Abstract
The hereditary sensory and autonomic neuropathies (HSAN, also known as the hereditary sensory neuropathies) are a clinically and genetically heterogeneous group of disorders, characterised by a progressive sensory neuropathy often complicated by ulcers and amputations, with variable motor and autonomic involvement. To date, mutations in twelve genes have been identified as causing HSAN. To study the frequency of mutations in these genes and the associated phenotypes, we screened 140 index patients in our inherited neuropathy cohort with a clinical diagnosis of HSAN for mutations in the coding regions of SPTLC1, RAB7, WNK1/HSN2, FAM134B, NTRK1 (TRKA) and NGFB. We identified 25 index patients with mutations in six genes associated with HSAN (SPTLC1, RAB7, WNK1/HSN2, FAM134B, NTRK1 and NGFB); 20 of which appear to be pathogenic giving an overall mutation frequency of 14.3%. Mutations in the known genes for HSAN are rare suggesting that further HSAN genes are yet to be identified. The p.Cys133Trp mutation in SPTLC1 is the most common cause of HSAN in the UK population and should be screened first in all patients with sporadic or autosomal dominant HSAN.
Introduction
The hereditary sensory and autonomic neuropathies (HSAN, also known as the hereditary sensory neuropathies, HSN) are a clinically and genetically heterogeneous group of disorders. The cardinal clinical feature is a predominantly sensory axonal neuropathy characterised by loss of sensation including pain and temperature and in some subtypes positive sensory symptoms such as pain and paraesthesiae. The sensory loss frequently results in complications such as ulcers, infections, osteomyelitis, selfmutilation and spontaneous or surgical amputations. Motor and autonomic involvement occur to a variable degree [8, 21]. HSAN has been traditionally classified into five groups (HSAN I–V) [2] based on mode of inheritance and clinical features. More recently the classification has included the known causative genes. To date, mutations in 12 genes have been identified as being responsible for HSAN (Table 1). Genetic analysis is essential in the diagnosis of patients with HSAN and has clarified the phenotypic spectrum and helped further the understanding of the mechanisms of sensory neuron degeneration. Rotthier et al. [24] investigated a cohort of 100 European patients with HSAN and found mutations in 19%; most frequently in RAB7 and NTRK1. Mutations were also found in HSN2/WNK1 and SPTLC1; however, no mutations were present in CCT5 or NGFB.
Table 1.
Classification of the hereditary sensory and autonomic neuropathies
| Type | Inheritance | Gene/locus | Specific phenotype |
|---|---|---|---|
| HSAN I | AD | SPTLC1 | Predominantly sensory neuropathy, frequent later motor involvement, neuropathic pain, |
| ulcero-mutilating complications | |||
| HSAN I | AD | SPTLC2 | As for SPTLC1 |
| HSAN I | AD | ATL1 | Sensory neuropathy without motor involvement, reflexes may be brisk, ulcero-mutilating complications. Spasticity has been described (allelic with hereditary spastic paraplegia |
| HSP3A) | |||
| HSAN I | AD | DNMT1 | Sensory neuropathy, sensorineural deafness with dementia developing in 4th decade |
| CMT2B | AD | RAB7 | Sensorimotor neuropathy, ulcero-mutilating complications |
| HSAN1B | AD | 3p22-p24 | Sensory neuropathy, cough, gastro-oesophageal reflux |
| HSAN II | AR | HSN2/WNK1 | Sensory neuropathy, severe ulcero-mutilating complications, frequent autonomic |
| dysfunction, onset first two decades | |||
| HSAN II | AR | FAM134B | Sensory neuropathy, severe ulcero-mutilating complications, variable autonomic |
| and motor involvement | |||
| HSAN II | AR | KIF1A | Sensory neuropathy, severe ulceromutilating complications, mild motor involvement |
| HSAN III | AR | IKBKAP | Familial dysautonomia or Riley-Day syndrome, prominent autonomic dysfunction, |
| absent fungiform papillae of the tongue | |||
| HSAN IV | AR | NTRK1 | Congenital insensitivity to pain with anhydrosis (CIPA), severe sensory neuropathy, anhidrosis, mental retardation, unmyelinated fibers mainly affected |
| HSAN V | AR | NGFB | Congenital insensitivity to pain, minimal autonomic dysfunction, no mental retardation, mainly small myelinated fibers affected (one case of HSAN V described due to NTRK1 mutations) |
| HSAN with spastic Paraplegia | AR | CCT5 | Mutilating sensory neuropathy with spastic paraplegia |
AD autosomal dominant, AR autosomal recessive, SPLTC1 serine palmitoyltransferase, long chain base subunit-1, SPTLC2 serine palmitoyltransferase, long chain base subunit-2, ATL1 atlastin-1, RAB7 member RAS oncogene family, DNMT1 DNA methyltransferase 1, HSN2/WNK1 nerve specific isoform of WNK lysine deficient protein kinase 1, FAM134B family with sequence similarity 134, member B, KIF1A kinesin family member 1A, IKBKAP inhibitor of kappa light polypeptide gene enhancer in B-cells, kinase complex-associated protein, NTRK1 neurotrophic tyrosine kinase receptor type 1, NGFB nerve growth factor beta polypeptide, CCT5 chaperonin containing T-complex polypeptide 1, subunit 5
This study was designed to determine if the frequency of mutations in the genes associated with HSAN is similar in our mainly UK cohort and to describe the associated phenotypes. A total of 140 index cases with HSAN/sensory predominant CMT2 were screened for mutations in the coding regions of SPTLC1, RAB7, WNK1/HSN2, FAM134B, NTRK1 and NGFB. We did not test for IKBKAP or CCT5 as none of our patients had the distinctive phenotype of Riley-Day syndrome (HSAN III) and none had spastic paraplegia. We did not screen SPTLC2, ATL-1, DNMT1 or KIF1A as these recently described genes were reported subsequent to completion of this project.
Methods
This study was carried out in the National Hospital for Neurology and Neurosurgery (NHNN) and Institute of Neurology. Ethical approval was obtained from the joint medical and ethics committee at the NHNN. Written informed consent was obtained from all patients.
Patients
We selected a cohort of 140 patients from our inherited neuropathy database. These were patients presenting with a phenotype compatible with any of the forms of HSAN or CMT2 with a predominant sensory phenotype (Supplementary table). Forty-one patients (29%) had definite autosomal dominant (AD) inheritance, 12 patients (9%) had presumed autosomal recessive (AR) inheritance and the remainder were either sporadic cases or the referring physician did not document inheritance. The database includes patients seen in the peripheral neuropathy clinics in the NHNN as well as patients’ DNA referred from other hospitals for diagnostic and research testing. External referring physicians were neurologists with experience in neuromuscular disease. Sixty-seven patients (48%) included in this study were seen within our own department, the remainder were external DNA samples. When mutations were found in patients we had not seen personally, we either reviewed the patient or obtained detailed clinical information from the referring physician. Most patients presented with distal progressive sensory loss, with or without ulceromutilating complications or autonomic dysfunction. Because of the overlap between CMT2B and HSAN I we included patients who had motor involvement; however, sensory features were always predominant. Diagnosis was based on clinical phenotype in addition to neurophysiology.
DNA extraction, PCR and sequencing
DNA was extracted from blood using Flexigene extraction kit and Autopure LS (Qiagen) extraction system. Coding regions and flanking introns were amplified using Roche or Qiagen PCR reagents. Primers and PCR conditions are available upon request. Reference sequences used were SPTLC1: NM_006415.2, RAB7: NM_0004637.5, HSN2/WNK1: NM_213655.1, FAM134B: NM_001034850.1, NGFB: NM_002506.2 and NTRK1 (TRKA): NM_002529.3.
Sequence reactions were performed using Big Dye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) and resolved on an ABI 3730xl Sequencer. Sequence variants were confirmed by repeat sequencing. We considered variants pathogenic if they were absent from controls, segregated within families or, in the case of homozygous variants, if unaffected parents were heterozygous. We also considered conservation of amino acids among species and used three commonly used prediction programmes PolyPhen (http://genetics.bwh.harvard.edu/pph/), SIFT (http://blocks.fhcrc.org/sift/SIFT.html) and aGVGD (http://agvgd.iarc.fr/). In addition, we checked the 1000genomes and NHLBI exome sequencing project databases (http://www.1000genomes.org and http://evs.gs.washington.edu/EVS) to determine whether novel variants had been reported previously. One hundred seventy chromosomes from British controls and 180 chromosomes from Asian controls were screened for all novel variants depending on ethnicity of the patient.
Results
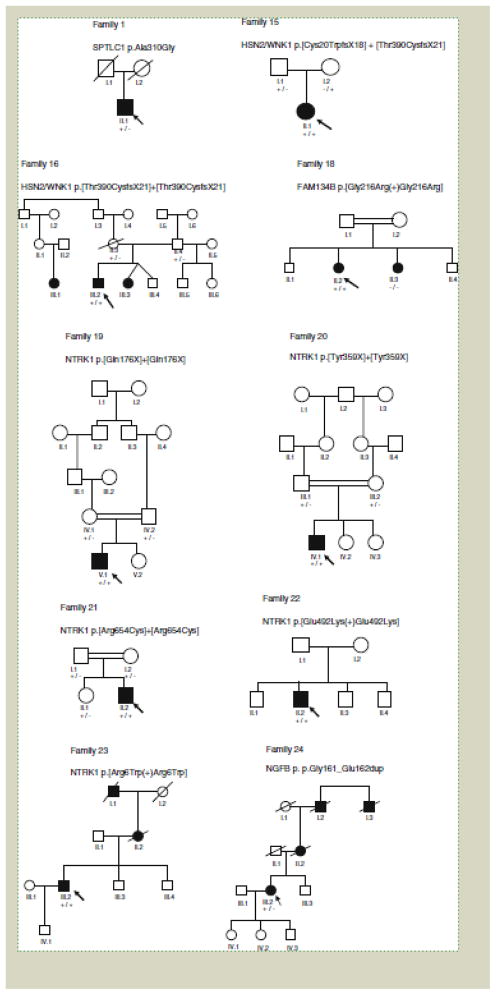
We identified 25 patients with sequence variants in our cohort of 140 patients (17.9%), 20 of which (14.3%) are likely to be pathogenic. Mutations were found in all genes tested (Table 2). Details on some of the patients with the RAB7, FAM134B and SPTLC1 mutations have been reported previously [9, 10, 18]. Patients with mutations varied in their clinical phenotype, although as expected there was more consistency in the phenotypes associated with individual genes (Table 3). Pedigrees of the families are shown in Fig. 1 (families reported previously by us and newly diagnosed families with p.Cys133Trp SPTLC1 have not been included). All novel variants reported here were not detected in the control cohorts.
Table 2.
Variants found in HSAN patients
| Gene | Total patients screeneda | No. of patients with mutations (%) | Family no. | Mutation(s) |
|---|---|---|---|---|
| SPTLC1b | 107 | 13 (12.1) | 1 | c.929C[G; p.Ala310Gly (1 patient)c |
| - | c.399T[G; p.Cys133Trp (12 index patients) [9] | |||
| RAB7b | 115 | 1 (0.9) | – | c.482A[C; p.Asn161Thr [10] |
| HSN2/WNK1 | 129 | 2 (1.5) | 15 | c.[60_61delTG] ? [1168_1171delACAG]; p.[Cys20TrpfsX18] ? [Thr390CysfsX21] |
| 16 | c.[1168-1171delACAG] ? [1168-1171delACAG]; p.[Thr390CysfsX21] ? [Thr390CysfsX21] | |||
| FAM134Bb | 108 | 2 (1.9) | 17 | c.[433C[T(?)433C[T]; p.[Gln145X(?) Gln145X] [18] |
| 18 | c.[646 G[A(?)646 G[A]; p.[Gly216Arg(?)Gly216Arg]c | |||
| NGFB | 138 | 2 (1.4) | 24 | c.482_487dupGAGAGG; p.Gly161_Glu162dupc |
| – | c.560G[A; p.Ser187Asnc | |||
| NTRK1 (TRKA) | 88 | 5 (5.7) | 19 | c.[526C[T] ? [526C[T]; p.[Gln176X] ? [Gln176X] |
| 20 | c.[1069_1076dupGGCAACTA] ? [1069_1076dupGGCAACTA]; p.[Tyr359X] ? [Tyr359X] | |||
| 21 | c.[1960 C[T] ? [1960 C[T]; p.[Arg654Cys] ? [Arg654Cys] | |||
| 22 | c.[1474G[A(?)1474G[A]; p.[Glu492Lys(?)Glu492Lys] | |||
| 23 | c.[16C[T(?)16C[T]; p.[Arg6Trp(?)Arg6Trp]c |
Genetic sequence variations are described according to the Human Genome Variation Society’s recommended nomenclature (http://www.hgvs.org/mutnomen)
Total number of patient samples fully sequenced for each gene
Some of these patients have previously been reported as referenced above
Uncertain significance
Table 3.
Phenotypes of index patients with genetic variants
| Gene | Amino acid change | Origin | AAO (year) | Inheritance | Sensory involvement | Motor involvement | Reflexes | Ulceromutilating complications | Autonomic symptoms | NCS | Other features | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SPTLC1 family 1 | p.Ala310Glyba,b | UK | 50s | U | Pin prick absent throughout, vibration reduced to costal margins Sensory ataxia | None | Present | 5th digits of both feet amputated | No | Sensory axonal neuropathy | ||
| SPTLC1 family 2 | p.Cys133Trp | UK | 20 | AD | Pinprick reduced to forearm and knee, vibration to ankles | Distal UL and LL weakness | Absent in LL | Ulcers on feet | No | Sensory motor axonal neuropathy | [9] | |
| SPTLC1 family 3 | p.Cys133Trp | UK | 29 | AD | Sensory loss in feet | None | All reduced | None | No | Sensory motor axonal neuropathy | Lancinating pain | [9] |
| SPTLC1 family 4 | p.Cys133Trp | UK | 24 | AD | Pinprick reduced above elbows and thighs, vibration to hips | Severe distal UL and LL weakness Hand contractures, Wheelchair | Absent in LL | Osteomyelitis | No | Sensory motor demyelinating neuropathy | Lancinating pain | [9] |
| SPTLC1 family 5 | p.Cys133Trp | UK | 16 | AD | Pinprick reduced in hands and feet | Distal UL and LL hand | Absent at ankle | Ulcers on feet | No | Sensory motor axonal neuropathy | [9] | |
| SPTLC1family 6 | p.Cys133Trp | UK | 20 | AD | Pinprick reduced to wrists and thighs, vibration to ankle | Distal weakness UL and LL | Absent at ankle | Ulcers on hands and feet, fingers amputated | No | Sensory motor axonal neuropathy | Lancinating pain | [9] |
| SPTLC1family 7 | p.Cys133Trp | UK | 18 | AD | Sensory loss in UL and LL | Sever UL and LL weakness | Absent | Ulcers on feet, toes amputated | No | Sensory motor axonal neuropathy | Lancinating pain | [9] |
| SPTLC1 family 8 | p.Cys133Trp | UK | 60 | AD | Pinprick reduced above elbows and mid thigh, vibration to costal margin | Severe distal UL and LL weakness Wheelchair | Absent in LL | Ulcers on feet | No | Sensory motor demyelinating neuropathy | Lancinating pain | [9] |
| SPTLC1 family 9 | p.Cys133Trp | UK | 18 | AD | Pinprick reduced above elbows and upper thighs, vibration to hips | Severe distal wasting and weakness UL and LL | Absent in LL | Ulcers on feet | No | Sensory motor axonal neuropathy | Lancinating pain | [9] |
| SPTLC1 family 10 | p.Cys133Trp | UK | Teens | AD | Sensation reduced in feet | Presentc | – | Trophic changes in fingers | No | Sensory motor demyelinating neuropathy | ||
| SPTLC1 family 11 | p.Cys133Trp | UK | Teens | AD | Presentc | Presentc | – | Trophic changes in fingers | Mild bladder and bowel disturbances | N/A | Lancinating pain | |
| SPTLC1 family 12 | p.Cys133Trp | UK | – | AD | Presentc | – | – | – | – | N/A | ||
| SPTLC1 family 13 | p.Cys133Trp | UK | 18 | U | Pinprick reduced to elbows and knees, vibration to ankle | Distal weakness and wasting UL and LL | Absent in LL | Ulcers in lower legs and eye bulb | Mild bowel disturbances | Sensory motor axonal neuropathy | Lancinating pain | |
| RAB7 family 14 | p.Asn161Thr | UK | 16 | AD | Pinprick reduced to ankles, vibration to costal margin | None | Absent at ankles | Scoliosis amputation middle left toe, deformity left foot | No | Sensory motor neuropathy | [10] | |
| HSN2 family 15 | p.[Cys20TrpfsX18] + [Thr390CysfsX21]a | Malta | Congenital | AR | CIP | No | – | Ulcers un noticed fractures osteomecrosis of ankle | No | Sensory motor axonal neuropathy | ||
| HSN2 family 16 | p.[Thr390CysfsX21] + [Thr390CysfsX21]a | Malta | Congenital | AR | CIP | No | – | Ulcerations, Finger tips amputated due to repeated trauma | – | N/A | ||
| FAM134B family 17 | p.[Gln145X(+) Gln145X] | Somalia | 5 | AR | Glove and stocking loss to pin | Significant weakness requiring wheelchair | Absent/diminished | Ulcers, right forefoot amputation scoliosis, ankle deformity acro-osteolysis of finger and toes | No | Sensory motor axonal neuropathy | [18] | |
| FAM134B family 18 | p.[Gly216Arg(+) Gly216Arg]a,b | 10 | AR | Pin prick reduced in toes | No | Diminished at ankles | Ulcerations, pes cavas scoliosis | No | Sensory axonal neuropathy | |||
| NTRK1 family 19 | p.[Gln176X] + [Gln176X] | Saudi Arabia | 1st month | AR | CIP | No | Normal | Osteomyelitis, injury to lips and tongue | Anhidrosis bouts of fever | N/A | Cognitive delay | |
| NTRK1 family 20 | p.[Try359X] + [Try359X] | Saudi Arabia | Birth | AR | CIP | No (wheelchair bound due to osteomylitis resulting in deformities of feet) | Normal | Charcot joint knee, osteomylitis, injury to lips and tongue | Anhidrosis bouts of fever | Sensory axonal neuropathy | ||
| NTRK1 family 21 | p.[Arg654Cys] + [Arg654Cys] | Saudi Arabia | Birth | AR | CIP | No | Normal | Injury to lips and tongue | Anhidrosis bouts of fever | Sensory axonal neuropathy | Cognitive delay | |
| NTRK1 family 22 | p.[Glu492Lys] + [Glu492Lys]a | UK/USA | Congenital | AR | CIP | Delayed motor milestones | Absent/diminished | Multiple injuries and burns on hands | Anhidrosis bouts of fever | Sensory axonal neuropathy | Deafness, cognitive delay | |
| NTRK1 family 23 | p.[Arg6Trp(+)Arg6Trp]a,b | UK | Adult | AD | Yes | No | – | No | No | N/A | Dementia, deafness | |
| NGFB family 24 | p.Gly161_Glu162dupa,b | UK | 48 | AD | Glove and stocking loss to pin. vibration reduced to costal margins | No | Absent ankle reflexes | Charcot joints in both ankles, deformities in both knees | No | Sensory motor axonal neuropathy | ||
| NGFB family 25 | p.Ser187Asna,b | Ireland | Congenital | Sporadic | CIP | – | – | – | – | N/A |
AAO Age at onset, AD autosomal dominant, AR autosomal recessive, CIP congenital insensitivity to pain, U unknown, N/A not available, UL upper limbs, LL lower limbs
Novel
Uncertain significance
Limited details available
Figure 1.
Pedigrees of families with mutations. An arrow indicates the proband; a square a male; a circle a female; a filled symbol indicates affected; a slanted line through a symbol indicates the individual is deceased; +/+ homozygous for mutation; +/− heterozygous for mutation; −/− homozygous normal
SPTLC1 mutations
We found mutations in SPTLC1 in 13 index patients with HSAN, all but one of which was the common p.Cys133Trp mutation. Seven of these families have been reported previously [9]. Patients presented in the second or third decades with decreased sensation in the feet; lancinating pain and paraesthesiae were common. Painless ulcers occurred in all families, with Charcot joints and amputations in some individuals, often with motor involvement. Neurophysiology demonstrated absent or reduced sensory action potentials (SAPs) with variable motor studies, usually demonstrating axonal loss but occasionally showing intermediate or demyelinating motor conduction velocities as we have previously described.
There is insufficient evidence of pathogenicity for the novel variant p.Ala310Gly; this amino acid is only moderately conserved among species and two of three prediction programs suggested that this change would be tolerated (Table 4). This patient was adopted and is not available to investigate the pathogenicity of the mutation further with deoxysphingoid base (DSB) levels.
Table 4.
Analysis of novel missense variants
| Variant | Amino acid conservation | Amino acid change | Grantham distance | aGVGD | PolyPhen2 | SIFT |
|---|---|---|---|---|---|---|
| SPTLC1 c.929C>G; p.Ala310Gly | Moderately conserved | Non-polar/hydrophobic–non-polar/hydrophobic | 60 | C0 | 0.827 Possibly damaging |
Tolerated |
| FAM134B c.646 G>A; p.Gly216Arg | Highly conserved | Non-polar/hydrophobic–positively charged/hydrophilic | 125 | C65 | 0.997 Probably damaging |
Affect protein function |
| NGFB c.560G>A; p.Ser187Asn | Highly conserved | Polar/hydrophilic–polar/hydrophilic | 46 | C45 | 0.048 Benign |
Affect protein function |
| NTRK1 c.1474G>A; p.Glu492Lys | Highly conserved | Negatively charged/hydrophilic–positively charged/hydrophilic | 56 | C55 | 0.995 Probably damaging |
Affect protein function |
| NTRK1 c.16C>T; p.Arg6Trp | Highly conserved | Positively charged/hydrophilic-non-polar/hydrophobic | 101 | C0 | 0.106 Benign |
Affect protein function |
aGVGD uses alignment and amino-acid similarity scores to classify variants in order of likelihood of affecting protein function from C65 (most likely to affect protein function); C55; C45; C35; C25; C15; C0 (least likely)
PolyPhen2 uses alignments and (where possible) information on known functional protein domains to score variants from 0 (benign) to 1 (pathogenic)
SIFT uses protein sequence alignments to calculate the probability that an amino acid change will affect protein function
RAB7 mutations
We identified one family with a mutation in RAB7, p.Asn161Thr. This family has been described in detail previously [10].
WNK1/HSN2 mutations
We identified two novel frameshift mutations in WNK1/HSN2; a compound heterozygous mutation p.[Cys20TrpfsX18] + [Thr390CysfsX21] in the index patient of family 15, and homozygous p.Thr390CysfsX21 in the proband of family 16. Unaffected parents were heterozygous (Fig. 1, families 15 and 16). The proband from family 15 had congenital insensitivity to pain (CIP) and is from a non-consanguineous Maltese family. She had loss of toe-nails due to repeated trauma, and fractured her left leg without pain. The index patient from family 16 was also of Maltese descent with CIP. He had trophic ulcers on the fingers which were shortened due to repeated trauma. His parents were not consanguineous, he had an affected sister and cousin; DNA was not available from the other affected members of this family.
FAM134B mutations
We found a homozygous nonsense mutation in FAM134B (p.Gln145X), in a patient with HSAN II, which we and others have previously reported [13, 18]. A second variant, p.Gly216Arg, is most likely a rare polymorphism as the affected sibling did not carry the variant (Fig. 1, family 18).
NTRK1 mutations
Five variants were found within the NTRK1 coding region; all were homozygous and two are novel. Four of these are considered pathogenic.
One patient from Saudi Arabia (family 19, V.1), with consanguineous parents, had a homozygous nonsense mutation (p.Gln176X) [11]; parents were heterozygous. Sural nerve biopsy demonstrated a reduction in small myelinated fibres and unmyelinated fibres, consistent with HSAN V [20]. In family 20, also from Saudi Arabia, there was a homozygous nonsense mutation (c.1069_1076dup-GGCAACTA) leading to a stop mutation, p.Tyr359X [17] in the proband; both his consanguineous, unaffected parents were heterozygous carriers. The third Saudi Arabian family, family 21, had a homozygous missense mutation (p.Arg654Cys) [17]. His parents were first cousins and unaffected, they as well as his unaffected sister were heterozygous (Fig. 1).
The index patient from family 22 was found to have a novel apparently homozygous missense mutation, p.Glu492Lys. Born to an unrelated American mother and British father, this patient had a congenital sensory neuropathy with associated anhidrosis, seizures, deafness and developmental delay. Neurophysiology demonstrated a sensory neuropathy with additional involvement of the central sensory pathways. Family members were unavailable for segregation analysis; however, this amino acid is highly conserved across species and all prediction programs suggested that this amino acid change would be damaging (Table 4). This variant has been published on the http://evs.gs.washington.edu/EVS website as being present in eight of 7,020 European/American and three of 3,738 African/American alleles (i.e. 0.1% of chromosomes); thus this may represent a rare recessive pathogenic allele.
The variant p.Arg6Trp is unlikely to be relevant as the family have dominant inheritance and have subsequently been found to have a mutation in DNMT1 [12]. In addition, this variant has been reported to occur in 15/3,668 (0.4%) Europeans (http://evs.gs.washington.edu/EVS).
NGFB mutations
A heterozygous duplication, p.Gly161_Glu162dup, was found in a British female (Fig. 1, family 24). This patient presented at 48 years with a progressive sensory axonal neuropathy with Charcot joints. There was a dominant family history of a similar neuropathy; however, all affected family members were deceased. Although we cannot prove that this duplication is pathogenic the phenotype is similar to the previously reported heterozygous NGFB patients.
A second heterozygous variant (p.Ser187Asn) was found in a patient with CIP, family DNA was not available. We are unsure of the pathogenicity of this variant; we cannot rule out a non-coding mutation or deletion on the other allele.
Discussion
Our genetic screen of 140 patients with HSAN found 25 index patients with mutations in six genes (SPTLC1, RAB7, WNK1/HSN2, FAM134B, NTRK1 and NGFB), of which at least 20 are considered pathogenic; a frequency of 14.3%, similar to the 19% described by Rotthier et al. [24]. Our control groups were negative for all variations described, suggesting that none of these variants are common polymorphisms. None of the variants were reported on the 1000genomes database, although two of the NTRK1 variants were reported on the NHLBI database. We did not have enough evidence to support pathogenicity for five of the variants found (SPTLC1 p.Ala310Gly, FAM134B p.Gly216Arg, NTRK1 p.Arg6Trp and NGFB p.Gly161_- Glu162dup and p.Ser187Asn).
The purpose of this study was to determine the frequency of mutations in the genes known to cause HSAN in a mainly UK cohort. Mutations were most frequent in SPTLC1 (12%), found in British Caucasian patients with dominant inheritance and adult-onset disease. Mutations in NTRK1 were next most common (6%) and found predominantly in patients of Saudi Arabian descent with consanguineous parents. Mutations in all other genes were rare, accounting for <2% each. These results suggest that many more genes responsible for causing HSAN have yet to be identified. Since this study was completed, mutations in four further genes (ATL-1 [6], SPTLC2 [23], DNMT1 [12] and KIF1A [22]) have been reported to cause HSAN; it is unknown whether mutations in these additional genes would contribute significantly more patients than that found in our cohort given the small number of patients reported to date.
When we analysed the number of cases with a genetic diagnosis based on inheritance pattern, we found causative mutations in 10/41 (24.4%) patients with AD inheritance and 5/12 (41.7%) patients with AR inheritance, versus 5/87 (5.7%) patients with unknown or sporadic inheritance; thus the likelihood of establishing a genetic diagnosis is higher in patients with a definite family history.
Mutations in SPTLC1 accounted for 12% of our patients with HSAN. All but one were the common p.Cys133Trp mutation, presenting with the typical HSAN I phenotype. We have previously demonstrated that this mutation occurs due to a founder effect in the UK [9]. Of the known disease- causing mutations in SPTLC1, all but two lie within exons 5 and 6, a region of the protein important for substrate specificity [19]. Altered substrate-specificity of the enzyme results in the formation and accumulation of toxic DSBs [3, 19]. Of the three reported mutations outside exons 5 and 6, p.Gly387Ala was subsequently demonstrated to be a polymorphism [7]. Our finding of mutations in SPTLC1 being the commonest cause of HSAN is in contrast to Rotthier et al. [24] who found mutations in RAB7 to be the most common in a European cohort. We only found one patient with a RAB7 mutation in our cohort [10]. This likely reflects the fact that in the European cohort, there was a founder effect for the p.Leu129Phe RAB7 mutation in Austrian patients [24], while in the UK population there is a founder effect for the common p.Cys133Trp SPTLC1 mutation [9].
Two patients with recessive mutations in HSN2/WNK1 were found. Both mutations cause a shift in the open reading frame, resulting in premature stop codons which likely render the protein non-functional. The phenotype of these patients was similar to previously reported patients with CIP [14]. Both of these mutations are novel but their absence in controls and segregation within both families supports pathogenicity.
The homozygous nonsense mutation in FAM134B, p.Gln145X [18], was previously reported in a Turkish individual [13]. Both patients had onset in the first decade, with impaired nociception, ulcerations and amputations. Our patient did not have any autonomic dysfunction, and had significant motor involvement, in contrast to the patient previously reported.
Mutations in NTRK1 accounted for 6% of our HSAN cohort. The phenotype in the four families with pathogenic mutations was consistent with that reported in the literature with congenital insensitivity to pain with anhidrosis (CIPA), learning disability and sensory complications. Three of the mutations were heterozygous in unaffected parents/siblings and homozygous in affected individuals, supporting pathogenicity. p.Tyr359X, found in a Saudi Arabian patient with CIPA, was previously described in a Japanese patient [17]. It causes a frameshift, resulting in a premature stop codon which likely renders the protein nonfunctional. p.Arg654Cys, found in a consanguineous Saudi Arabian family, was homozygous in the affected individual while unaffected family members were heterozygous. This mutation was previously described by Miura et al. as p.Arg648Cys, using an alternative amino acid numbering system, who demonstrated that this amino acid is conserved among the three human TRK families, as well as among at least 14 other tyrosine kinase receptors [17]. The homozygous p.Gln176X NTRK1 mutation has been described previously in two patients from Kuwait with CIPA [11]. This nonsense mutation lies within the extracellular domain of the TRKA protein and would likely disrupt its function as a receptor for NGF [11]. Of the two novel variants, p.Glu492Lys is considered pathogenic. This was found in a patient with CIPA and cognitive delay; the amino acid is highly conserved across species and all three prediction programs suggested a damaging effect of the change, supporting pathogenicity.
We did not find any patients with homozygous mutations in NGFB, but found two patients with heterozygous variants. To date only one NGFB point mutation has been described in a family with HSAN V (p.Arg221Trp) [4, 16], one family with HSAN IV was reported with a homozygous frameshift mutation due to a point mutation and two base pair deletion on the same allele (p.Val232fs) [1] and one patient with a sensory and autonomic neuropathy has been described with heterozygous deletion of 12 genes one of which was NGFB [5]. Although HSAN V is usually recessive, there is a suggestion that heterozygotes have a higher incidence of Charcot joints and neuropathy, as reported in a large family with p.Arg221Trp [15]. Our patient with a heterozygous duplication (p.Gly161_Glu162dup) has a similar phenotype; however, it is currently not possible to confirm whether this variant is pathogenic.
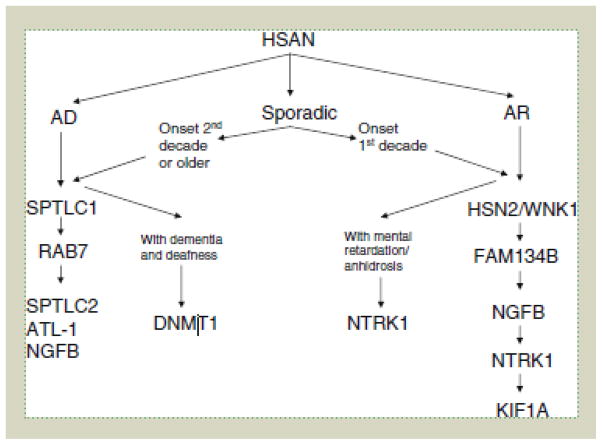
Mutations in the genes known to cause HSAN remain rare. Only 14.3% of this cohort of patients had a pathogenic mutation in a causative gene; thus there are clearly many more HSAN genes which remain unknown. Rapid advances in genetics have already led to the discovery of four additional HSAN genes since the completion of this study. The results of this study have allowed us develop an algorithm for genetic testing of patients with HSAN in the UK (Fig. 2). Although the majority of the mutations found in our cohort are likely to be pathogenic, the exact mechanisms of how these mutations disrupt neural processes require further investigation. Functional studies may provide further understanding of these genetic effects giving insight into possible treatments.
Figure 2.
Algorithm for genetic testing of patients with HSN
Overall, this study helps broaden the spectrum of HSAN, confirms the predominance of the p.Cys133Trp SPTLC1 mutation in the UK population, provides additional insights for molecular and clinical diagnosis and illustrates the need for further study into disease-causing mutations in HSAN.
Supplementary Material
Acknowledgments
We are grateful to the patients and families who support our research. We would like to thanks the following for essential grant support; The Medical Research Council (MRC) MMR and HH (MRC fellowship, G108/638 and G0802760), The Brain Research Trust (BRT) (HH), Ataxia UK (HH), The BMA Vera Down Award (HH) and the Muscular Dystrophy Campaign UK and association USA (HH and MMR). SMM and MMR are grateful to the NINDS/ORD (1U54NS065712-01) for their support. MAS was supported by the College of Medicine Research Center (CMRC, Project No. 05-495), College of Medicine, King Saud University, Riyadh, Saudi Arabia. This work was undertaken at University College London Hospitals/University College London, which received a proportion of funding from the Department of Health’s National Institute for Health Research Biomedical Research Centres funding scheme. This study was funded by a UCLH/UCL Comprehensive Biomedical Research Centre (CBRC) Grant. We would like to thank the following for essential grant support; The Medical Research Council (MRC) MMR and HH (MRC fellowship, G108/638 and G0802760), The Brain Research Trust (BRT) (HH), Ataxia UK (HH), The BMA Vera Down Award (HH) and the Muscular Dystrophy Campaign UK and association USA (HH and MMR). SMM and MMR are grateful to the NINDS/ORD (1U54NS065712-01) for their support. MAS was supported by the College of Medicine Research Center (CMRC, Project No. 05-495), College of Medicine, King Saud University, Riyadh, Saudi Arabia. FM is supported by the Great Ormond Street Hospital Children’s charity. This work was undertaken at University College London Hospitals/University College London, which received a proportion of funding from the Department of Health’s National Institute for Health Research Biomedical Research Centres funding scheme.
Footnotes
Contribution:
G. L. Davidson and S. M. Murphy contributed equally to the manuscript.
Conflicts of interest GL Davidson, JM Polke, M Laura, MAM Salih, J Blake, S Brandner, N Davies, R Horvath, S Price, M Donaghy, M Roberts, N Foulds, G Ramdharry, D Soler, MP Lunn, H Manji, MB Davis have no disclosures. SM Murphy is the recipient of a Post Doctoral training fellowship from the Inherited Neuropathy Consortium Rare Disease Clinical Research Consortium supported by the NINDS/ORD (1U54NS065712-01). F Muntoni serves on a scientific advisory board for AVI BioPharma, Inc.; serves on the editorial boards of Neuromuscular Disorders and Neuropaediatrics; is listed as an author on a pending patent re: Tailed antisense nucleotides to redirect splicing; receives publishing royalties for Duchenne Muscular Dystrophy (Oxford University Press, 2003); and receives research support from AVI BioPharma, Inc., PTC Therapeutics, Inc., GlaxoSmithKline, Wellcome Trust, the European Union, Medical Research Council UK, Muscular Dystrophy Campaign, Muscular Dystrophy Association USA, Spinal Muscular Atrophy Foundation, the NIH, the Association Francaise contre les myopathies (AFM), the NIHR, and the Great Ormond Street Hospital Children’s Charity. H Houlden receives research support from The Medical Research Council (MRC fellowship, G108/638 and G0802760), The Brain Research Trust, Ataxia UK, The BMA Vera Down Award and the Muscular Dystrophy Campaign UK and association USA. MM Reilly receives research support from The Medical Research Council, the Muscular Dystrophy Campaign and NINDS/ORD (1U54NS065712-01). This work was undertaken at University College London Hospitals/University College London, which received a proportion of funding from the Department of Health’s National Institute for Health Research Biomedical Research Centres funding scheme.
Contributor Information
G. L. Davidson, Email: gd339@cam.ac.uk, Neurogenetics Unit, National Hospital for Neurology and Neurosurgery, London, UK. MRC Centre for Neuromuscular Diseases and Department of Molecular Neuroscience, UCL Institute of Neurology and National Hospital for Neurology and Neurosurgery, Queen Square, London WC1N 3BG, UK
S. M. Murphy, Email: sinead.murphy@amnch.ie, MRC Centre for Neuromuscular Diseases and Department of Molecular Neuroscience, UCL Institute of Neurology and National Hospital for Neurology and Neurosurgery, Queen Square, London WC1N 3BG, UK
J. M. Polke, Email: james.polke@uclh.nhs.uk, Neurogenetics Unit, National Hospital for Neurology and Neurosurgery, London, UK. MRC Centre for Neuromuscular Diseases and Department of Molecular Neuroscience, UCL Institute of Neurology and National Hospital for Neurology and Neurosurgery, Queen Square, London WC1N 3BG, UK
M. Laura, Email: m.laura@ucl.ac.uk, MRC Centre for Neuromuscular Diseases and Department of Molecular Neuroscience, UCL Institute of Neurology and National Hospital for Neurology and Neurosurgery, Queen Square, London WC1N 3BG, UK
M. A. M. Salih, Email: Mustafa_salih05@yahoo.com, Division of Pediatric Neurology, Department of Pediatrics, College of Medicine, King Saud University, Riyadh, Saudi Arabia
F. Muntoni, Email: f.muntoni@ich.ucl.ac.uk, The Dubowitz Neuromuscular Centre, UCL Institute of Child Health, 30 Guildford St, London, UK
J. Blake, Email: julian.blake@nnuh.nhs.uk, Department of Clinical Neurophysiology, National Hospital for Neurology and Neurosurgery, London, UK. Department of Clinical Neurophysiology, Norfolk and Norwich University Hospital, Norwich, UK
S. Brandner, Email: Sebastian.brandner@prion.ucl.ac.uk, Division of Neuropathology, Department of Neurodegenerative Disease, Institute of Neurology, Queen Square, London, UK
N. Davies, Email: Nicholas.davies@uhb.nhs.uk, Department of Neurology, Queen Elizabeth Hospital, Birmingham, UK
R. Horvath, Email: rita.horvath@newcastle.ac.uk, Institute of Genetic Medicine, Newcastle University, Newcastle upon Tyne, UK
S. Price, Email: sue.price@nhg.nhs.uk, Department of Clinical Genetics, Oxford Radcliffe Hospital, Oxford, UK
M. Donaghy, Email: Michael.donaghy@clneuro.ox.ac.uk, Department of Clinical Neurology, University of Oxford, John Radcliffe Hospital, Oxford, UK
M. Roberts, Email: markrob@doctors.org.uk, Department of Neurology, University Hospital of South Manchester, Manchester, UK
N. Foulds, Email: nichola.foulds@suht.swest.nhs.uk, Clinical Genetics Service, Southampton University Hospitals Trust, Southampton, UK
G. Ramdharry, Email: g.ramdharry@ucl.ac.uk, MRC Centre for Neuromuscular Diseases and Department of Molecular Neuroscience, UCL Institute of Neurology and National Hospital for Neurology and Neurosurgery, Queen Square, London WC1N 3BG, UK
D. Soler, Email: doriette.m.soler@gov.mt, Department of Paediatrics, Mater Dei Hospital, Msida, Malta
M. P. Lunn, Email: Michael.lunn@uclh.nhs.uk, MRC Centre for Neuromuscular Diseases and Department of Molecular Neuroscience, UCL Institute of Neurology and National Hospital for Neurology and Neurosurgery, Queen Square, London WC1N 3BG, UK
H. Manji, Email: hadi.manji@btinternet.com, MRC Centre for Neuromuscular Diseases and Department of Molecular Neuroscience, UCL Institute of Neurology and National Hospital for Neurology and Neurosurgery, Queen Square, London WC1N 3BG, UK
M. B. Davis, Email: davismarytring@gmail.com, Neurogenetics Unit, National Hospital for Neurology and Neurosurgery, London, UK. MRC Centre for Neuromuscular Diseases and Department of Molecular Neuroscience, UCL Institute of Neurology and National Hospital for Neurology and Neurosurgery, Queen Square, London WC1N 3BG, UK
H. Houlden, Email: h.houlden@ucl.ac.uk, Neurogenetics Unit, National Hospital for Neurology and Neurosurgery, London, UK. MRC Centre for Neuromuscular Diseases and Department of Molecular Neuroscience, UCL Institute of Neurology and National Hospital for Neurology and Neurosurgery, Queen Square, London WC1N 3BG, UK
M. M. Reilly, Email: m.reilly@ucl.ac.uk, MRC Centre for Neuromuscular Diseases and Department of Molecular Neuroscience, UCL Institute of Neurology and National Hospital for Neurology and Neurosurgery, Queen Square, London WC1N 3BG, UK
References
- 1.Carvalho OP, Thornton GK, Hertecant J, Houlden H, Nicholas AK, Cox JC, Reilly MM, Al-Gazali L, Woods CG. A novel NGF mutation clarifies the molecular mechanism and extends the phenotypic spectrum of the HSAN5 neuropathy. J Med Genet. 2011;48:131–135. doi: 10.1136/jmg.2010.081455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dyck PJ. Neuronal atrophy and degeneration predominantly affecting peripheral sensory and autonomic neurons. In: Dyck PJ, Thomas PK, Griffin JW, Low PA, Poduslo JF, editors. Peripheral neuropathy. W.B Saunders; Philadelphia: 1993. pp. 1065–1093. [Google Scholar]
- 3.Eichler FS, Hornemann T, McCampbell A, Kuljis D, Penno A, Vardeh D, Tamrazian E, Garofalo K, Lee H-J, Kini L, Selig M, Frosch M, Gable K, von Eckardstein A, Woolf CJ, Guan G, Harmon JM, Dunn TM, Brown RH., Jr Overexpression of the wild-type SPT1 subunit lowers deoxysphingolipid levels and rescues the phenotype of HSAN1. J Neurosci. 2009;29:14646–14651. doi: 10.1523/JNEUROSCI.2536-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Einarsdottir E, Carlsson A, Minde J, Toolanen G, Svensson O, Solders G, Holmgren G, Holmberg D, Holmberg M. A mutation in the nerve growth factor beta gene (NGFB) causes loss of pain perception. Hum Mol Genet. 2004;13:799–805. doi: 10.1093/hmg/ddh096. [DOI] [PubMed] [Google Scholar]
- 5.Fitzgibbon GJ, Kingston H, Needham M, Gaunt L. Haploinsufficiency of the nerve growth factor beta gene in a 1p13 deleted female child with an insensitivity to pain. Dev Med Child Neurol. 2009;51:833–837. doi: 10.1111/j.1469-8749.2008.03173.x. [DOI] [PubMed] [Google Scholar]
- 6.Guelly C, Zhu P-P, Leonardis L, Papic L, Zidar J, Schabhuttl M, Strohmaier H, Weis J, Strom TM, Baets J, Willems J, de Jonghe P, Reilly MM, Frohlich E, Hatz M, Trajanoski S, Pieber TR, Janecke AR, Blackstone C, Auer-Grumbach M. Targeted high-throughput sequencing identifies mutations in atlastin-1 as a cause of hereditary sensory neuropathy type I. Am J Hum Genet. 2011;88:99–105. doi: 10.1016/j.ajhg.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hornemann T, Penno A, Richard S, Nicholson G, van Dijk FS, Rotthier A, Timmerman V, von Eckardstein A. A systematic comparison of all mutations in hereditary sensory neuropathy type I (HSAN I) reveals that the G387A mutation is not disease associated. Neurogenetics. 2009;10:135–143. doi: 10.1007/s10048-008-0168-7. [DOI] [PubMed] [Google Scholar]
- 8.Houlden H, Blake J, Reilly MM. Hereditary sensory neuropathies. Curr Opin Neurol. 2004;17:569–577. doi: 10.1097/00019052-200410000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Houlden H, King R, Blake J, Groves M, Love S, Woodward C, Hammans S, Nicoll J, Lennox G, O’Donovan DG, Gabriel C, Thomas PK, Reilly MM. Clinical, pathological and genetic characterization of hereditary sensory and autonomic neuropathy type 1 (HSAN I) Brain. 2006;129:411–425. doi: 10.1093/brain/awh712. [DOI] [PubMed] [Google Scholar]
- 10.Houlden H, King RH, Muddle JR, Warner TT, Reilly MM, Orrell RW, Ginsberg L. A novel RAB7 mutation associated with ulceromutilating neuropathy. Ann Neurol. 2004;56:586–590. doi: 10.1002/ana.20281. [DOI] [PubMed] [Google Scholar]
- 11.Indo Y. Molecular basis of congenital insensitivity to pain with anhidrosis (CIPA): mutations and polymorphisms in TRKA(NTRK1) gene encoding the receptor tyrosine kinase for nerve growth factor. Hum Mutat. 2001;18:462–471. doi: 10.1002/humu.1224. [DOI] [PubMed] [Google Scholar]
- 12.Klein CJ, Botuyan MV, Wu Y, Ward CJ, Nicholson GA, Hammans S, Hojo K, Yamanishi H, Karpf AR, Wallace DC, Simon M, Lander C, Boardman LA, Cunningham JM, Smith GE, Litchy WJ, Boes B, Atkinson EJ, Middha S, Dyck PJB, Parisi JE, Mer G, Smith DI, Dyck PJ. Mutations in DNMT1 cause hereditary sensory neuropathy with dementia and hearing loss. Nat Genet. 2011;43:95–600. doi: 10.1038/ng.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurth I, Pamminger T, Hennings JC, Soehendra D, Huebner AK, Rotthier A, Baets J, Senderek J, Topaloglu H, Farrell SA, Nurnberg G, Nurnberg P, De Jonghe P, Gal A, Kaether C, Timmerman V, Hubner CA. Mutations in FAM134B, encoding a newly identified Golgi protein, cause severe sensory and autonomic neuropathy. Nat Genet. 2009;41:1179–1181. doi: 10.1038/ng.464. [DOI] [PubMed] [Google Scholar]
- 14.Lafreniere RG, MacDonald ML, Dube MP, MacFarlane J, O’Driscoll M, Brais B, Meilleur S, Brinkman RR, Dadivas O, Pape T, Platon C, Radomski C, Risler J, Thompson J, Guerra-Escobio AM, Davar G, Breakefield XO, Pimstone SN, Green R, Pryse-Phillips W, Goldberg YP, Younghusband HB, Hayden MR, Sherrington R, Rouleau GA, Samuels ME. Identification of a novel gene (HSN2) causing hereditary sensory and autonomic neuropathy type II through the Study of Canadian Genetic Isolates. Am J Hum Genet. 2004;74:1064–1073. doi: 10.1086/420795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minde J, Anderson T, Fulford M, Aguirre M, Nennesmo I, Remahl IN, Svensson O, Holmberg M, Toolanen G, Solders G. A novel NGFB point mutation: a phenotype study of heterozygous patients. J Neurol Neurosurg Psychiatry. 2009;80:188–195. doi: 10.1136/jnnp.2007.136051. [DOI] [PubMed] [Google Scholar]
- 16.Minde J, Toolanen G, Andersson T, Nennesmo I, Remahl IN, Svensson O, Solders G. Familial insensitivity to pain (HSAN V) and a mutation in the NGFB gene. A neurophysiological and pathological study. Muscle Nerve. 2004;30:752–760. doi: 10.1002/mus.20172. [DOI] [PubMed] [Google Scholar]
- 17.Miura Y, Mardy S, Awaya Y, Nihei K, Endo F, Matsuda I, Indo Y. Mutation and polymorphism analysis of the TRKA (NTRK1) gene encoding a high-affinity receptor for nerve growth factor in congenital insensitivity to pain with anhidrosis (CIPA) families. Hum Genet. 2000;106:116–124. doi: 10.1007/s004390051018. [DOI] [PubMed] [Google Scholar]
- 18.Murphy SM, Davidson GL, Brandner S, Houlden H, Reilly MM. Mutation in FAM134B causing severe hereditary sensory neuropathy. J Neurol Neurosurg Psychiatry. 2012;83:119–120. doi: 10.1136/jnnp.2010.228965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Penno A, Reilly MM, Houlden H, Laura M, Rentsch K, Niederkofler V, Stoeckli E, Nicholson G, Eichler F, Brown RH, Jr, von Eckardstein A, Hornemann T. Hereditary sensory neuropathy type 1 is caused by the accumulation of two neurotoxic sphingolipids. J Biol Chem. 2010;285:11178–11187. doi: 10.1074/jbc.M109.092973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rafel E, Alberca R, Bautista J, Navarrete M, Lazo J. Congenital insensitivity to pain with anhidrosis. Muscle Nerve. 1980;3:216–220. doi: 10.1002/mus.880030305. [DOI] [PubMed] [Google Scholar]
- 21.Reilly MM, Shy ME. Diagnosis and new treatments in genetic neuropathies. J Neurol Neurosurg Psychiatry. 2009;80:1304–1314. doi: 10.1136/jnnp.2008.158295. [DOI] [PubMed] [Google Scholar]
- 22.Riviere JB, Ramalingam S, Lavastre V, Shekarabi M, Holbert S, Lafontaine J, Srour M, Merner N, Rochefort D, Hince P, Gaudet R, Mes-Masson AM, Baets J, Houlden H, Brais B, Nicholson GA, Van Esch H, Nafissi S, De Jonghe P, Reilly MM, Timmerman V, Dion PA, Rouleau GA. KIF1A, an axonal transporter of synaptic vesicles, is mutated in hereditary sensory and autonomic neuropathy type 2. Am J Hum Genet. 2011;89:219–230. doi: 10.1016/j.ajhg.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rotthier A, Auer-Grumbach M, Janssens K, Baets J, Penno A, Almeida-Souza L, Van Hoof K, Jacobs A, De Vriendt E, Sclotter-Weigel B, Loscher WN, Vondracek P, Seeman P, De Jonghe P, Van Dijck P, Jordanova A, Hornemann T, Timmerman V. Mutations in the SPTLC2 subunit of serine palmitoyltransferase cause hereditary sensory and autonomic neuropathy type I. Am J Hum Genet. 2010;87:513–522. doi: 10.1016/j.ajhg.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rotthier A, Baets J, De Vriendt E, Jacobs A, Auer-Grumbach M, Levy N, Bonello-Palot N, Kilic SS, Weis J, Nascimento A, Swinkels M, Kruyt MC, Jordanova A, De Jonghe P, Timmerman V. Genes for hereditary sensory and autonomic neuropathies: a genotype-phenotype correlation. Brain. 2009;132:2699–2711. doi: 10.1093/brain/awp198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.