Abstract
Intrasensory interference during visual working memory (WM) maintenance by object stimuli (such as faces and scenes), has been shown to negatively impact WM performance, with greater detrimental impacts of interference observed in aging. Here we assessed age-related impacts by intrasensory WM interference from lower-level stimulus features such as visual and auditory motion stimuli. We consistently found that interference in the form of ignored distractions and secondary task i nterruptions presented during a WM maintenance period, degraded memory accuracy in both the visual and auditory domain. However, in contrast to prior studies assessing WM for visual object stimuli, feature-based interference effects were not observed to be significantly greater in older adults. Analyses of neural oscillations in the alpha frequency band further revealed preserved mechanisms of interference processing in terms of post-stimulus alpha suppression, which was observed maximally for secondary task interruptions in visual and auditory modalities in both younger and older adults. These results suggest that age-related sensitivity of WM to interference may be limited to complex object stimuli, at least at low WM loads.
INTRODUCTION
External interference has been evidenced to negatively impact the ability to maintain information in WM (Baddeley, 1986, Sakai, 2003, Sakai and Passingham, 2004, Yoon et al., 2006, Sreenivasan and Jha, 2007, Clapp et al., 2010, 2011, Clapp and Gazzaley, 2012). Clapp et al. (2010) classified external interference as either distracting or interrupting: distractions involve task-irrelevant stimuli intended to be ignored, while interruptions are attended as part of a secondary task. Conceptually, engaging with interruptions while simultaneously maintaining information in WM can be considered dual tasking (Salvucci and Taatgen, 2008). Thus, in the present study, interference effects are investigated for both distractions and secondary task interruptions.
In a visual WM task consisting of object stimuli such as faces and scenes, Clapp et al. (2010) found distinct mechanisms of WM interference for distractions versus secondary task interruptions in young adults. Interestingly, behavioral performance in older adults compared to younger adults, was more negatively impacted by interference (Clapp et al., 2011, Clapp and Gazzaley, 2012). Using EEG and fMRI based neuroimaging measures it was additionally shown that distractor-related early visual processing in extrastriate cortex was suppressed in younger but not older adults, when compared to a passive baseline with non-distracting stimuli. Furthermore, the exacerbated impact of secondary task interruptions in aging was shown to be due to deficits in dynamically engaging the functionally connected prefrontal and visual cortical memory maintenance networks that emerge during the task period.
While the greater impact of WM interference in older relative to younger adults has been behaviorally and neurally dissected for complex visual object stimuli, the age-related impacts of interference on WM maintenance for visual or auditory motion is not known. Here, we define auditory motion as a sound sweep across a frequency range. Although the real-world utility of auditory motion WM is diverse, it is commonly used in processing speech patterns where intonations are held in WM while new sounds are received that subsequently form words and sentences. Similarly, visual motion WM is prevalent in every day cognition, such as when trying to cross a busy street. This scenario requires the memory maintenance of vehicular motion in one direction while traffic in the other direction is assessed. Thus, WM for both visual and auditory motion are critical cognitive operations that permits tracking of our environment and the impact of interference on motion WM can have serious consequences.
Since visual motion is processed via the dorsal visual stream, distinct from object processing that predominantly engages the ventral visual stream (Ungerleider and Mishkin, 1982, Goodale and Milner, 1992), it is not clear that the recent evidence of an age-related impact on visual object-based interference in WM is generalizable to visual motion. Interestingly, only certain aspects of global visual motion perception are affected by aging. Motion perception studies in aging using random dot kinematogram (RDK) stimuli show age-related deficits in motion perception specific to very slow dot motion speeds, high spatial dot displacements and low dot contrasts, but not otherwise (reviewed in Hutchinson et al., 2012). Given such differences in motion perception in aging, it is best practice that a study assessing the impact of motion as interference on WM first equates the perception of the motion stimuli across individuals. If participants were to engage in the task with perceptually non-thresholded motion stimuli, it would be unclear whether the interference effects are truly due to intrusions in the primary WM task or due to differences in motion perception abilities across individuals and age groups. Thus, in the present study we investigate whether interference differentially impacts visual motion WM in aging after equating motion stimulus perception across individuals using thresholding procedures. Use of perceptually thresholded stimuli ensures that the study findings are truly driven by interference effects.
In parallel to studies on intrasensory visual interference during WM, research on auditory interference during auditory WM has also progressed. However, a debate exists as to whether auditory distractibility is exacerbated in aging. Auditory distraction equally affects younger and older adults in some listening-in-noise experiments and simultaneous speech studies that require selective attention to one of the speech streams (Murphy et al., 1999, Schneider et al., 2000, Li et al., 2004) as well as in an auditory n-back task study (Gurreiro et al., 2013). Yet, other experiments suggest an age-related decline of intrasensory auditory interference control with age (Sommers and Danielson, 1999, Tun and Wingfield, 1999, Tun et al., 2002, Chao and Knight, 1997, Alain and Woods, 1999, Fabiani et al., 2006, Passow et al., 2012). Of note, while the effect of auditory distractions on audition-based cognitive tasks has been explored, no study to our knowledge has investigated the impact of auditory secondary task interruptions on WM performance in aging. Such auditory dual-tasking, for example, occurs when evaluating approaching traffic auditory cues or attending to auditory speech while being interrupted by a cell phone conversation. In contrast, there are a handful of studies of auditory and visual dual-tasking in the context of aging that generally suggest greater impairments with age (Andres et al., 2006, Chaparro et al., 2005, Parmentier and Andres, 2010, Thompson et al., 2012 but see Schneider et al., 2000). In the present research we exclusively focus on behavioral and neural influences of intrasensory interference, and hence investigate auditory distractions and auditory dual-tasking impacts on WM. Again, we use perceptually thresholded auditory stimuli in each individual, similar to the visual task, to ensure that the results are driven by WM interference effects and not perceptual differences.
To summarize, in the visual domain, we sought to investigate the influence of visual distractions and secondary task interruptions on visual motion WM. As noted above, age impacts of interference in visual motion WM are unexplored. As a parallel experiment in the auditory modality, we investigated age impacts of auditory interference on auditory motion WM. The experiments were based on a delayed-recognition task design with dot motion kinematograms in the visual modality and sound sweeps across a frequency range in the auditory modality as the to-be-remembered stimuli. Thus, in the context of auditory and visual motion stimulation, we specifically sought to investigate: (1) do distractions and secondary task interruptions affect WM performance? (2) are interference effects different in older relative to younger adults? and finally, (3) how do the observed intrasensory interference effects and associated age impacts compare across the auditory and visual modalities? In addition to addressing these questions in human behavior, we used EEG recordings concurrent with the behavioral tasks to investigate neural correlates underlying the interference effects on WM performance.
Neural processing of interfering stimuli, both distractions and interruptions, was analyzed relative to a baseline condition when these same interfering stimuli were passively perceived without concurrent WM goals. This was done to facilitate interpretation whether neural representations of distractions and secondary task interruptions are enhanced or suppressed relative to a passive baseline. Enhanced representations would suggest attentional allocation to the interfering stimuli. As we aimed to compare interference processing in the auditory and visual modalities, we focused on neural modulations in the spectral domain. Spectral measures especially in the alpha (8–14 Hz) range are known to be sensitive to changes in attention allocation irrespective of sensory modality (Klimesch et al., 2007, Foxe and Snyder, 2011), and hence, were analyzed as a common marker for attention to both types of interference (distraction/interruption) in each modality (auditory/visual) and age group (younger/older). In contrast to our prior studies (Clapp et al., 2010, 2011, Clapp and Gazzaley, 2012), event-related potentials (ERPs) were not analyzed here as early ERP components, such as the P1-N1-P2, in the auditory and visual modalities are not known to have similar underlying neural activities across the senses, and thus are not easily amenable to cross-sensory comparisons. We hypothesized that intrasensory interference would indeed impact visual and auditory motion-based WM, and based on prior evidence, aging may exacerbate these interference effects.
MATERIALS & METHODS
Participants
A total of seventy-nine healthy volunteers participated in the study. All participants gave written informed consent in accordance with the guidelines set by the Committee on Human Research at the University of California, San Francisco, and were monetarily compensated to participate in the study. Twenty-one younger adults (mean age 24 years, range 20–30 years, 11 females) and nineteen older adults (mean age 68 years, range 60–87 years, 14 females) participated in the auditory experiment, recruited from the San Francisco bay area community using print and web-based research study advertisements. All participant data for the visual experiment was obtained from prior studies (younger: Berry et al., 2009, older: Berry et al., 2010). Visual task raw (performance and neural) data for twenty younger adults (mean age 24 years, range 21–29 years, 9 females) was from a single assessment visit reported in Berry et al. (2009). Visual task raw data for nineteen older adults (mean age 71, range 62–82 years, 9 females) was from the first of two assessment visits conducted in the prior cognitive training study (Berry et al., 2010). Note that data for the nineteen older adults in the visual task is a subset of the thirty-two older adult cohort described in Berry et al. (2010), for which data across all visual task conditions was available. Also note that all age comparisons and statistical analyses on these previously acquired raw data are novel to the current study.
There was no participant overlap across the visual and auditory experiments. All participants included in the study had normal or corrected-to-normal vision examined using a Snellen chart, did not have any history of stroke, traumatic brain injury, psychiatric illness, substance abuse and none used any medication known to affect cognitive state. All participants had a minimum of 12 years of education. Participants in the auditory experiment were additionally screened for normal hearing. Prior to the lab visit, these participants answered a 12-point multiple-choice questionnaire regarding hearing abilities in daily life situations. To screen for normal hearing in the lab, audiometric thresholds in the 250 – 6000 Hz frequency range were determined in both ears by the method of ascending and descending limits. Individuals with mean audiometric thresholds greater than 50 dB at any test frequency in either ear, signifying moderate hearing loss, were excluded.
Neuropsychological testing
Prior to participation in the auditory experiment, all older adults underwent neuropsychological testing to ensure healthy executive and memory function. The neuropsychological test battery for the auditory experiment included: Geriatric Depression (Yesavage et al., 1982), Mini-Mental State Exam (MMSE: Folstein et al., 1975), WM and interruption through CVLT-II, visual-spatial function (copy of a modified Rey-Osterrieth figure), visual-episodic memory (details from a modified Rey-Osterrieth figure, Rey, 1941, Osterrieth, 1944), visual-motor sequencing through DKEFS trail making test A and B, phonemic fluency (words beginning with the letter ‘D’), semantic fluency (animal category), logical memory as per the Weschler Memory Scale (WMS-IV), WM and incidental recall (digit-span forward, backward and digit symbol), executive functioning (Stroop interference test; Stroop, 1935) and reading ability (Wide Range Achievement Test). Participants assessed as within 2 standard deviations of the age-matched normative values on each of the tests listed, were considered to have intact cognitive function.
Participants in the visual experiment were previously screened, and confirmed within two standard deviations of age-matched normative values, using the MMSE and the NeuroTrax (Mindstreams) measures of global cognition, memory, executive function, visuo-spatial and verbal function, and information processing speed (Berry et al., 2010). Older participants in the auditory and visual experiments did not differ in age, MMSE scores and z-score comparisons on all parallel neuropsychological function tests (Table 1); thus confirming that a direct comparison can be made between the results from these participant samples in the present study.
Table 1.
| Screening Metric | Auditory experiment |
Visual experiment |
|---|---|---|
| Age | 67.8 (±1.7) | 70.4 (±1.5) |
| MMSE | 29.7 (±0.1) | 29.4 (±0.2) |
| Working Memory Span | 6.3 (±0.3) | 5.9 (±0.4) |
| Long-term Memory* | 12.8 (±0.6) | 105.3 (±2.5) |
| Visuo-spatial Function* | 11.7 (±0.7) | 118.0 (±2.7) |
| Verbal Function* | 22.6 (±0.9) | 104.4 (±1.9) |
| Executive Function* | 73.7 (±3.0) | 105.1 (±2.8) |
| Processing Speed* | 64.4 (±2.9) | 105.3 (±2.3) |
Average (± standard error) age, MMSE and neuropsychological function raw scores for older adults in the auditory and visual experiment (* depict different tests in the auditory vs. visual experiment screening battery). Test results were compared across experiments after converting raw scores to age-normative z-scores (all t-test comparisons were p≥0.16). Younger adults did not undergo neuropsychological testing.
Stimuli
Auditory
The auditory stimuli used as the cue and probe stimuli in the delayed-recognition task were sound sweeps of 100 msec duration and mid-frequencies randomly chosen between 900–1100 Hz. The end frequencies for each sweep were 0.5 octaves away from the mid-frequency, starting at −0.5 octaves and ending at +0.5 octaves from the mid-frequency for ‘up’ sweeps and reversed for ‘down’ sweeps. 50% of all sweep stimuli were ‘up’ sweeps, while the other 50% were ‘down’ sweeps. A single 2 kHz high frequency tone of 100 msec duration was used as the interfering stimulus. All stimuli were presented to participants at a comfortable sound level of 65dB SPL using insert earphones (Cortech Solutions, LLC).
Visual
Delayed-recognition task cue and probe stimuli, as previously reported in Berry et al. (2009, 2010), consisted of a circular aperture presented for 800 msec containing 290 dots (0.08° × 0.08° each) that subtended 8° of visual angle at a 75 cm viewing distance and were centered at the fovea. This field of 290 spatially random gray scale dots moved with 100% coherence at an oblique angle at 10° per second. The longer 800 msec stimuli durations in the visual experiment, compared to 100 msec in the auditory experiment, were constrained by the time needed to discriminate directional motion. Stimuli were presented with a gray fixation cross in the center of the circular aperture with a black background of luminance level 0.32 cd/m2. All four sectors of the aperture were used (i.e. northeast, northwest, southeast, southwest) except the cardinal directions (up, down, left, right). The experimental stimuli consisted of 12 different directions of motion (3 per sector). A counter-clockwise circular motion stimulus of 800 msec duration was used as the interfering stimulus. Stimuli were presented through E-Prime software (Psychology Software Tools, Inc.) run on a Dell Optiplex GX620 and a ViewSonic G220fb CRT monitor.
Thresholding
Participants completed separate perceptual thresholding tasks for the auditory and visual features prior to the onset of the main experiment in that modality, in order to minimize the effects of individual differences in discriminability. The two motion stimuli (either both auditory or both visual) were separated by 2 sec. Participants determined whether the two motion stimuli were moving in the exact same direction on each trial of an adaptive procedure. For auditory thresholding an adaptive Zest procedure was used, which provides a psychometric function estimate that maintains an 85% performance across all trials (King-Smith et al., 1994). The auditory threshold was obtained as the octave difference between two sound sweep mid-frequencies at the end of 50 discrimination trials. Auditory thresholds did not significantly differ between younger and older adult participants (p=0.5). For visual thresholding a staircase procedure with fixed 2° steps was used. The largest angle at which discrimination performance between two directions of motion was less than 100% was selected as the discrimination angle in each participant. Visual thresholds were significantly reduced in older relative to younger adult participants (older: 27.8 ± 1.87°, younger: 20.25 ± 1.43°, p<0.001, Berry et al., 2010). Note that thresholding differed in the auditory and visual modalities in the adaptive procedure. An adaptive Zest procedure was adopted for auditory thresholding as it provided less variable results upon pilot repeat testing. Also, the Zest simulates a sigmoid psychometric function that plateaus in sensitivity at very high (near 100%) performance levels. Hence the auditory threshold was determined at 85% correct performance, while the visual threshold staircase was advanced to just under 100% performance.
Experimental procedure
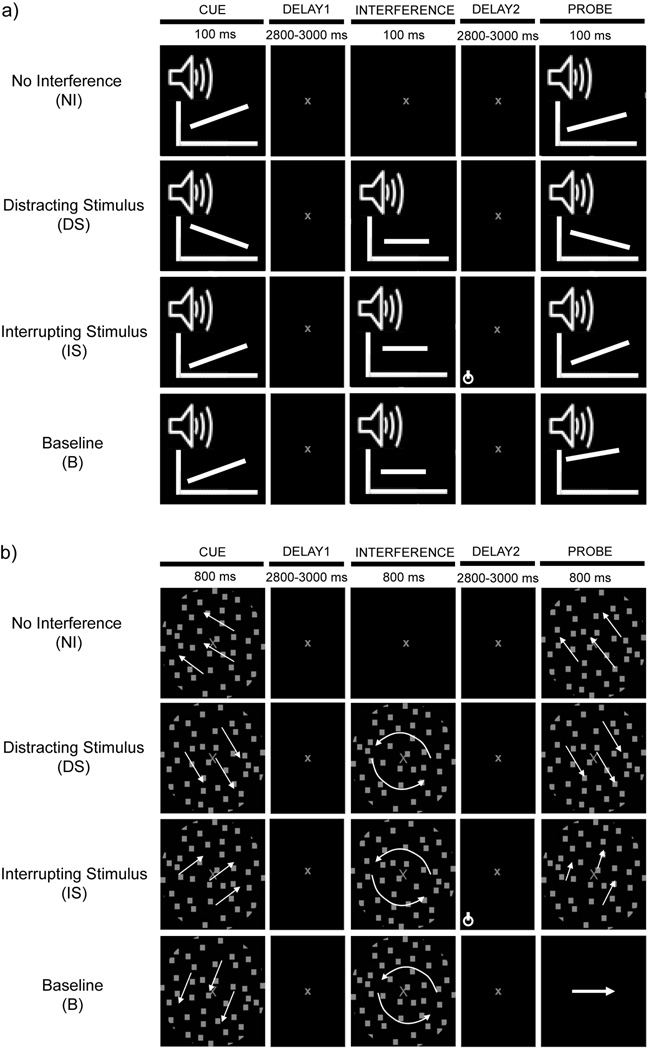
The auditory delayed-recognition task was modeled after Clapp et al. (2010, 2011), and was parametrically identical to the visual WM task previously described in Berry et al. (2009, 2010). In both auditory (Fig. 1a) and visual (Fig. 1b) experiments, participants were presented with four different tasks randomized across eight blocks, with two blocks per task. There were three WM tasks: No Interference (NI), distracting stimulus (DS), secondary task interrupting stimulus (IS), and a fourth baseline (B) that instructed participants to passively perceive the stimuli.
Figure 1.
Overview of the experimental block design in the (a) auditory and (b) visual modality. Cue and probe stimuli were sound sweeps in the auditory modality represented as slanted lines on power spectrum plot (PSP) depictions, interference stimuli were tones shown as PSP straight lines. In the visual modality, cue and probe stimuli were random dot kinematograms (RDKs) undergoing translational motion, interference stimuli were swirling RDKs. Four tasks were presented in a delayed-recognition design in each modality: three were WM tasks (NI, DS and IS), while the fourth passive listening/ viewing task served as baseline (B). The encircled symbol in IS delay 2 represents the button press response required to the IS targets.
For the NI task in both modalities, the cue stimulus (sound sweeps in the auditory experiment (Fig. 1a) and directional dot motion in the visual experiment (Fig. 1b)) was followed by a delay period (6 sec) in which participants were instructed to mentally rehearse the encoded cue, and the trial concluded with the presentation of a probe motion. Participants were instructed to make a match/non-match button press response as quickly and accurately as possible. For the two interference tasks, DS and IS, the interfering stimulus was inserted in the middle (400 msec jitter) of the delay period. In the DS task, participants were instructed to ignore the distractor. In the IS task, participants were asked to attend to the interrupter and detect whether the tone was a higher frequency target (2.3 kHz) or a 2 kHz non-target for auditory interrupters, or if the swirl was a fast motion target swirl or a slow non-target swirl for visual interrupters. A button press response was required only for auditory/visual target interrupters, which randomly occurred on 10% of the IS trials, but not otherwise. These 10% target IS trials were removed from further analyses and an additional 4 non-target trials (10%) were included in each IS block to account for the discarded trials. Participants were familiarized with the target/non-target interrupters used in the IS task prior to the start of either auditory/visual experiment. In the B task, participants were instructed not to remember either stimulus but to merely perceive them. For all WM tasks, 50% of the probe stimuli matched the previously presented cue stimulus, whereas the other 50% differed from the cue as per the discrimination threshold of each participant. Participants were instructed to indicate cue-probe match as quickly as possible without sacrificing accuracy during all tasks. Probes during the B task, were high or low frequency sound sweeps (of 2 kHz or 500 Hz mid-frequencies, respectively) in the auditory experiment, or a left or right pointing arrow in the visual experiment identical to previous visual WM experiments that incorporate a B task (Gazzaley et al., 2005, 2008). All participants easily discriminated the B task probes with 100% accuracy. In the auditory task version, participants responded to a response screen prompt immediately after probe presentation (presented at 400 msec from probe onset), while in the visual task responses were recorded ≤4 sec after probe onset. Participants were provided with correct/incorrect response feedback at the end of each trial in each task, and were instructed to maintain their gaze at central fixation throughout each trial. Each task was performed two times, with 40 trials per block (44 in the IS task); each experiment took approximately 1.5 hours to complete.
EEG: Electrophysiological Recordings
Electrophysiological signals were recorded at 1024 Hz through a 24-bit BioSemi ActiveTwo 64-channel Ag-AgCl–active electrode EEG acquisition system (Cortech Solutions, LLC, Wilmington, NC). Electrode offsets were maintained between ± 20mV. The three-dimensional coordinates of each electrode and of three fiducial landmarks (the left and right pre-auricular points and the nasion) were determined by means of a BrainSight (Rogue Research, Inc.) spatial digitizer. The mean Cartesian coordinates for each site were averaged across all subjects and used for topographic mapping.
Raw EEG data was digitally re-referenced off-line to the average reference. Eye artifacts were removed through independent component analyses by excluding components consistent with topographies for blinks and eye movements and the electro-oculogram time-series. Additionally, individual trials containing artifacts with a voltage threshold of ±75 µV were removed. Data was high-pass filtered at 0.1 Hz to exclude ultraslow DC drifts. This preprocessing was conducted in Matlab (The Mathworks, Inc.) and EEGLab toolbox (Swartz Center for Computational Neuroscience, UC San Diego).
Frequency domain analysis
As we aimed to analyze the neural impact of interference, we compared oscillatory cortical activity following interfering stimuli in the IS and DS conditions versus the same stimuli when they were passively perceived in the B condition. Note that the NI condition, by definition, did not contain a stimulus in the delay period that could be similarly analyzed. To analyze oscillatory cortical activity the single trial EEG signal on each channel was convolved with 6-cycle Morlet wavelets computed at each time point over a 2 sec window centered at stimulus onset. Six-cycle wavelets were chosen as they were found to be optimal for both temporal and frequency band resolution. Instantaneous power and phase were extracted at each time point over 85 frequency scales from 0.9 to 101 Hz incremented logarithmically (Lakatos et al., 2005, Mishra et al., 2012). Power was calculated as the sum of the squares of the real and imaginary Morlet components. The square roots of the power values, termed spectral amplitudes (in µV), were then averaged over single trials to yield the total averaged spectral amplitudes for each condition and electrode site. The averaged spectral amplitude at each time point and frequency was baseline corrected by subtracting the mean spectral amplitude over the −300 to −50 msec pre-stimulus interval (corrected separately for each frequency band in each individual subject) (Tallon-Baudry et al., 1998). Significant differences in spectral amplitude between interference conditions, age groups and sensory modalities were compared using an ANOVA (Kiebel et al., 2005) with a Greenhouse-Geisser correction when sphericity was violated. Modality (auditory/visual) and age (younger/older) were between-subjects factors in the repeated measures ANOVA, while interference (B/DS/IS) was a within-subjects factor. Post-hoc analyses of ANOVA interactions consisted of two-tailed t-tests. Two-tailed dependent sample t-tests were also used for planned comparisons between the different WM interference conditions within each age group for each modality with a significance threshold of 0.05. Note that processing of interfering stimuli was never directly compared across modalities (e.g. IS in the auditory versus visual experiment) given the physical differences between these stimuli.
Alpha frequency band (8–14 Hz) modulations were assessed across B, DS and IS interference conditions, in both auditory and visual modalities and across age groups. Post-stimulus alpha spectral amplitudes (square root of power) for both younger and older adults were measured at 350–650 msec latency over peak alpha desynchronization electrode sites (POz, PO3/4) in the auditory experiment, and at 350–850 msec latency over sites (PO7/8) in the visual experiment. These latencies were chosen as the time window during which post-stimulus alpha spectral amplitude showed peak desynchronization with stable scalp topographies across all conditions, measured separately in the auditory and visual modality.
Behavioral analysis
Percent accuracies were calculated within each of the WM conditions: NI, DS and IS, and analyzed using repeated measures ANOVAs. Modality (auditory/visual) and age (younger/older) were between subjects ANOVA factors and interference (NI/DS/IS) was a within subjects factor. Post-hoc analyses consisted of two-tailed t-tests with a significance threshold of 0.05. Additionally, two-tailed dependent sample t-tests were used for planned comparisons between the different WM interference conditions within each age group in each modality. Baseline (B) discrimination performance was consistently at 100% in all participants.
Response times (RTs) were also statistically compared across conditions in each modality and age group, although responding circumstances slightly differed in the auditory and visual task version. A response screen 400 msec after probe onset prompted responses in the auditory, but not the visual version of the task. In both tasks, RTs were calculated as the time between probe stimulus onset and correct response.
RESULTS
Behavioral Performance
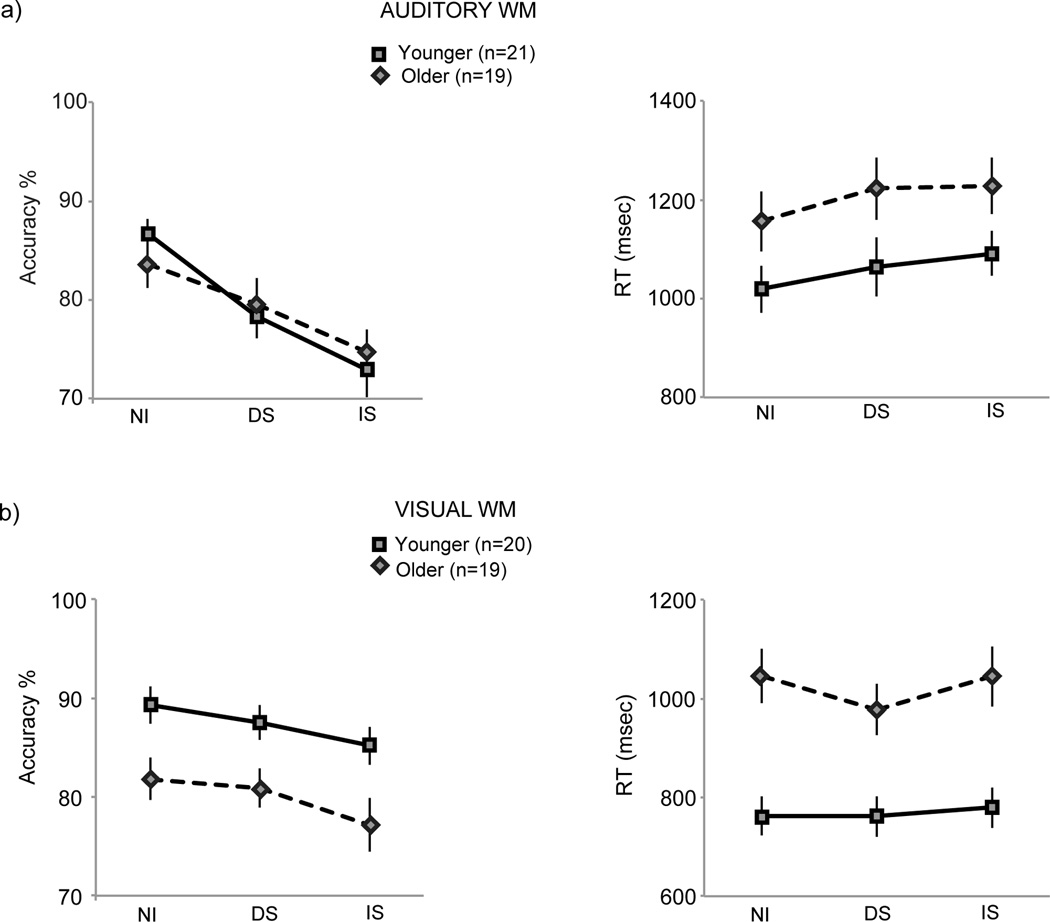
Percent accuracies in the WM conditions (NI, DS and IS) in the auditory and visual tasks are depicted in Figure 2, left column. WM performance data were entered into a 2 × 2 × 3 repeated measures ANOVA with between-subjects factors of modality (auditory/visual) and age (younger/older) and a within-subjects factor of interference (NI/DS/IS). This ANOVA showed a main effect of modality, with greater accuracy in the visual relative to auditory modality (F(1,75)=4.71, p=0.03), a trend towards, but a non-significant main effect of age (F(1,75)=3.42, p=0.07), and a main effect of interference (F(2,150)=46.31, p<0.0001) depicting greater accuracy decrements with secondary task interruptions versus ignored distractions. The ANOVA also showed a modality × interference interaction, such that interference in the auditory domain was more impactful on WM performance than in the visual domain (F(2,150)=9.55, p=0.0001). A modality × age interaction trended towards but did not reach significance (F(1,75)=3.41, p=0.07), suggesting age-related differences in average performance in the visual, but not auditory modality; indeed subsequent within modality ANOVAs supported the presence of an overall age difference in the visual (F(1,37)=7.75, p=0.008) but not auditory modality (p=0.99). Importantly, no other two or three way interactions were significant. Notably, there was no interaction between interference and age (F(2,150)=1.41, p=0.25) or interference × modality × age (F(2,150)=1.39, p=0.26). Moreover, the ANOVA within the visual modality that showed a main effect of age, did not reveal any further age × interference interaction (F(2,74)=0.22, p=0.81). This further confirmed that aside from the general WM accuracy decrement with age of approximately 7%, interference did not exacerbate visual WM to a greater extent in older relative to younger adults.
Figure 2.
Behavioral performance in the (a) auditory and (b) visual experiment tasks, accuracy and RT plotted in the left and right columns, respectively. WM accuracy was maximal during NI followed by DS and least during the IS condition in both younger and older adults, in both auditory and visual modalities. Older adults did not suffer relatively greater impacts of interference on WM performance relative to interference impacts observed in younger adults either for accuracy or RT measures.
Planned t-tests across WM conditions in the auditory modality for both age groups showed greater WM accuracies during NI relative to DS (younger: t(20)=4.80, p=0.0001, older: t(18)=2.96, p=0.008), NI relative to IS (younger: t(20)=6.39, p<0.0001, older: t(18)=5.90, p<0.0001) and DS relative to IS (younger: t(20)=2.96, p=0.008, older: t(18)=3.34, p=0.004). T-tests in the visual modality showed similar results as found in the auditory modality for younger adults: NI>DS (t(19)=2.12, p=0.05), NI>IS (t(19)=4.04, p=0.0007) and DS>IS (t(19)=2.11, p=0.05), and also in older adults for NI>IS (t(18)=2.38, p=0.03) though significance was not reached in older adults for NI relative to DS (t(18)=0.52, p=0.6) or DS relative to IS (t(18)=1.72, p=0.1). Additionally, polynomial contrasts showed significant linear trends from NI to DS to IS in both modalities and age groups (auditory, younger: F(1,20)=40.83, p<0.0001, older: F(1,18)=34.81, p<0.0001, visual, younger: F(1,19)=6.32, p=0.0007, older: F(1,18)=5.66, p=0.03).
RTs in the WM conditions (NI, DS, IS) were analyzed in ANOVAs similar to the accuracy data analysis (Figure 2, right column). The ANOVA showed a main effect of modality with slower RTs in the auditory versus visual modality (F(1,75)=23.51, p<0.0001), a main effect of age with slower RTs in older adults (F(1,75)=16.96, p<0.0001), and a main effect of interference with slower RTs in the IS relative to NI and DS conditions (F(2,150)=3.10, p=0.05). A modality × interference interaction emerged suggesting a different trend of RTs across interference conditions in the auditory versus visual modality (F(2,150)=3.85, p=0.02), parsed further in t-tests below. No interactions with age were found to be significant (age × modality: F(1,75)=1.3, p=0.26, age × interference: F(2,150)=0.24, p=0.79, age × modality × interference: F(2,150)=0.99, p=0.37). Finally, RTs in the baseline condition were faster than average RTs observed in the WM conditions in both modalities and age groups (baseline RT speeding relative to NI WM RT, auditory: younger: 155 ± 42 msec, older: 152 ± 55 msec, visual: younger: 353 ± 28 msec, older: 522 ± 37 msec).
T-tests across WM RTs in the auditory modality for both age groups showed slower RTs during IS relative to NI (younger: t(20)=2.69, p=0.01, older: t(18)=2.27, p=0.04), but not for DS relative to NI (younger: p=0.16, older: p=0.26) or IS relative to DS (younger: p=0.39, older: p=0.92). T-tests in the visual modality did not reveal RT differences across WM interference conditions for younger adults, while older adults showed significant RT speeding during DS relative to NI (t(18)=2.45, p=0.03) and no other significant RT results.
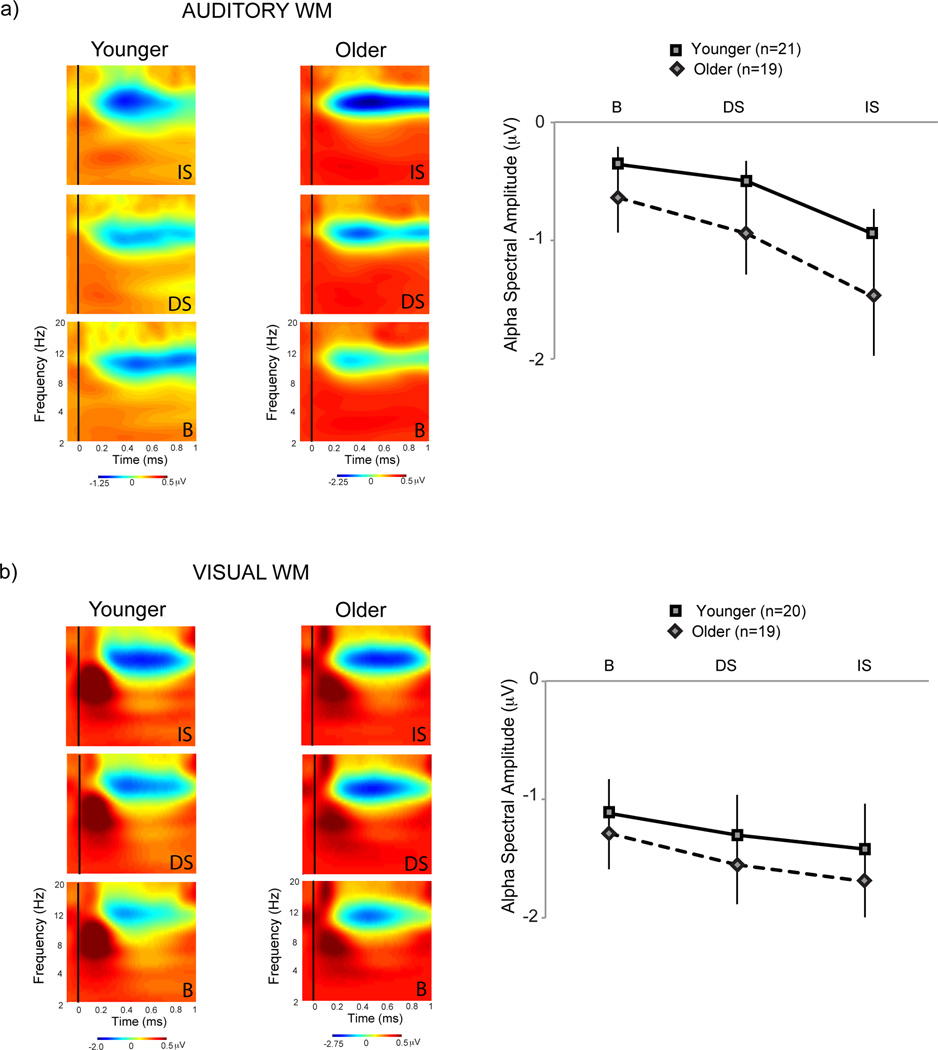
Spectral Responses
Alpha frequency band (8–14Hz) spectral measures time-locked to the onset of the interfering stimuli showed differential modulations across the DS, IS and B conditions. Significant post-stimulus alpha desynchronization was observed, which appeared maximal in the IS condition for both modalities and age groups (Figure 3). Akin to the behavioral analysis, a 2 × 2 × 3 repeated measures ANOVA, with between-subjects factors of modality (auditory/visual) and age (younger/older) and a within-subjects factor of interference (B/DS/IS), was conducted on the average alpha spectral amplitudes within the sensory specific time-windows as stated in the methods. This ANOVA showed a highly significant main effect of interference (F(2,150)=19.35, p<0.0001), a main effect of modality (F(1,75)=3.87, p=0.05), and no main effect of age (F(1,75)=1.20, p=0.28). No two or three way interactions with age were found to be significant (age × modality: F(1,75)=0.10, p=0.75, age × interference: F(2,150)=0.54, p=0.58, age × modality × interference: F(2,150)=0.09, p=0.92), though a trend towards significance was observed for the modality × interference interaction showing that the modulation of alpha spectral amplitudes across interference conditions may differ in the two modalities (F(2,150)=2.92, p=0.06).
Figure 3.
Alpha spectral amplitude modulations to the interfering stimuli in the (a) auditory and (b) visual tasks. Post-stimulus alpha desynchronization was maximal to the secondary task-based interrupting stimulus (IS) in both modalities and both age groups.
Planned t-tests in the auditory modality showed greater post-stimulus alpha desynchronization during IS relative to B for both age groups (younger: t(20)=2.77, p=0.01, older: t(18)=3.56, p=0.002). Alpha desynchronization during IS was also greater relative to DS in younger adults (t(20)=3.06, p=0.006), with a similar trend in older adults (t(18)=1.88, p=0.07). Alpha desynchronization during DS did not significantly differ from B (younger: t(20)=1.19, p=0.25, older: t(18)1.56, p=0.14). T-tests in the visual modality also showed significantly greater alpha desynchronization during IS relative to B (younger: t(19)=2.21, p=0.04, older: t(18)=2.10, p=0.05). In the visual modality, however, IS relative to DS differences did not emerge (younger: t(19)=1.30, p=0.21, older: t(18)=0.87, p=0.40). Significantly greater alpha desynchronization was observed in DS relative to B (younger: t(19)=2.38, p=0.03, older: t(18)=2.36, p=0.03).
The above post-stimulus alpha desynchronization measures were all normalized with respect to a pre-stimulus baseline in each participant. A 2 × 2 × 3 ANOVA with factors of modality (auditory/visual), age (younger/older) and interference (B/DS/IS) was also conducted on the ongoing pre-stimulus alpha data. A main effect of modality showed greater pre-stimulus alpha in the auditory versus visual modality (F(1,75)=4.22, p=0.04), and a main effect of interference showed less pre-stimulus alpha in the IS relative to B and DS conditions (F(2,150)=3.91, p=0.02). Less pre-stimulus alpha during IS suggests generally greater attention during this condition. Enhanced post-stimulus alpha desynchronization during IS relative to B, as found above, suggests even greater attentional allocation once the interrupting stimulus is presented. The greater post-stimulus alpha desynchronization result during IS above, is distinct from and cannot be simply accounted for by the pre-stimulus alpha modulations. Finally, similar to the post-stimulus alpha results, pre-stimulus alpha was also devoid of any main effect of age (p=0.87) or any age interaction with modality (p=0.48), with interference (p=0.37) or any 3-way interaction between these factors (p=0.74).
Peak alpha desynchronization (or minimum alpha) latencies were also tested in the post-stimulus time windows specified in the methods, using 2 × 2 × 3 repeated measures ANOVA, with between-subjects factors of modality (auditory/visual) and age (younger/older) and within-subjects factor of interference (B/DS/IS). This ANOVA showed a main effect of modality (F(1,75)=5.66, p=0.02) and a main effect of interference (F(2,150)=5.85, p=0.004), but no main effect of age (F(1,75)=1.22, p=0.27) and no significant two/three way factor interactions. The main effect of modality was driven by earlier minimum alpha latencies in the auditory relative to visual modality (auditory: 440±19 msec, visual: 473±18 msec), while the main effect of interference was due to longer minimum alpha latencies in the IS relative to DS and B conditions (IS: 477±19 msec, DS: 451±17 msec, B: 441±19 msec). Within modality and within age group t-test comparisons of minimum alpha latencies between interference conditions did not yield further significant results. Overall, while all alpha measures, post-stimulus desynchronization amplitude and latency as well as pre-stimulus ongoing alpha showed interference impacts, none showed an effect of age. This suggested general alpha-based neural preservation in the face of general age-related behavioral impacts of reduced accuracy in the visual modality and generally slower RTs.
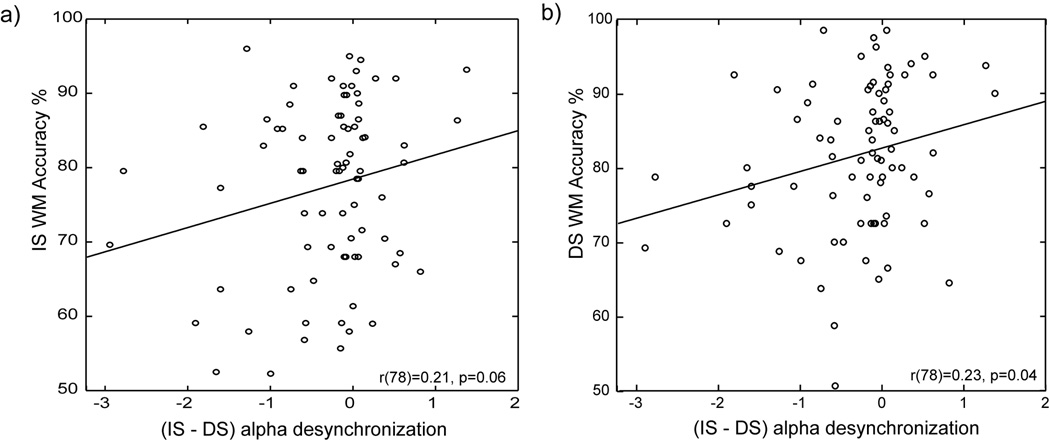
Neurobehavioral correlations between absolute alpha measures, spectral desynchronization/minimum alpha latencies, and WM performance accuracies/RTs, using Pearson's product moment correlations, were not found. There were also no neurobehavioral correlations with baseline subtracted WM RT indices. However, weak but significant correlations were observed between the degree of alpha spectral amplitude modulation for the interfering stimuli from DS to IS, and WM accuracy during IS (r(78)=0.21, p=0.06) and during DS (r(78)=0.23, p=0.04) across all 79 participants; individuals who showed greater alpha desynchronization modulations during interference exhibited poorer WM accuracies in the interfering DS and IS conditions (Fig. 4).
Figure 4.
Neurobehavioral correlations between the degree of alpha desynchronization modulated on interfering stimuli (IS-DS) and WM performance on the (a) IS and (b) DS tasks across all participants.
As Clapp et al. (2011) previously showed differential prefrontal neural modulations in response to different interference conditions, we also investigated prefrontal neural activations in the form of post-stimulus theta activations that have a midline frontal topography. No observable differences in frontal theta power modulation emerged between the B, DS and IS interference conditions.
DISCUSSION
Here, we used WM performance and neuro-oscillatory alpha band desynchronization measures to characterize the influence of intrasensory interference on auditory and visual motion based working memory in younger and older adults. For both auditory and visual modalities and in both age groups, interference in the form of ignored distractions and secondary task interruptions disrupted WM performance, with more negative impacts observed during interruptions relative to distracted WM. Neural oscillations in the alpha frequency range served to index the impact of interference via heightened post-stimulus desynchronization to interruptions, which was consistently observed across age and modality. Using face and scene based complex visual object stimuli as both WM stimuli and interference stimuli, our group previously showed that older adults experience greater interference-driven WM disruptions relative to younger adults (Clapp et al., 2010, 2011, Clapp and Gazzaley, 2012). Based on these findings, we initially hypothesized that interference effects would be globally exacerbated with aging, even for motion-based stimuli. In contrast, here we find that for auditory and visual motion, aging does not differentially impact WM interference effects by motion stimuli, at least at these WM loads.
That we did not find greater behavioral impacts of motion-based interference in WM across age groups for either the auditory or visual modality was unexpected given prior WM interference findings (Clapp et al., 2010, 2011, Clapp and Gazzaley, 2012). Older adults are known to have deficits in visual motion processing, although recent evidence suggests these age-related deficits may be specific to certain stimulus conditions, with some conditions even revealing enhanced global motion perception in older relative to younger adults (see Hutchinson et al., 2012 for a critical review). In this study, we did find visual motion discrimination thresholds, in the absence of WM, to be significantly worse (by a magnitude of 37%) in older relative to younger adults. Further, general visual motion-based WM, but not as a function of interference, was found to be deficient in aging (by a magnitude of 7%). Our WM findings are in line with prior age-related visual WM research that did not include an interference manipulation (Nielsen-Bohlman and Knight, 1995, Albert, 1997, Muller and Knight, 2002, Chen and Naveh-Benjamin, 2012). Also, it seems that the age effects observed for both visual motion thresholding and visual motion WM are independent given that the WM task probes (on cue-probe non-match trials) were presented at easier discrimination angles (>30°), beyond the perceptual threshold in all participants. Overall, the present study uniquely adds to the literature by showing that visual motion-based distractions and secondary task interruptions worsen motion-based WM performance in older adults, but not out of proportion to their impact in younger adults. Our results demonstrate relative preservation of the ability to contend with motion interference in older adults, highlighting an additional aspect of motion processing that seems to be unaffected by aging. Visual motion is processed by the dorsal processing stream separate from the ventral stream; the dorsal stream has faster stimulus response times and is less impacted by stimulus attention than the ventral stream (Mehta et al., 2000, Chen et al., 2007). These anatomical and physiological processing stream differences may also contribute to the different results in our study with motion stimuli vs. prior WM findings with complex object stimuli.
In the auditory domain, many studies suggest that intrasensory distractions do not differentially affect older compared to younger adults (Murphy et al., 1999, Schneider et al., 2000, Li et al., 2004). In all of these studies, as in ours, older adults were pre-screened to have normal or corrected-to-normal hearing. This is important as other studies in the literature have reported auditory interference-related deficits, but did not exclude older adults with impaired peripheral hearing. Auditory distractions have also been studied in the context of the irrelevant sound effect, wherein visual serial recall is probed in the presence of irrelevant speech distractions. This paradigm differs methodologically in the presentation of cross-modal distractions (auditory distractions in a visually presented WM task), but again older adults were not found to perform worse than younger adults (Belleville et al., 2003, Beaman 2005, Bell and Buchner, 2007, VanGerven et al., 2007). Here, we add to and extend this evidence by showing that intrasensory auditory distractions, as well as intrasensory auditory secondary task-based interruptions – investigated for the first time in older adults, do not generate differential WM impacts in aging.
It is important to note that although we find no age-differential impacts of interference on auditory/visual motion based WM performance, we cannot rule out the possibility that deteriorated performance with aging would emerge at higher cognitive loads, either higher memory or interference load (Groth and Allen, 2000, Gazzaley, 2011). For instance, Gazzaley et al. (2007) showed that age-related impairments of WM exclusively emerge during the combination of high memory load and distraction. Other manipulations that increase WM task challenges, such as longer delay intervals, might also result in interference effects that are out of proportion for older adults. Consistent with this, Chao and Knight (1997) showed that multiple auditory tone distractors presented during a 9 sec or longer, but not shorter, delay interval did indeed generate worse auditory WM in older adults. Meijer et al. (2006) showed similar findings using a sequence of auditory distractions, presented either rapidly or slowly, with greater age-related impacts on WM observed for the more challenging rapid distractor presentations. These studies raise the possibility that higher interference loads in the form of greater number of interfering stimuli, greater stimulus congruity and hence confusability between the WM and interference stimuli, and greater complexity of the interference task may indeed show differentially greater impacts on WM performance in older relative to younger adults. On the other hand, none of the previous studies that manipulated cognitive load (interference load or memory load) used perceptually thresholded stimuli as in the current study. The original studies by Clapp et al. (2010, 2011, Clapp and Gazzaley, 2012), which formed the bases of our experiments, used face and scene stimuli for which no perceptual thresholding was performed. Note that these studies found differential interference effects in aging while employing relatively low memory and interference loads, i.e., single item memory and single item interference. Also note that here we employed motion stimuli to serve as both memory and interference stimuli, but it is possible that a combination of object and motion stimuli, one type of stimulus serving as the WM stimulus and the other as the interference stimulus, may reveal WM interference impacts in aging. Thus, it is entirely possible that our results of preserved WM performance in aging are due to low memory and interference loads combined with perceptually-thresholded and exclusively motion stimulus presentations across age groups. The extent of cognitive load for perceptually-thresholded auditory/visual motion stimuli, which may cause greater interference-compromised WM performance in older relative to younger adults, needs to be clarified in future studies. Interestingly, our results emphasize a tight link between sensory and cognitive function with age as found in prior large scale aging studies (Berlin Aging Study: Baltes and Lindenberger, 1997); i.e., cognitive functions, such as WM interference effects, are preserved with aging when sensory perceptual differences are equated for.
In addition to WM performance, we also investigated the neural bases common to the interference effects observed in the auditory and visual domains. Alpha frequency band modulations relative to baseline consistently showed greatest post-stimulus alpha desynchronization for the secondary task interrupters in both younger and older adults and in both auditory and visual modalities. Enhanced alpha desynchronization has been commonly observed for attended stimuli (Muller and Keil, 2004, Gazzaley et al., 2008, Zanto et al., 2010, Mishra et al., 2012). Ongoing alpha oscillations have been inferred to inhibit stimulus processing, and attended stimuli release this inhibition via suppression or desynchronization of ongoing alpha oscillations (Klimesch et al., 2007). That most alpha desynchronization was observed to the attended interfering stimulus during the interrupted WM task is in line with the above findings. During distracted WM in our experiment, alpha desynchronization to the distractors either matched levels observed during passive listening in the auditory modality, or was also significantly greater relative to passive viewing in the visual modality. While this is not in line with findings in the attention literature, that of increased alpha levels or alpha synchronization for unattended stimuli (Kelly et al., 2006, Rihs et al., 2007, Händel et al., 2011), these prior studies investigated visuo-spatially irrelevant, unattended stimuli interspersed in a stream of attended relevant stimuli presented to the opposite hemifield. In our case, the distractor stimulus appeared alone and in the same spatial location as the attended stimuli, while participants internally retained the previously presented cue stimulus in memory. It is possible then that in the absence of other externally attended stimuli in our task, inattention to the isolated distractor was not optimal and associated alpha synchronization (or reduced alpha desynchronization relative to baseline) was not observed. The accuracy and RT performance statistics were also more variable during the distracted WM condition, further suggesting that this singular distractor may not be sufficiently unattended by all participants. Again, as discussed above, we hypothesize that at higher cognitive loads, distractor impacts on WM may become more prominent in behavior and in alpha-based neural synchrony. Of note, we did find that the degree of attention-related alpha modulation during interference, i.e. greater alpha desynchronization to the secondary task interrupter versus unattended distractor, significantly correlated with the extent of negative performance impacts on WM accuracy. This neurobehavioral correlation suggests that minimizing neural responses to interfering stimuli optimizes WM performance.
The age-invariance of the neural findings, i.e., similar extents of alpha modulation in younger and older adults, has been previously observed in a WM task setting (Gazzaley et al., 2008). However, other recent evidence suggests that older adults show deficits in modulation of WM-related alpha processing under varying memory loads (Sander et al., 2012, Vaden et al., 2012, Zanto et al., 2011). Older relative to younger adults have also been noted to exhibit latency differences in alpha (Zanto et al., 2010) and in non-alpha-based ERP processing in WM tasks (Jost et al., 2010, Störmer et al., 2013). That we did not observe different extents of alpha modulation with aging could be due to differences in the time range of alpha analyzed across studies. Specifically, we primarily focused on interference-related post-stimulus alpha desynchronization, while others have investigated pre-stimulus anticipatory alpha (Vaden et al., 2012, Zanto et al., 2011) or alpha during the WM maintenance but not stimulus-encoding period (Sander et al., 2012). Of note, ours was the only study that undertook perceptual thresholding of interference stimuli in each individual in either age group. That equivalent alpha modulations are obtained across age groups due to use of perceptually thresholded stimuli is certainly a possibility; this would underscore the benefits of a thresholding approach, yet needs to be verified in future research.
Overall, our study contributes to advancing our neurobehavioral understanding of how different types of intrasensory interference, distractions and secondary task interruptions, interact with WM in the auditory and visual domain. It shows that these interactions are not influenced by aging, at least not for perceptually-equivalent motion stimuli at low WM loads. While external interference diminished WM performance in both modalities, older adults did not suffer greater behavioral impacts in either accuracies or response times relative to younger adults. However, we refrain from overly broad generalization of our findings, as higher cognitive loads paired with real-world motion stimuli may reveal aging effects of intrasensory WM interference, and thus needs to be investigated in future studies. Finally, we show evidence for preserved alpha band neuro-oscillatory mechanisms across age, in ongoing pre-stimulus oscillations and in post-stimulus desynchronization, revealing that the behavioral preservation in aging does not seem to be due to additional compensatory neural processes (Grady, 2012).
HIGHLIGHTS.
Intrasensory interference from motion stimuli degrades working memory.
Motion interference impacts are observed in the auditory and visual domain.
Young and older adults are equally impacted by motion interference.
Alpha oscillations reveal preserved motion interference processing across age.
ACKNOWLEDGEMENTS
This work was supported by the National Institute of Health grants 5R01AG030395 (AG) and 5R24TW007988-05 subaward VUMC38412 (JM), and the Sandler Program for Breakthrough Biomedical Research grant (JM). We would like to thank Anne Berry, Pin-wei Chen, Ariane Ling and Melissa Nasiruddin for their assistance with data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
J.M., T.Z. and A.G. conceptualized the study. J.M., T.Z. and A.N. performed data collection and analysis. J.M. drafted the manuscript. J.M., T.Z., A.N. and A.G. edited and revised the manuscript.
The authors declare no competing financial interests.
REFERENCES
- Alain C, Woods DL. Age-related changes in processing auditory stimuli during visual attention: evidence for deficits in inhibitory control and sensory memory. Psychology and aging. 1999;14(3):507–519. doi: 10.1037//0882-7974.14.3.507. [DOI] [PubMed] [Google Scholar]
- Albert MS. The ageing brain: normal and abnormal memory. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 1997;352(1362):1703–1709. doi: 10.1098/rstb.1997.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrés P, Parmentier FBR, Escera C. The effect of age on involuntary capture of attention by irrelevant sounds: a test of the frontal hypothesis of aging. Neuropsychologia. 2006;44(12):2564–2568. doi: 10.1016/j.neuropsychologia.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working memory: looking back and looking forward. Nature reviews. Neuroscience. 2003;4(10):829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- Baltes PB, Lindenberger U. Emergence of a powerful connection between sensory and cognitive functions across the adult life span: a new window to the study of cognitive aging? Psychology and aging. 1997;12(1):12–21. doi: 10.1037//0882-7974.12.1.12. [DOI] [PubMed] [Google Scholar]
- Beaman P. European Journal of Cognitive Psychology. 2. Vol. 17. Psychology Press; 2005. Irrelevant sound effects amongst younger and older adults: Objective findings and subjective insights; pp. 241–265. [Google Scholar]
- Bell R, Buchner A. Equivalent irrelevant-sound effects for old and young adults. Memory & cognition. 2007;35(2):352–364. doi: 10.3758/bf03193456. [DOI] [PubMed] [Google Scholar]
- Belleville S, Rouleau N, Van der Linden M, Collette F. Effect of manipulation and irrelevant noise on working memory capacity of patients with Alzheimer’s dementia. Neuropsychology. 2003;17(1):69–81. [PubMed] [Google Scholar]
- Berry AS, Zanto TP, Clapp WC, Hardy JL, Delahunt PB, Mahncke HW, Gazzaley A. The Influence of Perceptual Training on Working Memory in Older Adults. In: Rogers N, editor. PLoS ONE. 7. Vol. 5. 2010. p. e11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry AS, Zanto TP, Rutman AM, Clapp WC, Gazzaley A. Practice-related improvement in working memory is modulated by changes in processing external interference. Journal of neurophysiology. 2009;102(3):1779–1789. doi: 10.1152/jn.00179.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LL, Knight RT. Prefrontal deficits in attention and inhibitory control with aging. Cerebral cortex (New York, N.Y. : 1991) 1997;7(1):63–69. doi: 10.1093/cercor/7.1.63. [DOI] [PubMed] [Google Scholar]
- Chaparro A, Wood JM, Carberry T. Effects of age and auditory and visual dual tasks on closed-road driving performance. Optometry and vision science : official publication of the American Academy of Optometry. 2005;82(8):747–754. doi: 10.1097/01.opx.0000174724.74957.45. [DOI] [PubMed] [Google Scholar]
- Chen C-M, Lakatos P, Shah AS, Mehta AD, Givre SJ, Javitt DC, Schroeder CE. Functional anatomy and interaction of fast and slow visual pathways in macaque monkeys. Cerebral cortex. 2007;17(7):1561–1569. doi: 10.1093/cercor/bhl067. [DOI] [PubMed] [Google Scholar]
- Chen T, Naveh-Benjamin M. Assessing the associative deficit of older adults in long-term and short-term/working memory. Psychology and aging. 2012;27(3):666–682. doi: 10.1037/a0026943. [DOI] [PubMed] [Google Scholar]
- Clapp WC, Gazzaley A. Distinct mechanisms for the impact of distraction and interruption on working memory in aging. Neurobiology of aging. 2012;33(1):134–148. doi: 10.1016/j.neurobiolaging.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp WC, Rubens MT, Gazzaley A. Mechanisms of working memory disruption by external interference. Cerebral cortex (New York, N.Y. : 1991) 2010;20(4):859–872. doi: 10.1093/cercor/bhp150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp WC, Rubens MT, Sabharwal J, Gazzaley A. Deficit in switching between functional brain networks underlies the impact of multitasking on working memory in older adults. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(17):7212–7217. doi: 10.1073/pnas.1015297108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiani M, Low KA, Wee E, Sable JJ, Gratton G. Reduced suppression or labile memory? Mechanisms of inefficient filtering of irrelevant information in older adults. Journal of cognitive neuroscience. 2006;18(4):637–650. doi: 10.1162/jocn.2006.18.4.637. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Snyder AC. The Role of Alpha-Band Brain Oscillations as a Sensory Suppression Mechanism during Selective Attention. Frontiers in psychology. 2011;2:154. doi: 10.3389/fpsyg.2011.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A. Influence of early attentional modulation on working memory. Neuropsychologia. 2011;49(6):1410–1424. doi: 10.1016/j.neuropsychologia.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Clapp W, Kelley J, McEvoy K, Knight RT, D’Esposito M. Age-related top-down suppression deficit in the early stages of cortical visual memory processing. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(35):13122–13126. doi: 10.1073/pnas.0806074105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D’Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nature neuroscience. 2005;8(10):1298–1300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- Gazzaley A, Sheridan MA, Cooney JW, D’Esposito M. Age-related deficits in component processes of working memory. Neuropsychology. 2007;21(5):532–539. doi: 10.1037/0894-4105.21.5.532. [DOI] [PubMed] [Google Scholar]
- Van Gerven PWM, Meijer WA, Vermeeren A, Vuurman EF, Jolles J. The irrelevant speech effect and the level of interference in aging. Experimental aging research. 2007;33(3):323–339. doi: 10.1080/03610730701319145. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends in Neurosciences. 1992;15(1):20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Grady C. The cognitive neuroscience of ageing. Nature reviews. Neuroscience. 2012;13(7):491–505. doi: 10.1038/nrn3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graewe B, Lemos R, Ferreira C, Santana I, Farivar R, De Weerd P, Castelo-Branco M. Impaired Processing of 3D Motion-Defined Faces in Mild Cognitive Impairment and Healthy Aging: An fMRI Study. Cerebral cortex (New York, N.Y. : 1991) 2012 doi: 10.1093/cercor/bhs246. [DOI] [PubMed] [Google Scholar]
- Groth KE, Allen PA. Visual attention and aging. Frontiers in bioscience : a journal and virtual library. 2000;5:D284–D297. doi: 10.2741/groth. [DOI] [PubMed] [Google Scholar]
- Guerreiro MJS, Murphy DR, Van Gerven PWM. Making sense of age-related distractibility: The critical role of sensory modality. Acta psychologica. 2013;142(2):184–194. doi: 10.1016/j.actpsy.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Händel BF, Haarmeier T, Jensen O. Alpha Oscillations Correlate with the Successful Inhibition of Unattended Stimuli. Journal of cognitive neuroscience. 2011;23(9):2494–2502. doi: 10.1162/jocn.2010.21557. [DOI] [PubMed] [Google Scholar]
- Hutchinson CV, Arena A, Allen HA, Ledgeway T. Psychophysical correlates of global motion processing in the aging visual system: a critical review. Neuroscience and biobehavioral reviews. 2012;36(4):1266–1272. doi: 10.1016/j.neubiorev.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Jost K, Bryck RL, Vogel EK, Mayr U. Are old adults just like low working memory young adults? Filtering efficiency and age differences in visual working memory. Cerebral cortex. 2011;21(5):1147–1154. doi: 10.1093/cercor/bhq185. [DOI] [PubMed] [Google Scholar]
- Kelly SP, Lalor EC, Reilly RB, Foxe JJ. Increases in alpha oscillatory power reflect an active retinotopic mechanism for distracter suppression during sustained visuospatial attention. J Neurophysiol. 2006;95(6):3844–3851. doi: 10.1152/jn.01234.2005. [DOI] [PubMed] [Google Scholar]
- Kiebel SJ, Tallon-Baudry C, Friston KJ. Parametric analysis of oscillatory activity as measured with EEG/MEG. Hum Brain Mapp. 2005;26(3):170–177. doi: 10.1002/hbm.20153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Smith PE, Grigsby SS, Vingrys AJ, Benes SC, Supowit A. Efficient and unbiased modifications of the QUEST threshold method: theory, simulations, experimental evaluation and practical implementation. Vision research. 1994;34(7):885–912. doi: 10.1016/0042-6989(94)90039-6. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res Rev. 2007;53(1):63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Kuba M, Kremláček J, Langrová J, Kubová Z, Szanyi J, Vít F. Aging effect in pattern, motion and cognitive visual evoked potentials. Vision research. 2012;62:9–16. doi: 10.1016/j.visres.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Lakatos P, Shah AS, Knuth KH, Ulbert I, Karmos G, Schroeder CE. An oscillatory hierarchy controlling neuronal excitability and stimulus processing in the auditory cortex. J Neurophysiol. 2005;94(3):1904–1911. doi: 10.1152/jn.00263.2005. [DOI] [PubMed] [Google Scholar]
- Li L, Daneman M, Qi JG, Schneider BA. Does the information content of an irrelevant source differentially affect spoken word recognition in younger and older adults? Journal of experimental psychology. Human perception and performance. 2004;30(6):1077–1091. doi: 10.1037/0096-1523.30.6.1077. [DOI] [PubMed] [Google Scholar]
- Mehta AD, Ulbert I, Schroeder CE. Intermodal selective attention in monkeys. I: distribution and timing of effects across visual areas. Cerebral cortex. 2000;10(4):343–358. doi: 10.1093/cercor/10.4.343. [DOI] [PubMed] [Google Scholar]
- Meijer WA, De Groot RHM, Van Boxtel MPJ, Van Gerven PWM, Jolles J. Verbal learning and aging: combined effects of irrelevant speech, interstimulus interval, and education. The journals of gerontology. Series B, Psychological sciences and social sciences. 2006;61(5):P285–P294. doi: 10.1093/geronb/61.5.p285. [DOI] [PubMed] [Google Scholar]
- Mishra J, Martínez A, Schroeder CE, Hillyard SA. Spatial attention boosts short-latency neural responses in human visual cortex. NeuroImage. 2012;59(2):1968–1978. doi: 10.1016/j.neuroimage.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller MM, Keil A. Neuronal synchronization and selective color processing in the human brain. Journal of cognitive neuroscience. 2004;16(3):503–522. doi: 10.1162/089892904322926827. [DOI] [PubMed] [Google Scholar]
- Müller NG, Knight RT. Age-related changes in fronto-parietal networks during spatial memory: an ERP study. Brain research. Cognitive brain research. 2002;13(2):221–234. doi: 10.1016/s0926-6410(01)00119-7. [DOI] [PubMed] [Google Scholar]
- Murphy DR, McDowd JM, Wilcox KA. Inhibition and aging: similarities between younger and older adults as revealed by the processing of unattended auditory information. Psychology and aging. 1999;14(1):44–59. doi: 10.1037//0882-7974.14.1.44. [DOI] [PubMed] [Google Scholar]
- Nielsen-Bohlman L, Knight RT. Prefrontal alterations during memory processing in aging. Cerebral cortex (New York, N.Y. : 1991) 5(6):541–549. doi: 10.1093/cercor/5.6.541. (n.d.). [DOI] [PubMed] [Google Scholar]
- Osterrieth P. Le test de copie d’une figure complexe. Archiv Psychologie. 1944;30:206–356. [Google Scholar]
- Parmentier FBR, Andrés P. The involuntary capture of attention by sound: novelty and postnovelty distraction in young and older adults. Experimental psychology. 2010;57(1):68–76. doi: 10.1027/1618-3169/a000009. [DOI] [PubMed] [Google Scholar]
- Passow S, Westerhausen R, Wartenburger I, Hugdahl K, Heekeren HR, Lindenberger U, Li S-C. Human aging compromises attentional control of auditory perception. Psychology and aging. 2012;27(1):99–105. doi: 10.1037/a0025667. [DOI] [PubMed] [Google Scholar]
- Rey A. L’examen psychologique dans les cas d’encephalopathie traumatique. Archiv Psychologie. 1941;28:286–340. [Google Scholar]
- Rihs TA, Michel CM, Thut G. Mechanisms of selective inhibition in visual spatial attention are indexed by alpha-band EEG synchronization. Eur J Neurosci. 2007;25(2):603–610. doi: 10.1111/j.1460-9568.2007.05278.x. [DOI] [PubMed] [Google Scholar]
- Sakai K. Reactivation of memory: role of medial temporal lobe and prefrontal cortex. Reviews in the neurosciences. 2003;14(3):241–252. doi: 10.1515/revneuro.2003.14.3.241. [DOI] [PubMed] [Google Scholar]
- Sakai K, Passingham RE. Prefrontal selection and medial temporal lobe reactivation in retrieval of short-term verbal information. Cerebral cortex (New York, N.Y. : 1991) 2004;14(8):914–921. doi: 10.1093/cercor/bhh050. [DOI] [PubMed] [Google Scholar]
- Salvucci DD, Taatgen NA. Threaded cognition: an integrated theory of concurrent multitasking. Psychological review. 2008;115(1):101–130. doi: 10.1037/0033-295X.115.1.101. [DOI] [PubMed] [Google Scholar]
- Sander MC, Werkle-Bergner M, Lindenberger U. Amplitude modulations and inter-trial phase stability of alpha-oscillations differentially reflect working memory constraints across the lifespan. NeuroImage. 2012;59(1):646–654. doi: 10.1016/j.neuroimage.2011.06.092. [DOI] [PubMed] [Google Scholar]
- Schneider BA, Daneman M, Murphy DR, See SK. Listening to discourse in distracting settings: the effects of aging. Psychology and aging. 2000;15(1):110–125. doi: 10.1037//0882-7974.15.1.110. [DOI] [PubMed] [Google Scholar]
- Sommers MS, Danielson SM. Inhibitory processes and spoken word recognition in young and older adults: the interaction of lexical competition and semantic context. Psychology and aging. 1999;14(3):458–472. doi: 10.1037//0882-7974.14.3.458. [DOI] [PubMed] [Google Scholar]
- Sreenivasan KK, Jha AP. Selective attention supports working memory maintenance by modulating perceptual processing of distractors. Journal of cognitive neuroscience. 2007;19(1):32–41. doi: 10.1162/jocn.2007.19.1.32. [DOI] [PubMed] [Google Scholar]
- Störmer VS, Li S-C, Heekeren HR, Lindenberger U. Normative shifts of cortical mechanisms of encoding contribute to adult age differences in visual-spatial working memory. NeuroImage. 2013;73:167–175. doi: 10.1016/j.neuroimage.2013.02.004. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- Tallon-Baudry C, Bertrand O, Peronnet F, Pernier J. Induced gamma-band activity during the delay of a visual short-term memory task in humans. The Journal of neuroscience. 1998;18(11):4244–4254. doi: 10.1523/JNEUROSCI.18-11-04244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KR, Johnson AM, Emerson JL, Dawson JD, Boer ER, Rizzo M. Distracted driving in elderly and middle-aged drivers. Accident; analysis and prevention. 2012;45:711–717. doi: 10.1016/j.aap.2011.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tun PA, O’Kane G, Wingfield A. Distraction by competing speech in young and older adult listeners. Psychology and aging. 2002;17(3):453–467. doi: 10.1037//0882-7974.17.3.453. [DOI] [PubMed] [Google Scholar]
- Tun PA, Wingfield A. One voice too many: adult age differences in language processing with different types of distracting sounds. The journals of gerontology. Series B, Psychological sciences and social sciences. 1999;54(5):P317–P327. doi: 10.1093/geronb/54b.5.p317. [DOI] [PubMed] [Google Scholar]
- Ungerleider L, Mishkin M. Two cortical visual systems. In: Ingle D, Goodale M, Mansfield R, editors. Analysis of Visual Behavior. MIT Press; 1982. pp. 549–586. [Google Scholar]
- Vaden RJ, Hutcheson NL, McCollum LA, Kentros J, Visscher KM. Older adults, unlike younger adults, do not modulate alpha power to suppress irrelevant information. NeuroImage. 2012;63(3):1127–1133. doi: 10.1016/j.neuroimage.2012.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: a preliminary report. Journal of psychiatric research. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Curtis CE, D’Esposito M. Differential effects of distraction during working memory on delay-period activity in the prefrontal cortex and the visual association cortex. NeuroImage. 2006;29(4):1117–1126. doi: 10.1016/j.neuroimage.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Zanto TP, Toy B, Gazzaley A. Delays in neural processing during working memory encoding in normal aging. Neuropsychologia. 2010;48(1):13–25. doi: 10.1016/j.neuropsychologia.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto TP, Pan P, Liu H, Bollinger J, Nobre AC, Gazzaley A. Age-related changes in orienting attention in time. The Journal of neuroscience. 2011;31(35):12461–12470. doi: 10.1523/JNEUROSCI.1149-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]