Micro RNAs (miRNAs) are small RNAs that play an important role in the negative regulation of gene expression by suppressing protein translation. Animal genomes contain an abundance of small genes that produce regulatory RNAs of about 22 nucleotides in length. The Ambros lab identified the first miRNAs in 1993 while characterizing a genetic locus involved in the control of developmental timing in C. elegans.1 It has since been shown that these miRNAs are diverse in sequence and expression patterns and are evolutionarily widespread, suggesting that they may participate in a wide range of genetic and regulatory pathways. Since their initial discovery, thousands of papers have been published characterizing miRNA properties, defining their expression, and demonstrating function. MiRNAs are initially transcribed as long primary miRNAs (pri-miRNAs) that are processed by the RNase III enzyme Drosha to generate stem-loop precursor miRNAs (pre-miRNAs) approximately 70 nucleotides in length.2 These precursors are exported into the cytoplasm and, subsequently, the cytoplasmic enzyme Dicer cleaves the pre-miRNA to release the mature miRNA.3 Binding of miRNA to a messenger RNA (mRNA) with Ago proteins inhibits protein translation. It is estimated that the human genome encodes about 1500 miRNAs that are thought to regulate more than 30% of protein-coding genes.4 As interindividual variation of miRNA expression levels influences the expression of a myriad of miRNA target genes; these processes likely contribute to phenotypic differences and susceptibility to common and complex disorders.
Consistent with the recent surge of studies characterizing the role of miRNAs in cellular function and disease relevance is the study by Engelhardt and colleagues in the current issue of Circulation.5 This interesting study focused on miR-378 and its’ involvement in repressing cardiomyocyte hypertrophy. The study identified a relevant regulatory pathway, specifically MAP kinase, as a target of miR-378. Importantly, the study also clearly characterizes the underlying pathways that govern repression of the hypertrophic response by miR-378. A strength of this study is that the initial target was identified from a broader screen of synthetic miRNAs for the induction of cardiomyocyte hypertrophy and not only based on prediction models. This is the initial description of miR-378 in cardiac hypertrophy and supports several recent publications that demonstrate a role of miRNAs in cardiomyopathy,6, 7 MAP kinase,8, 9 or, specifically, for miR-378 in the cardiac regulation of apoptosis, ischemic heart disease, and mitochondrial function.10, 11
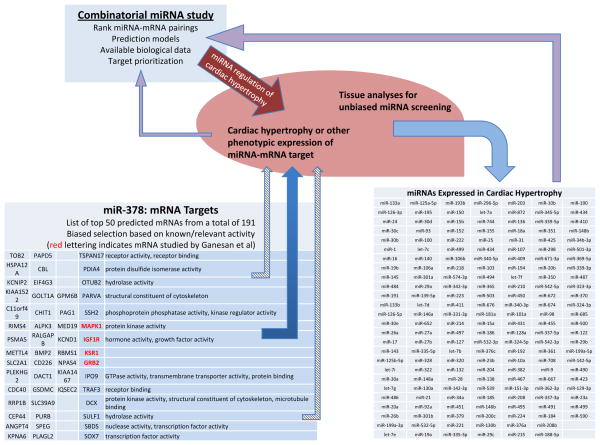
The findings of Engelhardt and colleagues provide an interesting and important mechanistic link between an individual miRNA, a specific signaling pathway, and a complex disease. However, as discussed above, miRNAs are generated through the concerted action of complexes that promote multi-step processing and loading of miRNA into silencing complexes, with individual classes of microRNAs differentially controlled through the association of regulatory factors. A growing number of studies suggest that each of these steps serves as potential points of regulation, adding to the complexity of miRNA-dependent gene modulation. Regulation of miRNAs is distinct from transcriptional or post-translational regulation of proteins as it modifies not only gene expression but cellular function. Importantly, as a single miRNA, such as miR-378, modulates the expression of many targets simultaneously (Figure 1), the co-regulation of multiple miRNAs could dramatically alter both gene expression and cellular function. This complexity is highlighted by large-scale profiling studies using tissue samples that reveal a somewhat consistent yet complex pattern of miRNA dysregulation in human disease12 as well as in cardiac hypertrophy.7, 13
Figure 1.
Utilizing both mechanistic and unbiased miRNA studies to understand disease. Using global miRNA profiling of ventricles during development of severe hypertrophic cardiomyopathy and heart failure7, 13 with mechanistic observations from specific miRNAs5 and predicted targets, combinatorial approaches can be pursued that could yield increasingly relevant in vivo data. These approaches acknowledge that there is both increased and decreased miRNA expression in disease settings and these miRNAs may target a broad number of compensatory and non-compensatory pathways.
In the setting of this complexity, the transcription of tissue and pathway-specific miRNAs may be directed by the same master regulatory factors controlling mRNA, such as with skeletal and cardiac muscle differentiation that may be characterized by the transcriptional activation of muscle specific genes.14 While “master regulation” likely occurs in specific settings, this cannot be assumed based on focused examination of miRNAs, gene expression, or tissue. Seeing a cluster of gene expression changes using a targeted assessment or biased prediction model does not preclude other relevant pathways being operational in complex systems. Simply put, if a relevant pathway or transcript is not studied, it cannot be assumed that changes did not occur.
As discussed, an individual miRNA can target multiple genes and each protein-coding gene can be regulated by several miRNAs. This complexity is compounded by the fact that many studies are performed with exogenous overexpressing miRNAs and it is not known, even in combinatorial studies, whether the miRNAs will be additive or redundant in their regulation.15 While single miRNA-single target studies and large-scale screening studies have become plentiful in the literature, there is a paucity of studies examining the combinatorial effect of multiple miRNAs on a single protein. One study that attempted this approach found that AKT1 and ERK2, two major kinases in the PI3K and RAS oncogenic pathways, might be co-downregulated by 30 miRNAs.16 This study used a combined strategy to analyze the multiple miRNA–protein interactions that regulate cell proliferation in response to epidermal growth factor receptor, an oncogenic pathway highly relevant in breast cancer.16 Such a study provides a more complete view of the combinatorial effort of miRNAs to control a signaling pathway at different levels and could be employed for cardiac hypertrophy (Figure 1).
Highlighting the limitations of individual miRNAs as targets, systematic genetic deletions of miRNAs have revealed grossly abnormal phenotypes in less than 10% of miRNA-mutant systems and genetic analyses of miRNAs in mice have revealed relatively minor functions under conditions of homeostasis.17 The paucity of strong loss-of-function miRNA phenotypes might be due to compensatory mechanisms that allow for re-calibration of protein expression. In addition, there is redundancy among homologous miRNAs within families or, possibly, the eventual targeting of individual mRNAs by several miRNAs could mitigate eventual phenotypic expression. Many believe the actions of miRNAs become more notable under conditions of injury or stress.18
The relevance of this balance goes well beyond a miRNA-mechanism-phenotype discussion. Therapeutics targeting a specific miRNA to target a specific disease are rapidly being developed.19, 20 Using knowledge gained from antisense technologies, oligonucleotides targeting miRNAs, known as anti-miRs, are being developed for therapeutic use as are pharmacologically active synthetic miRNAs, or miR mimics/mimetics.19, 20 The assumption is that the direct downstream targets of a single miRNA are commonly related genes that function in a comparable cellular process or signaling cascade. This infers that targeting of a single miRNA should result in a dramatic effect due to the combinatorial effect of gene expression changes in primarily related downstream targets. Whether this assumption is correct will likely depend on the setting. As discussed, a single miRNA can target many genes and also many cells suggesting that the off-target effects will be more complex as compared to many classic therapies.
Do these concerns mean a simple miRNA-mRNA-single phenotype targeted approach is invalid? Obviously, that is not the case. The majority of the disease-based studies currently in the literature are either single miRNA-few target or large scale screening without mechanism; but the true clinical relevance of both types of data will be realized by studies that meet in the middle; i.e. well-done, mechanistic, studies that utilize combinatorial approaches in relevant models. Given the importance of miRNAs in development, it is not surprising that alteration of miRNA expression is implicated in a variety of human diseases and that this has prompted copious investigation into the mechanism and function of miRNA-mediated repression. However, the mechanisms which govern the regulation of microRNA biogenesis and activity are just beginning to be understood and appreciated. Understanding the relative abundance and specific targeted effects in a variety of model systems, and defining them broadly in human disease, will be central in revealing the true complex function of miRNAs. Thus judiciously balancing mulit- and single-target approaches with broader screening methods, modeling, and bioinformatics will ultimately define the role of miRNAs in human cardiovascular disease.
Acknowledgments
Funding Sources: This work was partially supported by National Institutes of Health PO1 A1078894 (J.E.F.), and U54 HL12311 (J.E.F., K.T.).
Footnotes
Conflict of Interest Disclosures: None.
References
- 1.Lee RC, Feinbaum RL, Ambros V. The c. Elegans heterochronic gene lin-4 encodes small rnas with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 2.Ambros V. Development. Dicing up rnas. Science. 2001;293:811–813. doi: 10.1126/science.1064400. [DOI] [PubMed] [Google Scholar]
- 3.Ambros V. Microrna pathways in flies and worms: Growth, death, fat, stress, and timing. Cell. 2003;113:673–676. doi: 10.1016/s0092-8674(03)00428-8. [DOI] [PubMed] [Google Scholar]
- 4.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microrna targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 5.Engelhardt S, Ganesan J, Ramanujam D, Sassi Y, Ahles A, Jentzsch C, Werfel S, Leierseder S, Loyer X, Giacca M. Mir-378 controls cardiac hypertophy by combined repression of map kinase pathway factors. Circulation. 2013;xxx:xxx. doi: 10.1161/CIRCULATIONAHA.112.000882. [DOI] [PubMed] [Google Scholar]
- 6.Busk PK, Cirera S. Microrna profiling in early hypertrophic growth of the left ventricle in rats. Biochem Biophys Res Commun. 2010;396:989–993. doi: 10.1016/j.bbrc.2010.05.039. [DOI] [PubMed] [Google Scholar]
- 7.Bagnall RD, Tsoutsman T, Shephard RE, Ritchie W, Semsarian C. Global microrna profiling of the mouse ventricles during development of severe hypertrophic cardiomyopathy and heart failure. PLoS One. 2012;7:e44744. doi: 10.1371/journal.pone.0044744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen E, Diao X, Wang X, Chen R, Hu B. Micrornas involved in the mitogen-activated protein kinase cascades pathway during glucose-induced cardiomyocyte hypertrophy. Am J Pathol. 2011;179:639–650. doi: 10.1016/j.ajpath.2011.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JT, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engelhardt S. Microrna-21 contributes to myocardial disease by stimulating map kinase signalling in fibroblasts. Nature. 2008;456:980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 10.Fang J, Song XW, Tian J, Chen HY, Li DF, Wang JF, Ren AJ, Yuan WJ, Lin L. Overexpression of microrna-378 attenuates ischemia-induced apoptosis by inhibiting caspase-3 expression in cardiac myocytes. Apoptosis. 2012;17:410–423. doi: 10.1007/s10495-011-0683-0. [DOI] [PubMed] [Google Scholar]
- 11.Nagalingam RS, Sundaresan NR, Gupta MP, Geenen D, Solaro RJ, Gupta M. A cardiac enriched microrna, mir-378 blocks cardiac hypertrophy by targeting ras-signaling. J Biol Chem. 2013 doi: 10.1074/jbc.M112.442384. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Davis BN, Hata A. Regulation of microrna biogenesis: A miriad of mechanisms. Cell Commun Signal. 2009;7:18. doi: 10.1186/1478-811X-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Latronico MV, Condorelli G. Micrornas in hypertrophy and heart failure. Exp Biol Med (Maywood) 2011;236:125–131. doi: 10.1258/ebm.2010.010269. [DOI] [PubMed] [Google Scholar]
- 14.van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a microrna. Science. 2007;316:575–579. doi: 10.1126/science.1139089. [DOI] [PubMed] [Google Scholar]
- 15.Malumbres M. Mirnas versus oncogenes: The power of social networking. Mol Syst Biol. 2012;8:569. doi: 10.1038/msb.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uhlmann S, Mannsperger H, Zhang JD, Horvat EA, Schmidt C, Kublbeck M, Henjes F, Ward A, Tschulena U, Zweig K, Korf U, Wiemann S, Sahin O. Global microrna level regulation of egfr-driven cell-cycle protein network in breast cancer. Mol Syst Biol. 2012;8:570. doi: 10.1038/msb.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park CY, Choi YS, McManus MT. Analysis of microrna knockouts in mice. Hum Mol Genet. 2010;19:R169–175. doi: 10.1093/hmg/ddq367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Rooij E, Olson EN. Microrna therapeutics for cardiovascular disease: Opportunities and obstacles. Nat Rev Drug Discov. 2012;11:860–872. doi: 10.1038/nrd3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson AL, Levin AA. Developing microrna therapeutics: Approaching the unique complexities. Nucleic Acid Ther. 2012;22:213–225. doi: 10.1089/nat.2012.0356. [DOI] [PubMed] [Google Scholar]
- 20.van Rooij E, Purcell AL, Levin AA. Developing microrna therapeutics. Circ Res. 2012;110:496–507. doi: 10.1161/CIRCRESAHA.111.247916. [DOI] [PubMed] [Google Scholar]