Abstract
Background
The effectiveness of screening colonoscopy in average-risk adults is uncertain, particularly for right colon cancers.
Objective
Examine the association between screening colonoscopy and incident late-stage colorectal cancer (CRC) risk.
Design
Nested case-control study.
Setting
Four U.S. health plans
Patients
Average-risk adults with ≥5 years of enrollment in one of the health plans (n=1,039). Cases were 55–85 years old on their diagnosis date (reference date) of stage ≥IIB (late-stage) CRC during 2006–2008. We selected 1–2 controls for each case, matched on birth year, gender, health plan, and prior enrollment duration.
Measurements
Receipt of CRC screening between 3 months and up to 10 years before the reference date, ascertained through medical record audits. We compared cases and controls on receipt of screening colonoscopy or sigmoidoscopy using conditional logistic regressions that accounted for health history, socioeconomic status and other screening exposures.
Results
In analyses restricted to 471 eligible cases and their matched controls (n=509), 13 cases (2.8%) and 46 controls (9.0%) had undergone screening colonoscopy, which corresponded to an adjusted odds ratio (AOR) of 0.30 (95% confidence interval [CI]: 0.15–0.59) for any late-stage CRC, 0.37 (CI: 0.16–0.82) for right colon cancers, and 0.26 (CI: 0.06–1.11) for left-sided colon/rectum cancers. Ninety-two cases (19.5%) and 173 controls (34.0%) underwent screening sigmoidoscopy, corresponding to an AOR of 0.51 (CI: 0.36–0.71) overall, 0.80 (CI: 0.52–1.25) for right colon late-stage cancers, and 0.26 (CI: 0.14–0.49) for left colon/rectum cancers.
Limitations
The small number of screening colonoscopies affected the precision of our estimates.
Conclusions
Screening with colonoscopy in average-risk persons was associated with reduced risk of diagnosis with incident late-stage CRC in both the right colon and left colon/rectum. For sigmoidoscopy, this association was observed for left-sided CRC, but the association for right colon late-stage cancer was not statistically significant.
Primary Funding Source
National Cancer Institute of the National Institutes of Health.
INTRODUCTION
Evidence from randomized trials and observational studies has established that screening with sigmoidoscopy(1–4) or fecal occult blood tests (FOBT)(5–7) reduces the risk of colorectal cancer (CRC) incidence and death. In contrast, evidence for the effectiveness of screening colonoscopy is limited. There are no reports from randomized trials in average-risk individuals; and the few observational studies have not evaluated the effects of screening colonoscopy, as compared to unscreened controls, on CRC incidence or mortality.(8, 9) Microsimulation studies suggest that screening with colonoscopy every 10 years, sigmoidoscopy every 5 years with mid-interval FOBT, or annual FOBT alone, could all achieve comparable effects, based on assumptions about colonoscopy effectiveness.(10) Thus, the use of colonoscopy for screening in average-risk individuals remains controversial and its effectiveness in the right colon, the location of nearly 50% of new CRC cases in the US, has been questioned.(11–14)
Despite limited evidence of effectiveness, limited capacity, and higher cost and risk of potential complications relative to other screening tests,(15, 16) colonoscopy is rapidly replacing sigmoidoscopy and FOBT in the U.S.,(17) and its use is increasing in other countries.(18)
We conducted a case-control study to evaluate the association between receipt of screening colonoscopy and the risk of incident late-stage CRC diagnosis in average-risk adults, overall and separately for the right colon and left colon/rectum. Prevention of advanced CRC is an important attribute of effective CRC screening because advanced CRCs have a higher burden of cancer-related illness and mortality risk than early-stage disease.(14, 19) We also examined the effects of screening sigmoidoscopy, given the accumulated evidence of its efficacy, to help gauge the validity of our results for colonoscopy.
METHODS
Design and setting
We conducted a nested case-control study in four U.S. managed care organizations that participate in the HMO Cancer Research Network.(20) The study sites were Group Health Cooperative in western Washington State; Kaiser Permanente in Hawaii; Kaiser Permanente Northwest in Oregon and southern Washington states; and Reliant Medical Group/Fallon Community Health Plan in central Massachusetts. All sites have electronic utilization data on their populations dating back to at least 1995 and have used electronic medical records since 2005 or earlier. Subjects’ clinical data were linked to data from state or local tumor registries and to socioeconomic status (SES) information from the U.S. Census Bureau using a unique study identifier. This allowed us to define the study’s base population, evaluate the demographic and clinical characteristics of eligible subjects retrospectively for 10 years, and accrue cases and controls from an historical cohort. The Institutional Review Boards at the University of Massachusetts Medical School and participating sites approved the project.
Study subjects
Subjects included in this study were 55–85 years old between January 1, 2006 and December 31, 2008 and enrolled in their health plan for ≥5 years before their reference date (defined below). Because we wanted to study adults at average-risk for CRC,(21, 22) we excluded persons with a history of total colectomy, inflammatory bowel disease, or CRC in one or more first-degree relatives before age 50 or in two or more second-degree relatives at any age, or other familial CRC syndromes (strong family history).(23, 24)
Case definition and control selection
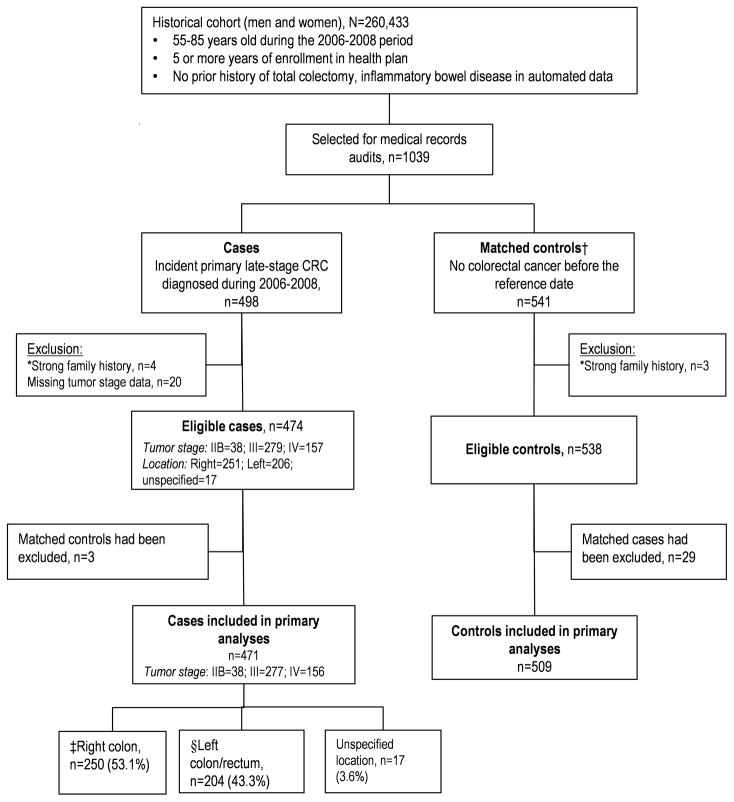
Diagnosis date and tumor stage of incident CRCs were ascertained from tumor registry data. Cases were subjects with primary late-stage CRC, defined as stage IIB or higher at the time of diagnosis, based on American Joint Commission on Cancer (AJCC) criteria. We included stage IIB tumors because their mortality risk is higher than that of some stage III CRCs.(25) Cancer location was categorized as: ‘right’ colon if proximal to the splenic flexure; ‘left’ if in or distal to the splenic flexure; or ‘unspecified’. The diagnosis date was set as the reference date for ascertaining test history and selecting controls. Each case was individually matched to 1–2 controls using incidence-density matching(26) according to calendar year of birth (±1 year), gender, health plan, and length of continuous health plan enrollment before the reference date (±1 year). With incidence-density matching, a person’s eligibility to be a control for a case is determined on the case’s diagnosis date; also, the same person can be selected as a control for more than 1 patient and/or become a case later on in the study. To increase sample size at the health plan with the fewest cases, two controls were selected for each case there only. Four cases and three controls initially included were later excluded because a strong family history of CRC was found during the medical records audit; 20 additional cases were excluded because of insufficient information for assigning an AJCC cancer stage (Figure 1).
Figure 1.
Selection of Cases and Control for the Study, 2006–2008
*Strong family history refers to colorectal cancer diagnosed in one or more first-degree relatives before age 50 or 2 or more relatives of any age, or other familial syndromes.
†Controls were matched on the reference date to cases according to study site on the calendar year of birth, gender, and length of continuous health plan enrollment (5–10 years) prior to the reference date.
‡Cancer in segments proximal to the splenic flexure were classified as right colon cancer.
§Left colon/rectum refer to the splenic flexure, descending colon, sigmoid and rectum.
Exposures
Receipt of screening colonoscopy and sigmoidoscopy in the 10-year period before the reference date was ascertained in a multistep data collection process. First, we collected data on CRC tests using automated searches for procedure codes in electronic administrative and clinical databases, as described previously.(27) With these data as a guide, trained auditors collected the dates of, reasons for, and findings of all relevant CRC tests received from medical records using a structured electronic data collection tool.
We applied an algorithm with blinded adjudication of selected tests by a 5-member committee to classify the indication for each test into seven mutually exclusive categories (excluding ‘high-risk’): surveillance, ‘definite’ diagnostic, ‘probable’ diagnostic, ‘possible’ diagnostic, ‘probable’ screening, ‘definite’ screening, or unknown. We considered a subject to be screened by a particular modality if exposed to a ‘definite’ or ‘probable’ screening test. In sensitivity analyses, we used a more restrictive definition of exposure to only ‘definite’ screening tests because of potential for misclassification in the ‘probable’ screening group.(28)
We sought to restrict screening exposures to tests received before the onset of late-stage CRC, since tests performed during the preclinical phase of late-stage CRC would not prevent the study outcome.(29) Hereafter, we refer to the period from the time at which a cancer transitioned from early- to late-stage, to the date of clinical diagnosis (reference date) as the ‘preclinical period’. We estimated the length of the preclinical period a priori to be 3 months. Thus, for our primary analyses, we defined the relevant screening exposure window as a period extending from up to 10 years to 3 months before the reference date (hereafter referred to as ‘observation period’).
Covariates
Data collection on CRC tests, as described above, included information on double contrast barium enemas (BE), CT colonographies, and FOBTs. None of six CT colonographies identified in the sample were for screening. Receipt of screening FOBT was defined using tests received within 2 years of the reference date, but before the preclinical period, based on evidence of the duration of effectiveness.(5–7) We also collected information on the following (‘other covariates’): number of preventive health care visits in the 5-year period before the reference date using Common Procedural Terminology codes as an indicator of health-seeking behavior; baseline modified Charlson comorbidity index(30) categorized as 0, 1, or 2+ (as an indicator of wellness to undergo screening); and SES (in quartiles) measured by the percentage of households in the census block-group with incomes below the 1999 federal poverty level based on the 2000 decennial census.(27, 31) Family history of CRC (relationship, number and diagnosis age) was collected from specific sections in the charts and from clinical notes in the 2-year period before the reference date. We created a dichotomous variable for any family history of CRC documented during the observation period that did not meet the exclusion criteria.
We also considered the following factors in sensitivity analyses: the specialty of the performing provider (categorized as gastroenterologist vs. others); whether the quality of bowel preparation was adequate;(32) and whether a colonoscopy was complete to the cecum.(32, 33) Examinations that found a pathological obstruction were considered complete. Bowel preparation was classified as adequate if described as ‘good’ to ‘excellent’, or obscuring <5 mm polyps or <10% of the mucosa; and inadequate if described as ‘poor’, ‘fair’, ‘suboptimal’, ‘borderline’, or not described in the procedure report.(33) We classified a complete colonoscopy with an adequate bowel preparation as a ‘high-quality’ test.
Statistical analysis
We estimated the association between receipt of screening and any late-stage CRC and, separately, for cancers in the right colon and left colon/rectum, with colonoscopy being the key predictor of interest: sigmoidoscopy was of secondary interest and was estimated in the same models as colonoscopy.
In our primary analyses, screening by either of these modalities was defined as receipt of a ‘definite’ or ‘probable’ screening test, without regard to exam quality or provider specialty. The analyses first adjusted for receipt of other screening tests (Model I) and then further adjusted for the other covariates (Model II). We evaluated our choice of a 3-month preclinical period by varying its length from 1 to 15 months.(29) These analyses were performed using conditional logistic regression on matched cases (n=471) and controls (n=509).
In secondary analyses, we assessed the impact of test quality on the effect of colonoscopy using the primary screening definition, but with restriction, separately, to ‘high-quality’ tests and tests performed by gastroenterologists. We further assessed the sensitivity of our results to various definitions of screening and to the exclusion of persons screened by multiple tests, colonoscopies or sigmoidoscopies of unknown indication, or tests other than either colonoscopy or sigmoidoscopy (endoscopy) alone. First, we used the more restrictive exposure definition of “definite” screening only, and sequentially excluded 311 subjects who had: ‘definite’ or ‘probable’ screening by both colonoscopy and sigmoidoscopy (n=11); any ‘probable’ screening endoscopy (n=38); screening BE (n=15) or FOBT (n=191); surveillance endoscopy (n=23); diagnostic colonoscopy for positive FOBT (n=21); or endoscopies with unknown indication (n=12).
All secondary analyses were performed using unconditional logistic regression, with adjustment for matching variables (age, sex, health plan, and enrollment duration), to retain patients whose matched case or controls had been excluded;(34) conditional logistic regression produced similar, but less precise, results because of exclusion of discordant cases and controls in the estimations. Therefore, we were able to consider all eligible subjects (n=1,012) including the unmatched controls and cases who were not in the primary analyses because of post-match exclusions (Figure 1).
The analyses were performed using SAS software 9.2 (SAS Institute Inc., Cary, NC; 2008) and Stata Statistical Software: Release 12.1 (StataCorp. 2011. College Station, TX: StataCorp LP). Tests with unknown indication (see Appendix A) were included in the reference group in the primary analyses but excluded in secondary analyses. Missing values for SES were imputed. The exclusion of subjects with missing SES data did not change our results. Sensitivity analyses that retained patients with ambiguous tumor stage data found similar results as our main analyses (see Appendix B). Model fit was evaluated using Hosmer-Lemeshow goodness-of-fit tests and was found to be reasonable (P-value>0.93).
Role of the Funding Source
This study was supported by an award (number CA148576) from the National Cancer Institute of the National Institutes of Health. The Institute did not play a role in study design or interpretation of the results.
RESULTS
Subject characteristics
A total of 1,039 subjects were selected for the study, of whom 1,012 eligible subjects (474 cases and 538 controls) with an average age of 71.7 years were analyzed for this report (Figure 1). Figure 1 also shows the distribution of cases by tumor location and stage; most cancers were stage III and few were IIB. Of the 474 cases, 53.0% were right colon cancers, 43.4% left CRCs and 17 (3.6%) had no specified locations. Our primary analyses excluded the three cases and 29 controls who did not have matched subjects.
Table 1 shows the characteristics of the primary analytic sample (n=980) by case-control status. The distribution of age, sex, enrollment duration, family history of CRC, and comorbidity index was similar between cases and controls, but controls were more likely to have had preventive health care visits. The majority had been enrolled in their health plan for ≥10 years before the reference date. During the observation period, 224 (22.9 %) eligible study subjects had at least one colonoscopy, 59 of which were screening; 340 (34.7%) subjects had at least one sigmoidoscopy, 265 of which were screening (Table 2); 11 subjects received screening by both sigmoidoscopy and colonoscopy. Fifty-four (90%) of the screening colonoscopies reached the cecum, of which 37 also had an adequate bowel preparation. These factors did not differ between cases and controls (data not shown).
Table 1.
Demographic and clinical characteristics of cases and controls, 2006–2008
| Characteristics, % | Cases (n= 471) | Controls (n=509) |
|---|---|---|
| Age, yr | ||
| 55–64 | 115 (24.4) | 126 (24.8) |
| 65–74 | 158 (33.5) | 169 (33.2) |
| 75–85 | 198 (42.0) | 214 (42.0) |
| Female | 229 (48.6) | 254 (49.9) |
| Poverty levels, quartiles* | ||
| 1 | 97 (21.3) | 142 (28.5) |
| 2 | 129 (28.4) | 108 (21.7) |
| 3 | 122 (26.8) | 116 (23.3) |
| 4 | 107 (23.5) | 132 (26.5) |
| Poverty level, % (median [interquartile range]) | 6.52 (3.77–10.06) | 6.37 (3.13–10.87) |
| Length of enrollment with health plan before reference date, yr | ||
| 5.0–7.4 | 69 (14.6) | 88 (17.3) |
| 7.5–9.9 | 47 (10.0) | 64 (12.6) |
| >10 | 355 (75.4) | 357 (70.1) |
| Study site | ||
| A | 95 (20.2) | 95 (18.7) |
| B | 189 (40.1) | 189 (37.1) |
| C | 38 (8.1) | 76 (14.9) |
| D | 149 (31.6) | 149 (29.3) |
| Number of preventive outpatient health care visits within 5 years of reference date | ||
| 0 | 215 (45.6) | 177 (34.8) |
| 1 | 127 (27.0) | 113 (22.2) |
| 2–3 | 89 (18.9) | 137 (26.9) |
| 4+ | 40 (8.5) | 82 (16.1) |
| Family history of colorectal cancer (CRC)† | 39 (8.3) | 45 (8.8) |
| Charlson comorbidity index at baseline‡ | ||
| 0 | 393 (83.4) | 407 (80.0) |
| 1 | 56 (11.9) | 79 (15.5) |
| 2+ | 22 (4.7) | 23 (4.5) |
| Had a healthcare visit during the 2-year period at baseline‡ | 381 (80.9) | 440 (86.4) |
Households below 1999 federal poverty levels within the block-group from 2000 decennial census measures. Analysis was based on 455 case patients and 498 control patients with non-missing data. Higher quartiles correspond to higher levels of household poverty in the census block-group
This variable refers to family history that did not meet the exclusion – those with a history of colorectal cancer diagnosed in any first degree relative before age 50 or 2 or more relatives of any age, or other familial syndromes.
Baseline refers to the 2-year period at the beginning of each subject’s observation period.
Table 2.
Association between receipt of screening colonoscopy or sigmoidoscopy and late-stage colorectal cancers, 2006–2008: Matched Analysis
| Receipt of screening colonoscopy or sigmoidoscopy according to colon location | Sample size (n) and % by cases and controls | Odds ratios and 95% confidence intervals | ||
|---|---|---|---|---|
|
| ||||
| Cases | Controls | Model I* | Model II*† | |
| Colonoscopy | ||||
| All late-stage colorectal cancers | ||||
| Screening colonoscopy | 13 (2.8) | 46 (9.0) | 0.32 (0.17–0.61) | 0.30 (0.15–0.59) |
| No screening colonoscopy | 458 (97.2) | 463 (91.0) | — | — |
| Right colon late-stage cancers | ||||
| Screening colonoscopy | 10 (4.0) | 29 (10.6) | 0.40 (0.19–0.86) | 0.37 (0.16–0.82) |
| No screening colonoscopy | 240 (96.0) | 244 (89.4) | — | — |
| Left colon/rectum late-stage cancers | ||||
| Screening colonoscopy | 3 (1.5) | 14 (6.4) | 0.33 (0.09–1.22) | 0.26 (0.06–1.11) |
| No screening colonoscopy | 201 (98.5) | 204 (93.6) | — | — |
| Sigmoidoscopy | ||||
| All late-stage colorectal cancers | ||||
| Screening sigmoidoscopy | 92 (19.5) | 173 (34.0) | 0.46 (0.33–0.63) | 0.51 (0.36–0.71) |
| No screening sigmoidoscopy | 379 (81.5) | 336 (66.0) | — | — |
| Right colon late-stage cancers | ||||
| Screening sigmoidoscopy | 68 (27.2) | 89 (32.6) | 0.72 (0.48–1.08) | 0.80 (0.52–1.25) |
| No screening sigmoidoscopy | 182 (72.8) | 184 (67.4) | — | — |
| Left colon/rectum late-stage cancers | ||||
| Screening sigmoidoscopy | 23 (11.3) | 78 (35.8) | 0.24 (0.13–0.42) | 0.26 (0.14–0.49) |
| No screening sigmoidoscopy | 181 (88.7) | 140 (64.2) | — | — |
Note: Screening was defined as exposure to a ‘definite or probable’ screening test. Analyses were performed on matched case-controls sets using conditional logistic regression. Twelve subjects had screening by both colonoscopy and sigmoidoscopy; 16 had ‘definite’ screening by barium enema and 191 by fecal occult blood test (FOBT); 18 subjects had both FOBT and colonoscopy, and 73 had both FOBT and sigmoidoscopy. Seventeen cases and 18 controls had an unknown location of cancer.
Model I was estimated with indicator variables for colonoscopy and sigmoidoscopy and receipt of ‘definite’ screening barium enema and FOBT.
Model II was further adjusted for census block-group poverty levels (as a continuous variable), number of preventive health care visits, family history of colorectal cancer, and comorbidity index at baseline. Missing values of poverty level were imputed using predictive mean matching.
Association between screening colonoscopy and late-stage CRC
Table 2 shows the results of our primary analyses. Thirteen cases (2.8%) and 46 controls (9.0%) had undergone screening colonoscopy, which corresponded to an odds ratio of 0.32 (95% confidence interval (CI): 0.17–0.61) after adjustment for other screening exposures; further adjustment for other covariates had a relatively small effect on this association (adjusted odds ratio (AOR)=0.30, CI: 0.15–0.59). These estimates were stable to varying the estimated duration of the preclinical period from 1 to 15 months (Figure 2). In analyses stratified by cancer location, the AOR was 0.37 (CI: 0.16–0.82) for the right colon (cases=250, controls=273) and 0.26 (CI: 0.06–1.11) for the left colon/rectum (cases=204, controls=218).
Figure 2.
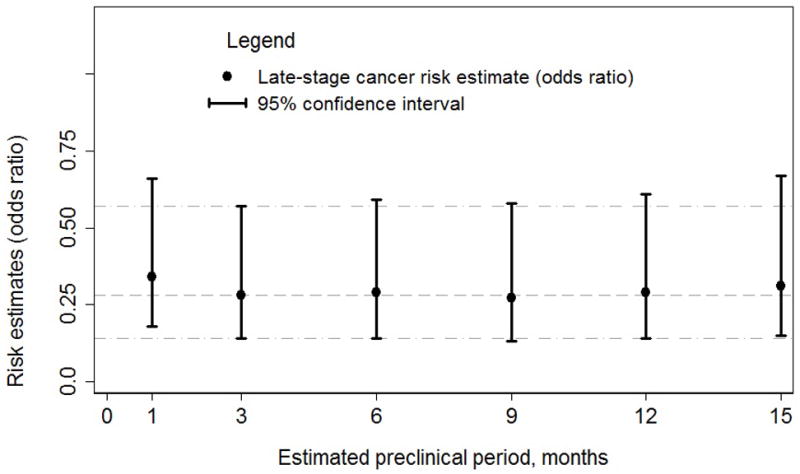
Sensitivity of the odds ratio estimates for screening colonoscopy on any late-stage colorectal cancer to varying the assumed preclinical period* for excluding tests
Note: Odds ratios and 95% confidence intervals (CI) were obtained with conditional logistic regression with screening defined as exposure to a ‘definite or probable’ screening test. Analyses were performed on matched case-controls sets using conditional logistic regression which adjusted for receipt of other screening tests (sigmoidoscopy, barium enema and fecal occult blood test), census block-group poverty levels (as a continuous variable), number of preventive health care visits, family history of colorectal cancer, and comorbidity index at baseline. Missing values of poverty level were imputed using predictive mean matching.
*The preclinical period refers to the time from the assumed onset of preclinical late-stage disease to the date of its clinical diagnosis, which we varied from 1 to 15 months.
Association between screening sigmoidoscopy and late-stage CRC
We performed analyses on screening sigmoidoscopy similar to those described for, and in the same models as, colonoscopy. In the primary analyses shown in Table 2, 92 (19.5%) cases and 173 (34.0%) controls had screening sigmoidoscopy, which corresponded to an AOR of 0.51 (CI: 0.36–0.71). Receipt of screening sigmoidoscopy was associated with a similar AOR as colonoscopy in the left colon/rectum (AOR=0.26, CI: 0.14–0.49), but only a modest reduction in the right colon (AOR=0.80, CI: 0.52–1.25, p=0.13). Analyses that varied the length of the preclinical period (data not shown) also yielded results similar to our primary analyses.
Sensitivity analysis of the Association between screening colonoscopy and late-stage CRC
In analyses restricted to ‘high-quality’ colonoscopies (cases=469, controls=520) using the primary definition of ‘definite’ or ‘probable’ screening, the AOR was 0.28 (CI: 0.12–0.65) overall, 0.47 (CI: 0.19–1.19) for right colon cancers, and 0.07 (CI: 0.01–0.53) for left CRC. Analyses restricted to tests performed by gastroenterologists (cases=471, controls=532) also yielded similar results as our primary analyses (overall, AOR=0.24, CI: 0.11–0.53; right colon, AOR=0.33, CI: 0.14–0.80; and left colon/rectum, AOR=0.15, CI: 0.04–0.53) (data not shown).
We also examined the association between screening colonoscopy and late-stage CRC risk using the more restrictive definition of screening (Table 3). In analyses of 365 cases and 336 controls, seven (1.9%) cases compared to 16 (4.8%) controls had screening colonoscopy. In unconditional logistic regressions, the effect of screening colonoscopy was unchanged, even after adjustment for all covariates (AOR=0.36, CI: 0.11–0.81). Analyses stratified by tumor location also yielded similar results as our primary analyses (right colon [cases=182]: AOR=0.72, CI: 0.25–2.05; left colon/rectum [cases=169]: AOR=0.09, CI: 0.01–0.72). Analysis of the effect of sigmoidoscopy based on the more restrictive definition of screening yielded similar results as the primary analyses (Table 3).
Table 3.
Association between use of ‘definite’ screening colonoscopy or sigmoidoscopy and late-stage colorectal cancers, 2006–2008: Unmatched Analysis
| Receipt of screening colonoscopy or sigmoidoscopy according to colon location | Sample size (n) and % by cases and controls | Odds ratios and 95% confidence intervals | ||
|---|---|---|---|---|
|
| ||||
| Cases | Controls* | Model I† | Model II‡ | |
| All late-stage colorectal cancers | ||||
| Screening colonoscopy | 7 (1.9) | 16(4.8) | 0.36 (0.14–0.91) | 0.36 (0.14–0.95) |
| Screening sigmoidoscopy | 62 (17.0) | 96 (28.6) | 0.47 (0.33–0.68) | 0.51 (0.35–0.75) |
| No screening | 296 (81.1) | 224 (66.7) | — | — |
| Right colon late-stage cancers | ||||
| Screening colonoscopy | 6 (3.3) | 16 (4.8) | 0.75 (0.28–2.04) | 0.72 (0.25–2.05) |
| Screening sigmoidoscopy | 48 (26.4) | 96 (28.6) | 0.89 (0.58–1.35) | 0.94 (0.60–1.45) |
| No screening | 128 (70.3) | 224 (66.7) | — | — |
| Left colon/rectum late-stage cancers | ||||
| Screening colonoscopy | 1 (0.6) | 16 (4.8) | 0.08 (0.01–0.65) | 0.09 (0.01–0.72) |
| Screening sigmoidoscopy | 13 (7.7) | 96 (28.6) | 0.18 (0.10–0.33) | 0.20 (0.11–0.38) |
| No screening | 155 (91.7) | 224 (66.7) | — | — |
Note: Screening was defined as exposure to a ‘definitely’ screening test only, and excluded cases and controls who had: screening by both colonoscopy and sigmoidoscopy; or screening by barium enema and fecal occult blood test (FOBT); surveillance colonoscopy or sigmoidoscopy; diagnostic colonoscopy for positive FOBT; or unknown colonoscopy or sigmoidoscopy indications. Fourteen cases with unknown cancer location were not included in the right or left-sided analyses.
The analyses in this table used the same sample of controls; we used unconditional logistic regressions in order to retain all eligible subjects, including those whose matched controls or case had been excluded.
In Model I, odds ratios and confidence intervals were obtained using unconditional logistic regression that adjusted for matching variables (study site, age, sex, and health plan enrollment duration).
Model II was further adjusted for census block-group poverty levels (as a continuous variable), number of preventive health care visits, family history of colorectal cancer, and comorbidity index at baseline. Missing values of poverty level were imputed using predictive mean matching.
We also performed conditional logistic regressions to evaluate the effect of non-screening tests on late-stage CRC risk using the following alternative exposure definitions: 1) a single variable for screening and non-screening combined; 2) one variable each for screening and non-screening tests; and 3) separate variables for screening and each of the non-screening test categories. The non-screening tests were surveillance, ‘possible’ diagnostic and ’probable’ diagnostic; screening was based on our primary exposure definition and the comparison group was comprised of ‘definite’ diagnostic tests, tests with unknown indication, and no colonoscopy or sigmoidoscopy. We found that the effect for screening colonoscopy on overall late-stage CRC risk was similar to the results of our primary analyses, but was stronger than the estimates for the individual non-screening indications (AOR of 0.38 [0.15–1.00] for surveillance, 0.40 [0.18–0.87] for ‘possible’ diagnostic, and 0.48 [0.18–1.24] for ‘probable’ diagnostic). The effect of screening was also stronger than the estimate for the non-screening tests considered together as a single variable (AOR: 0.42 [0.24–0.71]) (Appendix B).
DISCUSSION
We found that screening average-risk persons with colonoscopy was associated with a large reduction in risk of diagnosis with new-onset late-stage CRC. Receipt of screening colonoscopy was associated with reduction in risk of diagnosis with incident late-stage CRC diagnosis in both the right colon and left colon/rectum. Our results remained unchanged when we only considered ‘high-quality’ tests or tests performed by gastroenterologists. Although there are few studies of screening colonoscopy and the prevention of advanced CRC, our results of about a 70% reduction for any late-stage CRC associated with receipt of colonoscopy were similar to those of a German case-control study, published in 2011, which found a 78% and 83% reduction in risk of stage III and IV CRC, respectively, in relation to receipt of ‘any’ colonoscopy during the 1–10 years prior to the diagnosis date.(9)
Our study also simultaneously examined the association between screening sigmoidoscopy and late-stage CRC risk. Screening sigmoidoscopy receipt was associated with a similar reduction in risk of left-sided late-stage CRC as colonoscopy, but showed only a modest, statistically non-significant effect on risk of right-sided late-stage colon cancers. These results were similar to those of Selby et al.’s case-control study, which observed reduced risk of death from left-sided, but not right-sided, CRC with receipt of screening rigid sigmoidoscopy.(4) Also, each of the two randomized trials that reported on CRC site-specific mortality associated with screening sigmoidoscopy observed about a 50% mortality reduction for distal tumors; for fatal tumors arising in the proximal (right) colon, the relative risks were close to the null − 0.78 (CI 0.45–1.35)(1) and 0.97 (CI 0.77–1.22).(3)
Our study defined cases as persons with advanced CRC at the time of diagnosis. Thus, so long as colonoscopy can detect CRC at an early or precancerous stage, we can expect to observe a case-control difference in receipt of screening, regardless of whether treatment is effective. There is ample evidence that detection and treatment of precancerous colorectal lesions or early-stage CRCs in the left colon/rectum reduces the risk of death from CRC as reported by both case-control studies(4) and randomized trials of screening sigmoidoscopy.(1–3) In contrast, evidence on the benefits of detection and treatment of CRC precursors or early-stage cancers in the right colon is limited.(13)
A Canadian case-control study by Baxter et al. did not observe reduced mortality for right colon cancers associated with receipt of ‘any’ colonoscopy.(8) That study did not distinguish screening from diagnostic tests and its analyses focused on tests done well before diagnosis, most of which would be expected to be negative for cancer. Thus, it could be used to gauge the association between a negative exam and the risk of fatal CRC, but not the potential effectiveness of screening colonoscopy in the right colon. Future randomized trials and well-designed observational studies of the effect of screening colonoscopy on CRC mortality in average-risk individuals are needed to understand if the reduced risk of late-stage CRC in the right colon associated with receipt of screening colonoscopy that we observed in this study translates to lowered CRC mortality risk.
Mortality risk reduction is the most direct outcome for gauging efficacy of screening in non-randomized studies. A valid comparison in case-control studies of fatal disease considers all screening tests performed from the estimated time of onset of precursor lesions or cancer until disease is diagnosed.(29) Because the outcome in the present study was late-stage CRC, we considered only those tests that occurred before the estimated onset of late-stage disease. Our results did not change when we used estimates of this preclinical period that varied from 1–15 months.
There are some limitations of our study. Screening colonoscopy was relatively uncommon during our study’s observation period, which limited the precision of our risk estimates, particularly for subgroup analyses. Also, because this is an observational study, unmeasured confounders may have affected our results. Familial risk of cancer is not consistently or comprehensively documented in medical records.(35) It is therefore possible that a few subjects with familial syndromes were included in the analyses. However, this is unlikely to affect our results: the proportion with a family history of CRC was similar between cases and control and any misclassification is likely to have biased our results towards the null. Although our analyses adjusted for use of preventive health care, residual confounding by healthy behaviors or other confounders can affect the associations we observed. However, the similarity of our results for sigmoidoscopy to results of prior observational studies and recent clinical trials provides some assurance that our findings are fundamentally sound.(2, 4)
Another limitation of case-control studies of CRC screening effectiveness arises because the screening tests are also used to evaluate symptomatic disease, and the medical records on which this study is based may not reliably distinguish screening from non-screening tests. There may be greater degree of misclassification of diagnostic tests as screening in cases than in controls, thus, biasing the effect of screening towards the null. Our sensitivity analyses assessing the potential impact of such misclassification, including restriction to tests that were classified unambiguously as screening, produced similar effect sizes as in the primary analyses. We also found that non-screening colonoscopies, particularly tests done for non-specific gastrointestinal conditions, had a strong effect on late-stage CRC risk but was slightly weaker than the effect of average-risk screening. Additionally, our analyses on screening sigmoidoscopy produced results that were similar to those from randomized and observational studies.(1–4, 9)
In conclusion, screening for CRC in average-risk persons using colonoscopy was associated with a substantially reduced risk of diagnosis with new-onset primary late-stage CRC, including a reduced risk for right-sided colon cancers. Receipt of screening sigmoidoscopy was also associated with a substantially reduced risk of incident late-stage disease overall and in the left colon/rectum, but not the right colon.
Supplementary Material
Acknowledgments
This study was performed as part of a multicenter cancer screening comparative effectiveness research project, SEARCH (Screening Effectiveness and Research in Community-based Healthcare), which was supported by Grant Number UC2 CA148576 from the National Institutes of Health/National Cancer Institute. Dr. Doubeni’s time was also supported by the following grants from the National Institutes of Health/National Cancer Institute: K01-CA127118, K01-CA127118-S1 and U01-CA151736. The contents of this report are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health/National Cancer Institute. Data collection on cancer incidence for this study was supported in part by data infrastructure developed by the HMO Cancer Research Network at participating sites. Group Health Research Institute’s (GHRI) Cancer Surveillance System is funded in part by Contract # N01-CN-67009 and N01-PC-35142 from the Surveillance, Epidemiology and End Results Program of the National Cancer Institute with additional support from the State of Washington. We are grateful to SEARCH coordinators (Debra Bonollo, Shawn Gagne, Gabrielle Gundersen, Denise Schwarzkopf and Cyndee Yonehara) for coordinating data collection on this project. We thank medical records auditors (Amy Stone Murai, RN, MS, APRN-C, CHRC, at Kaiser Permanente Hawaii; Janet Guilbert and Doris Hoyer at Reliant Medical Group; Elie Castro, Melanie Currier and Karina Klepach at GHRI; and Kim Olsen at Kaiser Permanente Northwest). We are also grateful to Hirut Fassil for help with manuscript preparation.
Funding:
This research was supported by an award from the National Cancer Institute of the National Institutes of Health, grant number UC2CA148576. Additional support was provided through awards numbers K01-CA127118, K01-CA127118-S1 and U01-CA151736 to Dr. Doubeni, also from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is the prepublication, author-produced version of a manuscript accepted for publication in Annals of Internal Medicine. This version does not include post-acceptance editing and formatting. The American College of Physicians, the publisher of Annals of Internal Medicine, is not responsible for the content or presentation of the author-produced accepted version of the manuscript or any version that a third party derives from it. Readers who wish to access the definitive published version of this manuscript and any ancillary material related to this manuscript (e.g., correspondence, corrections, editorials, linked articles) should go to www.annals.org or to the print issue in which the article appears. Those who cite this manuscript should cite the published version, as it is the official version of record.
Contributor Information
Chyke A. Doubeni, Email: chyke.doubeni@uphs.upenn.edu.
Sheila Weinmann, Email: Sheila.Weinmann@kpchr.org.
Kenneth Adams, Email: Kenneth.F.Adams@HealthPartners.com.
Aruna Kamineni, Email: kamineni.a@ghc.org.
Diana SM Buist, Email: buist.d@ghc.org.
Arlene S. Ash, Email: Arlene.Ash@umassmed.edu.
Carolyn M. Rutter, Email: rutter.c@ghc.org.
V. Paul Doria-Rose, Email: doriarop@mail.nih.gov.
Douglas A. Corley, Email: Douglas.Corley@kp.org.
Robert T. Greenlee, Email: Greenlee.Robert@mcrf.mfldclin.edu.
Jessica Chubak, Email: chubak.j@ghc.org.
Andrew Williams, Email: Andrew.E.Williams@kp.org.
Aimee R. Kroll-Desrosiers, Email: Aimee.Kroll@umassmed.edu.
Eric Johnson, Email: johnson.ex@ghc.org.
Joseph Webster, Email: webster.jc@ghc.org.
Kathryn Richert-Boe, Email: Kathryn.E.Richert-Boe@kpchr.org.
Theodore R. Levin, Email: Theodore.Levin@kp.org.
Robert H. Fletcher, Email: robert_fletcher@hms.harvard.edu.
Noel S. Weiss, Email: nweiss@u.washington.edu.
References
- 1.Segnan N, Armaroli P, Bonelli L, et al. Once-only sigmoidoscopy in colorectal cancer screening: follow-up findings of the Italian Randomized Controlled Trial--SCORE. J Natl Cancer Inst. 2011;103(17):1310–22. doi: 10.1093/jnci/djr284. [DOI] [PubMed] [Google Scholar]
- 2.Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375(9726):1624–33. doi: 10.1016/S0140-6736(10)60551-X. [DOI] [PubMed] [Google Scholar]
- 3.Schoen RE, Pinsky PF, Weissfeld JL, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med. 2012;366(25):2345–57. doi: 10.1056/NEJMoa1114635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Selby JV, Friedman GD, Quesenberry CP, Jr, Weiss NS. A case-control study of screening sigmoidoscopy and mortality from colorectal cancer. N Engl J Med. 1992;326(10):653–7. doi: 10.1056/NEJM199203053261001. [DOI] [PubMed] [Google Scholar]
- 5.Mandel JS, Bond JH, Church TR, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328(19):1365–71. doi: 10.1056/NEJM199305133281901. [DOI] [PubMed] [Google Scholar]
- 6.Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348(9040):1472–7. doi: 10.1016/S0140-6736(96)03386-7. [DOI] [PubMed] [Google Scholar]
- 7.Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348(9040):1467–71. doi: 10.1016/S0140-6736(96)03430-7. [DOI] [PubMed] [Google Scholar]
- 8.Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150(1):1–8. doi: 10.7326/0003-4819-150-1-200901060-00306. [DOI] [PubMed] [Google Scholar]
- 9.Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med. 2011;154(1):22–30. doi: 10.7326/0003-4819-154-1-201101040-00004. [DOI] [PubMed] [Google Scholar]
- 10.Zauber AG, Lansdorp-Vogelaar I, Knudsen AB, Wilschut J, van Ballegooijen M, Kuntz KM. Evaluating test strategies for colorectal cancer screening: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149(9):659–69. doi: 10.7326/0003-4819-149-9-200811040-00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doubeni CA, Field TS, Buist DS, et al. Racial differences in tumor stage and survival for colorectal cancer in an insured population. Cancer. 2007;109(3):612–20. doi: 10.1002/cncr.22437. [DOI] [PubMed] [Google Scholar]
- 12.Rabeneck L, Davila JA, El-Serag HB. Is there a true “shift” to the right colon in the incidence of colorectal cancer? Am J Gastroenterol. 2003;98(6):1400–9. doi: 10.1111/j.1572-0241.2003.07453.x. [DOI] [PubMed] [Google Scholar]
- 13.Harris R, Kinsinger LS. Less is more: not “going the distance” and why. J Natl Cancer Inst. 2011;103(23):1726–8. doi: 10.1093/jnci/djr446. [DOI] [PubMed] [Google Scholar]
- 14.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 17 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2009 Sub (1973–2007 varying), National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2010, based on the November 2009 submission. Last accessed: July 29, 2012.
- 15.Whitlock EP, Lin JS, Liles E, Beil TL, Fu R. Screening for colorectal cancer: a targeted, updated systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149(9):638–58. doi: 10.7326/0003-4819-149-9-200811040-00245. [DOI] [PubMed] [Google Scholar]
- 16.Levin TR, Zhao W, Conell C, et al. Complications of colonoscopy in an integrated health care delivery system. Ann Intern Med. 2006;145(12):880–6. doi: 10.7326/0003-4819-145-12-200612190-00004. [DOI] [PubMed] [Google Scholar]
- 17.Doubeni CA, Laiyemo AO, Reed G, Field TS, Fletcher RH. Socioeconomic and racial patterns of colorectal cancer screening among Medicare enrollees in 2000 to 2005. Cancer Epidemiol Biomarkers Prev. 2009;18(8):2170–5. doi: 10.1158/1055-9965.EPI-09-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stock C, Ihle P, Schubert I, Brenner H. Colonoscopy and fecal occult blood test use in Germany: results from a large insurance-based cohort. Endoscopy. 2011;43(9):771–81. doi: 10.1055/s-0030-1256504. [DOI] [PubMed] [Google Scholar]
- 19.Compton CC, Greene FL. The staging of colorectal cancer: 2004 and beyond. CA Cancer J Clin. 2004;54(6):295–308. doi: 10.3322/canjclin.54.6.295. [DOI] [PubMed] [Google Scholar]
- 20.Wagner EH, Greene SM, Hart G, et al. Building a research consortium of large health systems: the Cancer Research Network. J Natl Cancer Inst Monogr. 2005;(35):3–11. doi: 10.1093/jncimonographs/lgi032. [DOI] [PubMed] [Google Scholar]
- 21.Screening for colorectal cancer: U S. Preventive Services Task Force recommendation statement. Annals of internal medicine. 2008;149(9):627–37. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 22.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570–95. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Lipton LR, Johnson V, Cummings C, et al. Refining the Amsterdam Criteria and Bethesda Guidelines: testing algorithms for the prediction of mismatch repair mutation status in the familial cancer clinic. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2004;22(24):4934–43. doi: 10.1200/JCO.2004.11.084. [DOI] [PubMed] [Google Scholar]
- 24.Umar A, Boland CR, Terdiman JP, et al. Revised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instability. Journal of the National Cancer Institute. 2004;96(4):261–8. doi: 10.1093/jnci/djh034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96(19):1420–5. doi: 10.1093/jnci/djh275. [DOI] [PubMed] [Google Scholar]
- 26.Rothman KJ, Greenland S. Modern Epidemiology. 2. Philadelphia, PA: Lippincott Williams & Wilkins; 1998. [Google Scholar]
- 27.Doubeni CA, Jambaulikar GD, Fouayzi H, et al. Neighborhood socioeconomic status and use of colonoscopy in an insured population--a retrospective cohort study. PLoS One. 2012;7(5):e36392. doi: 10.1371/journal.pone.0036392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiss NS. Analysis of case-control studies of the efficacy of screening for cancer: How should we deal with tests done in persons with symptoms? Am J Epidemiol. 1998;147(12):1099–102. doi: 10.1093/oxfordjournals.aje.a009407. [DOI] [PubMed] [Google Scholar]
- 29.Weiss NS. Case-control studies of the efficacy of screening tests designed to prevent the incidence of cancer. Am J Epidemiol. 1999;149(1):1–4. doi: 10.1093/oxfordjournals.aje.a009721. [DOI] [PubMed] [Google Scholar]
- 30.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 31.Doubeni CA, Schootman M, Major JM, et al. Health status, neighborhood socioeconomic context, and premature mortality in the United States: The National Institutes of Health-AARP Diet and Health Study. Am J Public Health. 2012;102(4):680–8. doi: 10.2105/AJPH.2011.300158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lieberman D, Nadel M, Smith RA, et al. Standardized colonoscopy reporting and data system: report of the Quality Assurance Task Group of the National Colorectal Cancer Roundtable. Gastrointest Endosc. 2007;65(6):757–66. doi: 10.1016/j.gie.2006.12.055. [DOI] [PubMed] [Google Scholar]
- 33.Rex DK, Bond JH, Winawer S, et al. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2002;97(6):1296–308. doi: 10.1111/j.1572-0241.2002.05812.x. [DOI] [PubMed] [Google Scholar]
- 34.Prentice R. Use of the logistic model in retrospective studies. Biometrics. 1976;32(3):599–606. [PubMed] [Google Scholar]
- 35.Berg AO, Baird MA, Botkin JR, et al. National Institutes of Health State-of-the-Science Conference Statement: Family History and Improving Health. Ann Intern Med. 2009;151(12):872–7. doi: 10.7326/0003-4819-151-12-200912150-00165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.