Abstract
We introduced a novel humanized HLA-A*0201 transgenic (HLA Tg) rabbit model to assess the protective efficacy of a human CD8+ T cell epitope-based vaccine against primary ocular herpes infection and disease. Each of the three immunodominant human CD8+ T cell peptide epitopes from HSV-1 glycoprotein D (gD53–61, gD70–78, and gD278–286) were joined with a promiscuous human CD4+ T cell peptide epitope (gD49–82) to construct three separate pairs of CD4–CD8 peptides. Each CD4–CD8 peptide pair was then covalently linked to an Nε-palmitoyl–lysine residue via a functional base lysine amino group to construct CD4–CD8 lipopeptides. HLA Tg rabbits were immunized s.c. with a mixture of the three CD4–CD8 HSV-1 gD lipopeptides. The HSV-gD–specific T cell responses induced by the mixture of CD4–CD8 lipopeptide vaccine and the protective efficacy against acute virus replication and ocular disease were determined. Immunization induced HSV-gD49–82–specific CD4+ T cells in draining lymph node (DLN); induced HLA-restricted HSV-gD53–61, gD70–78, and gD278–286–specific CD8+ T cells in DLN, conjunctiva, and trigeminal ganglia and reduced HSV-1 replication in tears and corneal eye disease after ocular HSV-1 challenge. In addition, the HSV-1 epitope-specific CD8+ T cells induced in DLNs, conjunctiva, and the trigeminal ganglia were inversely proportional with corneal disease. The humanized HLA Tg rabbits appeared to be a useful preclinical animal model for investigating the immunogenicity and protective efficacy of human CD8+ T cell epitope-based prophylactic vaccines against ocular herpes. The relevance of HLA Tg rabbits for future investigation of human CD4–CD8 epitope-based therapeutic vaccines against recurrent HSV-1 is discussed.
Herpes simplex virus type 1 remains one of the most prevalent viral infections of the eye worldwide (1–3). The clinical manifestations of ocular herpes infections extend from unnoticed asymptomatic disease to highly symptomatic blepharitis, conjunctivitis, dendritic keratitis, disciform stromal edema, and blinding herpetic stromal keratitis (1, 2, 4). In the United States alone, over 450,000 people have a history of symptomatic recurrent ocular herpes requiring doctor visits (5). Despite antiviral drug therapy, ocular herpes infections are still a major health problem, and no vaccines are available. Developing an effective vaccine against ocular HSV-1 would represent a powerful and cost-effective means for controlling this blindingdisease (3, 6–9). However, progress toward a human vaccine faces significant challenges, including the lack of an appropriate animal model that mounts humanized immune responses and mimics human ocular herpes.
HLA transgenic mice, such as HLA-A*0201 and HLA-DR transgenic mice, are powerful models that develop robust T cell responses to human epitopes after immunization or upon ocular HSV-1 infection (10–12). Most investigators prefer to work on a mouse model because many well-characterized immunologic probes and inbred transgenic mouse strains with specific immune defects are commercially available to study a variety of immune parameters. However, serum neutralizing Ab can protect the mouse, but not the human or rabbit eye, against ocular HSV-1 disease (13, 14). The neutralizing Ab responses can mask protective effects of T cell-mediated responses that may be critical in humans and rabbits. In addition, herpetic conjunctivitis is similar in rabbits and humans, but differs in mice (1, 15–17). Thus, although mouse studies have provided much useful and important information regarding ocular HSV-1 infection, and despite the tremendous amount known about mouse immunology, humanized HLA transgenic rabbits can be a more powerful model to study protective immunity induced by HSV-1 prophylactic vaccination. In addition, for potential future therapeutic vaccine studies, rabbits have the advantage of a high HSV-1 spontaneous reactivation rate similar to that of humans, whereas spontaneous reactivation in mice is either extremely rare or does not occur (1).
We now have a humanized HLA-A*0201 transgenic (HLA Tg) rabbit model that develops acute herpetic ocular disease similar to humans and mounts HLA-restricted and specific T cell responses to human (rather than rabbit) HSV-1 CD8+ T cell epitopes. We report in this study that prophylactic immunization of these HLA Tg rabbits with a mixture of three human glycoprotein D (gD) lipopeptides induced HSV-1–specific CD8+ T cells and reduced HSV-1 ocular replication and corneal disease following ocular challenge.
Materials and Methods
Peptides and lipopeptides
Three different CD4–CD8 lipopeptide constructs were synthesized by Mgenex Biosciences (San Diego, CA). Each lipopeptide contains one fixed CD4+ T cell epitope (gD49–82) and three variable CD8+ T cells epitopes (gD53–61, gD70–78, and gD278–286) from HSV-1 gD. The structure of the three lipopeptides is shown in Fig. 1. All peptides and lipopeptides were HPLC purified with a purity range 95–98%.
FIGURE 1.
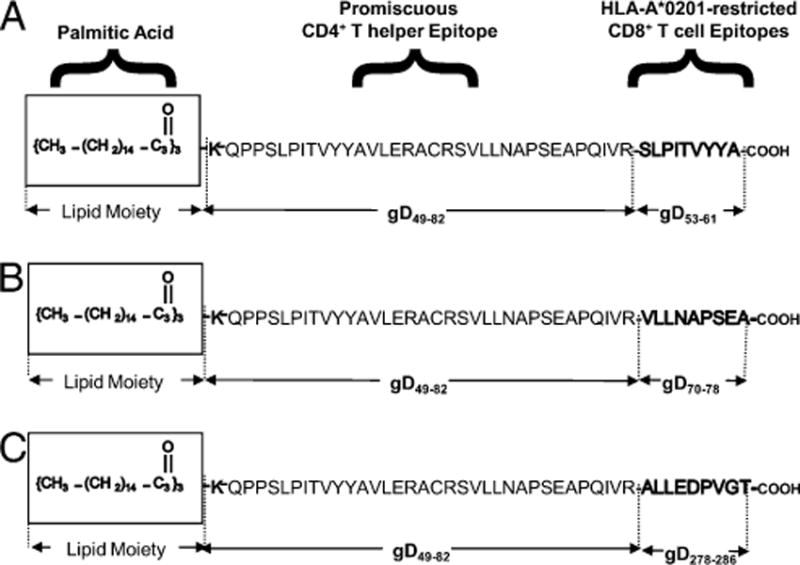
Schematic representation of prototypes human CD4–CD8 lipopeptide vaccines. The C-terminal end of a promiscuous CD4+ T cell peptide epitope (gD49–82) was joined in line with the N-terminal end of one of three different HSV-1 gD CD8 T cell epitopes: gD53–61 (A), gD70–78 (B), or gD278–286 (C). The N-terminal end of each resulting CD4–CD8 peptide was extended by a lysine covalently linked to one molecule of palmitic acid (a lipid). This results in three separate pairs of CD4–CD8 lipopeptides.
HLA-A*0201 transgenic rabbits
HLA Tg rabbits were derived from New Zealand White rabbits (18). The HLA Tg rabbits retain their endogenous rabbit MHC locus and express human HLA-A*0201 under the control of its normal promoter (18). Prior to this study, the expression of HLA-A*0201 molecules on the PBMC of each HLA Tg rabbit was confirmed by FACS analysis. In brief, PBMCs were stained with 2 μl anti–HLA-A2 mAb, BB7.2 (BD Pharmingen, San Diego, CA), at 4°C for 30 min, washed and analyzed by flow cy-tometry using a FACScan (Becton Dickinson, Mountain View, CA). New Zealand White rabbits (non-Tg control rabbits) were purchased from Irish Farms (Harlan Breeders, Indianapolis, IN).
Immunization
Rabbits were immunized s.c. with a mixture of three CD4+–CD8+ lip-opeptides (100 μg each) on days 0, 14, and 30, in a volume of 200 μl adjuvant-free saline (i.e., PBS). As a negative control, a group of HLA transgenic rabbits were injected with sterile PBS only. In some experiments, New Zealand White rabbits were included as a control for specificity to the human epitopes.
Preparation of single-cell suspension from rabbit conjunctiva
The bulbar and palpebral conjunctiva tissue from rabbit eyes was excised and collected in HBSS. Conjunctiva tissues were spun down at 1600 rpm for 5 min at 4°C and digested with collagenase type I (Life Technologies, Carlsbad, CA) at 3 mg/ml for 3 h at 37°C with occasional vortexing every 15 min. The digested tissue suspension was passed through a 70-μm nylon cell strainer and spun down at 1600 rpm for 5 min at 4°C. This process was repeated two times. The final pellet was resuspended in HBSS, passed through a 40-μm cell strainer, and spun down at 1600 rpm for 5 min at 4°C. This process was repeated two times. The final pellet was resuspended in complete RPMI 1640 medium and kept on ice. The live cells were counted using a hemocytometer.
Tetramer assay
Rabbits were euthanized, and draining lymph nodes (DLNs), conjunctiva, and trigeminal ganglia (TG) were individually harvested and single-cell suspensions were prepared. Cells were analyzed for the frequency of CD8+ T cells specific to each of the three immunizing CD8+ T cell epit-opes using the corresponding HLA-A2–peptide/Tetramer as previously described (10–12).
A human β-2–microglobulin was incorporated in the tetramers, because no rabbit β-2–microglobulins are currently available. Several critical staining parameters were considered (1): different tetramer concentrations were used to avoid the potential problem of missing CD8+ T cells with low binding avidities (e.g., CD8+ T cells expressing low affinity TCRs) (2). Because high temperatures (e.g., 37°C) can interfere with reliable enumeration of Ag-specific CD8+ T cells, CD8+ T cell staining is performed at 4°C in the presence of 5 mM EDTA and 5 mM sodium azide, which inhibits cell activation-dependent molecular events (3). The combination of tetramer staining along with anti-human CD8 mAb staining might have striking effects on tetramer staining, such as strong inhibition of several-fold increases. Therefore, the selected anti-human CD8 mAb has been pretested for not affecting tetramer binding. In addition, performing CD8 staining in the cold after tetramer staining reduces interference (4). Because tetramer staining strongly depends on cell viability and vitality, dead or dying cells are gated out using annexin V or propidium iodide staining. The cells were first incubated with 1 μg/ml PE-labeled HLA-A2–peptide/tetramer at 4°C for 30–45 min. The cells were washed twice and stained with 1 μg/ml FITC-conjugated mouse anti-rabbit CD8 mAb (clone 215B; Serotec, Oxford, U.K.). After two additional washings, cells were fixed with 1% formaldehyde in PBS. A total of 20,000 events were acquired by FACScan (Becton Dickinson) followed by analysis using Cell Quest software (BD Biosciences, San Jose, CA). The absolute numbers of gD-peptide–specific CD8+ T cells were calculated using the following formula: (No. of CD8+/Tetramer+ cells in the test) – (No. of CD8+/Te trame r+ cells in negative control).
T cell proliferation assay
CD4+ and CD8+ T cell proliferation was measured using CFSE assay as previously described (10, 19–21). DLN and conjunctiva cells were labeled with CFSE (2 μM) and incubated for 5 d with or without individual immunizing CD4+ or CD8+ T cell gD peptide (10 μg/ml). As a positive control, 2 μg/ml PHA was used to stimulate T cells for 4 d. The cells were then washed and stained with mAbs specific to rabbit CD4 and CD8 molecules (clone KEN-4 and clone 215B; Serotec). CellQuest was used to analyze the collected FACS data. The lymphocyte population was gated based on forward-scatter and side-scatter properties. The gated lymphocytes were analyzed for CFSE and CD4 (+) staining. The percentage of dividing lymphocytes = Nbr of gated CFSE(low) lymphocytes × Nbr of total lymphocytes—that is, (CFSE(low) + CFSE(high) lymphocytes)/Nbr of total gated lymphocytes) × 100. The ratio (CFSE(low) + CFSE(high) lymphocytes)/total lymphocytes) serves as a normalization factor between one sample to another and one experiment to another.
Immunohistochemistry
The immunostaining of rabbit tissues was performed as previously described (22). Rabbits were euthanized; corneas and TG were harvested, embedded in Tissue-Tek (OCT compound; VWR International, West Chester, PA), and snap-frozen. Approximately 10-μm-thick cryo sections were made, fixed in acetone (10 min, room temperature), air-dried, and stored at −80°C. For immunolocalization, tissue sections were rehydrated in PBS (10 min, room temperature), and stained with FITC-labeled anti–HLA-A2 (clone BB7.2; BD Biosciences) or with the isotype controls (FITC-labeled mouse IgG2b κ, clone 27–35; BD Biosciences) for 60 min at 4°C. After three successive washings in PBS (3 × 5 min), sections were stained with 14.3 μM DAPI (Molecular Probes, Eugene, OR) for 2 min at room temperature for staining of the cell nucleus. Excess DAPI was removed by washing with PBS (3 × 5 min) and mounted in 50% glycerol-PBS and analyzed under fluorescent microscope.
Viruses and cell lines
HSV-1 (strain McKrae) was used in this study. The virus was triple plaque purified and prepared as previously described (23, 24) using rabbit skin cell monolayes grown in MEM (1×) containing 10% FBS (HI), 2mML-glutamine, 2.5ug/ml amphotericin, and5%penicillin-streptomycin solution (fromastock of 10,000 IU penicillin and 10,000 μg/ml streptomycin).
Ocular infection of rabbits with HSV-1
Without making any corneal scarifice, rabbits were ocularly (both eyes) infected by dropping 5 μl HSV-1 (2 × 105 PFU) strain McKrae suspended in culture medium on day 12 from the last immunization (8).
Quantification of infectious virus
Tears from both eyes were collected by swabbing with a Dacron swab (type 1; Spectrum Laboratories, Los Angeles, CA) daily for 12 d. Individual swabs were transferred to a 2-ml sterile cryogenic vial containing 500 μl culture medium and stored at −80°C until use. The HSV-1 titers in the tear samples were determined by standard plaque assays on rabbit skin cells as previously described (8).
Monitoring and scoring ocular herpes disease
Eye disease was scored by slit lamp examination on days 3, 5, 7, 9, 11, 13, and 15 d postinfection (p.i.). Rabbits were lightly anesthetized, and a drop of 1% fluorescein dye was placed in each eye before taking pictures under blue light. The amount of corneal disease was expressed as the area of staining in square millimeters. These examinations were performed by investigators blinded to the treatment regimen of the rabbits.
Statistical analysis
Data are expressed as mean ± SE. Statistical significance was determined by ANOVA followed by Dunnett’s post test to identify differences between groups. Differences were considered significant when p < 0.05. All p values are two-tailed unless stated otherwise.
Results
Construction of CD4–CD8 lipopeptide candidate vaccines
CD4 and CD8 T cell epitopes were selected from HSV-1 gD, based on their strong recognition by T cells from HSV-1 seropositive individuals with no history of recurrent herpes (10, 11). One promiscuous CD4+ T cell peptide epitope (gD49–82) was linearly joined with one of three different immunodominant human CD8+ T cell peptide epitope (gD53–61, gD70–78, or gD278–286, to make three different pairs of CD4–CD8 peptides. To enhance their im-munogenic potential, each CD4–CD8 peptide was covalently linked at the N-terminal end to an Nε-palmitic acid-lysine moiety (Fig. 1). Construction of these CD4–CD8 lipopeptide vaccines was performed using a recently developed chemoselective ligation method (25), which provides higher-yield, purity, and solubility compared with the traditional method of lipopeptide synthesis. Unlike the first generation of lipopeptides that were synthesized using classic solid-phase methods, in which the fatty acyl moiety was introduced directly onto the crude peptide backbone before its purification, the lipopeptides used in this study were constructed by synthesizing and purifying the peptide and then ligating the lysine-lipid moiety with the peptide. This novel process yields lipopeptides that are fully soluble in water or PBS at concentrations up to 3 mg/ml.
Expression of HLA-A*0201 molecules in HLA Tg rabbits
Expression of HLA-A*0201 in spleen, ear, skin, kidney, liver, tongue, and peripheral blood-derived immune cells, including CD8+ T cells, was previously reported in the HLATg rabbits (4). Although this finding strongly suggests ubiquitous expression of HLA-A*0201 molecules in these rabbits, we further examined HLA-A*0201 expression in corneas, TG and PBMC. Specific expression of HLA-A*0201 molecules was detected by immunostaining of cornea and trigeminal ganglia of HLA Tg rabbits (Fig. 2A). As expected, no HLA-A*0201 expression was detected in the cornea or TG of wild type (nontransgenic) rabbits (negative controls). The specificity of anti-human HLA-A*0201 Ab was confirmed using an isotype IgG control (data not shown). In addition, up to 99% of PBMCs express a high levelofHLA-A*0201molecules (Fig. 2B). The HLA-A*0201 molecule was colocalized with the rabbit MHC class I (Fig. 2C).
FIGURE 2.
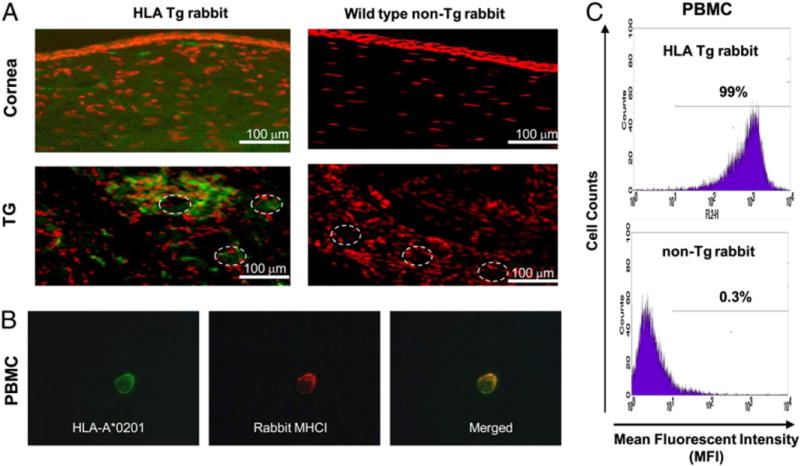
Detection of HLA-A*0201 molecules in cornea, TG, and PBMC of HLA transgenic rabbits. A, Cornea and TG sections from either HLA Tg rabbit or from wild type nontransgenic rabbits (control) were immunostained with FITC (green) conjugated BB7.2 mAb (anti-human HLA-A*0201) and analyzed by fluorescence microscopy (see Materials and Methods). Cell nuclei are shown in red. Dashed circles delineate neuron bodies in the TG (original magnification 320). B, Coexpression of HLA-A*0201 (green) and rabbit MHC class I (red) on the surface of a PBMC derived from an HLATg rabbit and detected by fluorescence microscopy. PBMCs from HLA Tg rabbit were double stained with two different primary Abs HLA-A2.1 mAb (BB7.2) and a rabbit MHCI followed by staining with secondary Abs conjugated with two different fluorescence probes, either Alexa Fluor 594 (green) or Alexa Fluor 488 (red), respectively. Merged yellow signals indicate the colocalization of these two molecules on the surface of PBMCs. C, PBMCs from either HLA Tg rabbit or from wild type nontransgenic rabbits were first stained with BB7.2 mAb, then with PE conjugated anti-mouse secondary IgG Ab, and analyzed by flow cytometry (original magnification ×20).
HLA Tg rabbits recall the same immunodominant CD8+ T cell epitopes identified in humans
To determine whether HSV-1 infected HLA Tg rabbits can recognize immunodominant human CD8+ T cell epitopes from HSV-1 gD, 10 rabbits were infected ocularly with HSV-1 (McKrae). Twenty-one days p.i., the frequencies of CD8+ T cells specific to a panel of 10 potential HLA-A*0201-restricted human gD epito-pes were determined in the cervical DLNs by standard HLA-A*0201 tetramer-staining assays. The frequencies of CD8+ T cells specific to the same 10 peptide panel were similarly determined in PBMCs of HSV-1 seropositive HLA-A*0201(+) humans with no history of recurrent herpes disease as well as from PBMCs of HSV-1/HSV-2 seronegative HLA-A*0201(+) humans. Three gD epitopes that were highly recognized by humans were also highly recognized by HLA Tg rabbits (Fig. 3, Supplemental Fig. 1). The dashed vertical line in each graph of Fig. 3 designates the maximum frequency levels of epitope-specific CD8+ T cells in un-infected HLA Tg rabbits, uninfected WT rabbits, and seronegative HLA-A2+ individuals. As shown, no significant frequency of CD8+ T cells specific to gD peptides was detected in uninfected HLA Tg rabbits. Thus, gD53–61, gD70–78, and gD278–286 that we recently found as the most immunodominant CD8+ T cell epitopes in HSV-1 seropositive HLA-A*0201(+) individuals with no history of recurrent herpes disease (10–12) were also highly recognized by HSV-1–infected HLA Tg rabbits. None of these epitopes were recognized by CD8+ T cells from nontransgenic wild type New Zealand rabbits. These results indicate: 1) these epitopes are HLA restricted; 2) the HLA-A*0201 Tg rabbits and HLA-A*0201(+) humans displayed similar pattern of T cell responses when the epitopes were presented by the same HLA-A*0201 molecule; and 3) as expected the HLA molecule plays a primary role in shaping the hierarchy of T cell immunodominance. These results indicate that the HLA Tg rabbits may be a useful model for preclinical assessment of immunogenicity and protective efficacy of HLA-A*0201–restricted epitope-based herpes vaccines.
FIGURE 3.
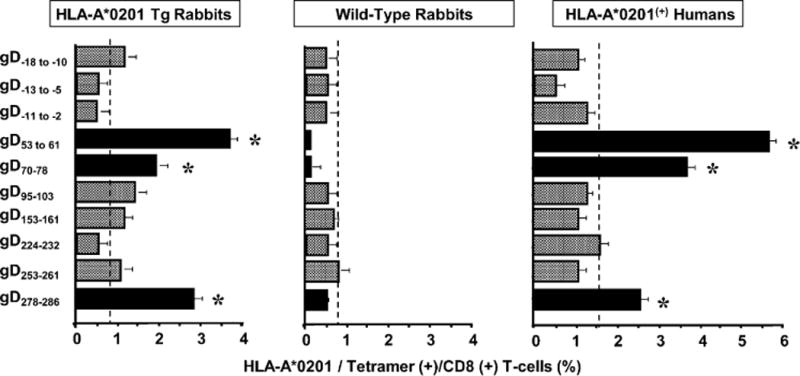
HLA-A*0201 Tg rabbit recognizes human HLA-A*0201–restricted CD8+ T cell epitopes from gD. Five HLA Tg rabbits and five wild type rabbits were infected ocularly with 2 × 105 PFU per eye of HSV-1. Twelve days p.i., spleens were harvested and the percentage HLA-A*0201/tetramer (+) CD8+ T cells specific to each of 10 HSV-1 gD epitopes was determined by FACS. In the numbering system used for gD peptides in this study, the first amino acid in the mature gD is indicated as AA1. Thus, amino acids in the 25 aa leader sequence are indicated as negative numbers relative to AA1. Similar tetramer analysis was performed in PBMCs derived from five HSV-seropositive HLA-A*0201(+) human blood samples. The results are representative of two independent experiments. Asterisk indicates a significant T cell response (p < 0.0005) compared with an irrelevant tetramer. The backgrounds were determined for all ten tetramers using PBMCs from each of the five different HLA-A2(+) HSV-uninfected individuals, five uninfected HLA Tg rabbits, and five uninfected wild type rabbits (controls). The dashed vertical lines in each panel designate maximum background cutoff levels of epitope-specific CD8+ T cells in uninfected HLA-Tg rabbits, uninfected WT rabbits, and seronegative HLA-A2+ individuals.
CD4+ T cell immunogenicity of CD4–CD8 lipopeptides in saline
We next evaluated the HSV-gD49–82–specific CD4+ T cell responses induced after s.c. immunization of HLA Tg rabbits with a mixture of all three CD4–CD8 lipopeptides, each of which has the same CD4 epitope. We used a mixture of the three lipopeptides rather than individual lipopeptides, because in mice immunization with a mixture of three gD lipopeptides induced better T cell-mediated protective immunity than did any individual lipopeptide (26, 27), a factor that could be advantageous when developing a vaccine for outbred populations, such as humans. As control for specificity, wild type New Zealand rabbits were similarly immunized.
Two weeks after the third immunization, cells were isolated from DLNs of each rabbit, labeled with CFSE and restimulated in vitro with the gD49–82 CD4+ Th epitope peptide. To selectively detect the CD4+ T cell response, the stimulated cells were stained with anti-rabbit CD4-PE and analyzed by FACS. Gated CD4+ T cells, from both lipopeptide- and mock-immunized HLA Tg rabbits and immunized wild type rabbits, were examined for CFSE incorporation in proliferated CD4+ T cells. Immunization with the mixture of three CD4–CD8 lipopeptides generated a substantial gD49–82 Th peptide-specific CD4+ T cell response in DLN of HLA Tg rabbits (Fig. 4A; 3.38% of CD4+ T cells responded in lip-opeptide immunized HLA Tg rabbits versus 1.88% of CD4+ T cells in mock-immunized HLA Tg rabbits [p < 0.005]). As expected, HSV-gD49–82–specific CD4+ T cell responses were also detected in nontransgenic wild type New Zealand rabbits, confirming that this is a promiscuous epitope (12).
FIGURE 4.
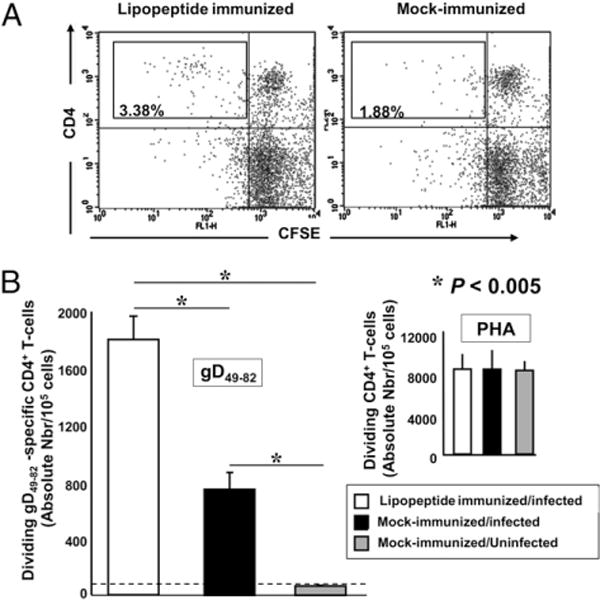
Induction of gD49–82-specific CD4+ T cell responses in HLA Tg rabbits immunized with human CD4–CD8 lipopeptides. HLATg rabbits (four in experiment 1, four in experiment 2) were immunized s.c. three times with the mixture of human CD4–CD8 lipopeptides as described in Materials and Methods. Ten days after the third immunization, all rabbits were ocularly challenged with 2 × 105 PFU per eyeof HSV-1 (McKrae). Mock-immunized HLA Tg rabbits (two in experiment 1, two in experiment 2) were ocularly challenged as above. Mock-immunized, uninfected HLATg rabbits (two in experiment 1, two in experiment 2) were used as negative controls. Two weeks after HSV-1 challenge, DLNs were collected from each rabbit, and cells suspension were prepared, labeled with CFSE (2 mM), and incubated with the promiscuous gD49–82 CD4+ T cell epitope peptide (10 mg/ml) for 5 d. The cells were washed and stained for CD4 molecule expression. Dividing CD4+ T cells were gated and analyzed by flow cytometry. A, Dot plot representation of CFSE intensity in dividing CD4+ T cells, from lipopeptide-immunized or mock-immunized rabbits, stimulated with gD49–82 peptide. CFSE staining is shown on the x-axis and CD4 staining on the y-axis. The percentage in the quadrant represents the fraction of dividing CD4+CFSElow T cells among total lymphocytes. B, Absolute number of dividing CD4+ T cells following in vitro stimulation with the promiscuous gD49–82 CD4+ T cell epitope (left panel). PHA stimulation (right panel, positive control). The percentage of dividing lymphocytes under stimulated conditions was calculated as: Nbr of dividing lymphocytes = Nbr of gated CD4(+) CFSE(low) lymphocytes × Nbr of total CD4+ lymphocytes—(CD4(+) CFSE(low) + CD4(+) CFSE(high) lymphocytes)/Nbr of total gated lymphocytes) × 100. The ratio ([CD4(+) CFSE (low) + CD4 (+) CFSE(high) lymphocytes]/total lymphocytes) serves as normalization factor between one sample to another and one experiment to another.
To confirm that HSV-1 infection boosts the gD49–82-peptide–specific CD4+ T cell response (9, 28), the CD4+ T helper responses were re-examined following infection of immunized HLA Tg rabbits. Two weeks after the third immunization with the three-lipopeptide mixture, five HLA Tg rabbits were ocularly challenged with 2 × 105 PFU per eye of HSV-1. Ten days later, the HSV-gD49–82–specific CD4+ T cell responses were measured in DLNs as above. Following HSV-1 ocular challenge, there was a 2-fold boost of HSV-gD49–82–specific CD4+ T helper responses in lipopeptide-immunized HLA Tg rabbits compared with nonimmunized HLA Tg rabbits (Fig. 4B; p < 0.005). PHA induced similar levels of stimulation of CD4+ T cells from both lipopeptide- and mock-immunized HLA Tg rabbits (Fig. 4B). These results suggest a natural boosting of HSV-gD49–82-specific CD4+ T helper responses by a native epitope of viral origin.
Multiepitopic CD8+ T cell responses generated in HLA Tg rabbits following immunization with a mixture of three CD4–CD8 lipopeptides
The CD8+ T cell responses induced following immunization of HLA Tg rabbits with a mixture of the three CD4–CD8 lip-opeptides were evaluated. Ten days after the third immunization, the frequency of HSV-1 gD53–61-, gD70–78-, and gD278–286-specific CD8+ T cells in the DLNs of HLA Tg rabbits were measured by standard HLA-A*0201 tetramer-staining assays and compared with mock-immunized HLA Tg rabbits. Fig. 5A shows representative results of three independent experiments, and the data are the average of the three experiments. Approximately 3.4% of HLA-A*0201/HSV-gD53–61, 2% of HLA-A*0201/HSV-gD70–78, and 2.4% of HLA-A*0201/HSV-gD278–286 tetramer positive CD8+ T cells were detected in the DLNs of immunized HLATg rabbits, compared with 0.7%, 0.5%, and 0.8% for mock-immunized rabbits (p < 0.05). As expected, little staining was seen with an irrelevant OVA tetramer (0.2–0.3%; data not shown). As expected, immunized nontransgenic wild type New Zealand rabbits had minimal CD8+ T cell responses (0.3–0.4%; data not shown), confirming that these responses were HLA-restricted.
FIGURE 5.
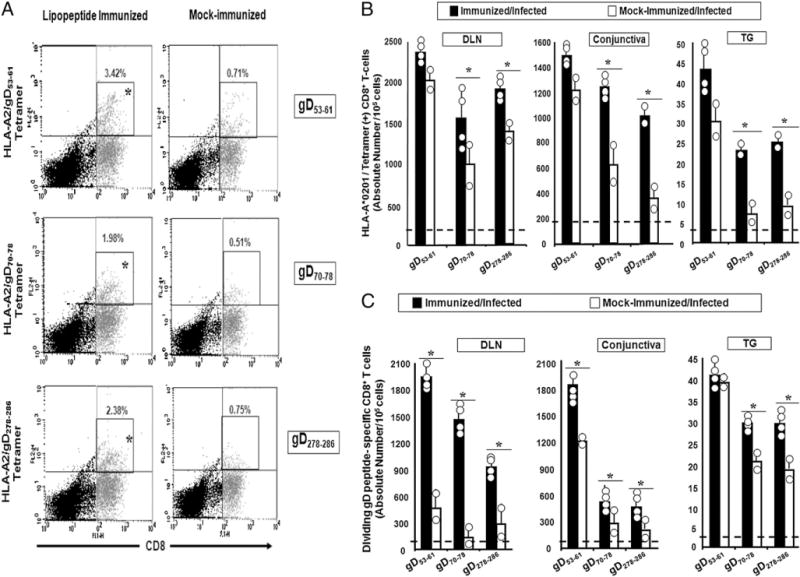
Frequency and absolute numbers of dividing HSV-gD epitope-specific CD8+ T cells in HLA Tg rabbits immunized with human CD4–CD8 lipopeptides. CD8 T cells were isolated from DLNs, conjunctiva, and TG from the rabbits described in Fig. 4. The cell suspensions were im-munostained with an FITC-labeled mAb specific to rabbit CD8 and with a PE-labeled human HLA-A*0201/tetramer specific to each of the three human CD8+ T cell epitopes. A, Representative FACS analysis from individual rabbits. The numbers show the average number of tetramer-positive/CD8+ T cells from all the rabbits in the indicated group. B, Average absolute numbers for each epitope-specific HLA-A*0201/tetramer/CD8+ T cells. The dashed horizontal lines designate the maximum frequency levels of epitope-specific CD8+ T cells in mock-immunized/uninfected HLA-Tg rabbits. C, Average absolute number of dividing CD8+ T cells in each group following in vitro stimulation with gD53–61, gD70–78, or gD278–286 epitope peptide. The number of dividing CD8+ lymphocytes under stimulated conditions was calculated as follows: Nbr of dividing lymphocytes = Nbr of gated CD8(+) CFSE(low) lymphocytes × Nbr of total CD8+ lymphocytes—(CD8(+) CFSE(low) + CD8(+) CFSE(high) lymphocytes)/Nbr of total gated lymphocytes. The dashed horizontal lines designate the maximum response levels of T cells in mock-immunized/uninfected HLA-Tg rabbits. The results are representative of two independent experiments.
Following ocular HSV-1 challenge of immunized HLA Tg rabbits, there was a trend toward an increase in the absolute number of tetramer-positive CD8+ T cells specific to each of the three epitopes to be boosted in DLNs, conjunctiva, and TG (Fig. 5B). However, this trend only reached significance for gD70–78 and gD278–286, but not for gD53–61. However, instead of absolute number of CD8+ T cells when the data analyzed as dividing CD8+ T cells, all three epitopes (i.e., gD53–61, gD70–78 and gD278–286) were significantly boosted in DLNs and conjunctiva after ocular challenge (Fig. 5C). In TG, only gD70–78 and gD278–286-specific CD8 T cells were significantly boosted. It is not clear why gD53–61-specific CD8 T cells in TG were not significantly boosted by infection when total CD8 T cells were examined or why gD53–61-specific CD8 T cells were not significantly boosted by infection when only dividing cells were examined. It is possible that there was little room to further boost the response to this epitope p.i., because this is the most immunodominant epitope in humans and the HLA tg rabbits (Fig. 3) and because the vaccine contained four copies of this epitope instead of one (this epitope overlaps with the CD4 epitope present in all three CD4–CD8 lipopeptides). Regardless, these results indicate a natural boosting of HSV-gD53–61, gD70–78 and gD278–286-specific CD8+ T cells by a native epitope of viral origin.
Immunization with human CD4–CD8 lipopeptides protects HLA Tg rabbits against replication of HSV-1 in eyes
Ten HLA Tg rabbits were immunized with the mixture of three CD4–CD8 lipopeptides and ocularly challenged with 2 × 105 PFU of HSV-1 per eye. HSV-1 replication in both eyes was monitored daily from day 1 to day 12 after challenge by collecting tears from both eyes. The amount of infectious virus in the tear samples was determined by standard plaque assays. During the period of peak replication (days 4 and 5 p.i.), the immunized HLA Tg rabbits had significantly less virus in their tears compared with mock-immunized rabbits (Fig. 6; p < 0.005).
FIGURE 6.
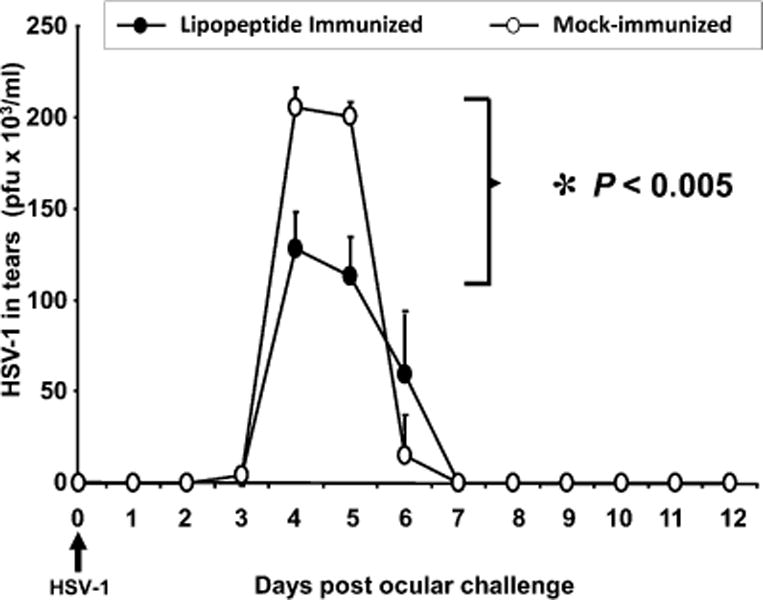
Immunization with human CD4–CD8 lipopeptides protects against HSV-1 replication in the eyes of HLA Tg rabbits. HLA Tg rabbits were immunized with the three human CD4–CD8 lipopeptides (four in experiment 1, four in experiment 2) or mock-immunized (two in experiment 1, two in experiment 2). Two weeks after the third immunization, all rabbits were ocularly challenged with 2 × 105 PFU per eye of HSV-1. Tears were collected daily from all eyes (20 eyes per group) on the days indicated, and the amount of infectious virus was determined by standard plaque assays. The p value is calculated using data from days 4 and 5 analyzed by ANOVA followed by Dunnett’s post test to identify differences between groups.
Immunization with human CD4–CD8 lipopeptides protects HLA Tg rabbits from acute herpes corneal disease
Twelve days after HSV-1 challenge, HLA-Tg rabbit eyes were examined by slit lamp after brief staining with fluorescein to reveal areas of epithelial damage (disease). Representative slitt lamp pictures of fluorescein stained eyes from HLATg rabbit are shown in Fig. 7A. The fluorescein-stained areas represent the epithelial damage and are outlined by dashed lines. Two independent experiments were performed using six immunized and four mock-immunized eyes in each experiment. Similar results were obtained in both experiments. The stained corneal areas were measured and are shown for each eye in a scatter plot. Mock-immunized rabbits had significantly more corneal disease than did immunized rabbits (Fig. 7B; p < 0.005).
FIGURE 7.
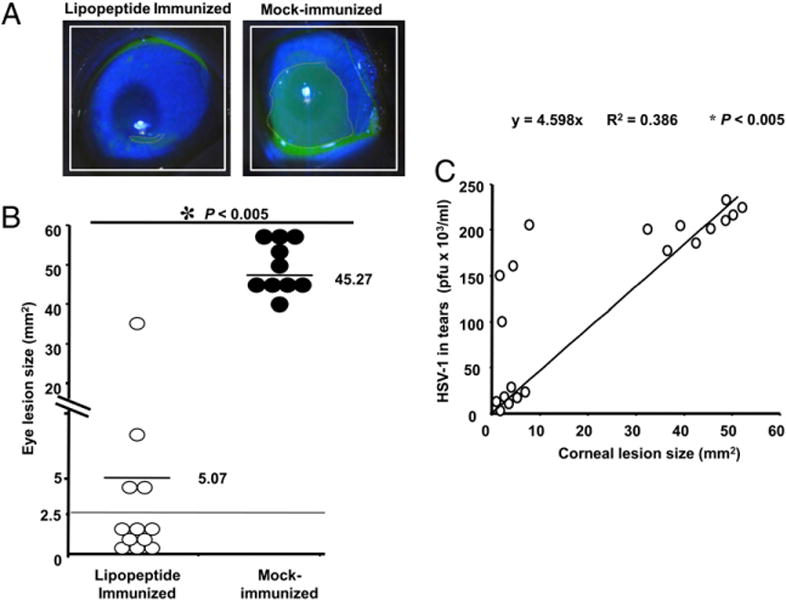
Immunization of HLA Tg rabbits with human CD4–CD8 lipopeptides protects the severity of ocular herpes disease. A, Representative slit lamp images of lipopeptide immunized/HSV-1 infected (four in experiment 1, four in experiment 2) and mock-immunized/HSV-1 (two in experiment 1, two in experiment 2). Infected HLA Tg rabbit eyes 12 d after ocular infection. One drop of 1% fluorescein dye was placed in each eye to identify the corneal damage under cobalt blue light. The amount of disease was estimated by determining the surface area stained, in square millimeters. B, The amount of corneal involvement in disease is plotted for each eye. C, Correlation between virus titers on day 5 and eye disease on day 12.
To compare the peak amount of virus in each eye (Fig. 6) with the amount of corneal disease, the day 5 virus titer for each eye (both immunized and mock-immunized, a total of 20 eyes) was plotted relative to the amount of corneal surface herpetic disease (mm2) on day 12 (Fig. 7C). The analysis shows that 1) eyes with minimal virus replication developed minimal corneal disease; 2) most eyes with high virus titers developed high levels of corneal disease; but 3) four eyes with high virus titers did not develop high levels of corneal disease. These findings suggest that acute herpetic corneal disease required a high level of virus in the eye, but that a high level of virus in the eye does not always result in acute herpetic corneal disease.
Correlation of herpes epitope-specific CD8+ T cells in the DLNs, conjunctiva, and TG with protection against corneal disease
In mouse studies, Ab depletion of CD8 T cells is often used to determine whether a protective immune response is CD8 T cell dependent. Unfortunately, in vivo CD8 T cell depletion studies are not feasible in rabbits because of the lack of a suitable Ab. In addition, although these rabbits are Tg for HLA class I, they are still outbred for MHC class II. Thus, adoptive transfer experiments would require autologous invitro expanded CD8 T cells, which was impractical. Therefore, we looked for correlations between the levels of epitope-specific CD8 T cells and protection. On day 12 p.i., the corneas of the immunized rabbits were divided into two groups based on low versus high corneal disease (< 10 versus > 30 mm2 of the corneal surface having corneal disease; Fig. 7C). The corresponding DLN, conjunctiva, and TG were excised and cell suspensions were prepared. The CD8 T cells specific to the gD53–61, gD70–78, or gD278–286 epitopes were detected by specific tetramer staining as described in Materials and Methods. The number of gD53–61 epitope-specific tetramer (+) CD8+ T cells was significantly higher in all three compartments of rabbits with low corneal disease compared with rabbits with high corneal disease (Fig. 8A; p < 0.005). The number of gD70–78 epitope-specific tetramer (+) CD8+ T cells was also significantly higher in the DLN and conjunctiva of rabbits with low disease compared with high corneal disease (p < 0.005). There was also a trend toward higher gD70–78 epitope tetramer staining in the TG and of higher gD278–286 epitope tetramer staining in all three compartments of rabbits with low versus high corneal disease. However, these trends did not reach statistical significance. Overall, the higher CD8+ T cell levels in rabbits with no corneal disease suggested that these cells may be involved in protection. In addition, there were significantly more HSV-specific, IFN-γ–producing CD8+ T cells in conjunctiva of eyes with low versus high corneal disease (Fig. 8B), suggesting that IFN-γ-producing CD8+ T cells might play a protective role.
FIGURE 8.
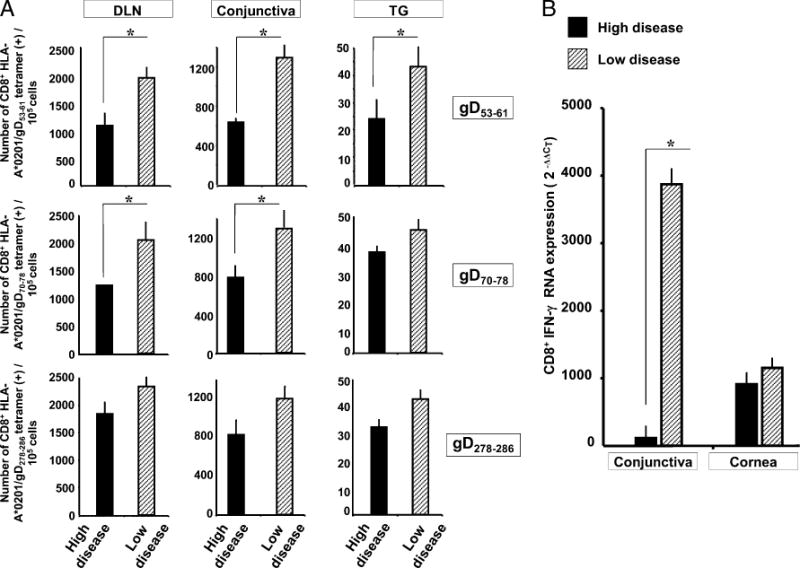
Immunized HLA Tg rabbits with more epitope-specific CD8+ T cells have less corneal disease. A, The results obtained from the HLA Tg rabbits that were immunized and ocularly challenged with HSV-1 (from Figs. 6 and 7) were divided into two groups, based on the amount of corneal disease on day 12 p.i. (low disease, < 10 mm2; high disease, > 30 mm2). The average absolute number of tetramer positive/CD8+ T cells specific to each of the three human CD8+ T cell epitopes are shown. The results are representative of two independent experiments. B, The relative amount of IFN-γ mRNA produced by conjunctiva- and cornea-derived CD8+ T cells of low and high corneal disease eyes was determined by quantitative RT-PCR.
Discussion
Several reports, mostly using murine models, have pointed to virus-specific, protective CD4+ and CD8+ T cells as critical effector cells for maintaining herpes immunoprotection (29–31). Paradoxically, virus-specific pathogenic T cells also cause her-petic corneal immunopathogenic disease in murine models (4, 32–35). A mechanism that is widely proposed as the basis of herpes immunity versus immunopathology is the imbalance of effector and regulatory T cell responses in the host (36, 37). We recently proposed a new hypothesis that a different spectrum of herpes disease, ranging from rare asymptomatic to frequent distressing symptomatic outbreaks, reflects different subsets of host effector CD4+ and CD8+ T cells reacting to different sets of HSV-1–specific T cell epitopes from one or several immediate early (ICP4, ICP0), tegument (UL35, UL46, UL47) and/or structural (gB, gD) proteins. Therefore, we believe that a distinct set of viral epitopes designated as symptomatic epitopes are recognized by pathogenic effector CD4+ and CD8+ T cells and associated with severe immunopathologic diseases, whereas recognition of a separate set of viral asymptomatic epitopes by protective effector CD4+ and CD8+ T cells might in turn lead to immunoprotection. This hypothesis is supported by our recent discovery showing that the two well-known HSV-1 glyco-proteins, gB and gD are targeted by different sets of asymptomatic and symptomatic human CD4+ and CD8+ T cells (10–12, 38–41). We found three immunodominant human HLA-A*0201–restricted CD8+ T cell epitopes, gD53–61, gD70–78, and gD278–286, and one promiscuous human CD4+ T cell epitope (gD49–82) from HSV-1 gD were selectively targeted in asymptomatic patients (10–12). However, the protective efficacy of these HLA-restricted asymptomatic epitopes against ocular herpes has never been tested, mainly because of a lack of an appropriate HLA transgenic animal model that displays ocular herpetic disease similar to humans.
In this study, we present a novel HLATg rabbit model of ocular herpes infection, disease, and immunity. Immunization of HLA Tg rabbits with a mixture of three immunodominant human CD4–CD8 lipopeptides induced: 1) HSV-gD49–82–specific CD4+ Th cell responses in DLNs; 2) HLA-restricted HSV-gD53–61-, gD70–78-, and gD278–286-specific CD8+ T cell responses in DLNs, conjunctiva, and TG; 3) an overall reduction in ocular herpes infection and disease after ocular challenge with HSV-1; and 4) a correlation between HSV-1 epitope-specific CD8 T cells and protection against ocular disease. Immunodominant CD8+ T cell epitopes, as used in this report, are those epitopes that are most frequently recognized in a group of HSV-1 seropositive HLA-A*0201 individuals (frequency of recognition) and that also induced the highest magnitude CD8+ T cell responses (strength of response) within a single individual (6, 7). Based on these two criteria, the three gD epitopes (gD53–61, gD70–78, and gD278–286) are labeled as immunodominant gD epitopes. To our knowledge, this report is the first to demonstrate the efficacy of a prophylactic HSV-1 vaccine against ocular herpes using human T cell epitopes in a humanized HLA Tg rabbit model.
Choosing the right animal model is a crucial first step in vaccine development. Some animal models are better at reflecting the human-pathogen interactions, including infection, disease, and immunity. A critical questionthatremains openfor herpes immunologistsis, which animal model would be the most appropriate to assess the efficacy of a human T cell epitope-based vaccine against ocular HSV-1 infection and disease? Theideal animal model shouldmountanHLA-restricted immune responses specific to human epitopes while mimicking as many aspects of ocular herpes infection and disease as possible (1). Mice have been the experimental animal model of choice for most immunologists, and the study of mouse immune responses has yielded tremendous insights into the human immune system (1, 10, 11, 42–45). Although mice are receptive to herpes infection, they do not mount specific T cell responses to HLA-restricted epitopes. In addition, ocular herpes disease in mice presents differently from that in humans (46, 47). Although HLA Tg mice can develop T cell responses to HLA-restricted epitopes, unlike humans, spontaneous HSV-1 reactivation either does not occur at all or occurs at low levels in mice (48). HSV-1–induced eye disease in rabbits is similar to that in humans and, like humans spontaneous reactivation and as measured by shedding of virus in tears, occurs at a high rate (in latently infected rabbits, ~10% of tears contain infectious virus) (49). To overcome the hurdle that rabbits do not mount T cell responses specific to HLA-restricted human epitopes, in this study we introduced a novel humanized HLATg rabbit model of ocular HSV-1 that is capable of 1) mounting T cell responses specific to human CD8+ T cell epitopes (Figs. 3–5); 2) developing ocular herpes disease similar to the human disease (Fig. 7); and 3) developing spontaneous HSV-1 reactivation (unpublished data). Thus, this HLA Tg rabbit model will allow us to investigate whether prophylactic and therapeutic immunization with human T cell epitopes can decrease primary ocular HSV-1 infection and disease and spontaneous reactivation, and ultimately reduce or eliminate recurrent ocular disease (i.e., herpetic stromal keratitis). In this study, we demonstrate for the first time the efficacy of a prophylactic human T cell-epitope–based vaccine against acute ocular herpes infection and disease using this novel HLA Tg rabbit model.
In mice, protection against ocular herpes correlates with the induction of high serum neutralizing Ab titers, but in rabbits and humans, serum neutralizing Ab titers do not correlate with ocular protection (50). The lipopeptide vaccines used in this study did not induce significant HSV-1 serum neutralizing Abs. Although pep-tide-specific Abs were induced in the serum (not shown) these Abs did not neutralize the virus in vitro. Therefore, neutralizing Abs were unlikely to have played a significant role in the observed protection. Virus-specific CD8+ T cells might be the critical effectors for maintaining herpes immunoprotection (29). Although in this report we have focused exclusively on IFN-γ producing CD8+ T cells, we do not mean to imply that other immune effectors are not playing important roles in the protective process.
T cell responses to peptide epitope-based vaccines are dependent on HLA molecules, which play a pivotal role in the selection of T cell repertoire in the thymus and presentation of T cell epitopes at the periphery. Because expression of the HLA Tg rabbits’ own MHC class I molecules might interfere with the human HLA-A*0201–restricted responses (51), the HLA-Tg rabbits were selected with high expression of HLA-A*0201 and low expression of rabbit class I molecules (52). The higher expression of HLA-A*0201 molecules in selected HLATg rabbits is expected to force rabbit CD8+ T cells to make use of the human molecules at both the thymic educational and peripheral effector levels (51). The results reported in this study show that the selected HLA Tg rabbits developed strong functional HLA-A*0201-restricted CD8+ T cell responses following HSV-1 infection (Fig. 3) and s.c. immunization with lipopeptide vaccine (Figs. 4, 5). Thus, simultaneous expression of high levels of HLA-A*0201 and low levels of rabbit class I molecules did not seem to cause any reduction of HLA-A*0201 education of rabbit CD8+ T cells. It should also be noted that the wild type nontransgenic rabbits failed to mount HLA-A*0201–restricted T cell responses to any human CD8+ T cell epitopes studies after either immunization or HSV-1 infection, pointing to the requirements of HLA-A*0201–restriction. Interestingly the pattern of CD8+ T cell responses detected in infected HLA Tg rabbits closely matched those of HLA-A*0201 positive HSV-1–seropositive individuals (Fig. 3). This finding suggests that, despite species differences between HLA-A*0201 Tg rabbits and HLA-A*0201(+) human individuals, the T cell repertoire in HLA-A*0201 Tg rabbits was able to display a similar pattern of CD8+ T cell response when the same HLA-A*0201 molecule presented the epitope. The results also confirm that in these HLA Tg rabbits the HLA molecule played a primary role in determining which human epitopes are recognized and in defining the repertoire of T cells induced and the hierarchy of im-munodominance. These results support the use of HLA-A*0201 Tg rabbits as a useful animal model for the assessment of the protective efficacy of HLA-A*0201–restricted epitope-based vaccines.
The human tetramers used in this study were previously used to detect CD8+ T cells specific to the same three gD epitopes in seropositive humans and in HLA-A*0201 Tg mice infected with HSV-1 (3). Therefore, these human tetramers were able to detect CD8+ T cells specific to three human epitopes in three different species: human, HLA Tg mice, and HLA Tg rabbits, suggesting that tetramer staining depends more on the epitope and HLA, rather than on the species. Although we do not have direct evidence that rabbit β-2–microglobulin binds to human HLA-A*A0201, the detection of CD8+ T cells specific to HLA-A*0201–restricted human epitopes in immunized and infected HLA Tg rabbits implies that the rabbit β-2–microglobulin formed heterodimers with human HLA-A*0201 molecules in vivo. In addition, no tetramer-positive CD8+ T cells were detected in either immunized or infected wild type (non-HLA transgenic) rabbits, confirming the specificity of the HLA-tetramers.
A question of practical importance is the translation of the current immunologic preclinical findings for the development of an epitope-based clinical vaccine for a genetically heterogeneous human population (9, 25, 53–56). A universal herpes vaccine should induce T cell responses in the context of many different HLA alleles. Although this report focused on HLA-A*0201, this allele is the most prevalent human HLA class I allele being present in ~50% of humans regardless of gender and ethnicity. In addition, the high degree of HLA polymorphism that might be a hindrance to epitope-based vaccines can be addressed by the inclusion of multiple HLA-restricted epitopes that are recognized in the context of diverse related HLA alleles, and by designing peptide-based vaccines with higher CD8+ T cell epitope densities. A combination of nine HLA supertypes has recently been defined to provide an almost perfect coverage (.99%) of the entire repertoire of HLA molecules (57). Because a mixture of multiple epitopes is likely to produce polyclonal T cell lines (one T cell clone for each epitope) and more effector T cells than a single epitope (26), we used multiple CD8+ T cell epitopes to induce a broader T cell response (9, 26). To further increase efficacy, a multiepitope-based lipopeptide vaccine should include several CD8+ T cell epitopes from several different glycoproteins and tegument proteins, each chosen to represent the HLA supertypes known to provide recognition in a large proportion of the global population, regardless of race and ethnicity.
Using a prophylactic vaccine strategy to immunize young children before their first encounter with HSV could greatly reduce the overall HSV infection in the general population, but it would not benefit the vast majority of adults (at least 80–90%) who already harbor latent HSV infections. Although the primary goal of this study was to evaluate the prophylactic efficacy of human HSV-1 gD T cell epitopes against primary infection and disease, our long-term goal is to expand the database of human T cell epitopes appropriate for a therapeutic vaccine. Because the immune response to primary infection is insufficient to prevent viral reactivation, a successful therapeutic vaccine will likely need to induce a different and/or much more vigorous immune response than natural immunity (29, 58). HSV-1–specific CD8+ T cells may block reactivation in vitro (explanted mouse TG) (59). In addition, CD8+ T cells have been found surrounding some neurons in (latently infected) human TG (42, 60) and rabbit TG (unpublished data). Thus, a CD8+ T cell epitope vaccine that induces vigorous HSV-1–specific CD8+ T cell responses at the site of latent infection (the TG) may prevent or significantly reduce HSV-1 shedding in tears and HSV-induced recurrent ocular disease.
Peptide epitope-based vaccines are molecularly defined and highly purified, and they offer potential advantages over traditional vaccines, including the safety and capacity of eliciting highly specific immune responses (9, 25, 27, 53, 54). Despite these advantages, many peptide vaccines tend to be poorly immunogenic and often require coadministration of external and potentially toxic immunoadjuvants (9, 25, 27, 53, 54, 61). In the current study, we demonstrate that immunization with self-adjuvanting CD4–CD8 lipopeptides induced strong T cell responses. We did not include a CD4 lipopeptide-only vaccine as a control because of the relatively small number of HLATg rabbits available. However, we did not include CD4 lipopetide-only vaccine as a control because of the relatively small number of HLA Tg rabbits available. We previously showed that immunization of mice with a mouse CD4–CD8 lipopeptide, but not with the CD4+ or CD8+ lip-opeptide alone, induced strong protection against ocular herpes infection and disease (1). Because the induction of CD8+ T cell responses, the optimization of effector cell functions, and the establishment of memory CD8+ T cell response need CD4+ T cell help (1, 2), but not vice versa, it is important to link CD4 T cell epitope with CD8+ T cell epitope in the vaccine construct. In addition, the effector CD8+ T cell mediated protection was further supported by in vivo depletion studies in mouse model (1). Based on these results, we incorporated the CD4+ T cell epitopes in the human CD4–CD8 lipopeptide vaccines to study the protective efficacy in HLATg rabbits. Because of the high percentage of the adult population that is latently infected with herpes, there is concern that a herpes vaccine, whether prophylactic or therapeutic and whether directed against genital, oral, or ocular herpes, may induce a T cell-mediated immunopathologic response that could exacerbate recurrent ocular disease. The new HLA Tg rabbit ocular HSV-1 model described in this study may be a valuable model for testing the safety issue of candidate human vaccines to ensure that the induced human HLA-restricted immune responses do not exacerbate recurrent corneal disease.
In conclusion, there are four principal findings in this study. First, a novel HLA-A*0201 Tg rabbit ocular HSV-1 model was introduced and shown to be a useful modelto study protective efficacy of human T cell epitope immunization against ocular HSV-1. Second, a human herpes lipopeptide vaccine formulation that contains three pairs of peptide epitopes (each with one variable CD8+ T cell epitope and one fixed CD4+ T cell epitope) derived from the sequence of HSV-1 gD was shown to provide protection in HLA-A*0201 Tg rabbits against ocular HSV-1. Third, the lipopeptide vaccine induced HLA-restricted T cell immune responses in conjunctiva, TG, and DLNs. Fourth, a correlation was seen between HSV-1 epitope-specific CD8 T cell responses and protection against ocular herpes. These initial studies demonstrated that prophylactic immunization with human CD8+ T cell epitopes can protect against primary HSV-1 infection and disease in a humanized HLA-A*0201 transgenic rabbit model and suggest the usefulness of HLA-A*0201 Tg rabbit to understand the immu-nopathologic mechanisms of ocular herpes diseases. Overall, this preclinical study in HLA-A*0201 Tg rabbits illustrates the feasibility of molecularly defined, chemically engineered, synthetic lip-opeptides as a chemically stable, hazard-free, and relatively low-cost approach for future herpes vaccines.
Supplementary Material
Acknowledgments
We thank Amy K. Stout from the National Institutes of Health Tetramer Core Facility (Atlanta, GA) for providing the tetramers.
This work was supported by: Public Health Service research Grants EY14900, EY14017, EY013191, EY018171, CA47622, and EY16663 from National Institutes of Health; the Discovery Eye Foundation; and an unrestricted grant from Research to Prevent Blindness. L.B.M. is a Research to Prevent Blindness Special Award Investigator.
Abbreviations used in this paper
- DLN
draining lymph node
- gD
glycoprotein D
- HLA Tg
HLA-A*0201 transgenic
- p.i.
postinfection
- TG
trigeminal ganglia
Footnotes
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Dasgupta G, Chentoufi AA, Nesburn AB, Wechsler SL, BenMohamed L. New concepts in herpes simplex virus vaccine development: notes and challenges from the battlefield. Expert Reviews. 2009;8:1023–1035. doi: 10.1586/erv.09.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001;20:1–13. doi: 10.1097/00003226-200101000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Richards CM, Case R, Hirst TR, Hill TJ, Williams NA. Protection against recurrent ocular herpes simplex virus type 1 disease after therapeutic vaccination of latently infected mice. J Virol. 2003;77:6692–6699. doi: 10.1128/JVI.77.12.6692-6699.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banerjee K, Biswas PS, Rouse BT. Elucidating the protective and pathologic T cell species in the virus-induced corneal immunoinflammatory condition herpetic stromal keratitis. J Leukoc Biol. 2005;77:24–32. doi: 10.1189/jlb.0904486. [DOI] [PubMed] [Google Scholar]
- 5.Thomas J, Rouse BT. Immunopathogenesis of herpetic ocular disease. Immunol Res. 1997;16:375–386. doi: 10.1007/BF02786400. [DOI] [PubMed] [Google Scholar]
- 6.Keadle TL, Laycock KA, Miller JK, Hook KK, Fenoglio ED, Francotte M, Slaoui M, Stuart PM, Pepose JS. Efficacy of a re-combinant glycoprotein D subunit vaccine on the development of primary and recurrent ocular infection with herpes simplex virus type 1 in mice. J Infect Dis. 1997;176:331–338. doi: 10.1086/514049. [DOI] [PubMed] [Google Scholar]
- 7.Nesburn AB, Burke RL, Ghiasi H, Slanina SM, Wechsler SL. A therapeutic vaccine that reduces recurrent herpes simplex virus type 1 corneal disease. Invest Ophthalmol Vis Sci. 1998;39:1163–1170. [PubMed] [Google Scholar]
- 8.Nesburn AB, Ramos TV, Zhu X, Asgarzadeh H, Nguyen V, BenMohamed L. Local and systemic B cell and Th1 responses induced following ocular mucosal delivery of multiple epitopes of herpes simplex virus type 1 glycoprotein D together with cytosine-phosphate-guanine adjuvant. Vaccine. 2005;23:873–883. doi: 10.1016/j.vaccine.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Issagholian A, Berg EA, Fishman JB, Nesburn AB, BenMohamed L. Th-cytotoxic T-lymphocyte chimeric epitopes extended by Nε-palmitoyl lysines induce herpes simplex virus type 1-specific effector CD8+ Tc1 responses and protect against ocular infection. J Virol. 2005;79:15289–15301. doi: 10.1128/JVI.79.24.15289-15301.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chentoufi AA, Zhang X, Lamberth K, Dasgupta G, Bettahi I, Nguyen A, Wu M, Zhu X, Mohebbi A, Buus S, et al. HLA-A*0201-restricted CD8+ cytotoxic T lymphocyte epitopes identified from herpes simplex virus glyco-protein D. J Immunol. 2008;180:426–437. doi: 10.4049/jimmunol.180.1.426. [DOI] [PubMed] [Google Scholar]
- 11.Chentoufi AA, Binder NR, Berka N, Durand G, Nguyen A, Bettahi I, Maillère B, BenMohamed L. Asymptomatic human CD4+ cytotoxic T-cell epitopes identified from herpes simplex virus glycoprotein B. J Virol. 2008;82:11792–11802. doi: 10.1128/JVI.00692-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X, Castelli FA, Zhu X, Wu M, Maillère B, BenMohamed L. Gender-dependent HLA-DR-restricted epitopes identified from herpes simplex virus type 1 glycoprotein D. Clin Vaccine Immunol. 2008;15:1436–1449. doi: 10.1128/CVI.00123-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perng GC, Maguen B, Jin L, Mott KR, Osorio N, Slanina SM, Yukht A, Ghiasi H, Nesburn AB, Inman M, et al. A gene capable of blocking apoptosis can substitute for the herpes simplex virus type 1 latency-associated transcript gene and restore wild-type reactivation levels. J Virol. 2002;76:1224–1235. doi: 10.1128/JVI.76.3.1224-1235.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shukla D, Dal Canto MC, Rowe CL, Spear PG. Striking similarity of murine nectin-1a to human nectin-1a (HveC) in sequence and activity as a glycoprotein D receptor for alphaherpesvirus entry. J Virol. 2000;74:11773–11781. doi: 10.1128/jvi.74.24.11773-11781.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trousdale MD, Dunkel EC, Nesburn AB. Effect of flurbiprofen on herpes simplex keratitis in rabbits. Invest Ophthalmol Vis Sci. 1980;19:267–270. [PubMed] [Google Scholar]
- 16.Nesburn AB. Common viral eye diseases and latent infections. Ophthalmology. 1980;87:1202–1207. doi: 10.1016/s0161-6420(80)35103-8. [DOI] [PubMed] [Google Scholar]
- 17.Metcalf JF, Kaufman HE. Herpetic stromal keratitis-evidence for cell-mediated immunopathogenesis. Am J Ophthalmol. 1976;82:827–834. doi: 10.1016/0002-9394(76)90057-x. [DOI] [PubMed] [Google Scholar]
- 18.Hu J, Peng X, Schell TD, Budgeon LR, Cladel NM, Christensen ND. An HLA-A2.1-transgenic rabbit model to study immunity to papillomavirus infection. J Immunol. 2006;177:8037–8045. doi: 10.4049/jimmunol.177.11.8037. [DOI] [PubMed] [Google Scholar]
- 19.Parish CR, Glidden MH, Quah BJ, Warren HS. Use of the intracellular fluorescent dye CFSE to monitor lymphocyte migration and proliferation. Curr Protoc Immunol. 2009;84:4.9.1–4.9.13. doi: 10.1002/0471142735.im0409s84. [DOI] [PubMed] [Google Scholar]
- 20.Quah BJ, Warren HS, Parish CR. Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye carbox-yfluorescein diacetate succinimidyl ester. Nat Protoc. 2007;2:2049–2056. doi: 10.1038/nprot.2007.296. [DOI] [PubMed] [Google Scholar]
- 21.Karandikar NJ, Crawford MP, Yan X, Ratts RB, Brenchley JM, Ambrozak DR, Lovett-Racke AE, Frohman EM, Stastny P, Douek DC, et al. Glatiramer acetate (Copaxone) therapy induces CD8+ T cell responses in patients with multiple sclerosis. J Clin Invest. 2002;109:641–649. doi: 10.1172/JCI14380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nesburn AB, Bettahi I, Dasgupta G, Chentoufi AA, Zhang X, You S, Morishige N, Wahlert AJ, Brown DJ, Jester JV, et al. Functional Foxp3+ CD4+ CD25(Bright+) “natural” regulatory T cells are abundant in rabbit conjunctiva and suppress virus-specific CD4+ and CD8+ effector T cells during ocular herpes infection. J Virol. 2007;81:7647–7661. doi: 10.1128/JVI.00294-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nesburn AB, Burke RL, Ghiasi H, Slanina SM, Wechsler SL. Therapeutic periocular vaccination with a subunit vaccine induces higher levels of herpes simplex virus-specific tear secretory immunoglobulin A than systemic vaccination and provides protection against recurrent spontaneous ocular shedding of virus in latently infected rabbits. Virology. 1998;252:200–209. doi: 10.1006/viro.1998.9454. [DOI] [PubMed] [Google Scholar]
- 24.Nesburn AB, Slanina S, Burke RL, Ghiasi H, Bahri S, Wechsler SL. Local periocular vaccination protects against eye disease more effectively than systemic vaccination following primary ocular herpes simplex virus infection in rabbits. J Virol. 1998;72:7715–7721. doi: 10.1128/jvi.72.10.7715-7721.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.BenMohamed L, Wechsler SL, Nesburn AB. Lipopeptide vaccines—yesterday, today, and tomorrow. Lancet Infect Dis. 2002;2:425–431. doi: 10.1016/s1473-3099(02)00318-3. [DOI] [PubMed] [Google Scholar]
- 26.Bettahi I, Nesburn AB, Yoon S, Zhang X, Mohebbi A, Sue V, Vanderberg A, Wechsler SL, BenMohamed L. Protective immunity against ocular herpes infection and disease induced by highly immunogenic self-adjuvanting glycoprotein D lipopeptide vaccines. Invest Ophthalmol Vis Sci. 2007;48:4643–4653. doi: 10.1167/iovs.07-0356. [DOI] [PubMed] [Google Scholar]
- 27.Bettahi I, Zhang X, Afifi RE, BenMohamed L. Protective immunity to genital herpes simplex virus type 1 and type 2 provided by self-adjuvanting lipopeptides that drive dendritic cell maturation and elicit a polarized Th1 immune response. Viral Immunol. 2006;19:220–236. doi: 10.1089/vim.2006.19.220. [DOI] [PubMed] [Google Scholar]
- 28.Rajasagi NK, Kassim SH, Kollias CM, Zhao X, Chervenak R, Jennings SR. CD4+ T cells are required for the priming of CD8+ T cells following infection with herpes simplex virus type 1. J Virol. 2009;83:5256–5268. doi: 10.1128/JVI.01997-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cunningham AL, Diefenbach RJ, Miranda-Saksena M, Bosnjak L, Kim M, Jones C, Douglas MW. The cycle of human herpes simplex virus infection: virus transport and immune control. J Infect Dis. 2006;194(Suppl 1):S11–S18. doi: 10.1086/505359. [DOI] [PubMed] [Google Scholar]
- 30.Ghiasi H, Cai S, Perng GC, Nesburn AB, Wechsler SL. Both CD4+ and CD8+ T cells are involved in protection against HSV-1 induced corneal scarring. Br J Ophthalmol. 2000;84:408–412. doi: 10.1136/bjo.84.4.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noisakran S, Carr DJ. Plasmid DNA encoding IFN-a1 antagonizes herpes simplex virus type 1 ocular infection through CD4+ and CD8+ T lymphocytes. J Immunol. 2000;164:6435–6443. doi: 10.4049/jimmunol.164.12.6435. [DOI] [PubMed] [Google Scholar]
- 32.Biswas PS, Banerjee K, Zheng M, Rouse BT. Counteracting corneal immunoinflammatory lesion with interleukin-1 receptor antagonist protein. J Leukoc Biol. 2004;76:868–875. doi: 10.1189/jlb.0504280. [DOI] [PubMed] [Google Scholar]
- 33.Stumpf TH, Case R, Shimeld C, Easty DL, Hill TJ. Primary herpes simplex virus type 1 infection of the eye triggers similar immune responses in the cornea and the skin of the eyelids. J Gen Virol. 2002;83:1579–1590. doi: 10.1099/0022-1317-83-7-1579. [DOI] [PubMed] [Google Scholar]
- 34.Maertzdorf J, Osterhaus AD, Verjans GM. IL-17 expression in human herpetic stromal keratitis: modulatory effects on chemokine production by corneal fibroblasts. J Immunol. 2002;169:5897–5903. doi: 10.4049/jimmunol.169.10.5897. [DOI] [PubMed] [Google Scholar]
- 35.Keadle TL, Morris JL, Pepose JS, Stuart PM. CD4+ and CD8+ cells are key participants in the development of recurrent herpetic stromal ker-atitis in mice. Microb Pathog. 2002;32:255–262. doi: 10.1006/mpat.2002.0506. [DOI] [PubMed] [Google Scholar]
- 36.Rouse BT, Sarangi PP, Suvas S. Regulatory T cells in virus infections. Immunol Rev. 2006;212:272–286. doi: 10.1111/j.0105-2896.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- 37.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6:353–360. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 38.BenMohamed L, Bertrand G, McNamara CD, Gras-Masse H, Hammer J, Wechsler SL, Nesburn AB. Identification of novel immunodo-minant CD4+ Th1-type T-cell peptide epitopes from herpes simplex virus gly-coprotein D that confer protective immunity. J Virol. 2003;77:9463–9473. doi: 10.1128/JVI.77.17.9463-9473.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim M, Taylor J, Sidney J, Mikloska Z, Bodsworth N, Lagios K, Dunckley H, Byth-Wilson K, Denis M, Finlayson R, et al. Im-munodominant epitopes in herpes simplex virus type 2 glycoprotein D are recognized by CD4 lymphocytes from both HSV-1 and HSV-2 seropositive subjects. J Immunol. 2008;181:6604–6615. doi: 10.4049/jimmunol.181.9.6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Posavad CM, Wald A, Hosken N, Huang ML, Koelle DM, Ashley RL, Corey L. T cell immunity to herpes simplex viruses in seronegative subjects: silent infection or acquired immunity? J Immunol. 2003;170:4380–4388. doi: 10.4049/jimmunol.170.8.4380. [DOI] [PubMed] [Google Scholar]
- 41.Koelle DM, Liu Z, McClurkan CL, Cevallos RC, Vieira J, Hosken NA, Meseda CA, Snow DC, Wald A, Corey L. Immunodominance among herpes simplex virus-specific CD8 T cells expressing a tissue-specific homing receptor. Proc Natl Acad Sci USA. 2003;100:12899–12904. doi: 10.1073/pnas.2131705100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Knickelbein JE, Khanna KM, Yee MB, Baty CJ, Kinchington PR, Hendricks RL. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science. 2008;322:268–271. doi: 10.1126/science.1164164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheridan BS, Cherpes TL, Urban J, Kalinski P, Hendricks RL. Reevaluating the CD8 T-cell response to herpes simplex virus type 1: involvement of CD8 T cells reactive to subdominant epitopes. J Virol. 2009;83:2237–2245. doi: 10.1128/JVI.01699-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thapa M, Carr DJ. CXCR3 deficiency increases susceptibility to genital herpes simplex virus type 2 infection: Uncoupling of CD8+ T-cell effector function but not migration. J Virol. 2009;83:9486–9501. doi: 10.1128/JVI.00854-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wuest TR, Carr DJ. Dysregulation of CXCR3 signaling due to CXCL10 deficiency impairs the antiviral response to herpes simplex virus 1 infection. J Immunol. 2008;181:7985–7993. doi: 10.4049/jimmunol.181.11.7985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willey DE, Trousdale MD, Nesburn AB. Reactivation of murine latent HSV infection by epinephrine iontophoresis. Invest Ophthalmol Vis Sci. 1984;25:945–950. [PubMed] [Google Scholar]
- 47.Margolis TP, Elfman FL, Leib D, Pakpour N, Apakupakul K, Imai Y, Voytek C. Spontaneous reactivation of herpes simplex virus type 1 in latently infected murine sensory ganglia. J Virol. 2007;81:11069–11074. doi: 10.1128/JVI.00243-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gebhardt BM, Varnell ED, Kaufman HE. Inhibition of cyclo-oxygenase 2 synthesis suppresses Herpes simplex virus type 1 reactivation. J Ocul Pharmacol Ther. 2005;21:114–120. doi: 10.1089/jop.2005.21.114. [DOI] [PubMed] [Google Scholar]
- 49.Perng GC, Maguen B, Jin L, Mott KR, Kurylo J, BenMohamed L, Yukht A, Osorio N, Nesburn AB, Henderson G, et al. A novel herpes simplex virus type 1 transcript (AL-RNA) antisense to the 5′ end of the latency-associated transcript produces a protein in infected rabbits. J Virol. 2002;76:8003–8010. doi: 10.1128/JVI.76.16.8003-8010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Richards CM, Aman AT, Hirst TR, Hill TJ, Williams NA. Protective mucosal immunity to ocular herpes simplex virus type 1 infection in mice by using Escherichia coli heat-labile enterotoxin B subunit as an adjuvant. J Virol. 2001;75:1664–1671. doi: 10.1128/JVI.75.4.1664-1671.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Firat H, Garcia-Pons F, Tourdot S, Pascolo S, Scardino A, Garcia Z, Michel ML, Jack RW, Jung G, Kosmatopoulos K, et al. H-2 class I knockout, HLA-A2.1-transgenic mice: a versatile animal model for preclinical evaluation of antitumor immunotherapeutic strategies. Eur J Immunol. 1999;29:3112–3121. doi: 10.1002/(SICI)1521-4141(199910)29:10<3112::AID-IMMU3112>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 52.Simpson E, Takacs K, Altmann DM. Thymic repertoire selection by superantigens: presentation by human and mouse MHC molecules. Thymus. 1994;23:1–13. [PubMed] [Google Scholar]
- 53.BenMohamed L, Belkaid Y, Loing E, Brahimi K, Gras-Masse H, Druilhe P. Systemic immune responses induced by mucosal administration of lipopeptides without adjuvant. Eur J Immunol. 2002;32:2274–2281. doi: 10.1002/1521-4141(200208)32:8<2274::AID-IMMU2274>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 54.BenMohamed L, Krishnan R, Auge C, Primus JF, Diamond DJ. Intranasal administration of a synthetic lipopeptide without adjuvant induces systemic immune responses. Immunology. 2002;106:113–121. doi: 10.1046/j.1365-2567.2002.01396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu X, Ramos TV, Gras-Masse H, Kaplan BE, BenMohamed L. Lipopeptide epitopes extended by Nε-palmitoyl-lysine moiety increase uptake and maturation of dendritic cells through a Toll-like receptor-2 pathway and trigger a Th1-dependent protective immunity. Eur J Immunol. 2004;34:3102–3114. doi: 10.1002/eji.200425166. [DOI] [PubMed] [Google Scholar]
- 56.Nesburn AB, Bettahi I, Zhang X, Zhu X, Chamberlain W, Afifi RE, Wechsler SL, BenMohamed L. Topical/mucosal delivery of sub-unit vaccines that stimulate the ocular mucosal immune system. Ocul Surf. 2006;4:178–187. doi: 10.1016/s1542-0124(12)70164-7. [DOI] [PubMed] [Google Scholar]
- 57.Sette A, Newman M, Livingston B, McKinney D, Sidney J, Ishioka G, Tangri S, Alexander J, Fikes J, Chesnut R. Optimizing vaccine design for cellular processing, MHC binding and TCR recognition. Tissue Antigens. 2002;59:443–451. doi: 10.1034/j.1399-0039.2002.590601.x. [DOI] [PubMed] [Google Scholar]
- 58.Gebhardt BM, Halford WP. Evidence that spontaneous reactivation of herpes virus does not occur in mice. Virol J. 2005;2:67. doi: 10.1186/1743-422X-2-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Derfuss T, Segerer S, Herberger S, Sinicina I, Hüfner K, Ebelt K, Knaus HG, Steiner I, Meinl E, Dornmair K, et al. Presence of HSV-1 immediate early genes and clonally expanded T-cells with a memory effector phenotype in human trigeminal ganglia. Brain Pathol. 2007;17:389–398. doi: 10.1111/j.1750-3639.2007.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Verjans GM, Hintzen RQ, van Dun JM, Poot A, Milikan JC, Laman JD, Langerak AW, Kinchington PR, Osterhaus AD. Selective retention of herpes simplex virus-specific T cells in latently infected human trigeminal ganglia. Proc Natl Acad Sci USA. 2007;104:3496–3501. doi: 10.1073/pnas.0610847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stanberry LR, Spruance SL, Cunningham AL, Bernstein DI, Mindel A, Sacks S, Tyring S, Aoki FY, Slaoui M, Denis M, et al. GlaxoSmithKline Herpes Vaccine Efficacy Study Group Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med. 2002;347:1652–1661. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


