Abstract
Eighteen normal women underwent pituitary down-regulation with leuprolide, followed by a 10-day treatment with 0.2 mg/d transdermal estradiol (E2) with subsequent allocation to one of two 10-day estradiol regimens plus 40 mg daily intramuscular P: supraphysiologic (0.2 mg/d transdermal E2 mg/d vaginal micronized E2) or subphysiologic (no exogenous E2 treatment). Average E2 and P in the supraphysiologic, physiologic, and subphysiologic groups were 1,175.9 pg/mL and 17.5 ng/mL, 136.9 pg/mL and 21.2 pg/mL, and 23.8 ng/mL and 22.0 ng/mL, respectively, and there were no differences between groups in endometrial histology or expression of biomarkers of receptivity.
Keywords: Endometrium, receptivity, estrogen, luteal phase, beta-3 integrin, osteopontin
Although the corpus luteum secretes both estradiol (E2) and progesterone (P), the effects of luteal E2 on endometrial function remain unclear (1–3). Data from experimentally designed studies as well as IVF studies have been conflicting. Some support the concept of estrogen-enhancing implantation, whereas others suggest no impact or even harm (1–11). In an effort to clarify the relevance of E2 levels during the luteal phase, we studied the effects of variations in serum E2 concentrations on secretory endometrial function in modeled cycles in normal women. We evaluated the functional state by assessing histologic progression as well as expression of several biomarkers suggested to have key roles in the implantation process including the β3 integrin subunit, osteopontin (OPN), and estrogen and P receptors (12–20). We hypothesized that wide variations in luteal phase E2 levels would have significant impact on these measures.
Approval for this study was obtained from the institutional review board of UNC Hospitals. Subjects were assigned to treatment groups using a blinded, block-randomized design. Subjects were healthy women, aged 18 to 34 years, having normal menstrual cycles and proven midsecretory phase endometrial β3 integrin subunit expression and histologic development. Power calculation determined that nine women were required in each treatment group for comparison with previously sampled control subjects to detect a significant difference in β3 integrin expression. All subjects were down-regulated with leuprolide acetate (Lupron, TAP Pharmaceuticals, Lake Forrest, IL) and then received two 0.1-mg E2 patches changed on alternate days for 10 days (18). This was followed by one of two investigational treatment regimens for an additional 10 days. One group received treatment with micronized E2 (2 mg twice daily, per vagina) and P in oil (40 mg daily, intramuscularly [IM]), in addition to continuing transdermal E2. The second group received the same exogenous P treatment regimen, but no further E2 treatment in any form. Subjects returned for serum E2 and P concentrations on alternate days during P treatment. On the tenth day, endometrial biopsy was performed using an endometrial Pipelle (Milex Products Inc., Chicago, IL). A portion of each specimen was fixed in formalin before paraffin embedding and staining for histologic dating; the remainder was flash frozen at −90 °C for analysis by immunohistochemistry and immunoblot.
Results obtained from study subjects were compared with those obtained in two other groups of tissue specimens obtained previously. One group of control tissue specimens derived from normally cycling women sampled on randomly assigned days after LH surge in natural cycles (representing early, mid, and late luteal phase). The second was from a group of women first suppressed by GnRH agonist and then treated with a physiologic E2 replacement regimen of two 0.1-mg patches changed on alternate days for 10 days followed by transdermal E2 and exogenous P in oil (40 mg daily, IM) for an additional 10 days before biopsy (18).
Sections of each tissue specimen were examined by a gynecologic pathologist (blinded to the day of sampling) and assigned a histologic date (19). Immunohistochemical staining was performed using monoclonal antibodies specific for each of the following molecular markers of endometrial function or receptivity: estrogen receptor (ER)-α, PR-A/B, PR-B, β3 integrin, and OPN. The staining intensity was assessed using the following equation: H-score = Σ Pi (i + 1), where I = intensity (1, 2, or 3, corresponding to weak, moderate, or strong, respectively) and Pi is the percentage of stained epithelial cells for each intensity (varying from 0–100%), as previously described (20).
Immunohistochemical stains were validated by immunoblot studies using antibodies specific for β3 integrin, ER-α, and OPN. Endometrial tissue specimens from all study and control groups were homogenized and pooled by each study condition (21). One hundred micrograms of each protein sample were denatured and fractionated using one-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis, then transferred to a polyvinylidene fluoride membrane using an electroblotter (Bio-Rad, Hercules, CA). Immunoblots were repeated using antibodies against β-actin (constitutive protein) for validation of findings.
Eighteen women were recruited and enrolled in the study and 100% of enrolled subjects completed the protocol. There were no differences between groups in age, body mass index, or race. As expected, spontaneous cycle control subjects had mean E2 and P concentrations that were low in the early luteal phase, peaked in midluteal, and fell in the late luteal phase. Endometrial histologic dating also was uniformly “in phase” in natural cycles. The mean serum E2 concentration in subjects receiving physiologic E2 treatment (136.9 pg/mL) was not different from those observed during the midluteal phase (128.3 pg/mL) in normally cycling controls. However, mean serum E2 level was significantly higher in subjects receiving supraphysiologic E2 treatment (1175.9 pg/mL; P<.0001) and lower in subjects receiving only P treatment (21.2 pg/mL; P<.0001). P concentrations were similar in all study groups, generally higher than midluteal phase levels in normally cycling controls, but uniformly within the range observed during the normal luteal phase. Thus, the characteristics of modeled cycles achieved targeted hormonal levels.
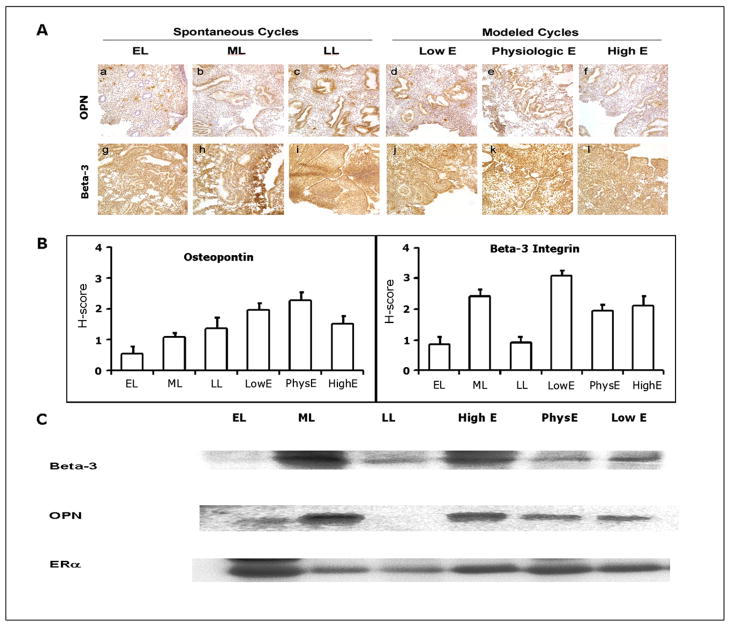
Immunostaining for β3 integrin subunit and OPN throughout the luteal phase in spontaneous cycles and in modeled cycles are shown in Figure 1A. Strong staining for β3 integrin and OPN, typical during the midsecretory phase of natural cycles, was observed in all study groups, and H-scores were not different between groups (Bartlett’s test for equal variance; Fig. 1B). We also observed no difference in H-scores for ER-α, PR-A, and PR-B during the midsecretory phase in natural cycles versus modeled cycles (Bartlett’s test for equal variance >0.05, data not shown). In samples derived from natural cycles, staining for the β3 integrin subunit and OPN by Western immunoblot was strong during the midsecretory phase; staining for ER-α was strong in the early secretory phase and decreased during the mid- and late secretory phase (Fig. 1C). Results from modeled cycles were similar to those for tissues from midsecretory phase of natural cycles. Expression of β-actin was similar in all tissues, demonstrating equivalent levels of total protein expression (data not shown).
FIGURE 1.
(A) Photomicrographs showing immunohistochemical localization of two biomarkers of receptivity in endometrium from normal and study women. β3 integrin subunit (β3) and osteopontin (OPN) are strongly expressed in the receptive midluteal phase as well as in all study conditions. Original magnification, 20×. (B) H-scores of immunohistochemcial stains. ML not different than study samples for β3 and OPN; (C) Immunoblots for β3, OPN, and ER-α. Note strong expression of β3 and OPN in the normal midluteal samples as well as in the altered hormonal conditions. EL: early luteal, ML: midluteal, LL: late luteal, LowE: subphysiologic estrogen, PhysE: physiologic estrogen, HighE: supraphysiologic estrogen.
Groll. No endometrial effect of luteal estrogen. Fertil Steril 2009.
It has been assumed that morphologic and functional endometrial maturation relate directly to the levels of circulating sex steroids and that abnormally low or high E2 and P concentrations or E2/P ratios during the luteal phase are likely to have important clinical consequences. However, data from this and our previous study of modeled cycles in normal women question that notion seriously. Previously, we demonstrated that secretory histologic endometrial development is not sensitive to variations in circulating P concentrations (18). Our current study extends those observations, revealing that histologic endometrial maturation also appears insensitive to widely varying E2 levels spanning the range between castrate and grossly supraphysiologic concentrations. These collected observations are striking, and suggest that the endometrium can tolerate a wide range in circulating E2 and P levels during the luteal phase without significant consequence. Although histologic endometrial dating cannot reliably define a specific luteal day, we expected the extremes in E2 concentrations imposed in our modeled cycles to result in discernible differences in endometrial histology (22, 23). That they did not supports the notion that histologic dating is not sufficiently sensitive to be an effective analytical tool for evaluating endometrial development and function.
To further investigate the effects of varying luteal phase E2 concentrations on the endometrium, we examined the expression of a number of putative biomarkers of endometrial function, using immunohistochemistry and immunoblotting. Interestingly, expression of the β3 integrin subunit and OPN were similar under all experimental conditions, with both methods of analysis. The expressions of β3 integrin subunit and OPN have been viewed as measures of endometrial receptivity. To the extent they are, these observations suggest that widely varying serum E2 levels also have no significant impact on endometrial receptivity and are consistent with studies finding no benefit from luteal phase E2 supplementation on IVF outcomes (9).
Acknowledgments
This research was supported by UNC Nova Carta Foundation and the Eunice Kennedy Shriver NICHD/NIH through cooperative agreement U54HD035041-11 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research.
Footnotes
J.M.G. has nothing to disclose. R.S.U. has nothing to disclose. B.A.L. has nothing to disclose. R.L. has nothing to disclose. S.L.Y. has nothing to disclose. M.A.F. has nothing to disclose.
References
- 1.de Ziegler D, Bergeron C, Cornel C, Medalie DA, Massai MR, Milgrom E, et al. Effects of luteal estradiol on the secretory transformation of human endometrium and plasma gonadotropins. J Clin Endocrinol Metab. 1992;74:322–31. doi: 10.1210/jcem.74.2.1730810. [DOI] [PubMed] [Google Scholar]
- 2.de Zeigler D, Bouchard P. Understanding endometrial physiology and menstrual disorders in the 1990s. Curr Opin Obstet Gynecol. 1993;5:378–88. [PubMed] [Google Scholar]
- 3.Fritz MA, Westfahl PK, Graham RL. The effect of luteal phase estrogen antagonism on endometrial development and luteal function in women. J Clin Endocrinol Metab. 1987;65:1006–13. doi: 10.1210/jcem-65-5-1006. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt CL, de Ziegler D, Gagliardi CL, Mellon RW, Taney FH, Kuhar MJ, et al. Transfer of cryopreserved-thawed embryos: the natural cycle versus controlled preparation of the endometrium with gonadotropin-releasing hormone agonist and exogenous estradiol and progesterone (GEEP) Fertil Steril. 1989;52:609–16. doi: 10.1016/s0015-0282(16)60973-1. [DOI] [PubMed] [Google Scholar]
- 5.Rosenwaks Z. Donor eggs: their application in modern reproductive technologies. Fertil Steril. 1987;47:895–909. doi: 10.1016/s0015-0282(16)59220-6. [DOI] [PubMed] [Google Scholar]
- 6.Meyer WR, Novotny DB, Fritz MA. Effect of exogenous gonadotropins on endometrial maturation in oocyte donors. Fertil Steril. 1999;71:109–14. doi: 10.1016/s0015-0282(98)00390-2. [DOI] [PubMed] [Google Scholar]
- 7.Valbuena D. Ovarian stimulation and endometrial receptivity. Hum Reprod. 1999;14(Suppl 2):107–11. doi: 10.1093/humrep/14.suppl_2.107. [DOI] [PubMed] [Google Scholar]
- 8.de Ziegler D, Fanchin R, de Moustier B, Bulletti C. The hormonal control of endometrial receptivity: estrogen (E) and progesterone. J Reprod Immun. 1998;39:149–66. doi: 10.1016/s0165-0378(98)00019-9. [DOI] [PubMed] [Google Scholar]
- 9.Kolibianakis EM, Venetis CA, Papanikolaou EG, Diedrich K, Tarlatzis BC, Griesinger G. Estrogen addition to progesterone for luteal phase support in cycles stimulated with GnRH analogues and gonadotrophins for IVF: a systematic review and meta-analysis. Hum Reprod. 2008;23:1346–54. doi: 10.1093/humrep/den115. [DOI] [PubMed] [Google Scholar]
- 10.Lukaszuk K, Liss J, Lukaszuk M, Maj B. Optimization of estradiol supplementation during the luteal phase improves the pregnancy rate in women undergoing in vitro fertilization-embryo transfer cycles. Fertil Steril. 2005;83:1372–6. doi: 10.1016/j.fertnstert.2004.11.055. [DOI] [PubMed] [Google Scholar]
- 11.Farhi J, Weissman A, Steinfeld Z, Shorer M, Nahum H, Levran D. Estradiol supplementation during the luteal phase may improve the pregnancy rate in patients undergoing in vitro fertilization–embryo transfer cycles. Fertil Steril. 2000;73:761–6. doi: 10.1016/s0015-0282(99)00632-9. [DOI] [PubMed] [Google Scholar]
- 12.Ilesanmi AO, Hawkins DA, Lessey BA. Immunohistochemical markers of uterine receptivity in the human endometrium. Microsc Res Tech. 1993;25:208–22. doi: 10.1002/jemt.1070250304. [DOI] [PubMed] [Google Scholar]
- 13.Lessey BA, Damjanovic L, Coutifaris C, Castelbaum A, Albelda SM, Buck CA. Integrin adhesion molecules in the human endometrium. Correlation with the normal and abnormal menstrual cycle. J Clin Invest. 1992;90:188–95. doi: 10.1172/JCI115835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yaron Y, Botchan A, Amit A. Endometrial receptivity in the light of modern assisted reproductive technologies. Fertil Steril. 1994;62:225–32. doi: 10.1016/s0015-0282(16)56868-x. [DOI] [PubMed] [Google Scholar]
- 15.Lessey BA, Castelbaum AJ, Sawin SJ, Sun J. Integrins as markers of uterine receptivity in women with primary unexplained infertility. Fertil Steril. 1995;63:535–42. [PubMed] [Google Scholar]
- 16.Lessey BA, Castelbaum AJ, Sawin SJ, Buck CA, Schinnar R, Wilkins B, et al. Aberrant integrin expression in the endometrium of women with endometriosis. J Clin Endocrinol Metab. 1994;79:643–9. doi: 10.1210/jcem.79.2.7519194. [DOI] [PubMed] [Google Scholar]
- 17.Meyer WR, Castelbaum AJ, Somkuti S, Sagoskin AW, Doyle M, Harris JE, et al. Hydrosalpinges adversely affect markers of endometrial receptivity. Hum Reprod. 1997;12:1393–8. doi: 10.1093/humrep/12.7.1393. [DOI] [PubMed] [Google Scholar]
- 18.Usadi RS, Groll JM, Lessey BA, Kowalik AI, Lininger R, Zaino R, et al. Endometrial development and function in experimentally-induced luteal phase deficiency. J Clin Endocrinol Metab. 2008;93:4058–64. doi: 10.1210/jc.2008-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noyes RW, Hertig AI, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1:3–25. doi: 10.1016/j.fertnstert.2019.08.079. [DOI] [PubMed] [Google Scholar]
- 20.Lessey BA. Adhesion molecules and implantation. J Reprod Immunol. 2002;55:101–12. doi: 10.1016/s0165-0378(01)00139-5. [DOI] [PubMed] [Google Scholar]
- 21.Carson DD, Lagow E, Thathiah A, Al-Shami R, Farach-Carson MC, Vernon M, et al. Changes in gene expression during the early to mid-luteal (receptive phase) transition in human endometrium detected by high-density microarray screening. Mol Hum Reprod. 2002;8:871–9. doi: 10.1093/molehr/8.9.871. [DOI] [PubMed] [Google Scholar]
- 22.Murray MJ, Meyer WR, Zaino RJ, Lessey BA, Novotny DB, Ireland K, et al. A critical analysis of the accuracy, reproducibility, and clinical utility of histologic endometrial dating in fertile women. Fertil Steril. 2004;81:1333–43. doi: 10.1016/j.fertnstert.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 23.Coutifaris C, Myers ER, Guzick DS, Diamond MP, Carson SA, Legro RS, et al. NICHD National Cooperative Reproductive Medicine Network. Histological dating of timed endometrial biopsy tissue is not related to fertility status. Fertil Steril. 2004;82:1264–72. doi: 10.1016/j.fertnstert.2004.03.069. [DOI] [PubMed] [Google Scholar]