Abstract
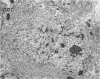
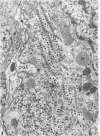
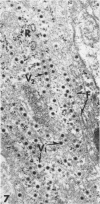
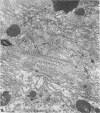
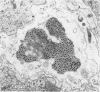
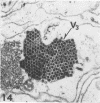
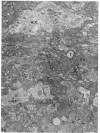
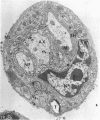
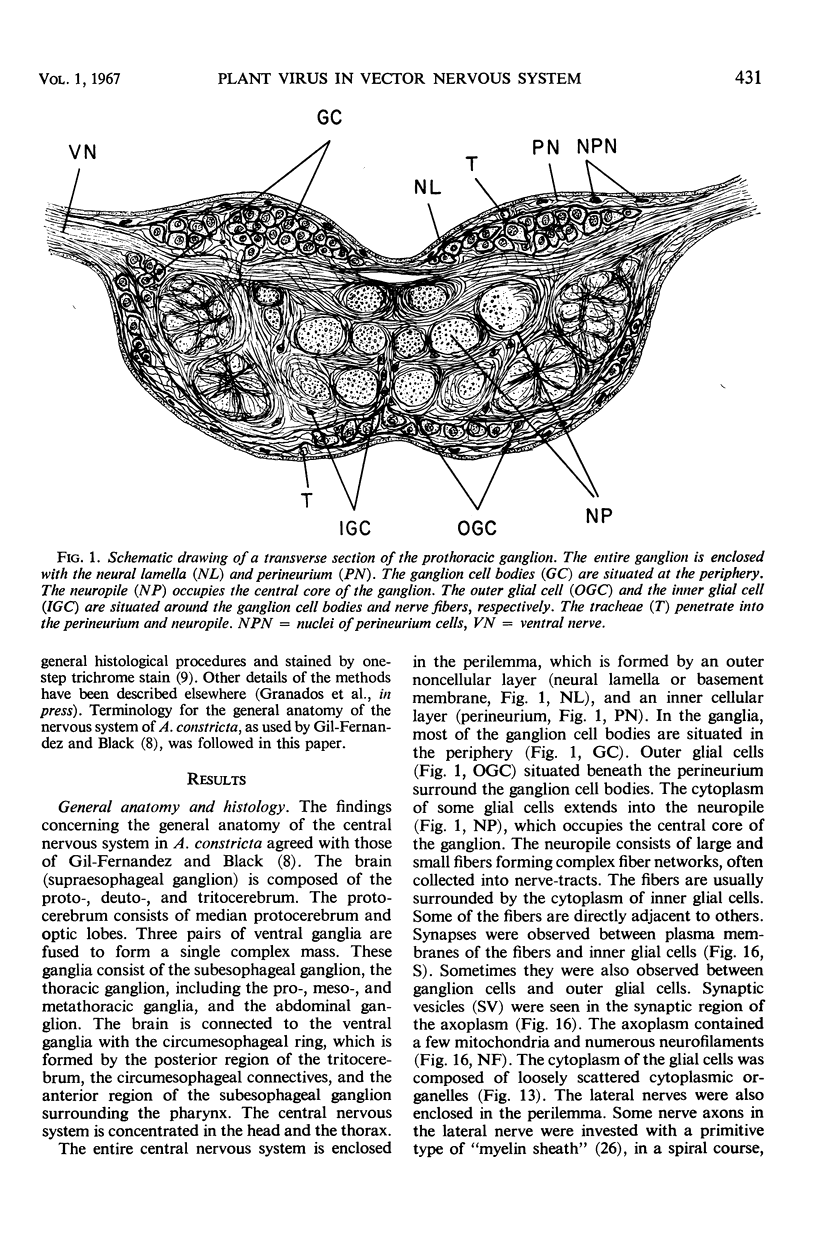
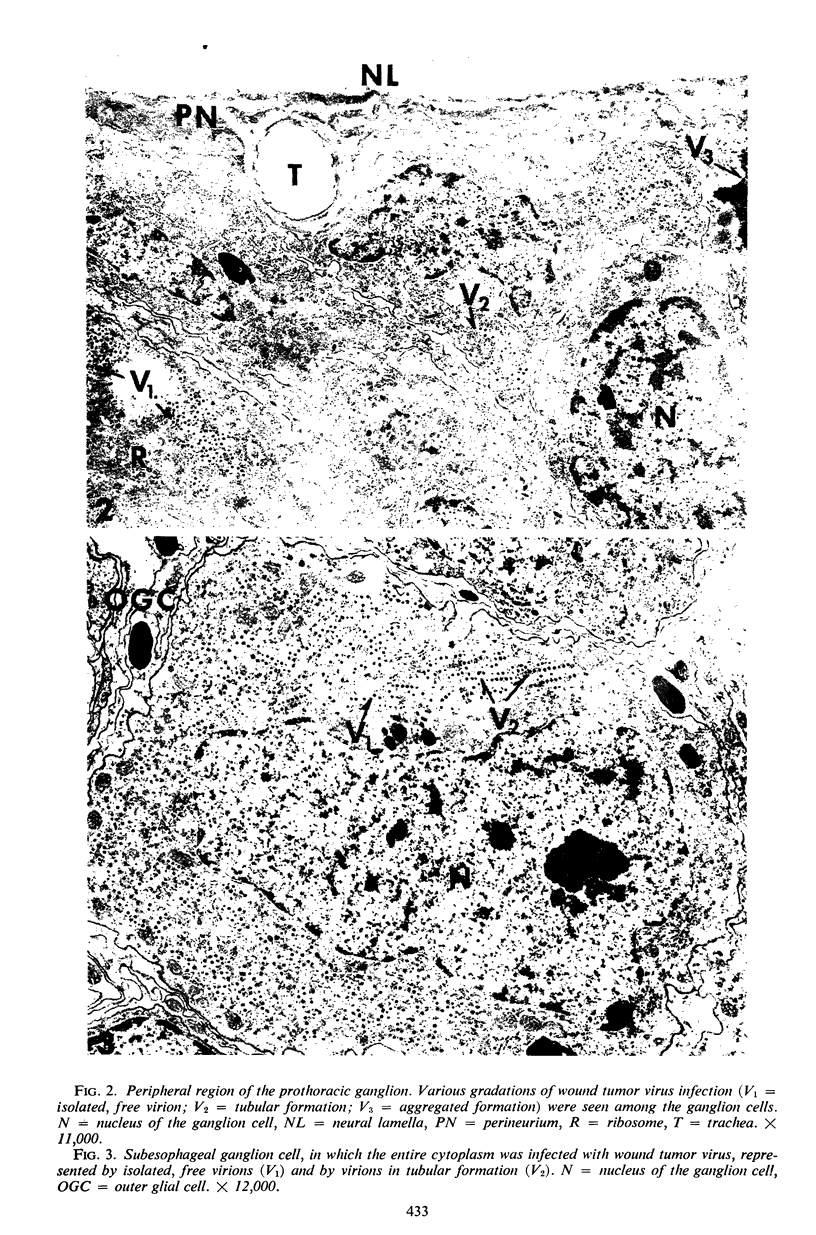
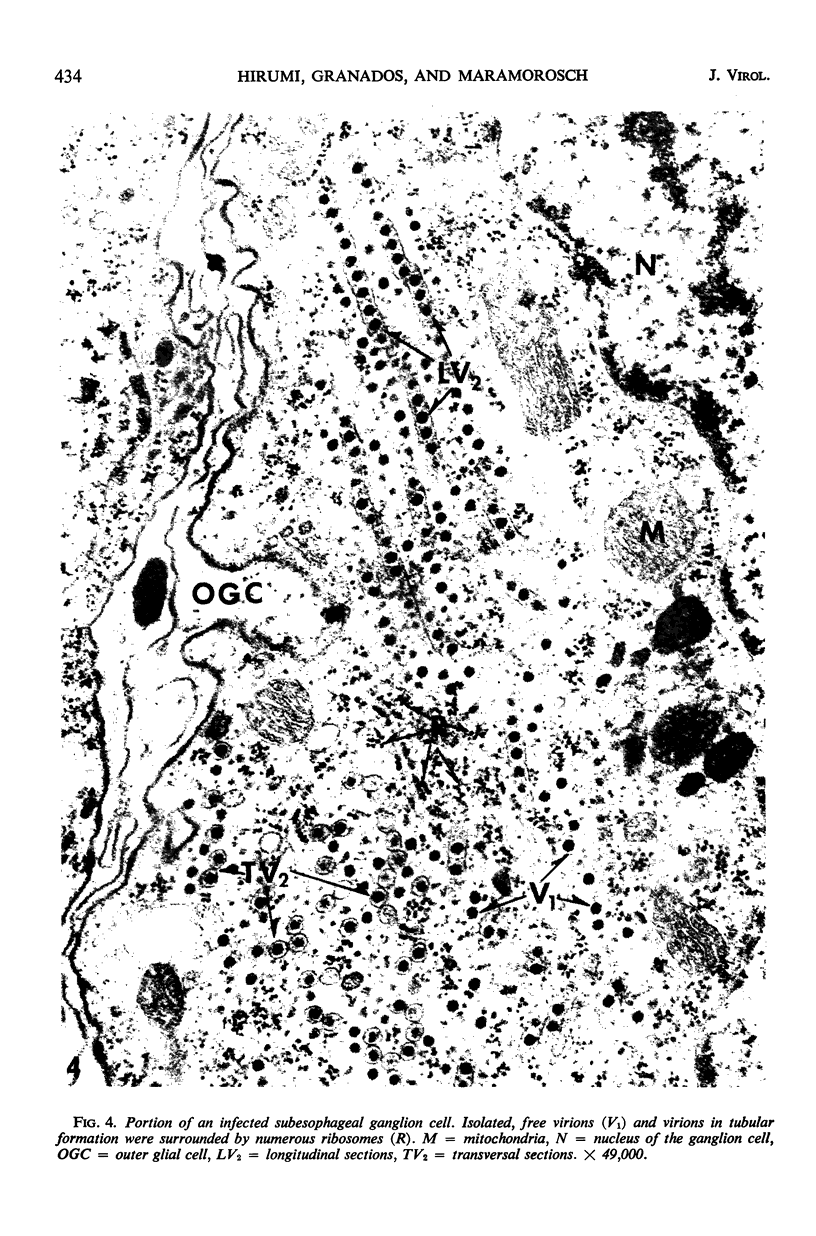
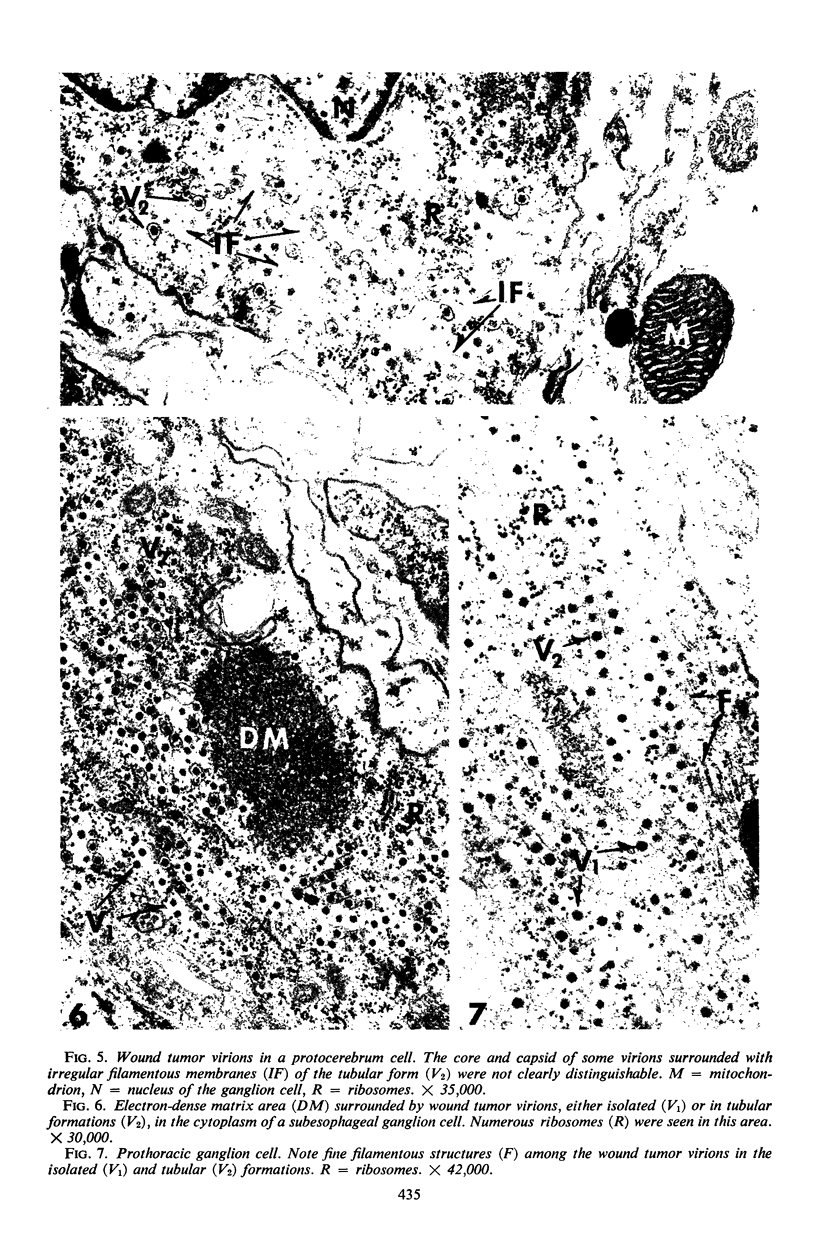
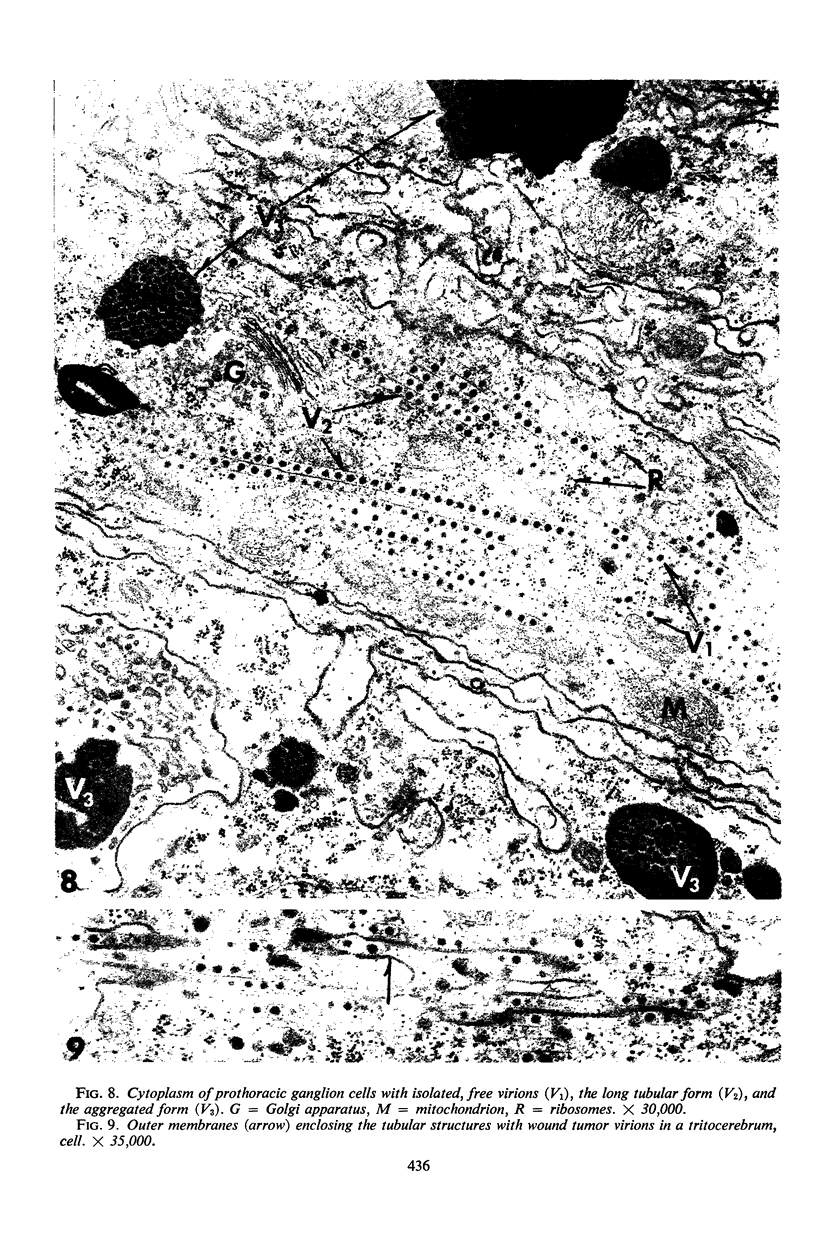
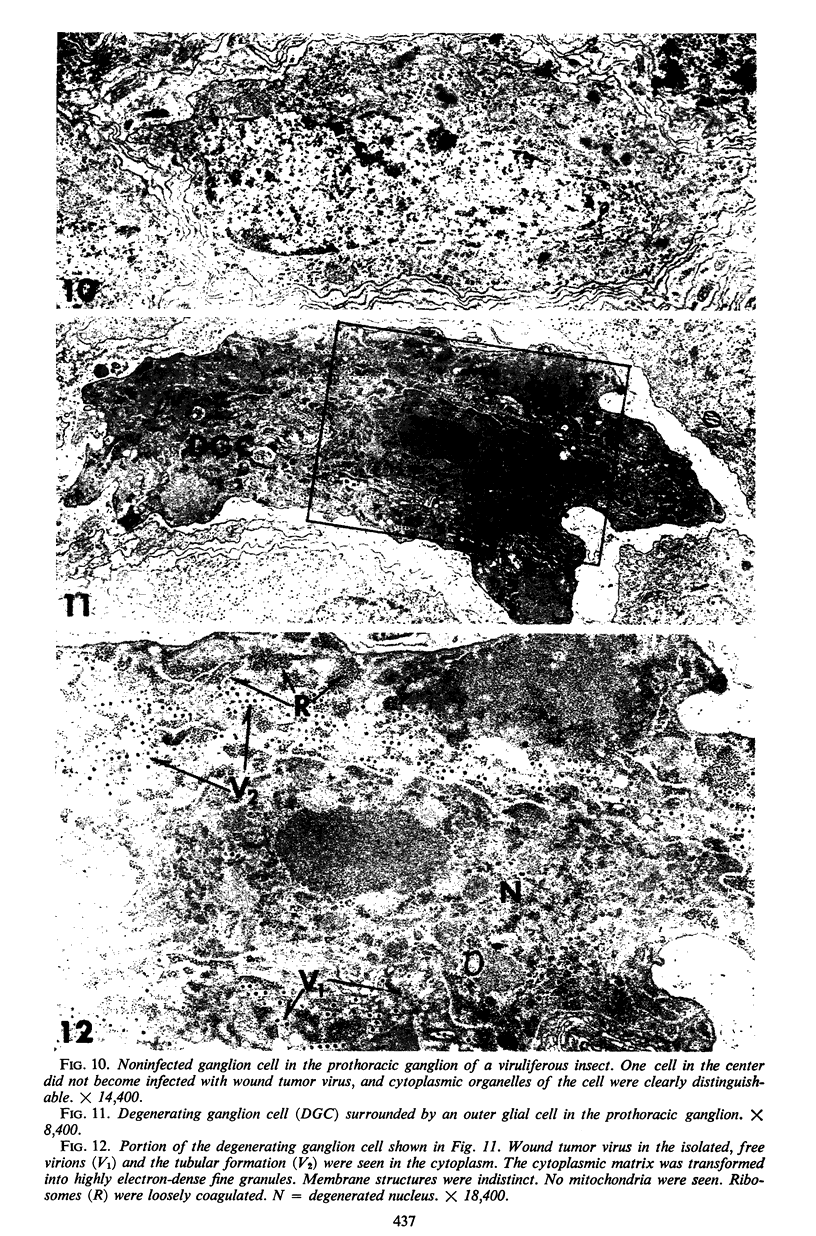
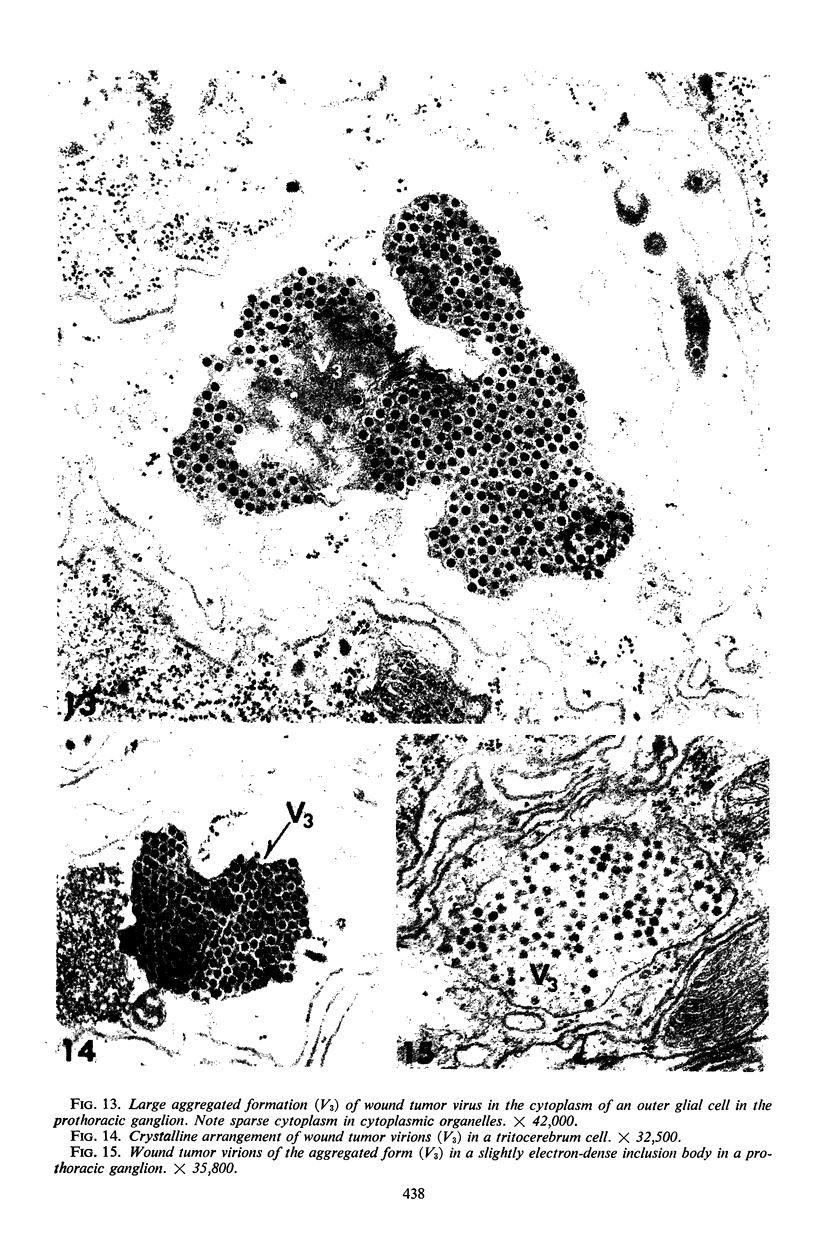
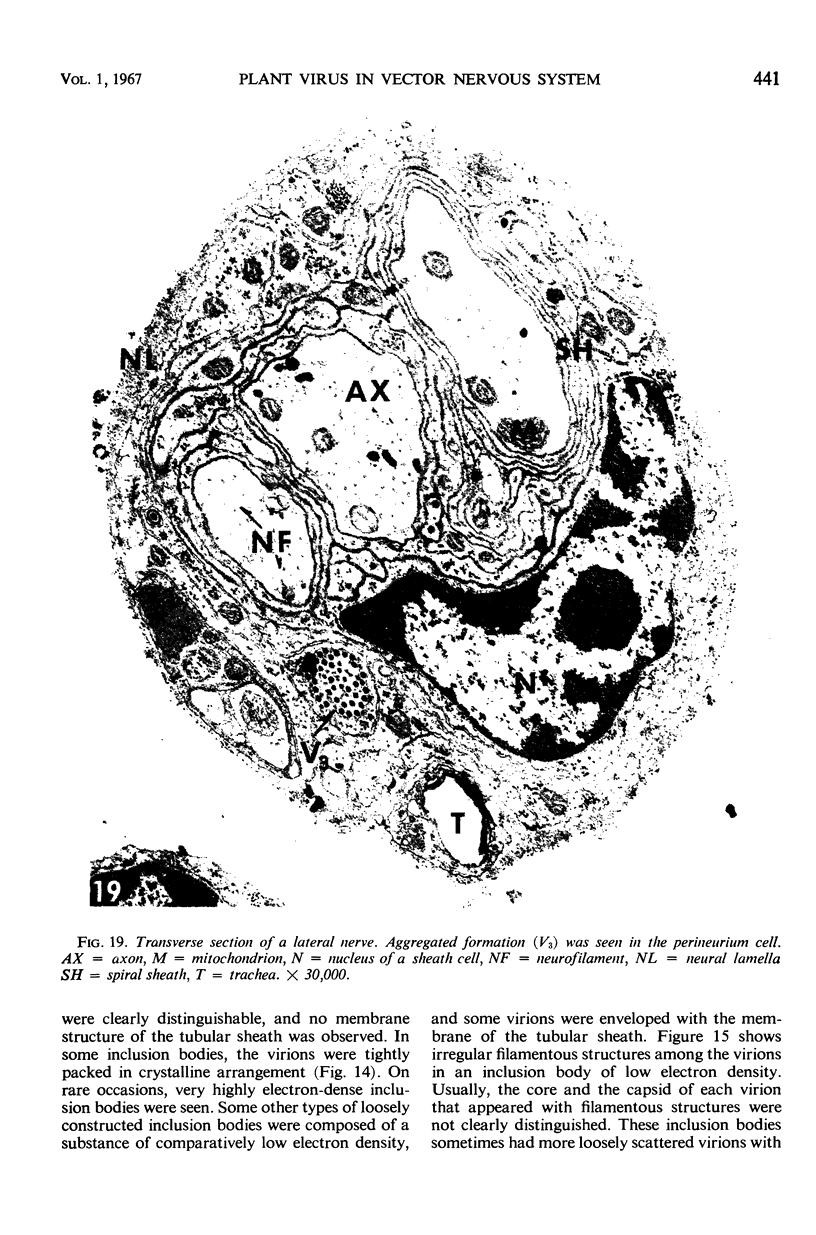
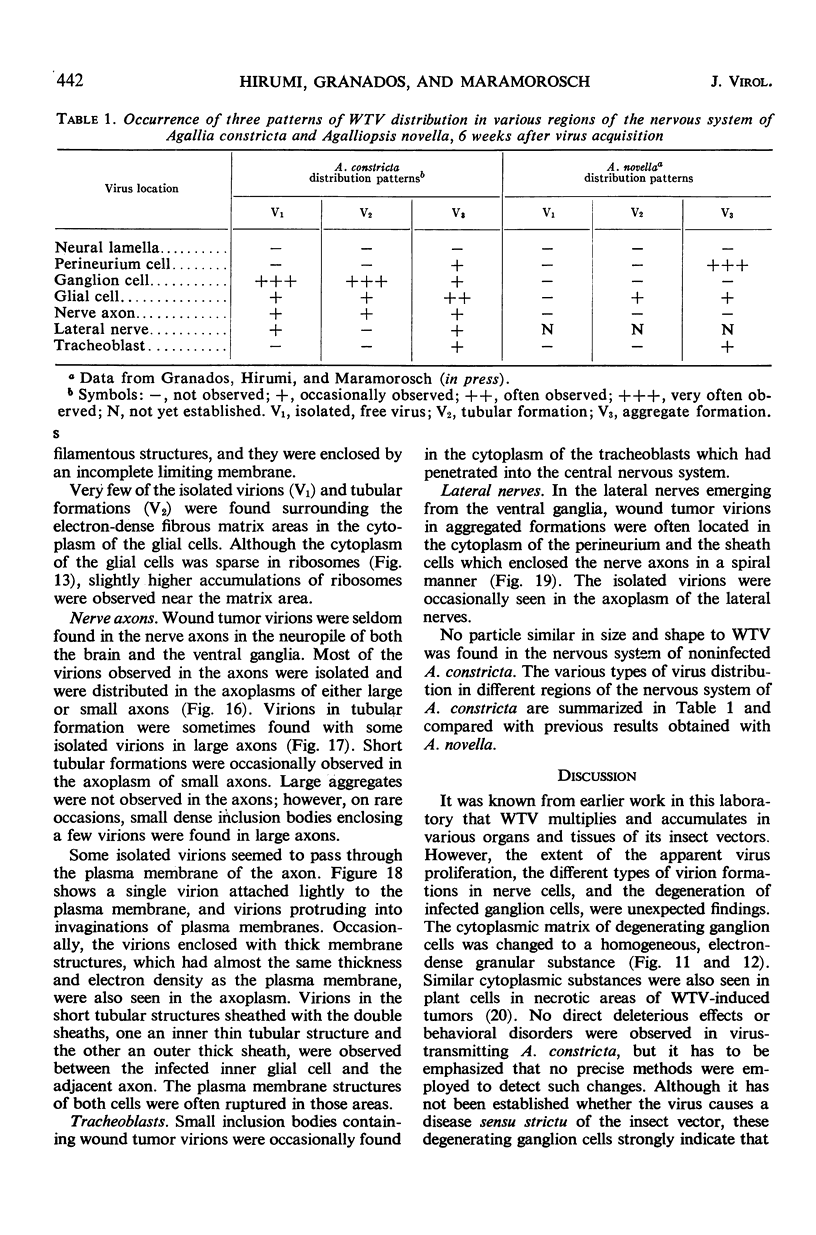
The central nervous system of wound tumor virus (WTV)-infected Agallia constricta was studied by electron microscopy to obtain information concerning the virus distribution in the nervous system. Wound tumor virions were mostly found in the cytoplasm of the ganglion cells and less frequently in the glial cells. WTV was occasionally observed in the perineurium cells, nerve axons, tracheoblasts, and lateral nerves. In the ganglion cells, virions appeared as individual isolated particles (V1), in tubular formation (V2), and occasionally in aggregates (V3). In the glial cells, the virions were mostly seen in the V3 formation, and very seldom in the V1 and V2 formations. In the perineurium cells and tracheoblasts, only small V3 formations were observed. The isolated virions were usually surrounded with polyribosomes, and often appeared around the foci of the viroplasm. Sometimes degenerating ganglion cells infected with the WTV were encountered. These damaged cells strongly indicated that WTV exerted a cytopathogenic effect on the nerve cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BILS R. F., HALL C. E. Electron microscopy of wound-tumor virus. Virology. 1962 May;17:123–130. doi: 10.1016/0042-6822(62)90089-2. [DOI] [PubMed] [Google Scholar]
- BRAKKE M. K., VATTER A. E., BLACK L. M. Size and shape of wound-tumor virus. Brookhaven Symp Biol. 1953 Aug;6:137-55; discussion, 155-6. [PubMed] [Google Scholar]
- CAULFIELD J. B. Effects of varying the vehicle for OsO4 in tissue fixation. J Biophys Biochem Cytol. 1957 Sep 25;3(5):827–830. doi: 10.1083/jcb.3.5.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALES S., EGGERS H. J., TAMM I., PALADE G. E. ELECTRON MICROSCOPIC STUDY OF THE FORMATION OF POLIOVIRUS. Virology. 1965 Jul;26:379–389. doi: 10.1016/0042-6822(65)90001-2. [DOI] [PubMed] [Google Scholar]
- DALES S., SIMINOVITCH L. The development of vaccinia virus in Earle's L strain cells as examined by electron microscopy. J Biophys Biochem Cytol. 1961 Aug;10:475–503. doi: 10.1083/jcb.10.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUKUSHI T., SHIKATA E., KIMURA I. Some morphological characters of rice dwarf virus. Virology. 1962 Oct;18:192–205. doi: 10.1016/0042-6822(62)90005-3. [DOI] [PubMed] [Google Scholar]
- GOMORI G. A rapid one-step trichrome stain. Am J Clin Pathol. 1950 Jul;20(7):661–664. doi: 10.1093/ajcp/20.7_ts.661. [DOI] [PubMed] [Google Scholar]
- Gil-Fernandez C., Black L. M. Some aspects of the internal anatomy of the leafhopper Agillia constricta (Homoptera: Cicadellidae). Ann Entomol Soc Am. 1965 May;58(3):275–284. doi: 10.1093/aesa/58.3.275. [DOI] [PubMed] [Google Scholar]
- HIGASHI N., OZAKI Y., ICHIMIYA M. Electron microscopy of pox virus-to-cell adsorption and the ultrastructure of developmental forms of pox virus. J Ultrastruct Res. 1960 Feb;3:270–281. doi: 10.1016/s0022-5320(60)80014-7. [DOI] [PubMed] [Google Scholar]
- KITAJIMA E. W. ELECTRON MICROSCOPY OF VIRA-CABECA VIRUS (BRAZILIAN TOMATO SPOTTED WILT VIRUS) WITHIN THE HOST CELL. Virology. 1965 May;26:89–99. doi: 10.1016/0042-6822(65)90029-2. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORGAN C., ELLISON S. A., ROSE H. M., MOORE D. H. Structure and development of viruses observed in the electron microscope. II. Vaccinia and fowl pox viruses. J Exp Med. 1954 Sep 1;100(3):301–310. doi: 10.1084/jem.100.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RHIM J. S., JORDAN L. E., MAYOR H. D. Cytochemical, fluorescent-antibody and electron microscopic studies on the growth of reovirus (ECHO 10) in tissue culture. Virology. 1962 Jun;17:342–355. doi: 10.1016/0042-6822(62)90125-3. [DOI] [PubMed] [Google Scholar]
- SHIKATA E., ORENSKI S. W., HIRUMI H., MITSUHASHI J., MARAMOROSCH K. ELECTRON MICROGRAPHS OF WOUND-TUMOR VIRUS IN AN ANIMAL HOST AND IN A PLANT TUMOR. Virology. 1964 Jul;23:441–444. doi: 10.1016/0042-6822(64)90272-7. [DOI] [PubMed] [Google Scholar]
- Shikata E., Maramorosch K. An electron microscope study of plant neoplasia induced by wound tumor virus. J Natl Cancer Inst. 1966 Jan;36(1):97–116. [PubMed] [Google Scholar]
- Shikata E., Maramorosch K. Electron microscopic evidence for the systemic invasion of an insect host by a plant pathogenic virus. Virology. 1965 Dec;27(4):461–475. doi: 10.1016/0042-6822(65)90171-6. [DOI] [PubMed] [Google Scholar]
- Shikata E., Maramorosch K., Granados R. R. Electron microscopy of pea enation mosaic virus in plants and aphid vectors. Virology. 1966 Jul;29(3):426–436. doi: 10.1016/0042-6822(66)90218-2. [DOI] [PubMed] [Google Scholar]
- Sinha R. C. Sequential infection and distribution of wound-tumor virus in the internal organs of a vector after ingestion of virus. Virology. 1965 Aug;26(4):673–686. doi: 10.1016/0042-6822(65)90330-2. [DOI] [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]