs, durable LVADs, and UNOS waiting list status. A multivariable Cox proportional hazards model was used to determine the independent effect of multiple risk factors on the hazard of the primary and secondary outcomes. Variables significant at the P<0.10 level in unadjusted analyses were considered for inclusion; only variables significant at the P<0.05 level on the basis of the likelihood ratio test were retained in the final model. UNOS waiting list status groups were included in all multivariable analyses independently of their significance level in univariable analyses. The 7 comparisons to the status 2 reference group were adjusted for multiple comparisons by use of the Holm procedure. Hazard ratios (HRs) and 95% confidence intervals (CIs) were generated for both univariable and multivariable analyses as measures of strength of association and precision, respectively. A 2-tailed value of P<0.05 was considered statistically significant. All analyses were performed with STATA software, version 12 (StataCorp LP, College Station, TX).
Results
There were 33 390 adult candidates registered for single-organ primary heart transplantation in the United States during the study period. We excluded transplant candidates who required MCS but the type of assist device was unknown (n=107) and registrations without an assist device that remained inactive on the list (n=210). In total, 33 073 patients met the inclusion criteria and comprised our study group. Of these, 23 217 candidates were registered in the first era and 9856 were registered in the current era.
Baseline Characteristics
Among the 23 217 heart transplant candidates registered in the first era, 5699 (24.5%) were status 1A, 7154 (30.8%) were status 1B, and 7585 (32.7%) were status 2. Circulatory support with durable LVADs was required in 2146 patients (9.2%), with most devices being PF-LVADs (84%). Other types of VADs were used less frequently and included TAH in 46 patients (0.2%), BIVADs in 295 patients (1.3%), and temporary VADs in 292 patients (1.3%). In the current era, the proportion of candidates registered as status 1A (28.3%) and 1B (31.8%) remained similar, whereas the percentage of status 2 patients decreased substantially (18.4%). The proportion of patients supported with durable LVADs doubled in the current era: 1763 patients or 17.9% were supported with mostly CF-LVADs (89%). The use of biventricular support with TAH (0.4%), BIVADs (2.4%), and temporary VADs (0.8%) remained infrequent. The distribution of VAD types and brands used in both eras is presented in the Table I of the online-only Data Supplement.
Baseline characteristics of the study groups, recorded at registration on the heart transplant waiting list, are summarized in Table 1. Although between-group comparisons for most baseline characteristics were statistically significant, the following were the most clinically relevant differences: Patients supported with LVADs were more likely to be male, had higher body mass index compared with status 1A and 1B listed patients, and were more likely to be of blood group O, particularly in the current era. Patients with restrictive and congenital heart disease were less likely to be supported with an LVAD compared with other groups. Patients on LVAD support in the first era were more likely to require support with mechanical ventilation and dialysis compared with the rest of the groups, and these differences were less pronounced in the current era. Patients on CF-LVAD support during the current era were less likely to be hospitalized or to require critical care and required inotropes or dialysis less frequently compared with other groups and LVAD patients from the first era.
Table 1.
Baseline Characteristics of Heart Transplant Candidates on the Waiting List
| Variable | UNOS status 1A | UNOS status 1B | UNOS status 2 | PF-LVAD | CF-LVAD | P |
|---|---|---|---|---|---|---|
| First era | ||||||
| n | 5699 | 7154 | 7585 | 1808 | 338 | |
| Age, y | 51±12 | 52±12 | 52±12 | 50±12§|| | 50±13§|| | <0.0001 |
| Male sex, n (%) | 4475 (78) | 5403 (76) | 5588 (74) | 1468 (81)* | 247 (73)‡# | <0.0001 |
| BMI, kg/m2 | 27±12 | 27±5 | 28±17 | 28±5§‡ | 28±5 | <0.0001 |
| ABO blood type, n (%) | <0.0001 | |||||
| A | 2131 (37.4) | 2875 (40.2) | 3136 (41.3) | 647 (35.8)§|| | 128 (37.9) | |
| B | 700 (12.3) | 941 (13.2) | 992 (13.1) | 232 (12.8) | 47 (13.9) | |
| AB | 193 (3.4) | 332 (4.6) | 357 (4.7) | 69 (3.8) | 14 (4.1) | |
| O | 2675 (46.9) | 3006 (42.0) | 3100 (40.9) | 860 (47.6)§|| | 149 (44.1) | |
| CMP type, n (%) | <0.0001 | |||||
| Nonischemic | 2704 (47.5) | 3280 (45.8) | 3034 (40.0) | 717 (39.6)‡§** | 163 (48.2)||# | |
| Ischemic | 2451 (43.0) | 3130 (43.8) | 3709 (49.0) | 998 (55.2)† | 162 (47.9) | |
| VHD | 138 (2.4) | 197 (2.8) | 208 (2.7) | 41 (2.3) | 3 (0.9) | |
| CHD | 155 (2.7) | 198 (2.8) | 290 (3.8) | 11 (0.6)† | 4 (1.2)|| | |
| Restrictive | 206 (3.6) | 291 (4.0) | 265 (3.5) | 23 (1.3)† | 3 (0.9)† | |
| Other | 45 (0.8) | 58 (0.8) | 79 (1.0) | 18 (1.0) | 3 (0.9) | |
| Diabetes mellitus, n (%) | 1432 (25) | 1759 (25) | 1784 (24) | 460 (25) | 93 (28) | 0.10 |
| Creatinine, mg/dL | 1.4±0.8 | 1.4±0.8 | 1.3±0.9 | 1.4±0.8§|| | 1.3±0.8# | <0.0001 |
| Mean PAP, mm Hg | 32±10 | 31±10 | 27±10 | 31±10|| | 32±10|| | <0.0001 |
| PCWP, mm Hg | 22±8 | 21±8 | 18±8 | 22±8§|| | 22±8|| | <0.0001 |
| Required support, n (%) | ||||||
| Hospitalized, ICU | 3533 (62) | 1574 (22) | 194 (2.6) | 506 (28)* | 64 (19)‡||# | <0.0001 |
| Inotropic agents | 3142 (55) | 3574 (50) | 540 (7) | 724 (40)† | 133 (39)† | <0.0001 |
| Ventilator | 358 (6.3) | 86 (1.2) | 52 (0.7) | 378 (21)* | 44 (13)* | <0.0001 |
| Dialysis | 237 (4.2) | 133 (1.9) | 125 (1.6) | 129 (7.1)† | 15 (4.4)§|| | <0.0001 |
| Current Era | ||||||
| n | 2789 | 3131 | 1813 | 190 | 1573 | |
| Age, y | 52±13 | 52±12 | 54±12 | 50±13|| | 52±12|| | <0.0001 |
| Male sex, n (%) | 2130 (76) | 2274 (73) | 1305 (72) | 157 (83)§|| | 1227 (78)† | <0.0001 |
| BMI, kg/m2 | 27±5 | 28±6 | 28±6 | 28±5‡ | 28±5‡§ | <0.0001 |
| ABO blood type, n (%) | 0.007 | |||||
| A | 1104 (39.6) | 1175 (37.5) | 701 (38.7) | 64 (33.7) | 552 (35.1)‡ | |
| B | 357 (12.8) | 441 (14.1) | 218 (12.0) | 25 (13.1) | 202 (12.8) | |
| AB | 104 (3.7) | 145 (4.6) | 92 (5.1) | 6 (3.2) | 54 (3.4) | |
| O | 1224 (43.9) | 1370 (43.8) | 802 (44.2) | 95 (50.0)† | 765 (48.6)† | |
| CMP type, n (%) | <0.0001 | |||||
| Nonischemic | 1461 (52.4) | 1570 (50.1) | 710 (39.2) | 90 (47.4) | 853 (54.2)§|| | |
| Ischemic | 977 (35.0) | 1193 (38.1) | 820 (45.2) | 94 (49.5)ठ| 675 (42.9)ठ| |
| VHD | 59 (2.1) | 53 (1.7) | 46 (2.5) | 1 (0.5) | 13 (0.8)‡|| | |
| CHD | 78 (2.8) | 131 (4.2) | 98 (5.4) | 0 (0.0)† | 6 (0.4)† | |
| Restrictive | 184 (6.6) | 154 (4.9) | 110 (6.1) | 3 (1.6)‡§ | 18 (1.1)† | |
| Other | 30 (1.1) | 30 (1.0) | 29 (1.6) | 2 (1.0) | 8 (0.5)|| | |
| Diabetes mellitus, n (%) | 760 (27) | 941 (30) | 531 (29) | 61 (32) | 492 (31)‡ | 0.04 |
| Creatinine, mg/dL | 1.3±0.6 | 1.3±0.8 | 1.4±1.0 | 1.3±0.6 | 1.2±0.7|| | 0.001 |
| Mean PAP, mm Hg | 32±10 | 31±10 | 27±10 | 29±11‡§ | 30±11† | <0.0001 |
| PCWP, mm Hg | 22±8 | 21±8 | 18±8 | 19±8‡§ | 20±9† | <0.0001 |
| Required support, n (%) | ||||||
| Hospitalized, ICU | 1813 (65) | 350 (11) | 35 (2) | 34 (18)* | 132 (8)* | <0.0001 |
| Inotropic agents | 1320 (47) | 1435 (46) | 120 (7) | 23 (12)† | 191 (12)† | <0.0001 |
| Ventilator | 61 (2.2) | 28 (0.9) | 7 (0.4) | 6 (3.2)§|| | 77 (4.9)† | <0.0001 |
| Dialysis | 98 (3.5) | 64 (2.0) | 54 (3.0) | 9 (4.7) | 35 (2.2) | 0.002 |
BMI indicates body mass index; CF-LVAD, continuous-flow left ventricular assist device; CHD, congenital heart disease; CMP, cardiomyopathy; ICU, intensive care unit; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PF-LVAD, pulsatile-flow left ventricular assist device; and VHD, valvular heart disease. Baseline characteristics were recorded at registration on the heart transplantation waiting list. First era: January 1999 to April 2008; current era: April 2008 to December 2011. Values are expressed as mean±SD when appropriate. Final column reflects overall group ANOVA, χ2 test, or Fisher exact test as appropriate. For between-group comparisons in LVAD recipients:
P<0.05 vs all the groups;
P<0.05 vs United Network for Organ Sharing (UNOS) status 1A, 1B, and 2;
P<0.05 vs UNOS status 1A;
P<0.05 vs UNOS status 1B;
P<0.05 vs UNOS status 2;
P<0.05 vs PF-LVAD; and
P<0.05 vs CF-LVAD. Multiple-group comparisons were adjusted by use of the Holm procedure.
Outcomes
First Era: January 1999 to April 2008
During this period, 3730 patients died while on the heart transplant waiting list, which represented a mortality rate of 2.9%/mo. Among patients without MCS, mortality of status 1A patients was the highest (21.8%/mo), mortality of status 1B patients was 4.6%/mo, and mortality of status 2 patients was the most favorable (1.2%/mo; Figure 1A). Waiting list mortality in patients with PF-LVADs was 4.5%/mo, which was similar to that of status 1B patients (4.6%/mo; P=0.71). Mortality of patients with PF-LVADs was significantly higher compared with that of status 2 patients (4.5%/mo versus 1.2%/ mo; P<0.0001) and lower than mortality of status 1A patients (4.5% versus 21.8%; P<0.0001). CF-LVADs were used infrequently in the first era, and the waiting list mortality of this group was 2.5%/mo (Figure 1A). The overall incidence of the secondary outcome of death or delisting for worsening clinical status was 3.2%/mo, and the differences between the groups mirrored the primary outcome results (Figure 1B). The incidence of the composite end point was similar in patients with PF-LVADs and status 1B listed patients (5.0%/mo versus 5.2%/mo; P=0.4). Patients listed in status 1A had the highest event rate (23.2%/mo; P<0.0001 for comparison with all groups), whereas patients in status 2 had the lowest rate of the secondary outcome (1.5%/mo). The event rate in patients with CF-LVADs was 2.8%/mo. The results of the univariable analyses for both outcomes (Table II of the online-only Data Supplement) were confirmed in multivariable Cox regression analyses that included the variables detailed in the Methods section (Table 2).
Figure 1.
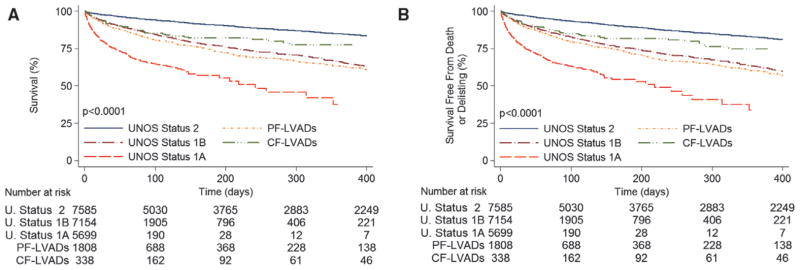
Outcomes for heart transplant candidates on the United Network for Organ Sharing (UNOS) waiting list in the first era. A, Unadjusted waiting list survival according to UNOS status and left ventricular assist device (LVAD) support type. B, Unadjusted waiting list survival free from death or delisting as a result of worsening clinical status according to UNOS status and LVAD support type. CF indicates continuous flow; and PF, pulsatile flow.
Table 2.
Multivariable Hazard Ratio Estimates for the Risk of Death on the Waiting List and for the Risk of Death or Delisting Among Heart Transplant Candidates in the First Era (1999–2008)
| Mortality | Mortality or Delisting | |||
|---|---|---|---|---|
|
| ||||
| Variable | HR (95% CI) | P | HR (95% CI) | P |
| Age (per year) | 1.01 (1.01–1.02) | <0.0001 | 1.01 (1.01–1.02) | <0.0001 |
| Restrictive vs nonischemic CMP | 1.50 (1.25–1.81) | <0.0001 | 1.43 (1.20–1.72) | <0.0001 |
| Valvular vs nonischemic CMP | … | … | 1.22 (1.00–1.48) | 0.049 |
| Diabetes mellitus (yes vs no) | 1.18 (1.08–1.27) | <0.0001 | 1.18 (1.10–1.28) | <0.0001 |
| Serum creatinine (per 1 mg/dL) | 1.21 (1.17–1.24) | <0.0001 | 1.21 (1.18–1.24) | <0.0001 |
| Mean PAP (per 1 mm Hg) | … | … | 1.01 (1.00–1.01) | 0.046 |
| PCWP (per 1 mm Hg) | 1.01 (1.01–1.02) | <0.0001 | 1.01 (1.00–1.02) | 0.003 |
| Inotropic support (yes vs no) | 1.24 (1.14–1.35) | <0.0001 | 1.24 (1.15–1.35) | <0.0001 |
| Mechanical ventilation (yes vs no) | 2.31 (2.02–2.65) | <0.0001 | 2.20 (1.92–2.51) | <0.0001 |
| UNOS status 1A vs 2 | 5.56 (4.92–6.29) | <0.0001 | 5.40 (4.80–6.06) | <0.0001 |
| UNOS status 1B vs 2 | 2.08 (1.86–2.31) | <0.0001 | 2.07 (1.87–2.29) | <0.0001 |
| PF-LVAD vs status 2 | 2.15 (1.87–2.47) | <0.0001 | 2.11 (1.85–2.40) | <0.0001 |
| CF-LVAD vs status 2 | 1.48 (1.13–1.93) | 0.01 | 1.45 (1.12–1.87) | 0.004 |
| TAH vs status 2 | 3.58 (1.34–9.59) | 0.01 | 4.05 (1.68–9.78) | 0.004 |
| BIVADs vs status 2 | 7.00 (4.89–10.03) | <0.0001 | 7.69 (5.52–10.70) | <0.0001 |
| Temporary VAD vs status 2 | 16.18 (11.95–21.92) | <0.0001 | 16.45 (12.32–21.96) | <0.0001 |
BIVAD indicates biventricular assist device; CF-LVAD, continuous-flow left ventricular assist device; CI, confidence interval; CMP, cardiomyopathy; HR, hazard ratio; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PF-LVAD, pulsatile-flow left ventricular assist device; TAH, total artificial heart; and UNOS, United Network for Organ Sharing. HRs, 95% CIs, and P values were generated by use of a Cox proportional hazard analysis. Multiple-group comparisons were adjusted by use of the Holm procedure.
Biventricular and Temporary Support
Patients with other forms of MCS—TAH, BIVADs, and temporary VADs—had a markedly (3- to 16-fold) increased risk of the primary and secondary outcomes compared with status 2 listed candidates (Table 2). Waiting list survival and survival free from death or delisting as a result of worsening clinical status in patients with the various forms of MCS are presented in Figure 2.
Figure 2.
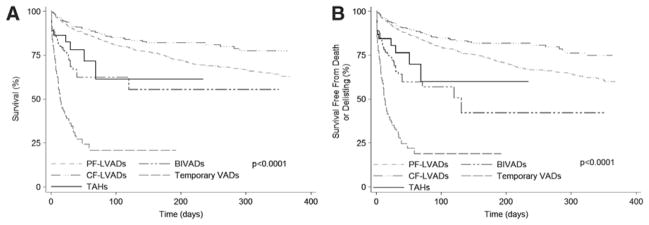
Outcomes for heart transplant candidates requiring mechanical circulatory support on the United Network for Organ Sharing (UNOS) waiting list in the first era. A, Unadjusted waiting list survival and B, unadjusted waiting list survival free from death or delisting as a result of worsening clinical status. BIVAD indicates biventricular assist device; CF, continuous flow; LVAD, left ventricular assist device; PF, pulsatile flow; and TAH, total artificial heart.
Current Era: April 2008 to December 2011
Waiting list mortality decreased significantly in the current era compared with the first era: 2.1%/mo versus 2.9%/ mo (P<0.0001). In patients without MCS, the listing status still discriminated well the risk of waiting list mortality: 9.6%/mo in status 1A, 2.6%/mo in status 1B, and 1.0%/mo in status 2 patients (P<0.0001 for all comparisons; Figure 3A). CF-LVADs were the most commonly used devices for bridging to transplantation in this era. The waiting list mortality in the CF-LVAD group was 1.0%/mo, similar to the mortality rate of 1.0% in the low-urgency status 2 patients (P=0.62). Results of a univariable analysis of waiting list mortality are shown in the Table III of the online-only Data Supplement. After adjustment for variables detailed in the Methods section with a multivariable Cox regression analysis (Table 3), mortality risk in patients bridged to transplantation with CF-LVADs was no longer higher than in the low-urgency status 2 patients; in fact, there was a trend toward lower mortality risk in CF-LVAD–supported patients (HR=0.80; P=0.12). The multivariable adjustment also confirmed that the high-urgency status 1A and the intermediate-urgency status 1B were independently associated with increased mortality risk compared with status 2 (P<0.0001 for both comparisons).
Figure 3.
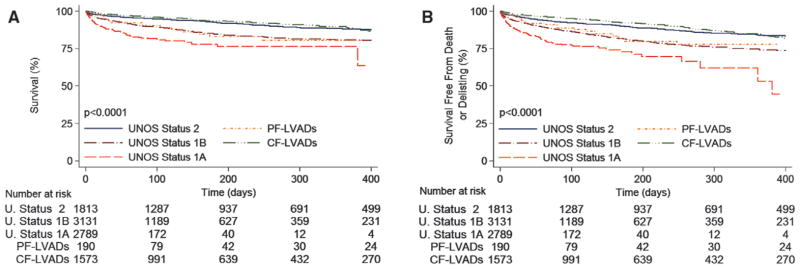
Outcomes for heart transplant candidates on the United Network for Organ Sharing (UNOS) waiting list in the current era. A, Unadjusted waiting list survival according to UNOS status and left ventricular (LVAD) support type. B, Unadjusted waiting list survival free from death or delisting as a result of worsening clinical status according to UNOS status and LVAD support type. CF indicates continuous flow; and PF, pulsatile flow.
Table 3.
Multivariable Hazard Ratio Estimates for the Risk of Death on the Waiting List and for the Risk of Death or Delisting Among Heart Transplant Candidates in the Current Era (2008–2011)
| Mortality | Mortality or Delisting | |||
|---|---|---|---|---|
|
| ||||
| Variable | HR (95% CI) | P | HR (95% CI) | P |
| Age (per year) | … | … | 1.01 (1.01–1.02) | <0.0001 |
| Restrictive vs nonischemic CMP | 1.47 (1.11–1.94) | 0.007 | 1.50 (1.17–1.91) | 0.001 |
| Diabetes mellitus (yes vs no) | 1.24 (1.08–1.41) | 0.002 | 1.20 (1.07–1.36) | 0.002 |
| Serum creatinine (per 1 mg/dL) | 1.23 (1.19–1.28) | <0.0001 | 1.21 (1.15–1.26) | <0.0001 |
| Mean PAP (per 1 mm Hg) | 1.02 (1.01–1.02) | <0.0001 | 1.01 (1.01–1.02) | 0.001 |
| Inotropic support (yes vs no) | 1.20 (1.04–1.38) | 0.01 | … | … |
| Mechanical ventilation (yes vs no) | 3.36 (2.58–4.38) | <0.0001 | 2.89 (2.25–3.71) | <0.0001 |
| UNOS status 1A vs 2 | 3.26 (2.62–4.05) | <0.0001 | 3.14 (2.60–3.80) | <0.0001 |
| UNOS status 1B vs 2 | 1.68 (1.38–2.04) | <0.0001 | 1.68 (1.42–1.98) | <0.0001 |
| PF-LVAD vs status 2 | 2.04 (1.34–3.08) | 0.003 | 1.97 (1.36–2.84) | <0.0001 |
| CF-LVAD vs status 2 | 0.80 (0.63–1.01) | 0.12 | 0.81 (0.66–0.99) | 0.08 |
| TAH vs status 2 | 2.36 (0.75–7.41) | 0.14 | 2.56 (0.81–8.03) | 0.11 |
| BIVADs vs status 2 | 5.00 (3.34–7.49) | <0.0001 | 5.31 (3.60–7.82) | <0.0001 |
| Temporary VAD vs status 2 | 7.72 (4.28–13.91) | <0.0001 | 8.53 (4.86–14.98) | <0.0001 |
BIVAD indicates biventricular assist device; CF-LVAD, continuous-flow left ventricular assist device; CI, confidence interval; CMP, cardiomyopathy; HR, hazard ratio; PAP, pulmonary artery pressure; PF-LVAD, pulsatile-flow left ventricular assist device; TAH, total artificial heart; and UNOS, United Network for Organ Sharing. HRs, 95% CIs, and P values were generated with a Cox proportional hazard analysis. Multiple-group comparisons were adjusted by use of the Holm procedure.
To ascertain that these results were not influenced by del-isting of patients whose condition worsened while awaiting transplantation, we evaluated the composite outcome of waiting list mortality or delisting as a result of worsening clinical status. The composite outcome occurred with an incidence rate of 2.8%/mo. The incidence rate was 11.7%/mo in status 1A patients, 3.4%/mo in status 1B patients, and 1.4%/mo in status 2 patients (P<0.0001 for all comparisons). The incidence rate of the composite outcome in the CF-LVAD group most closely approximated status 2 patients (1.4%/mo versus 1.4%/mo; P=0.93; Figure 3B). Only a minority of patients were bridged with PF-LVADs in the current era, and the rate of mortality or delisting in this group was similar to that of status 1B patients (3.2%/mo versus 3.4%/mo; P=0.79). The results of a univariable (Table III of the online-only Data Supplement) and a multivariable Cox regression analysis for the composite outcome (Table 3) confirmed the favorable risk profile of CF-LVADs, and similarly to the primary outcome, candidates with CF-LVADs had a trend toward lower risk of the secondary outcome compared with status 2 candidates in the current era (HR=0.81; P=0.08).
Biventricular and Temporary Support
Heart transplant candidates requiring support with BIVADs (HR=5.00; P<0.0001) and temporary VADs (HR=7.72; P<0.0001) had the highest risk of death compared with status 2 and other candidates in the current era. Patients requiring TAH had a >2-fold increase in mortality risk, but this did not reach statistical significance (HR=2.36; P=0.14; Table 3 and Figure 4A). For the composite outcome, candidates requiring the use of BIVADs (HR=5.31; P<0.0001) and temporary VADs (HR=8.53; P<0.0001) had the highest risk of death or delisting as a result of worsening clinical status. Similar to the primary outcome, support with TAH resulted in a trend for an increased risk of death or delisting compared with status 2 candidates (HR=2.56; P=0.11; Table 3 and Figure 4B).
Figure 4.
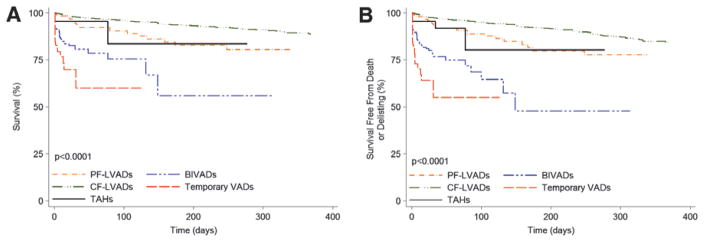
Outcomes for heart transplant candidates requiring mechanical circulatory support on the United Network for Organ Sharing (UNOS) waiting list in the current era. A, Unadjusted waiting list survival and (B) unadjusted waiting list survival free from death or delisting as a result of worsening clinical status. BIVAD indicates biventricular assist device; CF, continuous flow; LVAD, left ventricular assist device; PF, pulsatile flow; and TAH, total artificial heart.
LVAD Listing, Status 1A and 1B Subgroups
LVAD-supported patients are afforded a 30-day period of 1A status listing and, if not transplanted, are then downgraded to 1B status. It would appear that the risk of an adverse event on the waiting list during status 1A and 1B time would be similar in these LVAD-supported patients; therefore, we approached these patients as 1 group. To confirm that our assumption of risk was correct, we compared the risk of the primary and secondary outcomes in LVAD-supported patients during their status 1A and 1B time. There was no difference in survival free from the primary and secondary outcomes during LVAD patients’ status 1A and 1B listings in the first era (Figure I of the online-only Data Supplement) or the current era (Figure II of the online-only Data Supplement). The risk remained similar after adjustment in a multivariable model.
Listing Status Upgrade Resulting From Device-Related Complications
For patients with MCS-related complications, the UNOS allocation algorithm allows status 1A (high-urgency) listing to expedite transplantation. Such complications are defined by the OPTN policy as objective medical evidence of signifi-cant device-related complications (thromboembolism, device infection, mechanical failure, or life-threatening ventricular arrhythmias). Each such listing is reviewed by UNOS staff for appropriateness and has to be recertified by an attending physician every 14 days if extension of the status 1A listing is requested. Listing upgrade resulting from complications other than thromboembolism, device infection, mechanical failure, or life-threatening ventricular arrhythmias results in a review by the UNOS Heart Regional Review Board, which votes to approve or disapprove the listing upgrade.25
We also evaluated the risk of the primary and secondary outcomes in the candidates whose status was upgraded to status 1A owing to LVAD-related complications. In the first era, the status of 616 LVAD-supported candidates (29%) was upgraded to 1A as a result of LVAD-related complications. Compared with LVAD-supported candidates without a complication, these patients had higher rates of death (7.4%/mo versus 5.8%/mo; P=0.04) and death or delisting (8.3%/mo versus 6.3%/mo; P=0.02; Figure 5). However, in multivariable analysis considering variables detailed in the Methods section, the risk of death (HR=0.89; 95% confidence interval, 0.69–1.14; P=0.36) and death or delisting (HR=0.96; 95% confidence interval, 0.76–1.22; P=0.75) was similar between candidates listed with and without LVAD-related complications. In the current era, 491 candidates (28%) were upgraded to status 1A because of LVAD-related complications. Patients with complications had higher rates of death (2.3%/mo versus 1.2%/ mo; P<0.0001) and death or delisting (2.7%/mo versus 1.3%/ mo; P=0.0008) compared with patients without LVAD-related complications (Figure 6). These results were confirmed in multivariable analysis, in which candidates listed in status 1A as a result of an LVAD-related complication had a higher risk of death (HR=1.47; 95% confidence interval, 1.00–2.18; P=0.05) and death or delisting (HR=1.75; 95% confidence interval, 1.26–2.42; P=0.001) compared with candidates without an LVAD-related complication. In fact, their risk for both outcomes was now similar to that of status 1B listed candidates (P>0.38 for both comparisons; Figure 6). Survival free from listing status upgrade because of LVAD-related complications and the hazard function for such an upgrade in the current era are shown in Figure 7.
Figure 5.
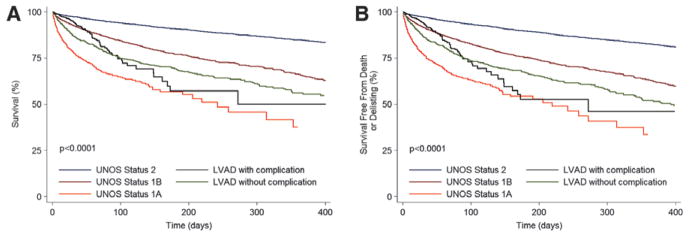
Waiting list outcomes in left ventricular assist device (LVAD) recipients with and without complications in relation to United Network for Organ Sharing (UNOS) status groups in the first era. A, Unadjusted waiting list survival. B, Unadjusted waiting list survival free from death or delisting as a result of worsening clinical status. Time 0 for LVAD recipients with complications is the time of status 1A upgrade resulting from an LVAD complication. Time 0 for LVAD recipients without complications is the time of listing for transplantation with an LVAD.
Figure 6.
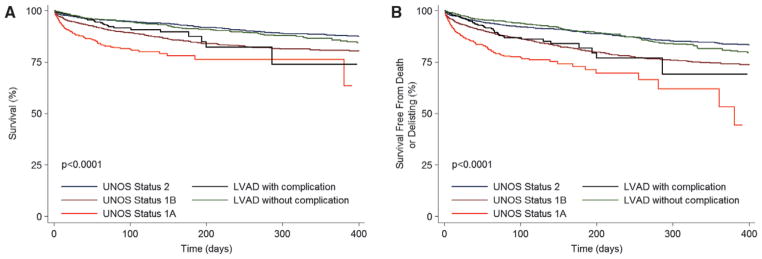
Waiting list outcomes in left ventricular assist device (LVAD) recipients with and without complications in relation to United Network for Organ Sharing (UNOS) status groups in the current era. A, Unadjusted waiting list survival. B, Unadjusted waiting list survival free from death or delisting as a result of worsening clinical status. Time 0 for LVAD recipients with complications is the time of status 1A upgrade resulting from an LVAD complication. Time 0 for LVAD recipients without complications is the time of listing for transplantation with an LVAD.
Figure 7.
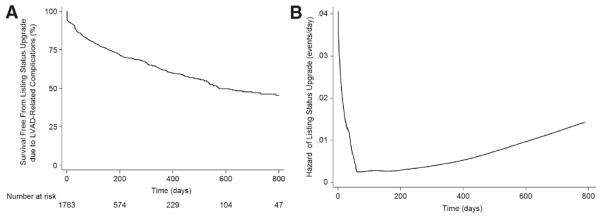
Listing status upgrade as a result of left ventricular assist device (LVAD)–related complications in the current era. A, Survival free from listing status upgrade resulting from LVAD-related complications. Patients were censored at the time of transplantation, death, or delisting. B, Hazard function for the risk of listing status upgrade owing to LVAD-related complications.
Competing Outcomes
To better illustrate the changing outcomes of LVAD-supported patients on the transplant waiting list, we display the different competing outcomes in Figure 8. In the first era, 83% of LVAD-supported patients at 180 days of listing and 80% of LVAD-supported patients at 365 days achieved a positive outcome of survival to transplantation, continued LVAD support, or delisting resulting from clinical recovery (Figure 8A). The outcomes were more favorable in the current era, in which 94% and 91% of the LVAD-supported patients were transplanted, continued on LVAD support, or delisted as a result of clinical recovery at 180 and 365 days (Figure 8B). The proportion of patients undergoing transplantation by 180 days in the current era was lower (46%) than in the first era (57%), and the number of patients who were not transplanted but remained alive on continued LVAD support at 180 days was higher in the current era (48% compared with 25% in the first era). The number of patients delisted as a result of worsening clinical status (1%) or clinical recovery (<1%) remained constant, whereas waiting list mortality of LVAD-bridged patients decreased by nearly 70% in the current era (16% versus 5%; P<0.01).
Figure 8.
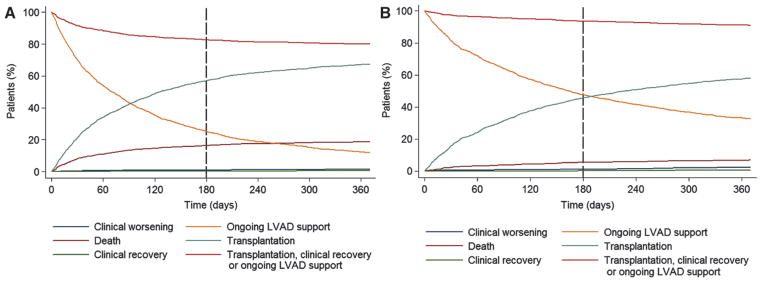
Competing outcomes for heart transplant candidates supported with left ventricular assist devices (LVADs) while on the United Network for Organ Sharing waiting list. A, In the first era, after 6 months of LVAD support, 83% of the patients were alive on ongoing LVAD support (25%), transplanted (57%), or delisted because of clinical recovery (0.3%). The remaining 17% of the patients had died (16%) or had been delisted as a result of worsening clinical status (1%). B, In the current era, after 6 months of LVAD support, 94% of the patients were alive on ongoing LVAD support (48%), transplanted (46%), or delisted because of clinical recovery (0.2%). The remaining 6% of the patients had died (5%) or had been delisted as a result of worsening clinical status (1%).
Discussion
This study evaluated the impact of MCS on heart transplant waiting list outcomes in an era of technological improvement and greater experience in the management of patients with VADs. The main finding of our study is the demonstration of markedly improved waiting list mortality and morbidity of heart transplant candidates bridged with durable LVADs in the current era. The mortality and morbidity risk in patients bridged with durable CF-LVADs is now similar to that in the status 2 listed patients (lowest priority). However, we also show that LVAD-supported transplant candidates whose status on the waiting list is upgraded as a result of an LVAD-related complication have a risk of mortality and mortality or delisting that is markedly higher compared with LVAD-supported candidates listed without complications and with status 2 listed patients. Interestingly, the proportion of patients who are listed in status 1A because of an LVAD-related complication is high and has remained without sig-nificant change between the 2 eras; 28% of LVAD-supported patients in the current era are listed in status 1A because of a device-related complication.
In the current era, the hazard of LVAD-related complications is highest early after listing for transplantation and nadirs at 80 days, after which it appears to again gradually increase (Figure 7). Patients requiring biventricular support and temporary VADs continue to have a very high risk of adverse outcomes on the waiting list.
Since its inception, OPTN/UNOS has strived to maintain a fair and balanced organ allocation system by prioritizing organ allocation to patients with the highest risk of death while waiting for a donor organ. Our analyses from the first era confirm the pertinence of the indefinite 1B status listing afforded to the LVAD-bridged candidates by the UNOS policies at the time. The use of the first-generation PF-LVADs was associated with reduced mortality in heart transplant candidates with a very high risk of death and allowed these patients to reach heart transplantation. The risk of death or delisting of these LVAD-supported candidates remained significant, however, and our study shows that this risk was similar to that of status 1B candidates without MCS. Assessment of more recent outcomes shows that waiting list mortality of patients bridged with CF-LVADs after 2008 has decreased significantly and is below the waiting list mortality of status 1B candidates not supported with LVADs. In fact, the waiting list mortality of CF-LVAD–supported patients without serious LVAD-related complications now is similar to the mortality of low-urgency status 2 transplant candidates. The impact of improving outcomes in LVAD-supported transplant candidates on reducing the wait-list mortality has been important. For illustration, the overall waiting list mortality has decreased in recent years, and much of this effect was attributed to the broader organ regional sharing implemented by UNOS in 2006.26 It is likely, however, that LVAD use has also contributed significantly to this trend; our findings show that the reduction of waiting list mortality in LVAD-bridged patients in this time frame (4.1%/mo to 1.2%/mo, a 71% reduction) has been far larger than in patients without MCS (2.6%/mo to 2.3%/mo, a 12% reduction).
The improved waiting list survival of LVAD-supported candidates is a remarkable achievement that affords positive outcome to patients who would have been at a high risk of mortality in the past. Technological advances, expanding clinical experience with mechanical assist, and refined patient selection approaches have all contributed to the better outcomes and to the fact that more patients are now being considered for LVAD support.1,27 However, with these uniformly positive developments, we are also faced with a question of whether the current UNOS heart allocation algorithm remains equitable.15,28 Our data suggest that this question is multifaceted. For patients who require biventricular support and support with pulsatile or nondurable mechanical assist devices, the adverse outcomes on the waiting list remain high. Therefore, affording high-urgency status to these patients appears appropriate. The markedly improved wait-list mortality for patients bridged with CF-LVADs raises new considerations. Should the risk of mortality on the waiting list be the sole determinant of the allocation priority? If the answer is yes, then the current UNOS allocation system would appear to be outdated because patients with LVADs might have an advantage over patients at a higher risk of wait-list mortality, including those who may not be LVAD candidates. Along these lines, there have been suggestions to revise the UNOS allocation algorithm and possibly align it more closely with the Eurotransplant allocation system, which does not grant high-urgency status to LVAD-supported heart transplant candidates unless a device-related complication occurs.15,29 In contrast, our data indicate that this approach might not necessarily improve outcomes on the UNOS waiting list. LVAD implantation often transforms a sick patient at very high risk of wait-list and posttransplant mortality into a good transplant candidate with improved organ function and nutritional and physical condition, so transplant in this favorable situation may be of the most benefit. Our data show that almost 30% of LVAD-supported transplant candidates develop a complication that justifies a higher-urgency status listing and that, once this occurs, the risk of death or delist-ing is markedly increased. Some of these complications (eg, stroke) may also have long-lasting effects on patient quality of life after transplantation. If, as a result of a change in the organ allocation algorithm, LVAD-supported patients were to remain on the waiting list for a longer period of time, the cumulative incidence of device-related complications would likely increase (Figure 7). Thus, the intent and the considerable expense that were dispensed to get an ill patient to transplant eligibility through the implantation of an LVAD could be negated, and the recent improvements in waiting list outcomes could be jeopardized.
Another consideration is that any organ allocation change is expected to result in changes in clinical decisions. It is conceivable that, if LVAD-supported patients were not given allocation priority, physicians and patients might opt to delay LVAD implantation for as long as possible in hopes of increasing the probability of receiving a heart transplant in high-urgency status on medical management. This, however, may expose the patients to higher risk of dying or becoming ineligible for transplantation (as our data for 1A and 1B status patients would suggest). In addition, those who decompensate on medical therapy may more likely require emergent LVAD implantation, increasing the risk profile of this group as well. These arguments would support continuation of the current allocation algorithm without change.
We recognize that there are limitations to our study. This was a retrospective analysis of a nationwide clinical registry. Although UNOS data collection is rigorous and undergoes periodic audits, some errors in data entry may be present. LVAD-specific morbidity outcomes and hospitalization data were not available and thus could not be contrasted with the morbidity outcomes and hospitalization rates of medically supported patients on the waiting list. Data on duration of LVAD support before registration on the waiting list were not available. Therefore, we were not able to accurately assess the effect of LVAD support duration on the risk of death. The Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) profile for the patients in our study was not available; therefore, we were unable to explore the effects of this characteristic on evaluated outcomes. Some patients who were already active on the waiting list later received mechanical assist devices. We assigned these patients to the medical therapy group because that was the original intent at the time of listing. Patients who underwent VAD implantation but died or had significant complications precluding their relisting contributed to the primary and secondary outcomes of the medical treatment groups. Patients who were relisted for transplantation with an LVAD in the current era had an exceptionally good outcome: waiting list mortality rate of 0.74%/mo and mortality or delisting rate of 1.0%/mo. For completeness, we performed ancillary analyses with the group of patients requiring LVAD placement while already on the waiting list. We determined that this study group assignment did not change the results of the study (data not shown). Finally, the prevalence of conditions that would preclude LVAD implantation such as significant right ventricular dysfunction or certain forms of congenital heart disease could not be determined from this registry. We are aware that not all the questions raised can be answered by our study. Nevertheless, as an allocation change is being considered, we believe our analysis provides important insights that can help inform policy.
Conclusions
Mortality and morbidity have decreased in patients awaiting heart transplant in the current era. Although the current allocation system accurately reflects the risk of mortality in medically managed patients awaiting heart transplantation, the issue of the most appropriate allocation priority in MCS- supported patients remains complex. Contemporary heart transplant candidates supported with CF-LVADs have the lowest risk of adverse outcomes while on the waiting list. However, serious LVAD-related complications occur frequently and are associated with an increased risk of death or delisting in these patients. Furthermore, transplant candidates requiring temporary and biventricular support represent a group at the highest risk of adverse outcomes on the waiting list. These results provide important information that can be helpful in guiding future allocation changes involving the complex and urgent topic of transplant prioritization.
Supplementary Material
CLINICAL PERSPECTIVE.
Heart transplant candidates supported with left ventricular assist devices (LVADs) are granted 30 days in high-urgency status 1A and indefinite time in intermediate-urgency status 1B. Improvement in outcomes observed with the new continuous-flow LVADs has brought into question whether current allocation policy, implemented in the pulsatile-flow LVAD era, is still justi-fied. The United Network for Organ Sharing (UNOS) registry was used to analyze the risk of death or delisting while on the heart transplant waiting list in 33 073 candidates listed from 1999 to 2011. Study groups were selected on the basis of the need for an LVAD and UNOS listing status. Two eras were defined on the basis of the approval date of the first continuous-flow LVAD for bridge to transplantation in the United States. Waiting list mortality decreased in the current compared with the first era. In the current era, patients with continuous-flow LVADs had a mortality risk that was similar to that of status 2 patients (lowest priority) and lower than for status 1B and 1A listed candidates. This was a significant change compared with the first era, in which the mortality of pulsatile-flow LVAD–supported patients was higher than that of status 2 patients and similar to that of status 1B patients. However, status upgrade for LVAD-related complications occurred frequently in both eras and significantly increased the risk of adverse outcomes. The risk of mortality and morbidity was highest in patients with biven-tricular assist devices and temporary VADs. These results may help to guide optimal allocation of donor hearts.
Acknowledgments
We are indebted to Gregory J. Stoddard, MPH, for his review and valuable contribution to the manuscript.
Sources of Funding
This work was supported in part by Health Resources and Services Administration contract 231-00-0115. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Footnotes
Disclosures
None.
References
- 1.Stehlik J, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dob-bels F, Kirk R, Rahmel AO, Hertz MI. The Registry of the International Society for Heart and Lung Transplantation: twenty-eighth adult heart transplant report—2011. J Heart Lung Transplant. 2011;30:1078–1094. doi: 10.1016/j.healun.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Renlund DG, Taylor DO, Kfoury AG, Shaddy RS. New UNOS rules: historical background and implications for transplantation management: United Network for Organ Sharing. J Heart Lung Transplant. 1999;18:1065–1070. doi: 10.1016/s1053-2498(99)00075-3. [DOI] [PubMed] [Google Scholar]
- 3.Zaroff JG, Rosengard BR, Armstrong WF, Babcock WD, D’Alessandro A, Dec GW, Edwards NM, Higgins RS, Jeevanandum V, Kauffman M, Kirklin JK, Large SR, Marelli D, Peterson TS, Ring WS, Robbins RC, Russell SD, Taylor DO, Van Bakel A, Wallwork J, Young JB. Consensus conference report: maximizing use of organs recovered from the cadaver donor: cardiac recommendations, March 28–29, 2001, Crystal City, Va. Circulation. 2002;106:836–841. doi: 10.1161/01.cir.0000025587.40373.75. [DOI] [PubMed] [Google Scholar]
- 4.Chen JM, Sinha P, Rajasinghe HA, Suratwala SJ, McCue JD, McCarty MJ, Caliste X, Hauff HM, John R, Edwards NM. Do donor characteristics really matter? Short- and long-term impact of donor characteristics on recipient survival, 1995–1999. J Heart Lung Transplant. 2002;21:608–610. doi: 10.1016/s1053-2498(01)00367-9. [DOI] [PubMed] [Google Scholar]
- 5.Shafer TJ, Wagner D, Chessare J, Zampiello FA, McBride V, Perdue J. Organ donation breakthrough collaborative: increasing organ donation through system redesign. Crit Care Nurse. 2006;26:33–42. 44. [PubMed] [Google Scholar]
- 6.Shafer TJ, Wagner D, Chessare J, Schall MW, McBride V, Zampiello FA, Perdue J, O’Connor K, Lin MJ, Burdick J. US organ donation breakthrough collaborative increases organ donation. Crit Care Nurs Q. 2008;31:190–210. doi: 10.1097/01.CNQ.0000325044.78904.9b. [DOI] [PubMed] [Google Scholar]
- 7.Johnson MR, Meyer KH, Haft J, Kinder D, Webber SA, Dyke DB. Heart transplantation in the United States, 1999–2008. Am J Transplant. 2010;10(pt 2):1035–1046. doi: 10.1111/j.1600-6143.2010.03042.x. [DOI] [PubMed] [Google Scholar]
- 8.Frazier OH, Rose EA, McCarthy P, Burton NA, Tector A, Levin H, Kayne HL, Poirier VL, Dasse KA. Improved mortality and rehabilitation of transplant candidates treated with a long-term implantable left ventricular assist system. Ann Surg. 1995;222:327–336. doi: 10.1097/00000658-199509000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frazier OH, Rose EA, Oz MC, Dembitsky W, McCarthy P, Radovancevic B, Poirier VL, Dasse KA HeartMate LVAS Investigators. Left Ventricular Assist System. Multicenter clinical evaluation of the HeartMate vented electric left ventricular assist system in patients awaiting heart transplantation. J Thorac Cardiovasc Surg. 2001;122:1186–1195. doi: 10.1067/mtc.2001.118274. [DOI] [PubMed] [Google Scholar]
- 10.Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, Conte JV, Naka Y, Mancini D, Delgado RM, MacGillivray TE, Farrar DJ, Frazier OH HeartMate II Clinical Investigators. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–896. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 11.Aaronson KDSM, McGee E, Cotts WG, Acker MA, Jessup ML, Gregoric ID, Loyalka P, Jeevanandam V, Aderson AS, Kormos RL, Teuteberg JJ, Pagani FD, Boyce S, Hathaway D, Miller LW, Acker MA. Evaluation of the Heartware® HVAD left ventricular assist device system for the treatment of advanced heart failure: results of the Advance Bridge to Transplant Trial. Circulation. 2010;122:2215–2226. [Google Scholar]
- 12.Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA, Ulisney KL, Baldwin JT, Young JB. Second INTERMACS annual report: more than 1,000 primary left ventricular assist device implants. J Heart Lung Transplant. 2010;29:1–10. doi: 10.1016/j.healun.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Starling RC, Naka Y, Boyle AJ, Gonzalez-Stawinski G, John R, Jorde U, Russell SD, Conte JV, Aaronson KD, McGee EC, Jr, Cotts WG, DeNofrio D, Pham DT, Farrar DJ, Pagani FD. Results of the post-U.S. Food and Drug Administration-approval study with a continuous flow left ventricular assist device as a bridge to heart transplantation: a prospective study using the INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support) J Am Coll Cardiol. 2011;57:1890–1898. doi: 10.1016/j.jacc.2010.10.062. [DOI] [PubMed] [Google Scholar]
- 14.Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA, Baldwin JT, Young JB. The fourth INTERMACS annual report: 4,000 implants and counting. J Heart Lung Transplant. 2012;31:117–126. doi: 10.1016/j.healun.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Moazami N, Sun B, Feldman D. Stable patients on left ventricular assist device support have a disproportionate advantage: time to re-evaluate the current UNOS policy. J Heart Lung Transplant. 2011;30:971–974. doi: 10.1016/j.healun.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Strueber M, O’Driscoll G, Jansz P, Khaghani A, Levy WC, Wieselthaler GM HeartWare Investigators. Multicenter evaluation of an intrapericar-dial left ventricular assist system. J Am Coll Cardiol. 2011;57:1375–1382. doi: 10.1016/j.jacc.2010.10.040. [DOI] [PubMed] [Google Scholar]
- 17.Frazier OH, Myers TJ, Westaby S, Gregoric ID. Use of the Jarvik 2000 left ventricular assist system as a bridge to heart transplantation or as destination therapy for patients with chronic heart failure. Ann Surg. 2003;237:631–636. doi: 10.1097/01.SLA.0000064359.90219.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haj-Yahia S, Birks EJ, Rogers P, Bowles C, Hipkins M, George R, Amrani M, Petrou M, Pepper J, Dreyfus G, Khaghani A. Midterm experience with the Jarvik 2000 axial flow left ventricular assist device. J Thorac Cardio-vasc Surg. 2007;134:199–203. doi: 10.1016/j.jtcvs.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Nativi JN, Drakos SG, Kucheryavaya AY, Edwards LB, Selzman CH, Taylor DO, Hertz MI, Kfoury AG, Stehlik J. Changing outcomes in patients bridged to heart transplantation with continuous- versus pulsatile-flow ventricular assist devices: an analysis of the registry of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2011;30:854–861. doi: 10.1016/j.healun.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 20.Box GEP. Some theorems on quadratic forms applied in the study of analysis of variance problems, I. effect of inequality of variance in the one-way classification. Ann Math Statist. 1954;25:290–302. [Google Scholar]
- 21.Lumley T, Diehr P, Emerson S, Chen L. The importance of the normality assumption in large public health data sets. Annu Rev Public Health. 2002;23:151–169. doi: 10.1146/annurev.publhealth.23.100901.140546. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statist Assn. 1958;53:457–481. [Google Scholar]
- 23.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 24.Cox DR. Regression models and life-tables. R Stat Soc Series B Stat Meth-odol. 1972;34:187–220. [Google Scholar]
- 25. [Accessed November 20, 2012];Organ procurement and transplantation network policy 3.7: allocation of thoracic organs. 2012 Sep 1; http://optn.transplant.hrsa.gov/Pol-iciesandBylaws2/policies/pdfs/policy_9.pdf.
- 26.Singh TP, Almond CS, Taylor DO, Graham DA. Decline in heart transplant wait list mortality in the United States following broader regional sharing of donor hearts. Circ Heart Fail. 2012;5:249–258. doi: 10.1161/CIRCHEARTFAILURE.111.964247. [DOI] [PubMed] [Google Scholar]
- 27.Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA, Ulisney KL, Baldwin JT, Young JB. Third INTERMACS annual report: the evolution of destination therapy in the United States. J Heart Lung Transplant. 2011;30:115–123. doi: 10.1016/j.healun.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Dardas T, Mokadam NA, Pagani F, Aaronson K, Levy WC. Transplant registrants with implanted left ventricular assist devices have insufficient risk to justify elective organ procurement and transplantation network status 1A time. J Am Coll Cardiol. 2012;60:36–43. doi: 10.1016/j.jacc.2012.02.031. [DOI] [PubMed] [Google Scholar]
- 29.Komoda T, Hetzer R, Lehmkuhl HB. Influence of new Eurotransplant heart allocation policy on outcome of heart transplant candidates in Germany. J Heart Lung Transplant. 2008;27:1108–1114. doi: 10.1016/j.healun.2008.07.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


