Abstract
Purpose of review
Ischemic preconditioning (IPC) is gaining attention as a novel neuroprotective therapy and could provide an improved mechanistic understanding of tolerance to cerebral ischemia. The purpose of this article is to review the recent work in the field of IPC and its applications to clinical scenarios.
Recent findings
The cellular signaling pathways that are activated following IPC are now better understood and have enabled investigators to identify several IPC mimetics. Most of these studies were performed in rodents, and efficacy of these mimetics remains to be evaluated in human patients. Additionally, remote ischemic preconditioning (RIPC) may have higher translational value than IPC. Repeated cycles of temporary ischemia in a remote organ can activate protective pathways in the target organ, including the heart and brain. Clinical trials are underway to test the efficacy of RIPC in protecting brain against subarachnoid hemorrhage.
Summary
IPC, RIPC, and IPC mimetics have the potential to be therapeutic in various clinical scenarios. Further understanding of IPC-induced neuroprotection pathways and utilization of clinically relevant animal models are necessary to increase the translational potential of IPC in the near future.
Keywords: cerebral ischemia, neuroprotection, tolerance
INTRODUCTION
Cerebral ischemia resulting from cardiac arrest and stroke is one of the leading causes of mortality and morbidity in the world. During decades of research, a large number of neuroprotective agents have shown efficacy in animal models but have failed in clinical trials. This failure may have been because of the use of wrong animal models, poor design of clinical trials, and the use of dosages that are much lower than those utilized in animal studies, among many others [1]. As a result, investigators have been pushed to rethink the strategies to develop new therapies for cerebral ischemia. One new strategy that has recently gained attention is ischemic preconditioning (IPC).
The phenomenon of IPC was discovered when studies in rabbit heart demonstrated that induction of mild ischemia followed by a period of reperfusion made the heart more resistant to a subsequent, ordinarily lethal ischemic insult [2]. IPC has since proven to be a powerful strategy to induce tolerance to ischemic insults in most organs studied and has been replicated in many laboratories around the world.
IPC in brain consists of an early and a late phase, during which different neuroprotective responses are elicited in specific time windows (time interval between the first sublethal insult and the second, ordinarily lethal insult). There is a rapid phase for which the cumulative protective effect of released factors is maximal if the window between initial and final insult is approximately 1h in duration [3–5]. The combination of released factors and activated pathways in the second phase, defined as delayed preconditioning [6,7], elicits maximum protection if the window is extended to several days after the preconditioning insult, and has been shown to provide more robust and longer lasting neuroprotection than the first phase. It is now understood that IPC comprises several key steps [8,9], during which triggering factors are released in response to the short-duration sublethal ischemic insult; signaling pathways are activated by the receptors of the triggering factors; and gene expression is orchestrated by the delayed preconditioning-activated signaling pathways. Activation of these pathways results in brain cells having a phenotype that is highly resistant to ischemic insults.
Although many triggering factors are activated by IPC, our laboratory has shown two such factors to be critical, and they appear to have opposite effects. We and others showed that adenosine is released after IPC and initiates a signaling pathway that promotes ischemic tolerance in brain via activation of A1 receptors (A1AR) [4,10,11]. Adenosine is a neuromodulator and vasomodulator that is normally released when ATP levels decline [12]. Inhibition of synaptic activity is observed when adenosine binds to the A1 receptor [4], which is believed to be the key receptor in the induction of ischemic tolerance.
In contrast to the inhibitory pathway activated by adenosine, we and others have shown that activation of excitatory postsynaptic NMDA receptors is also required for IPC-induced ischemic tolerance in brain [13–16]. It is now known that these two receptors (NMDA receptors and A1AR), via different mechanisms, activate a novel type of protein kinase C (εPKC) [13,17] which plays a key role in the induction of ischemic tolerance. Although there are multiple key signaling pathways that mediate preconditioning, it is plausible that significant cross-talk occurs among pro-survival kinases following IPC. As this area of research is beyond the scope of this review, the reader is directed to previously written reviews that provide an overview of these signaling pathways and their effect on IPC [8,9,18■■,19,20].
Cerebral ischemia targets many sensitive cellular sites that seldom recover thereafter. Owing to the excitotoxicity that ensues following cerebral ischemia, synaptic plasticity and functional recovery are significantly impaired. In addition, mitochondria are particularly sensitive to both the ischemic insult as well as the reperfusion phase. IPC, via activation of key survival signaling pathways, promotes synaptic and mitochondrial alterations that make neurons more resistant to ischemia. In the following sections, we will describe some of these effects mediated by IPC.
SYNAPTIC TARGETS OF ISCHEMIC PRECONDITIONING AND FUNCTIONAL RECOVERY
Excitotoxicity was identified as one of the first steps in the pathology of cerebral ischemia [21]. IPC was found to ameliorate excitotoxicity by inhibiting glutamate release [22,23], increasing inhibitory neurotransmitter gamma-aminobutyric acid (GABA) release [22], and enhancing GABA presynaptic and postsynaptic activities, thus making neurons more resistant to the excitotoxic insult [24–26]. These findings suggest that IPC promotes synaptic modifications that may preserve synaptic function and functional recovery following cerebral ischemia. This hypothesis is further supported by the findings that show IPC improves spatial memory, a well established memory system in humans and in animal models. In animals, spatial memory allows them to acquire and retain information about a surrounding environment. This type of memory resides in the hippocampus and entorhinal cortex [27]. In rats, spatial memory, evaluated by the Morris water maze, was shown to be impaired following asphyxial cardiac arrest [28]. However, IPC ameliorated spatial memory deficits after global cerebral ischemia [29], suggesting that IPC targets synaptic terminals in order to improve functional recovery.
ISCHEMIC PRECONDITIONING AND OXIDATIVE DAMAGE
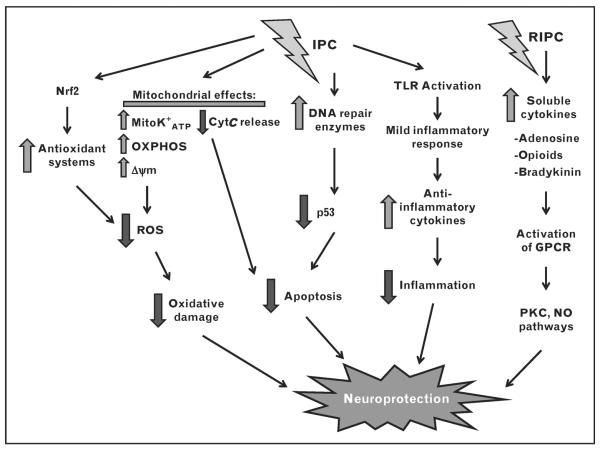
Cerebral ischemia can incite an array of pathologic events, many of which are precipitated by an increase in reactive oxygen species (ROS). IPC can ameliorate oxidative damage following cerebral ischemia through increased antioxidant production, DNA repair capacity, and suppression of inflammation (Fig. 1). The result is increased brain tolerance to ischemia, leading to neuroprotection.
FIGURE 1.
Summary of pathways involved in mediating IPC-induced and RIPC-induced neuroprotection. IPC can increase the recruitment of DNA repair enzymes and transcription factor Nrf2 involved in upregulating antioxidant enzymes. In addition, IPC can modulate the mitochondrial K+ATP channel and further suppress mitochondrial ROS production. Lastly, IPC can activate TLR to induce a mild inflammatory response, eventually triggering anti-inflammatory cytokines to suppress ischemia-induced recruitment of immune cells and thus inflammation. Alternatively, RIPC produces neuroprotection by increasing soluble cytokines in a vascular bed that is far removed from the desired location of cytoprotection. cyt C, cytochrome C; GPCR, G-protein-coupled receptor; IPC, ischemic preconditioning; mitoK+ATP, mitochondrial ATP-sensitive potassium channels; NO, nitric oxide; Nrf2, nuclear factor erythroid 2 related factor 2; OXPHOS, oxidative phosphorylation; PKC, protein kinase C; RIPC, remote ischemic preconditioning; ROS, reactive oxygen species; TLR, Toll-like receptor; Dcm, mitochondrial membrane potential.
Ischemic preconditioning and antioxidants
Oxidative stress in a cell can be sensed by the transcription factor Nrf2 (nuclear factor erythroid 2 related factor 2). Transient exposure of rat and human astrocytes to ischemia resulted in increased expression of Nrf2-targeted genes involved in maintaining the redox state of the cell, namely glutathione and glutathione-related enzymes [30]. Hypoxic preconditioning treatment was shown to increase thioredoxin, an Nrf2-regulated protein involved in reducing oxidized thiol groups in the cell. Increases in thioredoxin were observed in the rat neocortex [31] and hippocampus [32], which resulted in improved tolerance to a lethal hypoxic challenge. In addition, the transcription factor family of signal transducers and activators of transcription (STATs) have also been shown to be activated following IPC and confer neuroprotection through increases in antioxidants as well [19,33■■,34]. Thus, amelioration of oxidative stress through antioxidant expression may represent a potent neuroprotective mechanism of IPC.
Ischemic preconditioning and DNA repair
Oxidation of DNA is another pathologic consequence of oxidative stress. Not only does DNA damage exceeding an irreparable threshold instigate pro-apoptotic pathways, but also constitutive recruitment of the DNA repair enzyme poly(ADP-ribose) polymerase-1 (PARP-1) can deplete cellular stores of NAD+ and ATP [35,36]. Lastly, induction of IPC has been shown to increase β-polymerase-mediated and apurinic/apyrimidinic endonuclease-mediated base excision repair (BER), resulting in improved ischemic tolerance following focal cerebral ischemia in rats [37]. Thus, enhancing the cell's ability to repair DNA damaged from oxidative stress could be a potential therapeutic target for neuroprotection.
Ischemic preconditioning and attenuation of inflammation
A further consequence of cerebral oxidative damage can result in the activation of inflammatory mediators [38], resulting in disruption of the blood–brain barrier, cerebral edema, and inflammation-mediated tissue destruction in the brain [39]. IPC has been suggested to ameliorate the inflammatory response following focal cerebral ischemia through stimulation of Toll-like receptors (TLRs) [40■]. TLRs can activate many proinflammatory pathways, although the activation of TLR through lipopolysaccharide administration (TLR preconditioning) prior to ischemia confers robust neuroprotection. TLR preconditioning was previously shown to attenuate inflammation following focal cerebral ischemia by reducing activated circulating leukocytes, lymphocyte adhesion to endothelium, neutrophil infiltration, microglial activation, and induction of tumor necrosis factor alpha (TNFα, a proinflammatory cytokine) pathways [39,40■]. These functions of TLR preconditioning could ameliorate proinflammatory oxidative damage and dampen the pathological consequences of cerebral ischemia.
MITOCHONDRIAL AND OTHER ORGANELLE TARGETS OF ISCHEMIC PRECONDITIONING
As potent producers of ROS, mitochondria are key mediators of cell death following cerebral ischemia as well as in many neurological disorders, and therefore have become important targets for potential neuroprotective therapies [33■■]. In fact, mitochondria have been shown to play an important role in IPC-induced neuroprotection. IPC mitigates cerebral ischemia-induced neuronal death by several mechanisms, including prevention of decreased oxidative phosphorylation (OXPHOS) capacity, mitochondria-dependent cell death pathways, and ROS generation from the mitochondrial electron transport chain (ETC) [41–48] (Fig. 1). Because of their crucial role in IPC-induced neuroprotection, Dirnagl and Meisel [41] described mitochondria as `gatekeepers of preconditioning'.
In addition, IPC appears to activate several pathways, which in turn modify mitochondrial proteins. In a previous study, we observed that within 1 h after IPC, the mitochondrial ATP-sensitive K+ (mitoK+ATP) channel was phosphorylated and activated by εPKC. This pathway led to the induction of ischemic-tolerance pathways, potentially by sublethal mitochondrial release of ROS [49]. Our group observed delayed IPC-mediated increases in synaptosomal εPKC levels, which contributed to improved mitochondrial OXPHOS capacity following an ordinarily lethal ischemic insult. The increased OXPHOS capacity was attributed to εPKC-induced posttranslational modifications of mitochondrial ETC components [50].
IPC-induced improvement in brain mitochondrial OXPHOS capacity may also be because of IPC-mediated changes in OXPHOS gene expression. Several transcription factors have been shown to translocate to the nucleus following IPC, such as nuclear factor-kappa B (NF-κB) [51] and cyclic-AMP response element-binding protein (CREB) pathways [52], gene products of which can mediate IPC-induced neuroprotection; Liu et al. [53■] identified 19 differentially expressed microRNAs (miRNAs) in the brains of hypoxia-preconditioned mice following middle cerebral artery occlusion. Bioinformatics analysis of the miRNA target genes of two conventional PKC (βIIPKC and γPKC)-interacting and one novel PKC (εPKC)-interacting proteins predicted involvement of major energy-generating pathways (glycolysis or gluconeogenesis, citrate cycle, and OXPHOS) in hypoxia preconditioning-induced neuroprotection. However, two similar studies suggested that IPC-induced alterations in miRNA expression pattern had no effect on mitochondrial OXPHOS [54,55]. Gene ontology analysis in another study identified mitochondria as one of the cellular organelles affected by IPC [56]. These studies suggest that IPC and hypoxic preconditioning differentially affect miRNA expression in relation to mitochondrial OXPHOS pathways. It appears that IPC positively regulates mitochondrial functions by directly affecting gene expression of specific mitochondrial proteins rather than by altering miRNA expression.
Following cerebral ischemia-induced excitotoxicity, altered neuronal calcium (Ca2+) buffering capacity plays a key role in potentiating neuronal cell death. In-vitro models of excitotoxicity demonstrated that a tolerance-inducing stimulus protected neurons from lethal excitotoxicity by increasing mitochondrial Ca2+ buffering capacity and by decreasing mitochondrial Ca2+ uptake [57]. Iijima et al. [58] reported that brief oxygen–glucose deprivation increased mitochondrial calcium loading capacity. These studies highlighted another important role for mitochondria in protecting cells against lethal ischemia-induced excitotoxicity.
Effects of ischemic preconditioning on Golgi apparatus and endoplasmic reticulum
Other cellular organelles such as the Golgi apparatus also participate in cellular Ca2+ buffering. A previous study showed that IPC prevented lethal ischemia-induced suppression of secretory pathway Ca2+-ATPases (SPCA) in the Golgi apparatus by preventing hippocampal membrane lipid and protein oxidation. The same study also reported that IPC enhanced post-lethal ischemia expression of SPCA mRNA [59], suggesting IPC may mediate neuroprotection through Golgi functions preservation. The role of endoplasmic reticulum (ER) in Ca2+ buffering following IPC is not known; however, earlier studies established that IPC reduced neuronal death after lethal ischemia by reducing ER stress [60,61]. A recent study demonstrated that IPC treatment reduced ischemic damage by increasing molecular chaperones levels through activation of autophagy, ultimately lowering ER stress during lethal ischemia [62■]. Together with the ability of IPC to reduce oxidative damage, the previous studies suggest that IPC can reduce organelle stress, further contributing to ischemic tolerance.
CLINICAL SCENARIOS FOR ISCHEMIC PRECONDITIONING
There exist many clinical scenarios for when IPC treatment could be beneficial, most of which involve patients undergoing invasive or long surgeries and being subjected to procedures that could result in a relative state of ischemia. Therefore, patient outcome following surgery might be improved through IPC treatment. Additionally, chronic IPC treatments may afford ischemic and anti-inflammatory protection in patients who suffer from metabolic syndrome, cardiovascular and cerebrovascular disease, or in patients at risk for recurrent ischemic attacks.
Models of ischemic preconditioning in clinic
Most in-vivo models of IPC performed in rodent animals are usually invasive and thus impractical to translate into a clinical setting. However, a modified form of IPC known as remote ischemic preconditioning (RIPC) could prove to have high translational value. RIPC involves cycles of temporary occlusion and restoration of blood flow in a forelimb far removed from the desired sight of cytoprotection. Repeated cycles of temporary ischemia in this area can trigger the release of soluble protective factors into the blood, which can be delivered to the target organ and confer protection [63]. For example, clinical trials have already utilized blood pressure cuffs to induce temporary occlusion and restoration of blood flow in an arm or thigh of patients, thus constituting one model of RIPC [64]. A previous study reported that RIPC-treated patients showed improved ejection fraction, graft patency, and electrocardiogram parameters following coronary artery bypass surgery [65]. In stable angina pectoris patients, three 2-min coronary artery balloon inflation and deflation cycles demonstrated improved cardiac contractility and decreased chest pain [66]. In patients undergoing coronary angioplasty or coronary artery bypass procedures, the induction of carotid artery balloon inflations and deflations just prior to the main surgical procedure could mimic `early-window' IPC-induced cytoprotection. Lastly, RIPC has been shown to be safe and well tolerated in critically ill patients with subarachnoid hemorrhage [67■], suggesting that RIPC may represent a feasible, prophylactic therapy.
Pharmacologic therapies
Perhaps more clinically applicable is the use of a pharmacologic agent that can activate pathways critical for IPC-induced neuroprotection. Our laboratory has previously shown the contribution of Sirtuin 1 (SIRT1, a class III NAD+-dependent histone deacetylase) to mediate delayed IPC-induced neuroprotection [68]. Therefore, a potent activator of SIRT1 such as the polyphenol resveratrol could represent a potential therapy for cerebral ischemia. Although the safety of resveratrol in humans has already been profiled [69], further understanding of its mechanisms is imperative before utilizing this compound for clinical IPC treatment. Isoflurane, commonly used for the fast induction of anesthesia, has been shown to mimic IPC through the activation of mitoK+ATP channels [70]. Thus, brief patient exposures to isoflurane hours to days prior to invasive surgery could represent another clinical use of IPC.
CONCLUSION
As previously mentioned, use of rodent models may hinder translating IPC into clinical practice. Rodents lack similar brain architecture and their white matter and grey matter ratios are quite different from humans [71]. Additionally, the typical age of rodents used in IPC studies does not correlate with the age of the typical patient [72] who would necessitate IPC treatment (i.e., elderly patients at risk for cardiovascular or cerebrovascular disease), which poses a dilemma as evidence suggests that the ability to induce IPC diminishes with age [73]. In addition, rigorous exploration of IPC-mediated mechanisms may enable better understanding of a particular method or mimetic of IPC and ease of clinical translation. Rodent models also explore IPC using a reproducible, controlled ischemic insult, while cardiac arrest and stroke occur unpredictably with varying degrees of lethality in human patients [74]. Thus, future work is necessary to elucidate the mechanisms of IPC-induced neuroprotection moving into animal models that are closer to the real clinical scenarios as suggested by the STAIR criteria [75].
KEY POINTS
IPC treatment preserves mitochondrial OXPHOS functioning, maintains integrity of the membranous organelles, and reduces ER stress following lethal ischemia.
IPC mediates cytoprotection through increasing antioxidant expression, attenuating inflammation, and decreasing DNA damage.
RIPC, as well as pharmacologic IPC mimetics, present potential preventive clinical therapies to combat ischemia and metabolic syndrome.
Use of rodent models is a good first step to study IPC-mediated mechanisms in humans, but should be followed by the STAIR criteria for potential clinical translation.
Acknowledgements
This work was supported by the National Institutes of Health grants NS45676, NS054147, NS34773, NS073779, and F31 NS080344-01.
Footnotes
Conflicts of interest There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 102–103).
- 1.Ginsberg MD. Current status of neuroprotection for cerebral ischemia: synoptic overview. Stroke. 2009;40(3 Suppl.):S111–S114. doi: 10.1161/STROKEAHA.108.528877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 3.Schurr A, Reid KH, Tseng MT, et al. Adaptation of adult brain tissue to anoxia and hypoxia in vitro. Brain Res. 1986;374:244–248. doi: 10.1016/0006-8993(86)90418-x. [DOI] [PubMed] [Google Scholar]
- 4.Perez-Pinzon MA, Mumford PL, Rosenthal M, Sick TJ. Anoxic preconditioning in hippocampal slices: role of adenosine. Neuroscience. 1996;75:687–694. doi: 10.1016/0306-4522(96)00311-9. [DOI] [PubMed] [Google Scholar]
- 5.Perez-Pinzon MA, Xu GP, Dietrich WD, et al. Rapid preconditioning protects rats against ischemic neuronal damage after 3 but not 7 days of reperfusion following global cerebral ischemia. J Cereb Blood Flow Metab. 1997;17:175–182. doi: 10.1097/00004647-199702000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Kato H, Araki T, Kogure K. Preserved neurotransmitter receptor binding following ischemia in preconditioned gerbil brain. Brain Res Bull. 1992;29:395–400. doi: 10.1016/0361-9230(92)90074-8. [DOI] [PubMed] [Google Scholar]
- 7.Kato H, Araki T, Murase K, Kogure K. Induction of tolerance to ischemia: alterations in second-messenger systems in the gerbil hippocampus. Brain Res Bull. 1992;29:559–565. doi: 10.1016/0361-9230(92)90123-f. [DOI] [PubMed] [Google Scholar]
- 8.Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003;26:248–254. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- 9.Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci. 2006;7:437–448. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- 10.Lange-Asschenfeldt C, Raval A, Dave K, et al. Epsilon protein kinase C mediated ischemic tolerance requires activation of the extracellular regulated kinase pathway in the organotypic hippocampal slice. J Cereb Blood Flow Metab. 2004;24:636–645. doi: 10.1097/01.WCB.0000121235.42748.BF. [DOI] [PubMed] [Google Scholar]
- 11.Reshef A, Sperling O, Zorefshani E. Preconditioning of primary rat neuronal cultures against ischemic injury: characterization of the `time window of protection'. Brain Res. 1996;741:252–257. doi: 10.1016/s0006-8993(96)00939-0. [DOI] [PubMed] [Google Scholar]
- 12.Ghiardi GJ, Gidday JM, Roth S. The purine nucleoside adenosine in retinal ischemia–reperfusion injury. Vision Res. 1999;39:2519–2535. doi: 10.1016/s0042-6989(99)00038-3. [DOI] [PubMed] [Google Scholar]
- 13.Raval AP, Dave KR, Mochly-Rosen D, et al. Epsilon PKC is required for the induction of tolerance by ischemic and NMDA-mediated preconditioning in the organotypic hippocampal slice. J Neurosci. 2003;23:384–391. doi: 10.1523/JNEUROSCI.23-02-00384.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kato H, Liu Y, Araki T, Kogure K. MK-801, but not anisomycin, inhibits the induction of tolerance to ischemia in the gerbil hippocampus. Neurosci Lett. 1992;139:118–121. doi: 10.1016/0304-3940(92)90871-4. [DOI] [PubMed] [Google Scholar]
- 15.Bond A, Lodge D, Hicks CA, et al. NMDA receptor antagonism, but not AMPA receptor antagonism attenuates induced ischaemic tolerance in the gerbil hippocampus. Eur J Pharmacol. 1999;380:91–99. doi: 10.1016/s0014-2999(99)00523-3. [DOI] [PubMed] [Google Scholar]
- 16.Grabb MC, Choi DW. Ischemic tolerance in murine cortical cell culture: critical role for NMDA receptors. J Neurosci. 1999;19:1657–1662. doi: 10.1523/JNEUROSCI.19-05-01657.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lange-Asschenfeldt C, Raval AP, Dave KR, et al. Epsilon protein kinase C mediated ischemic tolerance requires activation of the extracellular regulated kinase pathway in the organotypic hippocampal slice. J Cereb Blood Flow Metab. 2004;24:636–645. doi: 10.1097/01.WCB.0000121235.42748.BF. [DOI] [PubMed] [Google Scholar]
- 18.Morris KC, Lin HW, Thompson JW, Perez-Pinzon MA. Pathways for ischemic cytoprotection: role of sirtuins in caloric restriction, resveratrol, and ischemic preconditioning. J Cereb Blood Flow Metab. 2011;31:1003–1019. doi: 10.1038/jcbfm.2010.229. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ This review describes how pharmacological and ischemic predoncitioning induces protection against cerebral ischemic damage by activating sirtuins.
- 19.Lin HW, Thompson JW, Morris KC, Perez-Pinzon MA. Signal transducers and activators of transcription: STATs-mediated mitochondrial neuroprotection. Antioxid Redox Signal. 2011;14:1853–1861. doi: 10.1089/ars.2010.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol. 2009;8:398–412. doi: 10.1016/S1474-4422(09)70054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi DW, Maulucci-Gedde M, Kriegstein AR. Glutamate neurotoxicity in cortical cell culture. J Neurosci. 1987;7:357–368. doi: 10.1523/JNEUROSCI.07-02-00357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dave KR, Lange-Asschenfeldt C, Raval AP, et al. Ischemic preconditioning ameliorates excitotoxicity by shifting glutamate/gamma-aminobutyric acid release and biosynthesis. J Neurosci Res. 2005;82:665–673. doi: 10.1002/jnr.20674. [DOI] [PubMed] [Google Scholar]
- 23.Douen AG, Akiyama K, Hogan MJ, et al. Preconditioning with cortical spreading depression decreases intraischemic cerebral glutamate levels and down-regulates excitatory amino acid transporters EAAT1 and EAAT2 from rat cerebal cortex plasma membranes. J Neurochem. 2000;75:812–818. doi: 10.1046/j.1471-4159.2000.0750812.x. [DOI] [PubMed] [Google Scholar]
- 24.DeFazio RA, Raval AP, Lin HW, et al. GABA synapses mediate neuroprotection after ischemic and epsilonPKC preconditioning in rat hippocampal slice cultures. J Cereb Blood Flow Metab. 2009;29:375–384. doi: 10.1038/jcbfm.2008.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sommer C, Fahrner A, Kiessling M. [3H]Muscimol binding to gamma-aminobutyric acid(A) receptors is upregulated in CA1 neurons of the gerbil hippocampus in the ischemia-tolerant state. Stroke. 2002;33:1698–1705. doi: 10.1161/01.str.0000016404.14407.77. [DOI] [PubMed] [Google Scholar]
- 26.Sommer C, Gass P, Kiessling M. Selective c-JUN expression in CA1 neurons of the gerbil hippocampus during and after acquisition of an ischemia-tolerant state. Brain Pathol. 1995;5:135–144. doi: 10.1111/j.1750-3639.1995.tb00587.x. [DOI] [PubMed] [Google Scholar]
- 27.Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci USA. 2004;101:14515–14520. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hickey RW, Akino M, Strausbaugh S, De Courten-Myers GM. Use of the Morris water maze and acoustic startle chamber to evaluate neurologic injury after asphyxial arrest in rats. Pediatr Res. 1996;39:77–84. doi: 10.1203/00006450-199601000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Plamondon H, Davignon G, Khan S, Charron C. Cerebral ischemic preconditioning induces lasting effects on CA1 neuronal survival, prevents memory impairments but not ischemia-induced hyperactivity. Behav Brain Res. 2008;189:145–151. doi: 10.1016/j.bbr.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 30.Bell KF, Fowler JH, Al-Mubarak B, et al. Activation of Nrf2-regulated glutathione pathway genes by ischemic preconditioning. Oxid Med Cell Longev. 2011;2011:689524. doi: 10.1155/2011/689524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stroev SA, Gluschenko TS, Tjulkova EI, et al. Preconditioning enhances the expression of mitochondrial antioxidant thioredoxin-2 in the forebrain of rats exposed to severe hypobaric hypoxia. J Neurosci Res. 2004;78:563–569. doi: 10.1002/jnr.20282. [DOI] [PubMed] [Google Scholar]
- 32.Stroev SA, Tyul'kova EI, Glushchenko TS, et al. Thioredoxin-1 expression levels in rat hippocampal neurons in moderate hypobaric hypoxia. Neurosci Behav Physiol. 2009;39:1–5. doi: 10.1007/s11055-008-9091-5. [DOI] [PubMed] [Google Scholar]
- 33.Perez-Pinzon MA, Stetler RA, Fiskum G. Novel mitochondrial targets for neuroprotection. J Cereb Blood Flow Metab. 2012;32:1362–1376. doi: 10.1038/jcbfm.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■■ This article provides in-depth review of articles on how mitochondrial targets play a role in neuroprotection specifically against cerebral ischemic damage.
- 34.Kim EJ, Raval AP, Perez-Pinzon MA. Preconditioning mediated by sublethal oxygen-glucose deprivation-induced cyclooxygenase-2 expression via the signal transducers and activators of transcription 3 phosphorylation. J Cereb Blood Flow Metab. 2008;28:1329–1340. doi: 10.1038/jcbfm.2008.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alano CC, Ying W, Swanson RA. Poly(ADP-ribose) polymerase-1-mediated cell death in astrocytes requires NAD+ depletion and mitochondrial permeability transition. J Biol Chem. 2004;279:18895–18902. doi: 10.1074/jbc.M313329200. [DOI] [PubMed] [Google Scholar]
- 36.Lavignon M, Tounekti N, Rayner B, et al. Inhibition of murine leukemia viruses by nuclease-resistant alpha-oligonucleotides. Antisense Res Dev. 1992;2:315–324. doi: 10.1089/ard.1992.2.315. [DOI] [PubMed] [Google Scholar]
- 37.Li W, Luo Y, Zhang F, et al. Ischemic preconditioning in the rat brain enhances the repair of endogenous oxidative DNA damage by activating the base-excision repair pathway. J Cereb Blood Flow Metab. 2006;26:181–198. doi: 10.1038/sj.jcbfm.9600180. [DOI] [PubMed] [Google Scholar]
- 38.Kelkka T, Hultqvist M, Nandakumar KS, Holmdahl R. Enhancement of antibody-induced arthritis via Toll-like receptor 2 stimulation is regulated by granulocyte reactive oxygen species. Am J Pathol. 2012;181:141–150. doi: 10.1016/j.ajpath.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 39.Yilmaz G, Granger DN. Cell adhesion molecules and ischemic stroke. Neurol Res. 2008;30:783–793. doi: 10.1179/174313208X341085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leung PY, Stevens SL, Packard AE, et al. Toll-like receptor 7 preconditioning induces robust neuroprotection against stroke by a novel type I interferon-mediated mechanism. Stroke. 2012;43:1383–1389. doi: 10.1161/STROKEAHA.111.641522. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ A novel study implicates the role of Toll-like receptor 7 in mediating IPC-induced neuroprotection through interferon. Preconditioning with specific TLR7 agonists implicates role of IPC in attenuating inflammation, as well as providing a possible pharmacologic IPC-mimetic.
- 41.Dirnagl U, Meisel A. Endogenous neuroprotection: mitochondria as gateways to cerebral preconditioning? Neuropharmacology. 2008;55:334–344. doi: 10.1016/j.neuropharm.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 42.Correia SC, Carvalho C, Cardoso S, et al. Mitochondrial preconditioning: a potential neuroprotective strategy. Front Aging Neurosci. 2010;2:138. doi: 10.3389/fnagi.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhan RZ, Fujihara H, Baba H, et al. Ischemic preconditioning is capable of inducing mitochondrial tolerance in the rat brain. Anesthesiology. 2002;97:896–901. doi: 10.1097/00000542-200210000-00022. [DOI] [PubMed] [Google Scholar]
- 44.Liu D, Lu C, Wan R, et al. Activation of mitochondrial ATP-dependent potassium channels protects neurons against ischemia-induced death by a mechanism involving suppression of Bax translocation and cytochrome c release. J Cereb Blood Flow Metab. 2002;22:431–443. doi: 10.1097/00004647-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 45.Miyawaki T, Mashiko T, Ofengeim D, et al. Ischemic preconditioning blocks BAD translocation, Bcl-xL cleavage, and large channel activity in mitochondria of postischemic hippocampal neurons. Proc Natl Acad Sci USA. 2008;105:4892–4897. doi: 10.1073/pnas.0800628105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Racay P, Chomova M, Tatarkova Z, et al. Ischemia-induced mitochondrial apoptosis is significantly attenuated by ischemic preconditioning. Cell Mol Neurobiol. 2009;29:901–908. doi: 10.1007/s10571-009-9373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang HX, Du GH, Zhang JT. Ischemic preconditioning preserves brain mitochondrial functions during the middle cerebral artery occlusion in rat. Neurol Res. 2003;25:471–476. doi: 10.1179/016164103101201878. [DOI] [PubMed] [Google Scholar]
- 48.Ding ZM, Wu B, Zhang WQ, et al. Neuroprotective effects of ischemic preconditioning and postconditioning on global brain ischemia in rats through the same effect on inhibition of apoptosis. Int J Mol Sci. 2012;13:6089–6101. doi: 10.3390/ijms13056089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raval AP, Dave KR, DeFazio RA, Perez-Pinzon MA. EpsilonPKC phosphorylates the mitochondrial K(+) (ATP) channel during induction of ischemic preconditioning in the rat hippocampus. Brain Res. 2007;1184:345–353. doi: 10.1016/j.brainres.2007.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dave KR, DeFazio RA, Raval AP, et al. Ischemic preconditioning targets the respiration of synaptic mitochondria via protein kinase C epsilon. J Neurosci. 2008;28:4172–4182. doi: 10.1523/JNEUROSCI.5471-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim EJ, Raval AP, Hirsch N, Perez-Pinzon MA. Ischemic preconditioning mediates cyclooxygenase-2 expression via nuclear factor-kappa B activation in mixed cortical neuronal cultures. Transl Stroke Res. 2010;1:40–47. doi: 10.1007/s12975-009-0006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Terasaki Y, Sasaki T, Yagita Y, et al. Activation of NR2A receptors induces ischemic tolerance through CREB signaling. J Cereb Blood Flow Metab. 2010;30:1441–1449. doi: 10.1038/jcbfm.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu C, Peng Z, Zhang N, et al. Identification of differentially expressed microRNAs and their PKC-isoform specific gene network prediction during hypoxic preconditioning and focal cerebral ischemia of mice. J Neurochem. 2012;120:830–841. doi: 10.1111/j.1471-4159.2011.07624.x. [DOI] [PubMed] [Google Scholar]; ■ In this research article, authors predicted the gene network affected during hypoxic preconditioning.
- 54.Lusardi TA, Farr CD, Faulkner CL, et al. Ischemic preconditioning regulates expression of microRNAs and a predicted target, MeCP2, in mouse cortex. J Cereb Blood Flow Metab. 2010;30:744–756. doi: 10.1038/jcbfm.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dharap A, Vemuganti R. Ischemic preconditioning alters cerebral microRNAs that are upstream to neuroprotective signaling pathways. J Neurochem. 2010;113:1685–1691. doi: 10.1111/j.1471-4159.2010.06735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Benardete EA, Bergold PJ. Genomic analysis of ischemic preconditioning in adult rat hippocampal slice cultures. Brain Res. 2009;1292:107–122. doi: 10.1016/j.brainres.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 57.Pivovarova NB, Stanika RI, Watts CA, et al. Reduced calcium-dependent mitochondrial damage underlies the reduced vulnerability of excitotoxicity-tolerant hippocampal neurons. J Neurochem. 2008;104:1686–1699. doi: 10.1111/j.1471-4159.2007.05080.x. [DOI] [PubMed] [Google Scholar]
- 58.Iijima T, Tanaka K, Matsubara S, et al. Calcium loading capacity and morphological changes in mitochondria in an ischemic preconditioned model. Neurosci Lett. 2008;448:268–272. doi: 10.1016/j.neulet.2008.10.056. [DOI] [PubMed] [Google Scholar]
- 59.Pavlikova M, Tatarkova Z, Sivonova M, et al. Alterations induced by ischemic preconditioning on secretory pathways Ca2+-ATPase (SPCA) gene expression and oxidative damage after global cerebral ischemia/reperfusion in rats. Cell Mol Neurobiol. 2009;29:909–916. doi: 10.1007/s10571-009-9374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hayashi T, Saito A, Okuno S, et al. Induction of GRP78 by ischemic preconditioning reduces endoplasmic reticulum stress and prevents delayed neuronal cell death. J Cereb Blood Flow Metab. 2003;23:949–961. doi: 10.1097/01.WCB.0000077641.41248.EA. [DOI] [PubMed] [Google Scholar]
- 61.Lehotsky J, Urban P, Pavlikova M, et al. Molecular mechanisms leading to neuroprotection/ischemic tolerance: effect of preconditioning on the stress reaction of endoplasmic reticulum. Cell Mol Neurobiol. 2009;29:917–925. doi: 10.1007/s10571-009-9376-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sheng R, Liu XQ, Zhang LS. Autophagy regulates endoplasmic reticulum stress in ischemic preconditioning. Autophagy. 2012;8:310–325. doi: 10.4161/auto.18673. [DOI] [PubMed] [Google Scholar]; ■ This research article describes how activation of autophagy by ischemic preconditioning stimuli supresses lethal ischemia-induced ER stress.
- 63.Lim SY, Yellon DM, Hausenloy DJ. The neural and humoral pathways in remote limb ischemic preconditioning. Basic Res Cardiol. 2010;105:651–655. doi: 10.1007/s00395-010-0099-y. [DOI] [PubMed] [Google Scholar]
- 64.Kharbanda RK, Mortensen UM, White PA, et al. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation. 2002;106:2881–2883. doi: 10.1161/01.cir.0000043806.51912.9b. [DOI] [PubMed] [Google Scholar]
- 65.Hoole SP, Heck PM, Sharples L, et al. Cardiac Remote Ischemic Preconditioning in Coronary Stenting (CRISP Stent) study: a prospective, randomized control trial. Circulation. 2009;119:820–827. doi: 10.1161/CIRCULATIONAHA.108.809723. [DOI] [PubMed] [Google Scholar]
- 66.Leesar MA, Stoddard MF, Dawn B, et al. Delayed preconditioning-mimetic action of nitroglycerin in patients undergoing coronary angioplasty. Circulation. 2001;103:2935–2941. doi: 10.1161/01.cir.103.24.2935. [DOI] [PubMed] [Google Scholar]
- 67.Koch S, Katsnelson M, Dong C, Perez-Pinzon M. Remote ischemic limb preconditioning after subarachnoid hemorrhage: a phase Ib study of safety and feasibility. Stroke. 2011;42:1387–1391. doi: 10.1161/STROKEAHA.110.605840. [DOI] [PMC free article] [PubMed] [Google Scholar]; ■ A clinical study indicating that RIPC is a feasible, noninvasive therapy and can be readily administered even to critically ill patients in a hospital setting.
- 68.Della-Morte D, Dave KR, DeFazio RA, et al. Resveratrol pretreatment protects rat brain from cerebral ischemic damage via a sirtuin 1-uncoupling protein 2 pathway. Neuroscience. 2009;159:993–1002. doi: 10.1016/j.neuroscience.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johnson WD, Morrissey RL, Usborne AL, et al. Subchronic oral toxicity and cardiovascular safety pharmacology studies of resveratrol, a naturally occurring polyphenol with cancer preventive activity. Food Chem Toxicol. 2011;49:3319–3327. doi: 10.1016/j.fct.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tanaka K, Weihrauch D, Ludwig LM, et al. Mitochondrial adenosine triphosphate-regulated potassium channel opening acts as a trigger for isoflurane-induced preconditioning by generating reactive oxygen species. Anesthesiology. 2003;98:935–943. doi: 10.1097/00000542-200304000-00021. [DOI] [PubMed] [Google Scholar]
- 71.Oberheim NA, Wang X, Goldman S, Nedergaard M. Astrocytic complexity distinguishes the human brain. Trends Neurosci. 2006;29:547–553. doi: 10.1016/j.tins.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 72.Quinn R. Comparing rat's to human's age: how old is my rat in people years? Nutrition. 2005;21:775–777. doi: 10.1016/j.nut.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 73.He Z, Crook JE, Meschia JF, et al. Aging blunts ischemic-preconditioning-induced neuroprotection following transient global ischemia in rats. Curr Neurovasc Res. 2005;2:365–374. doi: 10.2174/156720205774962674. [DOI] [PubMed] [Google Scholar]
- 74.Koch S, Sacco RL, Perez-Pinzon MA. Preconditioning the brain: moving on to the next frontier of neurotherapeutics. Stroke. 2012;43:1455–1457. doi: 10.1161/STROKEAHA.111.646919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fisher M, Feuerstein G, Howells DW, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]