Abstract
Active immunization with the amyloid β (Aβ) peptide has been shown to decrease brain Aβ deposition in transgenic mouse models of Alzheimer's disease and certain peripherally administered anti-Aβ antibodies were shown to mimic this effect. In exploring factors that alter Aβ metabolism and clearance, we found that a monoclonal antibody (m266) directed against the central domain of Aβ was able to bind and completely sequester plasma Aβ. Peripheral administration of m266 to PDAPP transgenic mice, in which Aβ is generated specifically within the central nervous system (CNS), results in a rapid 1,000-fold increase in plasma Aβ, due, in part, to a change in Aβ equilibrium between the CNS and plasma. Although peripheral administration of m266 to PDAPP mice markedly reduces Aβ deposition, m266 did not bind to Aβ deposits in the brain. Thus, m266 appears to reduce brain Aβ burden by altering CNS and plasma Aβ clearance.
Abundant evidence suggests that a key event in Alzheimer's disease (AD) pathogenesis is the conversion of the amyloid β (Aβ) peptide from soluble to aggregated forms in the brain. Aβ, the principal proteinaceous component of plaque core and cerebrovascular amyloid, is composed of aggregates of the 4-kDa Aβ peptide (1). Aβ is predominantly 40–42 aa in length and is a normal, soluble proteolytic product of the amyloid precursor protein (APP), a large integral membrane protein expressed at high levels in the brain (2). Studies of mutations in APP and the presenilins, which cause early-onset, autosomal dominant, familial AD have revealed one common molecular consequence; they all increase Aβ production or increase the ratio of Aβ42/Aβ40 (3–6). Because Aβ42 is more prone to aggregate, this appears to increase the probability that Aβ aggregation, amyloid deposition, and other downstream consequences will ensue, resulting in AD neuropathology.
Production of Aβ via APP processing, however, is not the only factor that can influence the probability of Aβ deposition. Evidence has accumulated that indicates that factors regulating Aβ catabolism (7), clearance (8, 9), and aggregation (10) are also critical in regulating Aβ metabolism. For example, the ɛ4 allele of apolipoprotein E (apoE) is a major AD risk factor, and apoE plays an important role in Aβ deposition (11). In vitro and in vivo studies indicate that apoE does not appear to play a role in Aβ production per se but influences Aβ clearance, aggregation, conformation, and toxicity (10–17). Other Aβ binding proteins may have similar or distinct effects (10). The transport of exogenous Aβ between the central nervous system (CNS) and plasma also may regulate brain Aβ levels (9). Recent studies have demonstrated that exogenous Aβ40 is rapidly transported from cerebrospinal fluid (CSF) to plasma with an elimination half-life from brain of ≤30 min (8, 9). Because “physiological” Aβ-binding proteins (e.g., apoJ/apoE) can influence the transport/flux of Aβ between CNS and/or plasma (9, 18, 19), we became interested in whether exogenous Aβ binding molecules might be able to change the dynamic equilibrium of Aβ between CNS and plasma. We now report that the “central domain” anti-Aβ antibody, monoclonal antibody 266 (m266), rapidly sequesters all plasma Aβ present in PDAPP mice and causes a large accumulation of centrally derived Aβ in the plasma. Peripherally administered m266 also causes rapid increases in CSF Aβ, part of which does not appear to be due to entry of the antibody into the CNS. Finally, chronic parenteral treatment with m266 results in marked suppression of Aβ deposition in brain, suggesting that certain anti-Aβ antibodies suppress AD-like pathology by altering Aβ clearance from CNS to plasma.
Materials and Methods
Aβ ELISA.
The measurement of plasma, brain, and CSF Aβ was performed in a similar fashion as that described (20). For measurement of Aβ40, the mAb m2G3, specific for Aβ40 was used (20). The ELISA described (20) was modified into an RIA by replacing the streptavidin-horseradish peroxidase reagent with 125I-strepavidin. For plasma and CSF samples, the procedure was performed under nondenaturing conditions that lacked guanidine in the buffers. The measurement of Aβ/m266 complex in plasma was performed by a modified RIA. Mice were injected with biotinylated m266 (m266B), and plasma was isolated at multiple time points. Total Aβ bound to m266B was measured by using 96-well Optiplates (Packard) coated with m3D6. Diluted plasma samples and standards (varying concentrations of Aβ40 and m266B) were incubated overnight in the coated plates, and the amount of total Aβ/m266B complex was determined with the use of 125I-streptavidin.
Denaturing Acid/Urea Gradient Polyacrylamide Gels.
Denaturing gradient PAGE followed by Aβ Western blotting was used to identify plasma/CSF Aβ. Plasma (20 μl) or CSF (15 μl) samples were denatured in formic acid to a final concentration of 80% (vol/vol) and reduced with β-mercaptoethanol (1%). Samples were electrophoresed (anode to cathode) in a 0.9 M acetic acid running buffer through a 4–35% polyacrylamide gradient gel containing 6 M urea, 5% (vol/vol) glacial acetic acid, and 2.5% N,N,N′,N′-tetramethylethylenediamine. The acidic pH of the gel was neutralized before transfer to nitrocellulose. Subsequently, standard Western blotting techniques were used to identify Aβ.
CSF Isolation.
CSF was isolated from the cisterna magna compartment. Mice were anesthetized with pentobarbital, and the musculature from the base of the skull to the first vertebrae was removed. CSF was collected by carefully puncturing the arachnoid membrane covering the cistern with a micro needle (Roboz, Rockville, MD) under a dissecting microscope and withdrawing the CSF into a polypropylene micropipette. Measurement of m266 access into the CNS was performed on Swiss–Webster mice injected i.v. with biotinylated m266. Twenty four hours postinjection, CSF and plasma were isolated, and the concentration of biotinylated m266 was measured by an RIA using 125I-streptavidin (Amersham Pharmacia). To measure the flux of CNS Aβ into the plasma compartment, Aβ was injected into the cisterna magna of Swiss–Webster mice that had biotinylated m266 circulating in the plasma. Human Aβ40 was solubilized in rat CSF and was diluted into PBS containing 10% glycerol. The weighted Aβ40 solution (5 μl) was injected into the cisterna magna compartment, and the appearance of human Aβ in the plasma was detected by RIA.
m266 Immunostaining.
To determine whether m266 injected i.p. over 5 months was bound to Aβ deposits, brain sections from 9-month-old PDAPP+/+ mice that contained Aβ deposits and had been treated with either m266, saline, or control IgG were used. Tissue processing and immunostaining was performed as described (15, 17). Tissue from all groups of animals was incubated with fluorescein-labeled anti-mouse IgG (Vector Laboratories) and then examined under a fluorescent microscope. No specific staining of Aβ deposits was seen in any of the groups. In contrast, when applying m266 to sections before incubation of the sections with anti-mouse IgG, Aβ deposits were clearly detected.
Results and Discussion
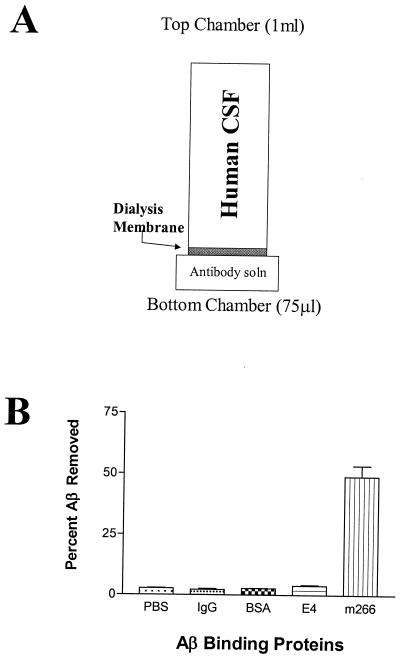
We first devised an in vitro dialysis system to test the ability of different Aβ binding proteins to influence interactions of Aβ with endogenous CSF binding molecules. By placing human CSF above a dialysis membrane separating it from a bottom chamber, we assessed the ability of Aβ binding proteins to act as an Aβ “sink” and influence the equilibrium of Aβ between one compartment and the other (Fig. 1). Either PBS or PBS containing apoE4, BSA, control IgG, or a mAb directed against central domain 13–28 of Aβ, m266 (21), were placed in the bottom chamber. With PBS in the bottom chamber, some Aβ moved across the membrane with peak amounts observed once the molecular mass cutoff of a dialysis membrane reached 25 kDa. We found that equilibrium of Aβ moving across the membrane was reached by 3 h. In the absence of Aβ binding proteins in the bottom chamber, the concentration of Aβ in the bottom chamber reached ≈½ the concentration in the top chamber. In this assay, astrocyte-secreted apoE4, purified as described (22, 23), had a small but statistically significant effect (P < 0.05) on increasing the mass of Aβ that reached the bottom chamber. Interestingly, when m266 was present in the bottom chamber, the amount of Aβ at equilibrium increased by more than 20-fold (Fig. 1), and 50% of the endogenous Aβ present in 1 ml of CSF was drawn into the 75-μl bottom chamber (Fig. 1). The difference between the effect of m266 vs. that of apoE may be due to the affinity of the molecules for Aβ as well as other factors. The affinity of m266 for Aβ is in the low pM range whereas the affinity of apoE is in the low nM range (24). Thus, m266 is able to act as a strong Aβ “sink” in the presence of physiological buffers and endogenous Aβ binding proteins. Other Aβ antibodies including m3D6 and m10D5, effective antibodies in decreasing Aβ deposition in vivo (25), were also able to act as Aβ sinks in this dialysis experiment, although they were not as effective as m266 (data not shown).
Figure 1.
Anti-Aβ antibody m266 acts as an Aβ sink. (A) An in vitro assay was developed to identify the relative efficiency of Aβ binding proteins on sequestering soluble CNS Aβ. One milliliter of human CSF was placed in the top chamber of a tube separated by a 25-kDa dialysis membrane from a bottom chamber that contained 75 μl of PBS. Human CSF used in these studies contained, on average, 10 ng/ml of AβTotal (2.5 pmol/ml). (B) The % Aβ removed from the top chamber was determined by Aβ ELISA analysis of both the top and bottom chamber (n = 4 per condition) after a 3-h incubation at 37°C. The bottom chamber contained PBS ± 20 μg of the listed proteins (IgG and m266, 133 pmol; BSA, 332 pmol; apoE4, 585 pmol). Affinity-purified, astrocyte-secreted apoE4 lipoproteins, a known Aβ binding protein, sequestered significantly more Aβ (3.86%, P < 0.05) to the bottom chamber than nonspecific mouse IgG (2.18%) or BSA (2.64%). The Aβ antibody m266 was dramatically more efficient at sequestering CSF Aβ (48.91%) to the bottom chamber as compared with all other conditions tested (P < 0.001). Data were analyzed with ANOVA followed by post hoc Tukey's test.
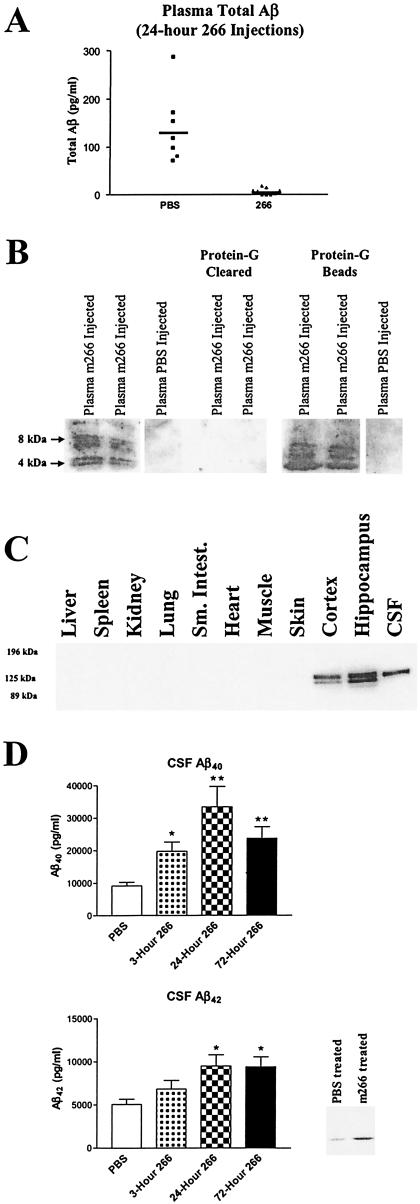
Because m266 was able to sequester Aβ in vitro, we next asked whether it would have similar effects in vivo. For these experiments, we used PDAPP+/+ transgenic mice (also referred to as APPV717F transgenic mice; ref. 13 and 15–17), a mouse model of AD in which a mutant human APP transgene (the amino acid at position 717 is phenylalanine instead of valine) is expressed and results in production of human Aβ in the CNS (26). In addition, we used an Aβ ELISA that specifically detects human and not mouse Aβ (see Materials and Methods) (20). We assessed the concentration of plasma and CSF Aβ both before and 24 h after i.v administration of 500 μg of m266 or PBS to PDAPP mice. Levels of plasma AβTotal were similar in both groups of mice before treatment (PBS: 160.5 ± 29 pg/ml, n = 6 vs. m266: 141.9 ± 12.3 pg/ml, n = 9; mean ± SEM). To assess the concentration of AβTotal that was not bound to m266 after injection, all plasma samples were treated with protein G, which binds IgG, to remove any m266-Aβ complexes. Mean plasma AβTotal levels in PBS-injected animals were 140 pg/ml and were unchanged as compared with the values before treatment (Fig. 2A). By contrast, in m266-treated mice, plasma Aβ not bound to m266 was virtually undetectable (Fig. 2A). To confirm the presence of Aβ bound to m266 in vivo, plasma samples of m266-treated mice were run on acid-denaturing gels either before or after immunoprecipitation with protein G followed by Western blotting with Aβ antibodies. A strong signal, which was depleted by protein G, was detected at 4–8 kDa consistent with the presence of monomers and probably dimers of Aβ (Fig. 2B). Based on standard curves, we estimated that the amount of Aβ complexed with m266 was ≈100 ng/ml of plasma after several days; an increase in Aβ mass of ≈1,000-fold over endogenous plasma Aβ in untreated PDAPP mice. Human APP and human Aβ in PDAPP mice are produced almost solely in the brain (Fig. 2C). Thus, the finding of a dramatic increase of plasma Aβ levels in m266-treated animals strongly suggests that circulating m266 acts as a peripheral Aβ sink, facilitating flux of Aβ from a central to peripheral compartment.
Figure 2.
m266 sequesters Aβ in vivo. (A) Three-month-old PDAPP+/+ mice were treated with PBS (n = 7) or 500 μg of m266 (n = 9) i.v. Twenty-four hours after m266 administration, plasma was analyzed for AβTotal by RIA. Before analysis, all plasma samples were first treated with protein G to remove Aβ complexed to m266. (B) In the first three lanes, 20 μl of plasma from PDAPP mice (24 h after administering 500 μg of m266 or PBS i.v.) was run on acid-urea gels followed by Western blotting for Aβ with m6E10 (Senetek, Napa, CA). Lanes 4 and 5 demonstrate that the plasma Aβ, from 20 μl of m266-injected mice, can be completely cleared with protein G treatment for animals treated with m266. In lanes 6 and 7, the protein-G beads from the 20 μl of plasma from lanes 4 and 5 were washed, denatured in formic acid, and analyzed. Lane 8 represents the protein G beads from 20 μl of plasma from a PBS-injected mouse. (C) Tissues collected from adult PDAPP+/+ mice were homogenized in a buffer containing 1% SDS. One hundred micrograms of total protein from each lysate was subjected to reducing 8% SDS/PAGE and analyzed by Western blotting for human APP with m6E10. (D) CSF Aβ40 and Aβ42 was determined by RIA from 3-month-old PBS- (n = 23) and m266-treated PDAPP mice 3 (n = 9), 24 (n = 5), and 72 h (n = 9) after i.v. injection of m266 as above. There was significantly greater Aβ40 in the CSF of the m266-treated mice at 3, 24, and 72 h and Aβ42 at 24 and 72 h (*, P < 0.05; **, P < 0.0001, ANOVA followed by post hoc Tukey's test.). Two microliters of CSF from each mouse per treatment group was collected, pooled (15 μl total), and subjected to acid urea gels followed by Western blotting with Aβ-specific antibodies.
If the presence of m266 in the circulation acutely alters the dynamic equilibrium of both plasma and CNS Aβ, we reasoned that there might be a rapid detectable change in the concentration of Aβ in the extracellular compartment within the CNS. Because the concentration of many molecules in the CSF reflects to some extent their concentration in the extracellular space of brain, we asked whether there were any changes in the concentration of CSF Aβ in m266-treated animals. As in Fig. 2A, 3-month-old PDAPP mice were injected with either PBS or m266 i.v., and Aβ40 and Aβ42 levels were assessed in CSF. By 3 h after m266 injection, there was a significant, 2-fold increase in Aβ40 levels and an insignificant increase in Aβ42 (Fig. 2D). At both 24 and 72 h, there was a significant 2- to 3-fold increase in both Aβ40 and Aβ42 (Fig. 2D). Denaturing gel analysis followed by Aβ Western blotting of pooled CSF revealed a similar increase in Aβ in m266-treated mice (Fig. 2D). The efflux of Aβ through brain interstitial fluid, which is reflected to some degree by CSF levels, may account for the observed increase in CSF Aβ. To assess the concentration of m266 that can access CSF, we injected biotinylated m266 (500 μg i.v.) into adult Swiss–Webster mice. Twenty four hours later, we determined that the concentration of the antibody was 12.0 ± 0.95 ng/ml (n = 4), which should only be able to account for an increase of Aβ of less than 1 ng/ml.
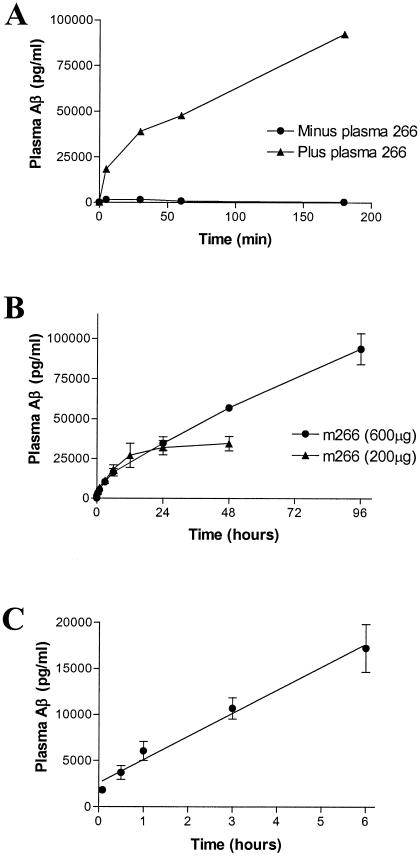
To directly assess whether m266 acts as a peripheral Aβ sink in vivo, we infused 1 μg of Aβ40 into the CSF compartment via the cisterna magna of wild-type (Swiss–Webster) mice. Just before intraventricular Aβ administration, the mice were treated with 200 μg of biotinylated m266 or PBS i.v. In the PBS-treated mice, Aβ could be detected in plasma after several minutes, with peak levels of 1,500 pg/ml after 30 min (Fig. 3A). Much greater levels of Aβ were detected in the plasma of m266-treated mice. The amount of plasma Aβ reached levels 64-fold higher (47,700 pg/ml) at 60 min, and there was a 365-fold increase (92,488 pg/ml) by 3 h as compared with PBS-treated mice at the same time points (Fig. 3).
Figure 3.
i.v. m266 detects rapid efflux of exogenous and endogenous Aβ from CNS into plasma. (A) One microgram of Aβ40 was dissolved into 5 μl of rat CSF to keep it soluble and was then injected into the subarachnoid space of the cisterna magna of wild-type Swiss–Webster mice that had previously received either PBS (n = 3) or 200 μg of biotinylated m266 (n = 3, i.v.). At different time points after treatment, plasma AβTotal was determined by Aβ RIA. (B and C) Either 200 μg (n = 3) or 600 μg (n = 3) of m266 was injected i.v. into 3-month-old PDAPP+/+ mice. Before and at different time points after i.v. injection, the plasma concentration of Aβ bound to m266 was determined by RIA and each value is presented as mean ± SEM. (B) The amount of Aβ bound to m266 is illustrated up to 4 days after treatment. (C) The time course over the first several hours for all animals is shown.
Given the effect of m266 on exogenously administered Aβ to the central compartment, we next determined the effect of m266 on endogenously produced Aβ. By administering biotinylated m266 into the plasma of PDAPP mice and use of an RIA, we were able to accurately quantify the time course and absolute levels of endogenous plasma Aβ bound to m266. After i.v. administration of 200 or 600 μg of m266, AβTotal bound to m266 rapidly increased from basal levels of 150 pg/ml to levels of over 100 ng/ml by 4 days (Fig. 3B). From analysis of the early time points of the curve (Fig. 3C), we determined that the rate of entry of AβTotal into the plasma of PDAPP mice was 42 pg/ml per min in the presence of saturating levels of m266. A component of this net increase in plasma Aβ could be due to a decrease in degradation or peripheral clearance. However, the effects of m266 on plasma Aβ levels in both wild-type and PDAPP mice along with the acute effects of m266 on CSF Aβ strongly suggests that the presence of circulating m266 results in a change in the equilibrium (increased efflux and/or decreased influx) of Aβ between the CNS and plasma.
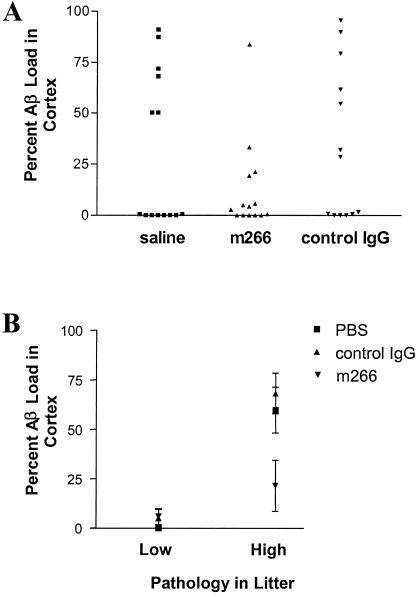
To determine whether administration of m266 could prevent Aβ deposition in the brain, PDAPP mice were treated with either PBS (n = 14), control IgG (n = 13), or m266 (n = 14) (randomly assigned) every other week beginning at 4 months of age (before Aβ deposition). Mice then were killed at 9 months of age, and Aβ deposition was determined by quantitative immunostaining. By 9 months of age, 6/14 and 5/13 mice in groups treated with PBS and control IgG, respectively, had more than 50% of the cortex overlying the dorsal hippocampus occupied by Aβ deposits. In contrast, only 1/14 mice treated with m266 had this level of Aβ deposition (P = 0.02, χ2 test, Fig. 4A). Additionally, the levels of Aβ in brain homogenates also were significantly reduced in m266 mice as assessed by ELISA (Fig. 4, legend). Unexpectedly, almost 50% of the animals in all groups still had not developed Aβ deposition by 9 months of age. The latter appears to be due to parental origin of individual mice in our cohort because even though all mice studied were confirmed to be PDAPP+/+, high levels of Aβ deposition at this age were observed only in mice derived from 4/8 breeding pairs (high pathology litters). Mice derived from the other four breeding pairs were virtually free of Aβ deposits (low pathology litters). Using parental origin as a covariate, there was a strong, significant effect of m266 in reducing Aβ deposition (P = 0.0082, Fig. 4B).
Figure 4.
Chronic administration of m266 decreases Aβ burden in PDAPP mice. Four-month-old PDAPP+/+ mice were treated every 2 weeks for 5 months with saline (n = 14), m266 (n = 14, 500 μg), or control mouse mAb (n = 13, control IgG, 100 μg, PharMingen), all administered i.p. The % area covered by Aβ immunoreactivity as identified with a rabbit pan-Aβ antibody (BioSource International, Camarillo, CA) was quantified in the cerebral cortex immediately overlying the dorsal hippocampus as described (35). (A) At 9 months of age, about half of each group had still not developed Aβ deposits. In PBS- and IgG-treated animals, 6/14 and 5/13 mice, respectively, had greater than 50% of the cortex covered by Aβ staining. In contrast, only 1/14 m266-treated mice had this level of Aβ staining. The proportion of mice with this level of Aβ staining was lowered by treatment with m266 (P = 0.02, χ2 test). Levels of PBS-soluble and insoluble Aβ in cortex of PBS and m266 treated groups were as follows (mean Aβ in ng/mg protein ± SEM): soluble AβTotal, PBS, 0.115 ± 0.019, m266, 0.06 ± 0.007, P = 0.01; insoluble AβTotal, PBS, 4.64 ± 1.62, m266, 1.4 ± 0.34, P = 0.06; soluble Aβ42, PBS, 0.026 ± 0.002, m266, 0.020 ± 0.002, P = 0.04; insoluble Aβ42, PBS, 2.66 ± 1.18, m266, 0.62 ± 0.166, P = 0.09. (B) The variability in Aβ deposition within the groups was related in some way to parental origin. Even though all mice used were PDAPP+/+ transgenic mice, plaque burdens of 50% or greater were only seen in mice derived from four of eight breeding pairs (high pathology litters). Most mice from the other breeding pairs were free of plaques (low pathology litters). Using parental origin as a covariate, there was a strong effect of m266 in reducing Aβ burden. When comparing m266 to controls in all groups (high and low pathology litters), P = 0.0082. When comparing m266 to controls in only the high pathology litters, P = 0.00025.
Previous studies have demonstrated rapid transport of exogenous Aβ between CNS and plasma (8, 9, 19). Here, we demonstrate that the central domain monoclonal Aβ antibody 266 can act as an Aβ sink both in vitro and in vivo, and that parenteral administration of m266 can alter the equilibrium of Aβ between the central and peripheral compartments. These findings combined with the marked reduction in Aβ deposition observed after m266 administration to PDAPP mice suggest that antibody-mediated change in Aβ efflux from (or influx to) brain is an important mechanism by which m266 suppresses AD pathology. Thus, augmentation of Aβ clearance via parenteral antibody administration may be a useful approach to preventing and treating amyloidoses of the CNS such as AD.
The current understanding of Aβ catabolic and clearance processes in vivo that lead to multiple CNS compartments of Aβ (cell associated, interstitial fluid, and CSF) are poorly understood. Recent in vivo work from Zlockovic and colleagues (9, 18, 19, 27) have identified efficient receptor-mediated transport mechanisms for Aβ at the blood brain barrier. They have shown that this transport of Aβ is bidirectional; Aβ is transported from the CNS to plasma and from plasma to the CNS. Thus peripheral Aβ must influence the overall CNS catabolic equilibrium. Our data suggest a novel mechanism for altering CNS Aβ is to either increase efflux of Aβ from CNS to plasma and/or decrease Aβ influx from plasma into CNS. Peripheral m266 administration almost certainly negates any Aβ influx into the CNS from the periphery, because unbound Aβ is virtually absent in treated animals. Acute m266 treatment of the transgenic mice results in a rapid 2- to 3-fold increase in CSF Aβ concentration. The increase in CSF Aβ levels is likely to represent, at least in part, a shift or efflux from a CNS pool of Aβ that normally would reside or be catabolized within the brain. In light of this, it is interesting to note that exactly the opposite occurs in AD patients, where CSF Aβ is decreased (28), and there is a progressive shift of Aβ from soluble to insoluble pools as the disease progresses (29). Additionally, there is an emerging literature of in vitro studies, demonstrating that plaque nucleation may be occurring intracellularly in the endocytic pathway before Aβ forms large extracellular aggregates in plaques in the extracellular space (30–33). We postulate that the presence of m266 in the peripheral compartment alters the transport and dynamic equilibrium of Aβ between brain and plasma. This altered equilibrium favors peripheral clearance and catabolism instead of deposition within the brain.
In contrast to our findings demonstrating effects of m266 on soluble Aβ, a recent study using PDAPP mice concluded that the anti-amyloid effects of certain anti-Aβ antibodies (not m266) are due to their entry into the CNS followed by local antibody (Fc)-mediated Aβ plaque clearance (25). Bard et al. (25) provide evidence from ex vivo and in vitro experiments in which the presence of added anti-Aβ antibody induced exogenously added microglia-mediated clearance of Aβ deposits in brain slices. In a recent in vivo study, anti-Aβ antibodies were applied directly on the surface of the cortex of PDAPP mice with a resulting decrease in Aβ deposits in the immediate vicinity of their application (34). Although it is conceivable that this mechanism contributes to the effects of some peripherally administered antibodies, our findings that demonstrate that m266 can rapidly sequester all plasma Aβ and change soluble Aβ metabolism in both nonplaque and plaque bearing animals suggest that this latter mechanism probably underlies the ability of parentally administered m266 to decrease Aβ burden in PDAPP mice. Importantly, we found no evidence that m266 bound to Aβ in plaques of m266-treated mice (see Materials and Methods). Further, other mAbs previously found to be effective at suppressing Aβ deposition in vivo (m3D6 and m10D5) (25) are able to act as Aβ sinks in our dialysis experiments (data not shown). In addition to these considerations, we cannot, however, exclude the possibility that small amounts of m266 enter the brain and sequester a soluble, toxic Aβ species.
Our findings have important implications for understanding the normal metabolic and clearance pathways for other brain-derived peptides in addition to Aβ. Our data suggest that antibodies as well as other binding proteins/molecules present outside the blood-brain barrier may serve to facilitate clearance of soluble peptides such as Aβ out of the CNS. Identification of other key molecules, which under physiological conditions influence plasma Aβ clearance, may lead to new insights into plasma Aβ clearance and additional ways to block CNS Aβ deposition. Given the relatively large volume of distribution of the peripheral (circulation) compared with central compartments, the addition of anti-Aβ or other antipeptide antibodies could serve to quickly and efficiently alter the clearance and effect of biologically active peptides in the brain. Generation of this peripheral sink mechanism via administration of humanized mAbs may be useful for treating a number of disorders characterized by abnormal protein accumulation in the extracellular space, both centrally and peripherally.
Acknowledgments
We thank Maia Parsadanian, Eric Foss, Malca Kierson, Nishant Shah, Cynthia DeLong, Xian Wu, and Mark O'Dell for their excellent technical assistance and Eugene Johnson, Sangram Sisodia, Dennis Choi, Trish Augsberger-Brown, and Anne Fagan for helpful comments.
Abbreviations
- Aβ
amyloid β
- m266
monoclonal antibody 266
- CNS
central nervous system
- AD
Alzheimer's disease
- APP
amyloid precursor protein
- apo
apolipoprotein
- CSF
cerebrospinal fluid
References
- 1.Selkoe D J. Science. 1997;275:630–631. doi: 10.1126/science.275.5300.630. [DOI] [PubMed] [Google Scholar]
- 2.Golde T E, Eckman C B, Younkin S G. Biochim Biophys Acta. 2000;1502:172–187. doi: 10.1016/s0925-4439(00)00043-0. [DOI] [PubMed] [Google Scholar]
- 3.Goate A, Chartier-Harlon M C, Mullan M, Brown J, Crawford F, Fidani L, Giuffra L, Haynes A, Irving N, James L, et al. Nature (London) 1991;349:704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- 4.St. George-Hyslop P H, Levesque G, Levesque L, Rommens J, Westaway D, Fraser P E. Cold Spring Harbor Symp Quant Biol. 1996;61:559–564. [PubMed] [Google Scholar]
- 5.Kim T W, Tanzi R E. Curr Opin Neurobiol. 1997;7:683–688. doi: 10.1016/s0959-4388(97)80089-x. [DOI] [PubMed] [Google Scholar]
- 6.Thinakaran G. J Clin Invest. 1999;104:1321–1327. doi: 10.1172/JCI8728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwata N, Tsubuki S, Takaki Y, Watanabe K, Sekiguchi M, Hosoki E, Kawashima-Marishima M, Lee H-J, Hama E, Sekine-Aizawa Y, Saido T C. Nat Med. 2000;6:143–150. doi: 10.1038/72237. [DOI] [PubMed] [Google Scholar]
- 8.Ghersi-Egea J-F, Gorevic P D, Ghiso J, Frangione B, Patlak C S, Fensternacher J D. J Neurochem. 1996;67:880–883. doi: 10.1046/j.1471-4159.1996.67020880.x. [DOI] [PubMed] [Google Scholar]
- 9.Shibata M, Yamada S, Kumar S R, Calero M, Bading J, Frangione B, Holtzman D M, Miller C A, Strickland D K, Ghiso J, Zlokovic B V. J Clin Invest. 2000;106:1489–1499. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wisniewski T, Ghiso J, Frangione B. Neurobiol Dis. 1997;4:313–328. doi: 10.1006/nbdi.1997.0147. [DOI] [PubMed] [Google Scholar]
- 11.Strittmatter W J, Roses A D. Annu Rev Neurosci. 1996;19:53–77. doi: 10.1146/annurev.ne.19.030196.000413. [DOI] [PubMed] [Google Scholar]
- 12.Du Y, Ni B, Glinn M, Dodel R C, Bales K R, Zhang Z, Hyslop P, Paul S M. J Neurochem. 1997;69:299–305. [PubMed] [Google Scholar]
- 13.Bales K R, Verina T, Dodel R C, Du Y, Altstiel L, Bender M, Hyslop P, Johnstone E M, Little S P, Cummins D J, et al. Nat Genet. 1997;17:263–264. doi: 10.1038/ng1197-263. [DOI] [PubMed] [Google Scholar]
- 14.Russo C, Angelini G, Dapino D, Piccini A, Piombo G, Schettini G, Chen S, Teller J K, Zaccheo D, Gambetti P, Tabaton M. Proc Natl Acad Sci USA. 1998;95:15598–15602. doi: 10.1073/pnas.95.26.15598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holtzman D M, Bales K R, Wu S, Bhat P, Parsadanian M, Fagan A M, Chang L K, Sun Y, Paul S M. J Clin Invest. 1999;103:R15–R21. doi: 10.1172/JCI6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bales K R, Verina T, Cummins D J, Du Y, Dodel J C, Saura J, Fishman C E, DeLong C A, Piccardo P, Petegnief V, et al. Proc Natl Acad Sci USA. 1999;96:15233–15238. doi: 10.1073/pnas.96.26.15233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holtzman D M, Bales K R, Tenkova T, Fagan A M, Parsadanian M, Sartorius L J, Mackey B, Olney J, McKeel D, Wozniak D, Paul S M. Proc Natl Acad Sci USA. 2000;97:2892–2897. doi: 10.1073/pnas.050004797. . (First Published February 29, 2000; 10.1073/pnas.050004797) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zlokovic B V, Martel C L, Mackic J B, Matsubara E, Wisniewski T, McComb J G, Frangione B, Ghiso J. Biochem Biophys Res Commun. 1994;205:1431–1437. doi: 10.1006/bbrc.1994.2825. [DOI] [PubMed] [Google Scholar]
- 19.Zlokovic B V, Martel C L, Matsubara E, McComb J G, Zheng G, McCluskey R T, Frangione B, Ghiso J. Proc Natl Acad Sci USA. 1996;93:4229–4234. doi: 10.1073/pnas.93.9.4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson-Wood K, Lee M, Motter R, Hu K, Gordon G, Barbour R, Khan K, Gordon M, Tan H, Games D, et al. Proc Natl Acad Sci USA. 1997;94:1550–1555. doi: 10.1073/pnas.94.4.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, David D, Sinha S, Schlossmacher M, Whaley J, Swindlehurst C, et al. Nature (London) 1992;359:325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- 22.DeMattos R B, Curtiss L K, Williams D L. J Biol Chem. 1998;273:4206–4212. doi: 10.1074/jbc.273.7.4206. [DOI] [PubMed] [Google Scholar]
- 23.Sun Y, Wu S, Bu G, Onifade M K, Patel S N, LaDu M J, Fagan A M, Holtzman D M. J Neurosci. 1998;18:3261–3272. doi: 10.1523/JNEUROSCI.18-09-03261.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tokuda T, Calero M, Matsubara E, Vidal R, Kumar A, Permanne B, Zlokovic B, Smith J D, Ladu M J, Rostagno A, et al. Biochem J. 2000;348:359–365. [PMC free article] [PubMed] [Google Scholar]
- 25.Bard F, Cannon C, Barbour R, Burke R-L, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, et al. Nat Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 26.Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, et al. Nature (London) 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- 27.Zlokovic B V, Ghiso J, Mackic J B, McComb J G, Weiss M H, Frangione B. Biochem Biophys Res Commun. 1993;197:1034–1040. doi: 10.1006/bbrc.1993.2582. [DOI] [PubMed] [Google Scholar]
- 28.Motter R, Vigopelfrey C, Kholodenko D, Barbour R, Johnsonwood K, Galasko D, Chang L, Miller B, Clark C, Green R, et al. Ann Neurol. 1995;38:643–648. doi: 10.1002/ana.410380413. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Dickson D W, Trojanowski J Q, Lee V M Y. Exp Neurol. 1999;158:328–337. doi: 10.1006/exnr.1999.7085. [DOI] [PubMed] [Google Scholar]
- 30.Knauer M F, Soreghan B, Burdick D, Kosmoski J, Glabe C G. Proc Natl Acad Sci USA. 1992;89:7437–7441. doi: 10.1073/pnas.89.16.7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang A J, Chandswangbhuvana D, Shu T, Henschen A, Glabe C G. J Biol Chem. 1999;274:20650–20656. doi: 10.1074/jbc.274.29.20650. [DOI] [PubMed] [Google Scholar]
- 32.Walsh D M, Tseng B P, Rydel R E, Podlisny M B, Selkoe D J. Biochemistry. 2000;39:10831–10839. doi: 10.1021/bi001048s. [DOI] [PubMed] [Google Scholar]
- 33.Ditaranto K, Tekirian T L, Yang A J. Neurobiol Dis. 2001;8:19–31. doi: 10.1006/nbdi.2000.0364. [DOI] [PubMed] [Google Scholar]
- 34.Backsai B J, Kajdasz S T, Christie R H, Carter C, Games D, Seubert P, Schenk D, Hyman B T. Nat Med. 2001;7:369–372. doi: 10.1038/85525. [DOI] [PubMed] [Google Scholar]
- 35.Holtzman D M, Fagan A M, Mackey B, Tenkova T, Sartorius L, Paul S M, Bales K, Ashe K H, Irizzary M C, Hyman B T. Ann Neurol. 2000;47:739–747. [PubMed] [Google Scholar]