Abstract
The role of transforming growth factor-β (TGF-β) during tumorigenesis complex and paradoxical, reflecting its ability to function as a tumor suppressor in normal and early-stage cancers, and has a tumor promoter in their late-stage counterparts. The switch in TGF-β function is known as the “TGF-β Paradox,” whose manifestations are intimately linked to the initiation of epithelial-mesenchymal transition (EMT) programs in developing and progressing carcinomas. Indeed, as carcinoma cells emerge from EMT programs stimulated by TGF-β, they readily display a variety of acquired phenotypes that provide a selective advantage to growing carcinomas, including (i) enhanced cell migration and invasion; (ii) heightened resistance to cytotoxic agents, targeted chemotherapeutic, and radiation treatments; and (iv) boosted expansion of cancer-initiating and stem-like cell populations that underlie tumor metastasis and disease recurrence. At present, the molecular, cellular, and microenvironmental mechanisms that enable post-EMT and metastatic carcinoma cells to hijack the oncogenic activities of TGF-β remain incompletely understood. Additionally, the molecular mechanisms that counter EMT programs and limit the aggressiveness of late-stage carcinomas, events that transpire via mesenchymal-epithelial transition (MET) reactions, also need to be further elucidated. Here we review recent advances that provide new insights into how TGF-β promotes EMT programs in late-stage carcinoma cells, as well as how these events are balanced by MET programs during the development and metastatic progression of human carcinomas.
Keywords: Epithelial plasticity, Metastasis, TGF-β
1. Introduction
Transforming growth factor-β (TGF-β) is the prototypical member of a large family of structurally-related and multifunctional cytokines, including the activins and inhibins, bone morphogenetic proteins (BMPs), Nodal, and growth and differentiation factors [1; 2]. TGF-β regulates a vast array of physiological processes ranging from embryonic development and tissue morphogenesis to cell differentiation and survival to cellular and organ homeostasis; it also inhibits the proliferation of epithelial, endothelial, and hematopoietic cell lineages [3; 4]. Aberrant TGF-β signaling has also been linked to the initiation, development, and progression of a variety of human pathologies, including fibrosis, autoimmune diseases, and cancer [1]. With respect to human malignancies, TGF-β typically exerts tumor suppressing activities in normal cells, as well as in early-stage carcinomas through its ability to induce cell cycle arrest and apoptotic reactions. Interestingly, as carcinomas continue to evolve and ultimately acquire metastatic phenotypes, the tumor suppressing functions of TGF-β are circumvented such that TGF-β is utilized as an oncogenic factor that promotes carcinoma growth, invasion, and metastasis. The paradoxical switch in TGF-β function during tumorigenesis has been linked to the ability of TGF-β to induce epithelial-mesenchymal transition (EMT) programs [2; 3; 4; 5], which reflect an evolutionarily cascade in which polarized epithelial cells adopt mesenchymal cell characteristics that include (i) reduced apicobasolateral polarity and cell adhesion; (ii) enhanced chemoresistance and evasion from host immunosurveillance; (iii) expanded stem-like and tumor-initiating activities; (iv) elevated resistance to apoptotic stimuli; and (v) acquired migratory, invasive, and metastatic phenotypes [5; 6]. During its induction of EMT programs, TGF-β signaling ultimately converges in the nucleus to regulate the expression and activity of a variety of master EMT transcription factors operant in maintaining EMT reactions. Amongst the EMT transcription factors targeted by TGF-β are members of the Snail (SNAI1 and SNAI2/Slug), ZEB (ZEB1 and ZEB2/SIP1), basic helix-loop-helix (Twist1 and Twist2), Six family of homeobox (Six1), and Forkhead (FOXC2), as well as members of the High Mobility Group proteins (HMG2a), which modify DNA structure to enhance transcription factor binding [3]. Recently, EMT reactions have been subcategorized into three distinct programs, including (i) Type 1 EMT, which transpires during embryonic development of the endocardial cushion, neural crest, and closure and fusion of the palate; (ii) Type 2 EMT, which transpires during tissue remodeling, wound healing, and fibrosis; and (iii) Type 3 EMT, which transpires during tumor metastasis [7]. In addition, EMT programs are countered and reversed by mesenchymal-epithelial transitions, which also play essential roles during embryogenesis and tissue morphogenesis, as well as during carcinoma progression and metastatic outgrowth [8]. TGF-β is a master regulator of all EMT subtypes and readers desiring a more thorough summary of the mechanisms whereby TGF-β drives EMT programs are directed to several comprehensive reviews [5; 9; 10]. Here we discuss recent findings related to the paradoxical role of TGF-β in regulating oncogenic Type 3 EMT-MET programs, as well as its function in creating EMT-permissive microenvironments during carcinoma development and metastatic progression.
2. TGF-β Signaling
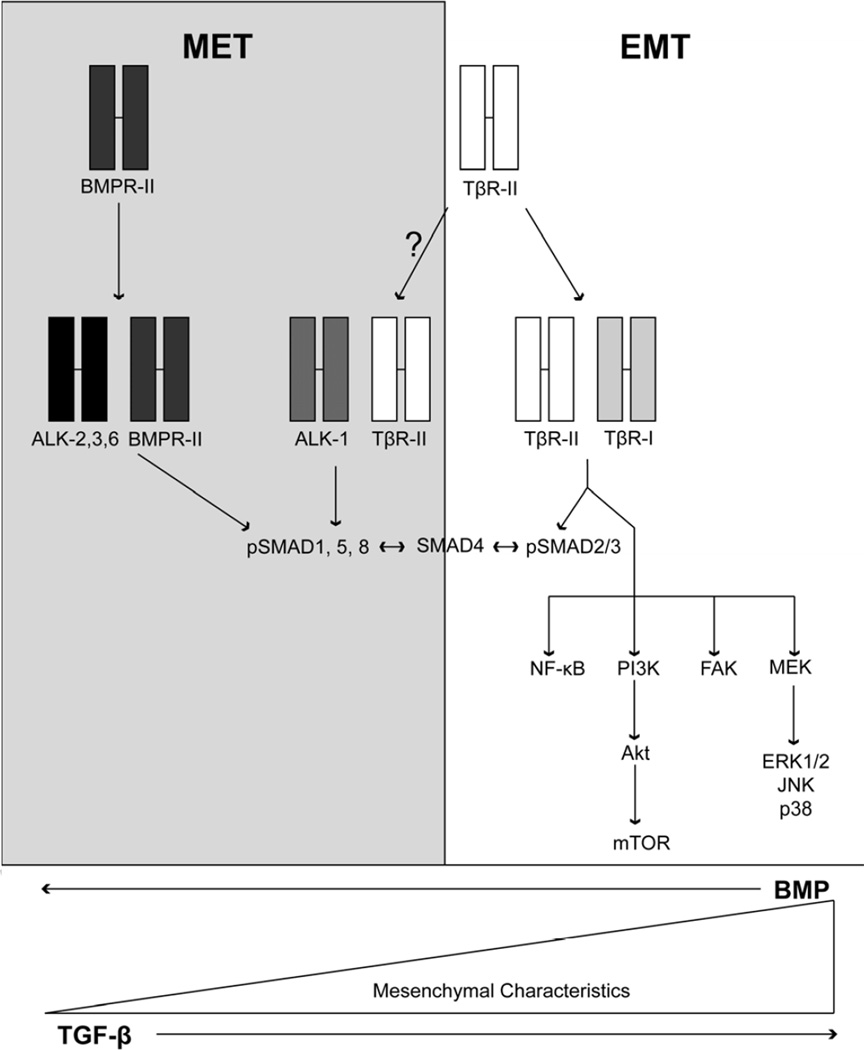
Mammals express three genetically distinct TGF-β ligands (TGF-βs 1–3), whose mature and biologically active forms are ~97% identical and exhibit redundant activities in vitro [2; 11]. TGF-β signaling commences upon binding to its high-affinity receptors, namely the TGF-β type I (TβR-I), type II (TβR-II), and type III (TβR-III or betaglycan) receptors [2; 11]. TβR-I and TβR-II both contain serine/threonine protein kinases in their cytoplasmic domains that produce intracellular signals in response to TGF-β. In contrast, the cytoplasmic domain of TβR-III lacks intrinsic protein kinase activity; however, this TGF-β receptor is highly expressed in cells and modulates the binding and presentation of TGF-β to its signaling receptors, as well as functions as a tumor suppressor in a variety of tissues, including the breast, ovary, prostate, lung, pancreas, and kidney [12]. The binding of TGF-β to TβR-II results in its transphosphorylation and activation of TβR-I, which phosphorylates and activates the latent transcription factors, Smad2 and Smad3 (Fig. 1). Once activated, Smad2/3 form heteromeric complexes with the common Smad, Smad4, which accumulate en masse in the nucleus to govern gene transcription, an event referred to as “canonical” TGF-β signaling. Recent evidence also indicates that TGF-β receptors can activate the BMP-regulated Smads, Smad1/5/8 (Fig. 1), leading to the acquisition of migratory and invasive phenotypes in carcinomas [13], and to the induction of proliferative and migratory phenotypes in endothelial cells (Fig. 1) [14; 15]; [16; 17]. The precise mechanisms and functional consequences of this unconventional coupling remain to be elucidated. Importantly, the diversity of canonical TGF-β signaling is mediated in part via the ability of Smad2/3/4 complexes to interact physically with a variety of transcriptional activators or repressors that are expressed in a cell- and context-specific manner. Canonical Smad2/3 signaling is also regulated by their being targeted by several adapter molecules, including SARA, Hgs, and Dab2 [5; 11], and by the inhibitory Smad, Smad7, which inhibits Smad2/3 phosphorylation by TβR-I and induces its internalization and degradation [5; 11].
Figure 1.
Schematic of TGF-β and BMP signaling pathways coupled to EMT and MET programs, respectively. TGF-β and BMP signaling systems typically oppose one another in normal epithelial cells. Shown are the receptors for TGF-β and BMP ligands, as well as their downstream effectors operant in mediating the initiation of EMT (TGF-β) and MET (BMP) reactions in normal and malignant cells. ALK-1 is primarily expressed on endothelial cells and functions to regulate proliferation, migration and angiogenesis. The precise signaling components required to mediate these physiological effects remain to be fully elucidated. See text for additional details.
Besides its ability to activate Smad2/3, TGF-β also alters cell behavior by stimulating an ever expanding list of noncanonical TGF-β effectors, including several nonreceptor protein tyrosine kinases (e.g., Src and FAK), mediators of cell survival (e.g., NF-κB and PI3K/Akt), mediators of immune responses (e.g., TAK1/TAB and TRAF6), small GTP-binding proteins (e.g., Ras, Rho, and Rac1), and MAP kinases (e.g., ERK1/2, p38 MAPK, and JNK), and; Fig. 1) [2; 11; 18]. Moreover, crosstalk between various noncanonical TGF-β effectors has been shown to drive the oncogenic activities of this multifunctional cytokine. Indeed, we recently defined a TβRI:xIAP:TAB1:TAK1:IKK complex that activates NF-κB and promotes Cox-2 expression that are essential in eliciting EMT program by TGF-β [19; 20; 21]. In addition, others demonstrated a physical interaction between TRAF6 and TβRI that activates TAK1, leading to the initiation of JNK and p38 MAPK signaling [22; 23]. TRAF6 also ubiquitinates TβRI, which promotes its cleavage by TACE and the accumulation of the intracellular domain of TβR-I (ICD) to accumulate in the nucleus where it functions in conjunction with other regulators of transcription to alter gene expression [24]. It should be noted that both canonical and noncanonical TGF-β effectors participate in driving EMT programs by TGF-β. Indeed, Smads 3 and 4 function as positive regulators of EMT programs, while Smad2 functions as a negative regulator of EMT [10; 25]. Along these lines, noncanonical TGF-β effectors are essential in initiating EMT reactions and maintaining phenotypes in cells that have emerged from EMT programs [2; 11; 18]. Although the precise mechanisms that coordinate the dynamics between the canonical and noncanonical TGF-β signaling systems remain to be fully elucidated, recent evidence has shown that imbalances between these two branches of the TGF-β signaling system, particularly those dominated by noncanonical TGF-β effectors, underscores the “TGF-β Paradox” and the acquisition of oncogenic activity by TGF-β in developing and progressing carcinomas [5; 11]. In the succeeding sections, we highlight recent findings that depict the function of TGF-β and its effector molecules as potent promoters of carcinoma EMT-MET programs and their role in mediating metastatic progression (Fig. 2).
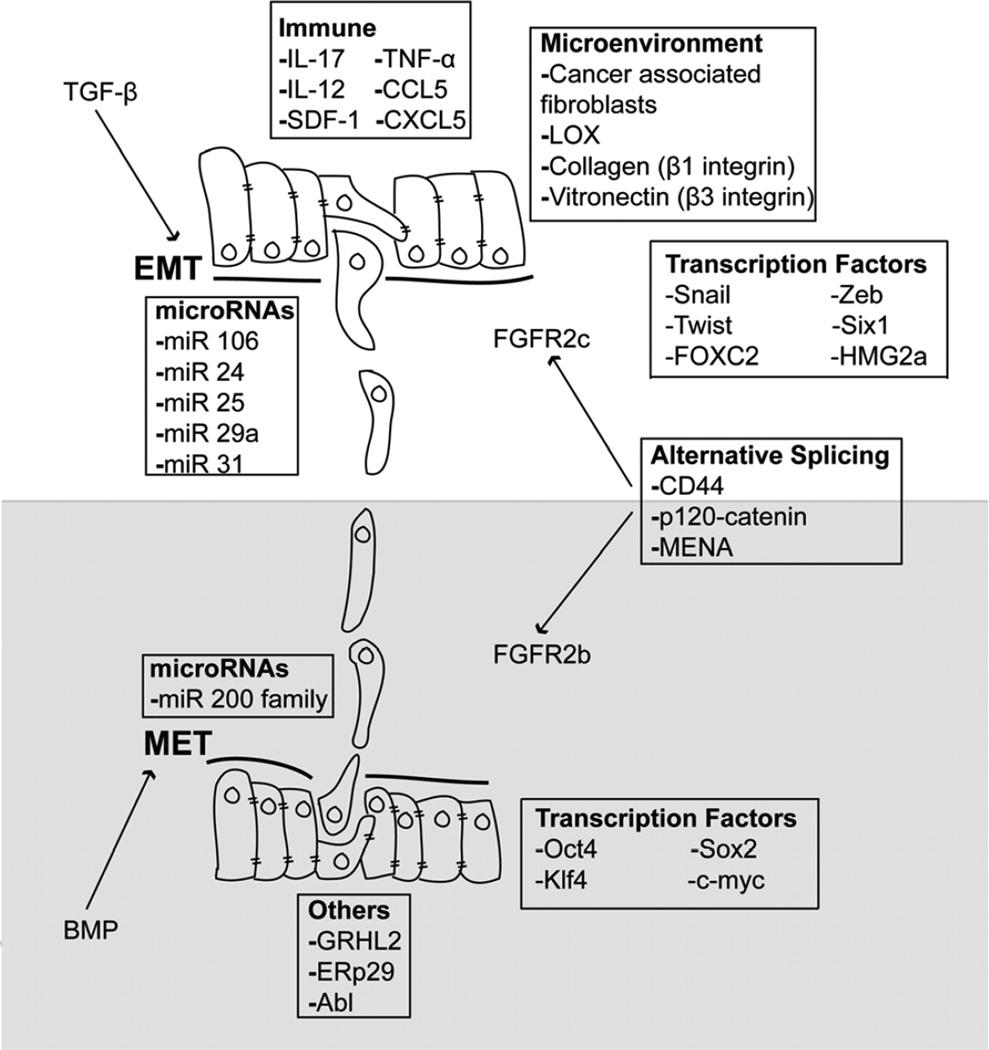
Figure 2.
Cell and environmental factors coupled to the induction of EMT and MET programs in carcinoma cells. Activation of TGF-β and BMP signaling systems couples to a complex cascade of expression and repression that is regulated by a variety of factors both within the carcinoma cells themselves, and within their accompanying host microenvironment. Targets of TGF-β and BMP signaling include microRNAs, infiltrating immune cells, and numerous microenvironmental factors and cytokines. Finally, transitioning carcinoma cells further fine tune the balance between EMT:MET reactions through employment of alternative gene splicing events, which gives rise to isoform specific expression of proteins in epithelial versus mesenchymal cell states. See text for additional details.
3. TGF-β and post-transcriptional regulation of EMT
3.1. TGF-β, microRNAs, and EMT
MicroRNAs (miRNAs) are small, noncoding RNAs that post-transcriptionally control gene expression by inhibiting mRNA translation, or by promoting mRNA degradation [26; 27]. Similar to the vast array of biological activities regulated by TGF-β, miRNAs also oversee a wide variety of physiological processes, such as cell proliferation, differentiation, and apoptosis. Additionally, accumulating evidence demonstrates that miRNAs can serve as tumor suppressors or tumor promoters, and as such, miRNA expression is typically dysregulated in numerous human diseases, including cancer [26; 27]. Recently, aberrant miRNA expression, including those responsive to TGF-β [26; 27; 28; 29], has been linked to the initiation of the “TGF-β Paradox” and its manifestation of oncogenic TGF-β signaling [30]. Indeed, the coupling of oncogenic TGF-β to miRNAs was first described by Goodall and colleagues who found TGF-β to inhibit the expression of miR-200 family members, which function in suppressing the expression of the EMT transcription factors, ZEB1 and ZEB2 (SIP1) [31; 32]. During EMT and metastasis stimulated by TGF-β, the loss of miR-200 family members leads to the stabilization of ZEB1 and ZEB2, resulting in their binding to and repression of the Cdh1 (E-cadherin) promoter [32]. More recently, aberrant miR-200 family member expression has been observed to induce a powerful feed-forward signaling loop between TGF-β and miR-200 family members to drive oncogenic EMT and carcinoma metastasis [33]. Finally, two recent studies demonstrated that highly metastatic 4T1 breast cancer cells are more epithelial-like as compared to their isogenic and nonmetastatic 4T07 counterparts [34; 35]. Amongst the many unique differences between these two isogeneic cell types is the re-expression of miR-200 in metastatic 4T1 cells, leading to the synthesis and secretion of metastasis-promoting proteins necessary metastatic outgrowth [35]. Collectively, these findings establish miR-200 family members as tumor suppressors capable of preventing oncogenic TGF-β signaling and its induction of EMT in normal epithelial cells [36].
Recently, the homeobox transcription factor Six1 has been shown to transform mammary epithelial cells and promote their metastasis by facilitating the conversion of TGF-β from a tumor suppressor to a tumor promoter [37; 38; 39]. The enhancement in TGF-β signaling mediated by Six1 transpires in part via its induction of the miR-106b-25 cluster, which represses Smad7 expression. The net effect of this event results in elevated TβR-I expression that not only promotes EMT programs in response to heightened autocrine TGF-β signaling, but also expands the population of tumor-initiating cells that significantly shortens the time to disease relapse [40]. Along these lines, miR-29a is highly expressed in mammary epithelial cells undergoing EMT reactions. Interestingly, miR-29a downregulates the expression of tristetraproline, which normally functions to degrade mRNAs whose 3’-UTRs are rich in AU-repeats. When combined with oncogenic Ras signaling, miR-29a-mediated repression of tristetraproline expression promotes EMT and metastasis of mammary epithelial cells in mouse models, and associates with the appearance of invasive ductal carcinomas in human breast cancers [41].
TGF-β and its activation of Smad4 also couple to the expression of miR-155 in mammary epithelial cells, which downregulates their expression of RhoA and promotes tight junction disassembly [42]. Along these lines, TGF-β also inhibits RhoA activity by inducing the expression of miR-24, which represses the expression of the Rho guanine nucleotide exchange factor (GEF), Net1 [43]. Similarly, TGF-β-mediated induction of Twist results in upregulation of miR-10b, which suppresses HOXD10 expression. The net-effect of these events results in the dramatic expression of the prometastatic gene, RhoC and elicits breast cancer invasion and metastasis [44]. Somewhat surprisingly, miR-10b expression has also been associated with diminished breast cancer invasion via its ability to target the Rac1 GEF, Tiam1 (T-cell lymphoma invasion and metastasis 1) [45]. Interestingly, Tiam1 expression is also governed by miRNAs miR-21 and miR-31 to enhance colon cancer cell EMT, migration, and invasion [46]. miRNAs 21 and 31 are both strongly induced by TGF-β [46], and a high expression of miR-21 in breast cancers associates with a poor clinical outcomes and predicts for increased TGF-β1 expression in cancers of the breast [47]. Lastly, Smad3 has recently been shown to associate with the p68/Drosha/DGCR8 miRNA processing complex during the maturation of miR-21 transcripts, a reaction that transpires independent of Smad4 expression and activity [48; 49]. Collectively, these findings highlight the cooperative manner in which canonical and noncanonical TGF-β effectors regulate miRNA expression in developing and progressing carcinomas, as well as demonstrate the importance of aberrant miRNA expression in facilitating the “TGF-β Paradox.”
3.2. TGF-β, alternative mRNA splicing, and EMT
Alternative mRNA splicing is now gaining acceptance as an important and integral mechanism operant in promoting EMT and MET programs [50; 51; 52], which greatly increases the variability, flexibility, and complexity of the human genome [53]. TGF-β signaling plays a major role in regulating mRNA splicing, particularly in developing carcinomas by suppressing their expression of epithelial splicing regulatory proteins (ESRPs) that bind RNA and govern the alternative splicing of epithelial gene transcripts [54; 55]. The ability of TGF-β to downregulated ESRPs transpires via a EF1- and ZEB2/SIP1-dependent manner [54]. Importantly, downregulated expression of ESRP results in the appearance of an EMT splicing signature capable of differentiating luminal versus basal breast cancer subtypes [50]. Moreover, the downregulation of ESRP by TGF-β also elicits differential isoform expression of CD44, p120 catenin, and hMENA [52; 54], events that further enhance the development of EMT programs induced by TGF-β. Accordingly, enforced expression of ESRP attenuates the coupling of TGF-β to EMT programs in epithelial cells [52]. Lastly, amongst the best studied examples of alternative splicing governed by TGF-β is that of the fibroblast growth factor receptor (FGFR) gene located on human chromosome 10q26, which is expressed as two alternative splice variants, namely FGFR-IIb and FGFR-IIc [8]. FGFR-IIb is primarily expressed in epithelial cells, while their mesenchymal counterparts primarily express FGFR-IIc, particularly in post-EMT cells stimulated by TGF-β [52; 56]. Along these lines, stable expression of FGFR-IIb variants in mesenchymal cells promotes their acquisition of MET phenotypes, while stable expression of FGFR-IIc variants in epithelial cells elicits EMT reactions reminiscent of those stimulated by TGF-β [8; 56]. In addition to continuing to identify novel splice variants operant in mediating EMT and metastasis stimulated by TGF-β, future studies also need to develop high-throughput methods to detect and assay the potential of essential splice variants to serve as novel predictive biomarkers and chemotherapeutic targets in carcinoma cells.
4. The influence of the tumor microenvironment on EMT programs induced by TGF-β
Studies over the last two decades have continually affirmed the overall importance of the tumor microenvironment (TME) in regulating carcinoma EMT and metastasis. Critical components of the tumor microenvironment include cancer-associated fibroblasts, endothelial cells, and a variety of infiltrating immune cells such as T and B cells, dendritic cells (DCs), and tumor-associated macrophages [57]. In addition, hypoxia and alterations in the ECM composition and its biomechanical properties also dictate the response of carcinoma cells to TGF-β and its ability to drive EMT reactions and metastatic progression [58; 59]. In the succeeding sections, we highlight the central role of the TME in facilitating EMT and oncogenic signaling by TGF-β.
4.1. TGF-β, cancer-associated fibroblasts, and EMT
Recent findings have established that normal and malignant cells respond differently to TGF-β in a manner that reflects alterations in the composition of the surrounding ECM [3]. Indeed, TGF-β potently regulates the activation status of adjacent cancer-associated fibroblasts (CAFs) and myofibroblasts, which are the predominant cell types housed within the TME. When activated by TGF-β, these cells produce and secrete a variety of growth factors, cytokines, chemokines, and ECM proteins capable of promoting tumor initiation and progression in adjacent epithelial cells [2; 58]. Indeed, conditional deletion of TβR-II expression to inactivate TGF-β signaling in stromal fibroblasts elicited carcinoma formation in the prostate and stomach, as well as generated significant expansion of the stromal compartment [60]. Similar initiation of carcinoma formation was observe following conditional deletion of TβR-II in mammary fibroblasts, which enhanced the growth and invasion of breast cancer cells via elevated fibroblast secretion of cytokines and growth factors, including TGF-β, MSP (macrophage stimulating protein), and HGF [61]. Likewise, genetic inactivation of TβR-II in as little as 50% of human prostate fibroblasts was sufficient to transform normal human prostate epithelial cells in mouse tissue recombination models of prostate cancer development and progression [62]. Importantly, these mouse-based findings have also been observed in the stroma of human colon cancers, whose tumor-associated fibroblasts exhibit downregulated expression of TβR-II that correlated with increased lymph node metastasis and decreased overall patient survival [63; 64]. Collectively, these findings highlight the tumor suppressing activities of TGF-β that transpire through its inhibition of fibroblast activities, such that loss of TGF-β signaling in tumor-associated fibroblasts upregulates their production of environmental factors capable of driving tumor formation and metastatic progression.
4.2. TGF-β and tissue compliance during EMT programs
TGF-β also regulates the differentiation status of fibroblasts, particularly their acquisition of myofibroblast phenotypes and altered expression patterns of ECM proteins and growth factors as compared to normal fibroblasts [65]. Along these lines, alterations in the biomechanics of the ECM within developing tumors has been linked to carcinoma development and metastasis. Indeed, tumor-associated myofibroblasts are responsible for manifesting desmoplastic reactions through their secretion of large amounts of collagen into the tumor microenvironment. Collagen becomes crosslinked to elastin through the activities of lysyl oxidases (LOXs) and tumor-associated myofibroblasts further contribute to enhancing tumor rigidity by their production of α-smooth muscle actin [65; 66; 67; 68]. Recently, increased microenvironmental rigidity has been shown to promote EMT programs stimulated by TGF-β. For instance, matrix rigidity converts TGF-β from a proapoptotic molecule to an inducer of EMT reactions in NMuMG and MDCK cells, doing so via enhanced coupling of TGF-β to the PI3K/Akt pathway [69]. We too observed matrix rigidity to not only enhance the ability of TGF-β to induce EMT programs in normal and malignant mammary epithelial cells, but also to underlie the manifestations of the “TGF-β Paradox,” such that the tumor suppressing functions of TGF-β are favored in compliant microenvironments, while its tumor promoting functions are favored in rigid microenvironments [68]. This dichotomous change in TGF-β behavior reflects the upregulated expression of LOX and its ability to enhance the coupling of TGF-β to p38 MAPK [68]. Along these lines, elevated LOX expression also elicits the activation of PI3K and increases the stiffness and tumorigenicity of mammary tumors that arose in MMVT-Neu transgenic mice [70]. It is interesting to note that biomechanically rigid tumors are typically more hypoxic than their compliant counterparts, and as such, rigid and hypoxic tumor microenvironments drive LOX expression in a hypoxia-inducible factor-1 (HIF-1α)-dependent manner [71]. In addition, hypoxia-dependent LOX expression is also essential in promoting breast cancer invasion and metastasis [71; 72; 73], and in engendering the formation of the premetastatic niche operant in receiving disseminated cells [72; 73]. Finally, LOX-like2 (LOXL2) was observed to interact physically with the EMT transcription factor Snail, leading to the activation of EMT programs and acquisition of aggressive phenotypes by human squamous cell carcinomas [74; 75]. Moreover, rendering basal-like human breast cancer cells deficient in LOXL2 expression not only prevented their pulmonary metastasis, but also induced these cells to undergo MET programs [76]. Collectively, these findings highlight the emerging importance of how biochemical changes within tumor microenvironments function in driving oncogenic TGF-β and its stimulation of EMT and metastasis in developing carcinomas.
4.3. TGF-β and hypoxia during EMT programs
Hypoxia is an integral part of tumorigenesis, which culminates in the expression of HIFs and the urokinase receptor (uPAR) that promote EMT programs [5; 77; 78]. As alluded to above, hypoxia induces breast cancer cells to exhibit the classical hallmarks of EMT, including E-cadherin downregulation and/or delocalization from the plasma membrane, as well as the nuclear accumulation of Snail and β-catenin [79]. In fact, reoxygenation enables post-EMT cells to undergo MET programs and reestablish junctional protein complexes [77]. Moreover, hypoxia-mediated EMT reactions are also associated with a gain in stem-like characteristics [80], which may in part be driven by the Notch signaling system [81]. Likewise, EMT induced by hypoxia is further characterized by the formation of NF-κB-HIF-1α complexes, which enhance the expression of c-Jun [82]. Collectively, these studies demonstrate a critical role for hypoxia in regulating the balance between EMT and MET states, particularly during the evolution of metastatic phenotypes in carcinomas. Given the striking parallels between hypoxia and oncogenic TGF-β signaling, future studies need to address the specific roles played by TGF-β and BMP during hypoxia-driven EMT programs and vice versa.
4.4. TGF-β and integrins during EMT programs
The differential expression and activity of integrins clearly modulates how normal and malignant cells sense and respond to TGF-β in compliant and rigid microenvironments. Integrins act as transmembrane scaffolds that link the ECM to the actin cytoskeletal system; they also function as mediators of mechanotransduction and regulate cell proliferation, migration, and invasion, as well as cell survival [67; 83]. For instance, expression of a 1 integrin mutant that mimics rigidity-induced integrin clustering (i.e., V737N-β1 integrin) readily enhanced the invasion of premalignant mammary epithelial cells by promoting the formation of focal adhesion complexes [70]. Even more remarkably, culturing V737N-β1 integrin-expressing mammary epithelial cells in compliant 3D-organotypic cultures elevated their activation of the PI3K pathway to levels typically observed in their rigid 3D-organotypic counterparts [70]. Thus, 1 integrin clustering plays a prominent role in eliciting oncogenic signaling events in rigid carcinoma microenvironments. Along these lines, we identified integrins as prominent mediators of oncogenic TGF-β signaling and its coupling to EMT programs. Indeed, upregulated expression of 3 integrin in response to TGF-β is essential for its initiation of EMT reactions [84; 85; 86]. Mechanistically, 3 integrin forms a complex with TβR-II that is bridged by FAK, leading to Src-mediated phosphorylation of TβR-II at Tyr284 that recruits ShcA and Grb2 necessary in coupling TGF-β to the amplified activation of p38 MAPK [84; 85; 86]. Inhibiting these events alleviates oncogenic TGF-β signaling, including its coupling to NF-κB, Cox-2, and PGE2 signaling [19; 20; 21; 87], as well as prevents the acquisition of EMT and metastatic phenotypes driven by TGF-β [5]. The formation of TβR-II: 3 integrin complexes also results in the activation of FAK, which mediates TGF-β stimulation of breast cancer cell invasion both in vitro and in vivo [88], and in the upregulated expression of p130Cas, which elicits diminished expression of Smad3 and enhances the pulmonary metastasis of breast cancers [89]. Thus, aberrant coupling of TGF-β to βv 3 integrin expression is sufficient to manifest the “TGF-β Paradox” and elicit the oncogenic activities of TGF-β in responsive cells.
Besides 3 integrin, TGF-β signaling is also impacted by its physical interaction with 1 integrin [84], which also regulates TGF-β stimulation of EMT programs and p38 MAPK [90]. Recently, we linked TGF-β stimulation of EMT to the initiation of proliferative programs and metastatic outgrowth of disseminated breast cancer cells. In doing so, we observed compliant microenvironments to prevent TGF-β from activating Smad4 [34; 91; 92], which blocks the expression of Pyk2 necessary to mediate escape from metastatic dormancy [91]. Interestingly, these events are also governed by the reciprocal expression patterns of E-cadherin and 1 integrin, such that dormant cells are typically E-cadherin high, 1 integrin low, while outgrowth proficient cells are typically 1 integrin high, E-cadherin low [34; 64; 91; 93; 94]. Importantly, the acquisition of EMT phenotypes represents the molecular switch that allows cells to toggle between metastatic dormancy and outgrowth due to diminished E-cadherin expression, which interacts in a heterotypic manner with the extracellular domain of 1 integrin [95], leading to a loss of 1 integrin and Pyk2 expression operant in mediating metastatic outgrowth [34; 91]. Finally, we recently identified “integrin switching” as a novel modulator of the oncogenic activities of TGF-β. Indeed, pharmacologic or genetic inactivation of 1 integrin elicits a dramatic compensatory upregulation of 3 integrin expression that not only restores the oncogenic functions of TGF-β, but also enhances its ability to drive metastasis at earlier stages of mammary tumor development (J.G. Parvani and W.P. Schiemann, unpublished observation). Thus, metastatic carcinoma cells and their acquisition of EMT programs provided the means necessary to evade single agent integrin-based therapies through “integrin-switching” mechanisms [96; 97]. As such, future studies need to identify the collection of integrins operant in mediating the oncogenic activities of TGF-β, as well as determine how EMT programs and chemotherapies alter the composition of integrins in TGF-β-responsive carcinoma cells.
5. TGF-β and cancer stem cells (CSCs) during EMT programs
Developing carcinomas are highly heterogeneous and contain numerous distinct cell populations, some of which can reinitiate tumor growth and development with varying degrees of efficiency. Amongst the most efficient tumor-initiating cells are cancer stem cells (CSCs) that comprise a small fraction of the primary tumor and possess self-renewing capabilities [98; 99]. Initial evidence linking EMT programs to the generation of CSCs was provided by Weinberg and colleagues [100] who demonstrated that engineering immortalized mammary epithelial cells to stably express Snail or Twist, or stimulating them with TGF-β produced a post-EMT population of cells that displayed the markers (e.g., CD44high/CD24low) and features (e.g., mammosphere and tumor-initiating behaviors) of stem-like cells. Subsequent studies showed that this same post-EMT population of cells can also assume the characteristics of mesenchymal stem cells (MSCs), including their ability to (i) differentiate into bone, adipocytes, or chrondrocytes; (ii) migrate to breast cancer cells; and (iii) home to wounded tissues [101]. Similar findings by others have clearly established EMT programs as major drivers of the selection and expansion of CSCs [100; 102; 103; 104], resulting in the appearance of chemoresistant phenotypes and disease recurrence [99; 105; 106; 107; 108]. The importance of TGF-β in mediating these events is highlighted by the finding that (i) TGF-β gene signatures associate with metastatic progression and poor clinical outcomes, and (ii) pharmacological inactivation of TGF-β signaling in metastatic breast cancers cells induced a MET program indicative of diminished tumorigenicity [102]. With respect to breast cancers, mesenchymal phenotypes derived from EMT programs are predominantly associated with basal-like or triple-negative breast cancer subtypes. However, a newly described rare breast cancer subtype termed “claudin-low” was observed to be enriched in post-EMT and stem-like properties [99; 109], and to exhibit significantly worse prognoses as compared to other breast cancer subtypes [110]. Consistent with its designation as a master regulator of EMT programs, TGF-β signaling drives the appearance of “claudin-low” post-EMT cell populations [111].
Conversely, recent evidence also suggests that EMT suppresses CSC formation, such that CSCs may reside in the epithelial-like basal cells in prostate cancer [112]. Along these lines, other studies have also shown that epithelial-like tumor cells are in fact capable of forming distant metastases [113; 114; 115; 116]. Accordingly, epithelial-like prostate cancer cells were enriched in subpopulations of metastatic tumor initiating cells, whereas their mesenchymal-like counterparts were observed to display reduced tumor initiating capabilities [115; 116]. Clearly, future studies are warranted to fully define the relationship between epithelial-like and mesenchymal-like cell types in promoting the expansion of CSCs and tumor-initiating cell populations during carcinoma development, metastasis, and recurrence.
6. Mesenchymal-epithelial transition (MET) programs
As alluded to above, MET is the process whereby mesenchymal-like cells transdifferentiate to establish polarized epithelial-like cells, complete with the formation of strong cell-cell junctions and adherens complexes. These junctional complexes are comprised of various transmembrane molecules and accessory proteins, which form intricate networks with the actin cytoskeleton. Additional descriptions related the to regulation of these junctional complexes and their role in EMT programs are directed to several recent reviews [11; 117; 118]. MET programs are also characterized by decreases in the expression of mesenchymal markers, such as N-cadherin and vimentin, and accompanying increases in epithelial markers, as E-cadherin and CK-19 [8; 119]. The induction of MET programs has been postulated as playing an integral part of the metastatic cascade because metastatic lesions typically appear more differentiated as compared to their primary tumor of origin [120]. Thus, the current paradigm states that EMT drives carcinoma cells to disseminate from the primary tumor, while MET drives disseminated carcinoma cells to reinitiate proliferative programs necessary for their formation of secondary tumors [120]. The latter response is important because TGF-β stimulation of EMT typically inhibits cell cycle progression, and as such, it appears that the ability of carcinoma cells to disseminate and ultimately develop into macroscopic lesions may in fact represent two mutually exclusive processes. Indeed, the ability of disseminated tumor cells to reestablish epithelial phenotypes via MET programs may in fact represent the rate-limiting step of metastasis, whose failure to transpire could potentially lock metastatic loci into a state of perpetual dormancy [120]. Here we highlight recent findings of the molecular mechanisms that underlie MET reactions and their relevance to TGF-β and metastasis.
6.1. Bone morphogenetic proteins (BMPs) and MET programs
BMPs are multifunctional cytokines of the TGF-β superfamily that were originally identified based on their ability to induce bone differentiation and formation [121]. Similar to TGF-β, BMP signaling transpires through its binding to specific type I and type II receptors [121]. However, while TGF-β binds exclusively to TβR-II, BMP ligands are more promiscuous and bind not only to the BMP type II receptor (BMPR2), but also to the activin type II receptors, ActRII and ActRIIB [121]. Once activated, BMP receptors activate Smads 1, 5, or 8, which translocate into the nucleus bound to Smad4 to bring about changes in gene expression [121]. In addition to regulating bone formation, BMPs have also been implicated in mediating MET programs. For instance, administration of BMP7 to human breast cancer cells results in their upregulated expression of E-cadherin, as well as inhibits TGF-β signaling, an event that culminates in decreased vimentin expression [122]. Along these lines, therapeutic administration of BMP7 diminished breast cancer metastasis to bone [122] and reduced the pool of CSCs associated with EMT reactions [123]. Furthermore, functional disruption of the BMPR2 dramatically enhanced pulmonary metastases in a MMTV-PyMT mouse model [124]. Interestingly, intravital imaging analyses have identified a reduction in TGF-β signaling in pulmonary metastases [92; 125], suggesting that BMPs may antagonize the activities of TGF-β at micrometastatic sites to facilitate metastatic outgrowth of secondary lesions [120]. Future studies need to investigate the spatiotemporal contexts that govern BMP signaling at distinct stages of metastasis, as well as how these events differ between primary tumors and their metastatic lesions.
6.2. Transcriptional Regulators of MET
While EMT programs are clearly required for the generation of stem-like CSCs and MSCs, the production of inducible pluripotent stem cells (iPSCs) requires the initiation of MET programs [126]. Indeed, the reprogramming of fibroblasts requires the simultaneous expression of four embryonic stem cell transcription factors, namely Oct4, Sox2, Klf4, and c-Myc. These transcription factors collectively induce a MET reaction that inhibits TGF-β by downregulating TβR-II expression, as well as that of Snail [126]. Conversely, augmenting TGF-β signaling significantly impeded the generation of iPSCs [126], indicating that EMT and MET programs oppose one another during the generation of iPSCs, and consequently, that EMT programs driven by TGF-β limit cellular reprogramming and the pluripotency of fully transitioned post-EMT cells. Future studies need to further our understanding of the dynamics and interplay that exist between EMT and MET programs in regulating cell reprogramming, and to determine the therapeutic potential of targeting these differences as a means to alleviate CSCs and disease recurrence.
6.3. The miR-200 family and MET programs
The miR-200 family of miRNAs (i.e., miR-200a, miR-200b, miR-200c, miR-141 and miR-429) have been implicated in promoting MET via their ability to repress the expression of ZEB1 and ZEB2, leading to upregulated E-cadherin expression [32]. In fact, enforced expression of the miR-200 family enhances the epithelial characteristics of breast cancer cells [127]. Besides their ability to alleviate the repression of essential epithelial gatekeepers, miR-200 family members also target cytoskeletal components, such as WAVE3 and ECM proteins, such as fibronectin, both of which are prominently associated with mesenchymal phenotypes [127]. Recently, the tumor suppressor p53 was shown to mediate the expression of miR-200c, as the loss of p53 expression in mammary epithelial cells resulted in the appearance of mesenchymal cell populations that possessed stem-like properties [128]. As mentioned previously, TGF-β is a potent suppressor of the miR-200 family, doing so by promoting the hypermethylation of the promoters for these miRNAs [127]. It should be noted that while miR-200 family members are clearly linked to maintaining epithelial cell integrity, aberrantly upregulated or downregulated expression of these miRNAs has also been associated with the acquisition of metastatic phenotypes [35; 127]. For instance, MET programs can be initiated by expression of miR-200 family members, or by inactivation of EMT-inducing miRNAs [27]. Importantly, the nuances of expressing MET-inducing miRNAs versus inhibiting EMT-inducing miRNAs reflects the summation of global changes in gene expression that transpire in response to these miRNAs. Along these lines, the interplay between miR-200s and other plasticity-associated miRNAs remain incompletely understood [27]. Future studies need to address these relationships, as well as to better define their role in driving metastatic progression.
6.4. Grainyhead-like-2 (GRHL2) and MET programs
GRHL2 is a developmentally regulated gene that is associated with neural tube closure [129]. Recently, decreased expression of GRHL2 has been observed in basal-like and claudinlow breast cancer subtypes, whose re-expression of GRHL2 induced cadherin switching consistent with the induction of MET programs (i.e., N-cadherin in GRHL2low to E-cadherin in GRHL2high; [129]. Indeed, GRHL2 promotes MET programs through its ability to inhibit TGF-β-mediated activation of Smad2/3, which leads to the loss of ZEB1 expression and accompanying upregulation of miR-200 expression [129]. Finally, GRHL2 reduces the expression of CD44, suggesting GRHL2 not only alleviates EMT phenotypes, but diminishes the proportion of carcinoma cells that possess CSC and stem-like characteristics [129]. Given the parallels between MET programs induced by BMPs and GRHL2, future studies need to determine the extent to which GRHL2 mediates MET reactions stimulated by BMPs, and conversely, how these events are inactivated by TGF-β in developing and progressing carcinoma cells.
6.5. Endoplasmic reticulum protein 29 (ERp29) and MET programs
ERp29 is a resident endoplasmic reticulum protein that functions as a chaperone during the unfolding and secretion of proteins through the vesicular transport system [130; 131]. With respect to developing carcinomas, endoplasmic reticulum homeostasis is often disrupted as a result of DNA damage or oxidative stress; however, the mechanistic role of ERp29 during carcinogenesis remains incompletely understood [130]. Interestingly, overexpression of ERp29 in mesenchymal-like and metastatic MDA-MB-231 cells induced a MET program that manifested in the appearance of cortical actin staining, elevated expression of epithelial markers (e.g., E-cadherin and CK-19), and decreased expression of mesenchymal markers (e.g., vimentin and fibronectin), events associated with diminished expression of Slug, Snail, ZEB2, and Twist [131]. Conversely, disruption of ERp29 expression in epithelial-like and nonmetastatic MCF-7 cells induced a loss of junctional integrity that was reminiscent of those observed in EMT programs [131]. Collectively, these findings demonstrate a novel role for ERp29 and endoplasmic reticulum homeostasis in driving MET programs, as well as in inhibiting TGF-β signaling in mammary carcinomas.
6.6. c-Abl and MET programs
c-Abl is a nonreceptor protein tyrosine kinase (PTK) that governs a variety of physiological process, including cell proliferation, motility, and survival, as well as cell and tissue homeostasis [132; 133; 134]. c-Abl is perhaps best known for its causative role in promoting hematologic cancer development, typically arising in chronic myelogenous leukemia (CML) cells that harbor the Philadelphia translocation on chromosome 22 wherein c-Abl is translocated and fused to the break-point cluster region, resulting in the production of a constitutively-active c-Abl kinase fusion protein [132; 133; 134]. Importantly, rational drug design led to the generation of the small molecule c-Abl antagonist, Imatinib, which ushered in the era of targeted drug therapies and significantly improved the clinical course of CML patients [132; 133; 134]. Interestingly, the success of Imatinib in treating liquid tumors has not been replicated in their solid tumor counterparts. For instance, three recent clinical trials designed to assess the therapeutic effects of Imatinib on advanced breast cancers were halted due to a lack of efficacy [135; 136; 137], findings we recapitulated in preclinical mouse models of mammary tumor development and metastasis [132]. Importantly, we recently established c-Abl as an essential gatekeeper charged with maintaining epithelial phenotypes in mammary epithelial cells. Indeed, heterologous expression of a constitutively-active c-Abl mutant (CST-Abl) in late-stage breast cancer cells prevented their acquisition of EMT and metastatic phenotypes in response to TGF-β [132]. In doing so, we observed CST-Abl expression in late-stage breast cancer cells to promote the formation of strong cell-cell junctions and reduce cell spreading in 2D cultures, as well as to elicit the normalization and hollowing of acinar structures in 3D cultures [132]. Conversely, pharmacologic or genetic inactivation of c-Abl in normal mammary epithelial cells initiated EMT programs, complete with the downregulated expression of epithelial markers (e.g., E-cadherin and MMPs) and upregulated expression of their mesenchymal counterparts (e.g., vimentin, N-cadherin, and Twist1) [132]. Thus, these morphological and phenotypic changes brought about by c-Abl activation clearly reflect the initiation of MET programs and their ability to render late-stage breast cancers benign when engrafted into mice [132]. Future studies need to develop the means to harness and exploit the tumor suppressing and MET-inducing functions of c-Abl as a novel means to alleviate carcinoma metastasis and disease recurrence.
7. Conclusion and future directions
The TGF-β superfamily regulates various aspects of EMT and MET programs in normal and malignant epithelial cells. With respect to the ability of TGF-β to induce EMT reactions, numerous studies have documented the integral role played by both canonical and noncanonical TGF-β effectors to elicit EMT and metastatic progression of developing carcinomas [5; 11]. Collectively, these signaling events coalesce to induce EMT programs by modulating the expression of miRNAs, by creating a permissive tumor microenvironment, and by inducing the acquisition of stem-like and chemoresistant phenotypes. Although our understanding of EMT programs seems to grow on a daily basis, our knowledge related to MET programs and their regulation by BMP continues to lag and remain highly elusive. Given the proposed role of MET programs in functioning as the rate-limiting step of metastasis [120], future studies need to elucidate the molecular mechanisms that enable TGF-β and BMP signals to diverge and oppose one another in normal epithelial cells, as well as identify the signaling defects that enable these pathways to converge in metastatic carcinoma cells. In doing so, science and medicine will undoubtedly glean valuable therapeutic insights capable of targeting and alleviating metastatic disease in carcinoma patients.
Acknowledgements
Members of the Schiemann Laboratory are thanked for critical reading of the manuscript. W.P.S. was supported in part by grants from the National Institutes of Health (CA129359), the Department of Defense (BC084561), and pilot funding from the Case Comprehensive Cancer Center (P30 CA043703).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors have declared that no conflict of interest exists.
References
- 1.Massague J. TGFβ in Cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor MA, Lee YH, Schiemann WP. Role of TGF-β and the tumor microenvironment during mammary tumorigenesis. Gene Expr. 2011;15:117–132. doi: 10.3727/105221611x13176664479322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wendt MK, Tian M, Schiemann WP. Deconstructing the mechanisms and consequences of TGF-β-induced EMT during cancer progression. Cell and tissue research. 2012;347:85–101. doi: 10.1007/s00441-011-1199-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tian M, Neil JR, Schiemann WP. Transforming growth factor-β and the hallmarks of cancer. Cell Signal. 2011;23:951–962. doi: 10.1016/j.cellsig.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor MA, Parvani JG, Schiemann WP. The pathophysiology of epithelial-mesenchymal transition induced by transforming growth factor-β in normal and malignant mammary epithelial cells. J Mammary Gland Biol Neoplasia. 2010;15:169–190. doi: 10.1007/s10911-010-9181-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yao D, Dai C, Peng S. Mechanism of the mesenchymal-epithelial transition and its relationship with metastatic tumor formation. Mol Cancer Res. 2011;9:1608–1620. doi: 10.1158/1541-7786.MCR-10-0568. [DOI] [PubMed] [Google Scholar]
- 9.Wendt MK, Allington TM, Schiemann WP. Mechanisms of the epithelial-mesenchymal transition by TGF-β. Future Oncol. 2009;5:1145–1168. doi: 10.2217/fon.09.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heldin CH, Vanlandewijck M, Moustakas A. Regulation of EMT by TGFβ in cancer. FEBS Lett. 2012;586:1959–1970. doi: 10.1016/j.febslet.2012.02.037. [DOI] [PubMed] [Google Scholar]
- 11.Parvani JG, Taylor MA, Schiemann WP. Noncanonical TGF-β signaling during mammary tumorigenesis. J Mammary Gland Biol Neoplasia. 2011;16:127–146. doi: 10.1007/s10911-011-9207-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gatza CE, Oh SY, Blobe GC. Roles for the type III TGF-β receptor in human cancer. Cell Signal. 2010;22:1163–1174. doi: 10.1016/j.cellsig.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bharathy S, Xie W, Yingling JM, Reiss M. Cancer-associated transforming growth factor type II receptor gene mutant causes activation of bone morphogenic protein-Smads and invasive phenotype. Cancer Res. 2008;68:1656–1666. doi: 10.1158/0008-5472.CAN-07-5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu IM, Schilling SH, Knouse KA, Choy L, Derynck R, Wang XF. TGFβ-stimulated Smad1/5 phosphorylation requires the ALK5 L45 loop and mediates the pro-migratory TGFβ switch. EMBO J. 2009;28:88–98. doi: 10.1038/emboj.2008.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goumans MJ, Valdimarsdottir G, Itoh S, Lebrin F, Larsson J, Mummery C, Karlsson S, ten Dijke P. Activin receptor-like kinase (ALK)1 is an antagonistic mediator of lateral TGF-β/ALK5 signaling. Mol Cell. 2003;12:817–828. doi: 10.1016/s1097-2765(03)00386-1. [DOI] [PubMed] [Google Scholar]
- 16.Goumans MJ, Valdimarsdottir G, Itoh S, Rosendahl A, Sideras P, ten Dijke P. Balancing the activation state of the endothelium via two distinct TGF-β type I receptors. EMBO J. 2002;21:1743–1753. doi: 10.1093/emboj/21.7.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ray BN, Lee NY, How T, Blobe GC. ALK5 phosphorylation of the endoglin cytoplasmic domain regulates Smad1/5/8 signaling and endothelial cell migration. Carcinogenesis. 2010;31:435–441. doi: 10.1093/carcin/bgp327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moustakas A, Heldin CH. Non-Smad TGF-β signals. J Cell Sci. 2005;118:3573–3584. doi: 10.1242/jcs.02554. [DOI] [PubMed] [Google Scholar]
- 19.Neil JR, Johnson KM, Nemenoff RA, Schiemann WP. Cox-2 inactivates Smad signaling and enhances EMT stimulated by TGF-β through a PGE2-dependent mechanisms. Carcinogenesis. 2008;29:2227–2235. doi: 10.1093/carcin/bgn202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neil JR, Schiemann WP. Altered TAB1:IκB kinase interaction promotes transforming growth factor β-mediated nuclear factor-βB activation during breast cancer progression. Cancer Res. 2008;68:1462–1470. doi: 10.1158/0008-5472.CAN-07-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neil JR, Tian M, Schiemann WP. X-linked inhibitor of apoptosis protein and its E3 ligase activity promote transforming growth factor-β-mediated nuclear factor-β B activation during breast cancer progression. J Biol Chem. 2009;284:21209–21217. doi: 10.1074/jbc.M109.018374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorrentino A, Thakur N, Grimsby S, Marcusson A, von Bulow V, Schuster N, Zhang S, Heldin CH, Landstrom M. The type I TGF-β receptor engages TRAF6 to activate TAK1 in a receptor kinase-independent manner. Nat Cell Biol. 2008;10:1199–1207. doi: 10.1038/ncb1780. [DOI] [PubMed] [Google Scholar]
- 23.Yamashita M, Fatyol K, Jin C, Wang X, Liu Z, Zhang YE. TRAF6 mediates Smad-independent activation of JNK and p38 by TGF-β. Mol Cell. 2008;31:918–924. doi: 10.1016/j.molcel.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mu Y, Sundar R, Thakur N, Ekman M, Gudey SK, Yakymovych M, Hermansson A, Dimitriou H, Bengoechea-Alonso MT, Ericsson J, Heldin CH, Landstrom M. TRAF6 ubiquitinates TGF-β type I receptor to promote its cleavage and nuclear translocation in cancer. Nat Commun. 2011;2:330. doi: 10.1038/ncomms1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moustakas A, Heldin CH. Induction of epithelial-mesenchymal transition by transforming growth factor β. Semin Cancer Biol. 2012;22:446–454. doi: 10.1016/j.semcancer.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 26.Ruan K, Fang X, Ouyang G. MicroRNAs: novel regulators in the hallmarks of human cancer. Cancer Lett. 2009;285:116–126. doi: 10.1016/j.canlet.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 27.Gregory PA, Bracken CP, Bert AG, Goodall GJ. MicroRNAs as regulators of epithelial-mesenchymal transition. Cell Cycle. 2008;7:3112–3118. doi: 10.4161/cc.7.20.6851. [DOI] [PubMed] [Google Scholar]
- 28.Bullock MD, Sayan AE, Packham GK, Mirnezami AH. MicroRNAs: critical regulators of epithelial to mesenchymal (EMT) and mesenchymal to epithelial transition (MET) in cancer progression. Biol Cell. 2012;104:3–12. doi: 10.1111/boc.201100115. [DOI] [PubMed] [Google Scholar]
- 29.Zavadil J, Narasimhan M, Blumenberg M, Schneider RJ. Transforming growth factor-β and microRNA:mRNA regulatory networks in epithelial plasticity. Cells Tissues Organs. 2007;185:157–161. doi: 10.1159/000101316. [DOI] [PubMed] [Google Scholar]
- 30.Tian M, Schiemann WP. The TGF-β paradox in human cancer: an update. Future Oncol. 2009;5:259–271. doi: 10.2217/14796694.5.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korpal M, Kang Y. The emerging role of miR-200 family of microRNAs in epithelial-mesenchymal transition and cancer metastasis. RNA Biol. 2008;5:115–119. doi: 10.4161/rna.5.3.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 33.Gregory PA, Bracken CP, Smith E, Bert AG, Wright JA, Roslan S, Morris M, Wyatt L, Farshid G, Lim YY, Lindeman GJ, Shannon MF, Drew PA, Khew-Goodall Y, Goodall GJ. An autocrine TGF-β/ZEB/miR-200 signaling network regulates establishment and maintenance of epithelial-mesenchymal transition. Mol Biol Cell. 2011;22:1686–1698. doi: 10.1091/mbc.E11-02-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wendt MK, Taylor MA, Schiemann BJ, Schiemann WP. Downregulation of epithelial cadherin is required to initiate metastatic outgrowth of breast cancer. Mol Biol Cell. 2011;22:2423–2435. doi: 10.1091/mbc.E11-04-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Korpal M, Ell BJ, Buffa FM, Ibrahim T, Blanco MA, Celia-Terrassa T, Mercatali L, Khan Z, Goodarzi H, Hua Y, Wei Y, Hu G, Garcia BA, Ragoussis J, Amadori D, Harris AL, Kang Y. Direct targeting of Sec23a by miR-200s influences cancer cell secretome and promotes metastatic colonization. Nat Med. 2011;17:1101–1108. doi: 10.1038/nm.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schliekelman MJ, Gibbons DL, Faca VM, Creighton CJ, Rizvi ZH, Zhang Q, Wong CH, Wang H, Ungewiss C, Ahn YH, Shin DH, Kurie JM, Hanash SM. Targets of the tumor suppressor miR-200 in regulation of the epithelial-mesenchymal transition in cancer. Cancer Res. 2011;71:7670–7682. doi: 10.1158/0008-5472.CAN-11-0964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farabaugh SM, Micalizzi DS, Jedlicka P, Zhao R, Ford HL. Eya2 is required to mediate the pro-metastatic functions of Six1 via the induction of TGF-β signaling, epithelial-mesenchymal transition, and cancer stem cell properties. Oncogene. 2012;31:552–562. doi: 10.1038/onc.2011.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Micalizzi DS, Christensen KL, Jedlicka P, Coletta RD, Baron AE, Harrell JC, Horwitz KB, Billheimer D, Heichman KA, Welm AL, Schiemann WP, Ford HL. The Six1 homeoprotein induces human mammary carcinoma cells to undergo epithelial-mesenchymal transition and metastasis in mice through increasing TGF-β signaling. J Clin Invest. 2009;119:2678–2690. doi: 10.1172/JCI37815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Micalizzi DS, Wang CA, Farabaugh SM, Schiemann WP, Ford H. Homeoprotein Six1 increases TGF-β type I receptor and converts TGF-β signaling from suppressive to supportive for tumor growth. Cancer Res. 2010;70:10371–10380. doi: 10.1158/0008-5472.CAN-10-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith AL, Iwanaga R, Drasin DJ, Micalizzi DS, Vartuli RL, Tan AC, Ford HL. The miR-106b-25 cluster targets Smad7, activates TGF-β signaling, and induces EMT and tumor initiating cell characteristics downstream of Six1 in human breast cancer. Oncogene. 2012 doi: 10.1038/onc.2012.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gebeshuber CA, Zatloukal K, Martinez J. miR-29a suppresses tristetraprolin, which is a regulator of epithelial polarity and metastasis. EMBO Rep. 2009;10:400–405. doi: 10.1038/embor.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kong W, Yang H, He L, Zhao JJ, Coppola D, Dalton WS, Cheng JQ. MicroRNA-155 is regulated by the transforming growth factor β/Smad pathway and contributes to epithelial cell plasticity by targeting RhoA. Mol Cell Biol. 2008;28:6773–6784. doi: 10.1128/MCB.00941-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Papadimitriou E, Vasilaki E, Vorvis C, Iliopoulos D, Moustakas A, Kardassis D, Stournaras C. Differential regulation of the two RhoA-specific GEF isoforms Net1/Net1A by TGF-β and miR-24: role in epithelial-to-mesenchymal transition. Oncogene. 2011;31:2862–2875. doi: 10.1038/onc.2011.457. [DOI] [PubMed] [Google Scholar]
- 44.Ma L, Teruya-Feldstein J, Weinberg RA. Tumour invasion and metastasis initiated by microRNA-10b in breast cancer. Nature. 2007;449:682–688. doi: 10.1038/nature06174. [DOI] [PubMed] [Google Scholar]
- 45.Moriarty CH, Pursell B, Mercurio AM. miR-10b targets Tiam1: implications for Rac activation and carcinoma migration. J Biol Chem. 2010;285:20541–20546. doi: 10.1074/jbc.M110.121012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cottonham CL, Kaneko S, Xu L. miR-21 and miR-31 converge on TIAM1 to regulate migration and invasion of colon carcinoma cells. J Biol Chem. 2010;285:35293–35302. doi: 10.1074/jbc.M110.160069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qian B, Katsaros D, Lu L, Preti M, Durando A, Arisio R, Mu L, Yu H. High miR-21 expression in breast cancer associated with poor disease-free survival in early stage disease and high TGF-β1. Breast Cancer Res Treat. 2009;117:131–140. doi: 10.1007/s10549-008-0219-7. [DOI] [PubMed] [Google Scholar]
- 48.Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454:56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davis BN, Hilyard AC, Nguyen PH, Lagna G, Hata A. Smad proteins bind a conserved RNA sequence to promote microRNA maturation by Drosha. Mol Cell. 2010;39:373–384. doi: 10.1016/j.molcel.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shapiro IM, Cheng AW, Flytzanis NC, Balsamo M, Condeelis JS, Oktay MH, Burge CB, Gertler FB. An EMT-driven alternative splicing program occurs in human breast cancer and modulates cellular phenotype. PLoS Genet. 2011;7:e1002218. doi: 10.1371/journal.pgen.1002218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dutertre M, Lacroix-Triki M, Driouch K, de la Grange P, Gratadou L, Beck S, Millevoi S, Tazi J, Lidereau R, Vagner S, Auboeuf D. Exon-based clustering of murine breast tumor transcriptomes reveals alternative exons whose expression is associated with metastasis. Cancer Res. 2010;70:896–905. doi: 10.1158/0008-5472.CAN-09-2703. [DOI] [PubMed] [Google Scholar]
- 52.Warzecha CC, Sato TK, Nabet B, Hogenesch JB, Carstens RP. ESRP1 and ESRP2 are epithelial cell-type-specific regulators of FGFR2 splicing. Mol Cell. 2009;33:591–601. doi: 10.1016/j.molcel.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, Kingsmore SF, Schroth GP, Burge CB. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008;456:470–476. doi: 10.1038/nature07509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Horiguchi K, Sakamoto K, Koinuma D, Semba K, Inoue A, Inoue S, Fujii H, Yamaguchi A, Miyazawa K, Miyazono K, Saitoh M. TGF-β drives epithelial-mesenchymal transition through EF1-mediated downregulation of ESRP. Oncogene. 2012;31:3190–3201. doi: 10.1038/onc.2011.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Warzecha CC, Jiang P, Amirikian K, Dittmar KA, Lu H, Shen S, Guo W, Xing Y, Carstens RP. An ESRP-regulated splicing programme is abrogated during the epithelial-mesenchymal transition. EMBO J. 2010;29:3286–3300. doi: 10.1038/emboj.2010.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shirakihara T, Horiguchi K, Miyazawa K, Ehata S, Shibata T, Morita I, Miyazono K, Saitoh M. TGF-β regulates isoform switching of FGF receptors and epithelial-mesenchymal transition. EMBO J. 2011;30:783–795. doi: 10.1038/emboj.2010.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fuxe J, Karlsson MC. TGF-β-induced epithelial-mesenchymal transition: A link between cancer and inflammation. Semin. Cancer Biol. 2012;22:455–461. doi: 10.1016/j.semcancer.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 58.Naber HP, ten Dijke P, Pardali E. Role of TGF-β in the tumor stroma. Curr Cancer Drug Targets. 2008;8:466–472. doi: 10.2174/156800908785699342. [DOI] [PubMed] [Google Scholar]
- 59.Jing Y, Han Z, Zhang S, Liu Y, Wei L. Epithelial-Mesenchymal Transition in tumor microenvironment. Cell Biosci. 2011;1:29. doi: 10.1186/2045-3701-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bhowmick NA, Chytil A, Plieth D, Gorska AE, Dumont N, Shappell S, Washington MK, Neilson EG, Moses HL. TGF-β signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 61.Cheng N, Bhowmick NA, Chytil A, Gorksa AE, Brown KA, Muraoka R, Arteaga CL, Neilson EG, Hayward SW, Moses HL. Loss of TGF-β type II receptor in fibroblasts promotes mammary carcinoma growth and invasion through upregulation of TGF-β-, MSP- and HGF-mediated signaling networks. Oncogene. 2005;24:5053–5068. doi: 10.1038/sj.onc.1208685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Franco OE, Jiang M, Strand DW, Peacock J, Fernandez S, Jackson RS, 2nd, Revelo MP, Bhowmick NA, Hayward SW. Altered TGF-β signaling in a subpopulation of human stromal cells promotes prostatic carcinogenesis. Cancer Res. 2011;71:1272–1281. doi: 10.1158/0008-5472.CAN-10-3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bacman D, Merkel S, Croner R, Papadopoulos T, Brueckl W, Dimmler A. TGF-β receptor 2 downregulation in tumour-associated stroma worsens prognosis and high-grade tumours show more tumour-associated macrophages and lower TGF-β1 expression in colon carcinoma: a retrospective study. BMC Cancer. 2007;7:156. doi: 10.1186/1471-2407-7-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barkan D, Kleinman H, Simmons JL, Asmussen H, Kamaraju AK, Hoenorhoff MJ, Liu ZY, Costes SV, Cho EH, Lockett S, Khanna C, Chambers AF, Green JE. Inhibition of metastatic outgrowth from single dormant tumor cells by targeting the cytoskeleton. Cancer Res. 2008;68:6241–6250. doi: 10.1158/0008-5472.CAN-07-6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Elenbaas B, Weinberg RA. Heterotypic signaling between epithelial tumor cells and fibroblasts in carcinoma formation. Exp Cell Res. 2001;264:169–184. doi: 10.1006/excr.2000.5133. [DOI] [PubMed] [Google Scholar]
- 66.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 67.Erler JT, Weaver VM. Three-dimensional context regulation of metastasis. Clin Exp Metastasis. 2009;26:35–49. doi: 10.1007/s10585-008-9209-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taylor MA, Amin J, Kirschmann DA, Schiemann WP. Lysyl oxidase contributes to mechanotransduction-mediated regulation of transforming growth factor-β signaling in breast cancer cells. Neoplasia. 2011;13:406–418. doi: 10.1593/neo.101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leight JL, Wozniak MA, Chen S, Lynch ML, Chen CS. Matrix rigidity regulates a switch between TGF-β1-induced apoptosis and epithelial-mesenchymal transition. Mol Biol Cell. 2012;23:781–791. doi: 10.1091/mbc.E11-06-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Postovit LM, Abbott DE, Payne SL, Wheaton WW, Margaryan NV, Sullivan R, Jansen MK, Csiszar K, Hendrix MJ, Kirschmann DA. Hypoxia/reoxygenation: a dynamic regulator of lysyl oxidase-facilitated breast cancer migration. J Cell Biochem. 2008;103:1369–1378. doi: 10.1002/jcb.21517. [DOI] [PubMed] [Google Scholar]
- 72.Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, Le QT, Giaccia AJ. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Erler JT, Bennewith KL, Nicolau M, Dornhofer N, Kong C, Le QT, Chi JT, Jeffrey SS, Giaccia AJ. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 74.Peinado H, Del Carmen Iglesias-de la Cruz M, Olmeda D, Csiszar K, Fong KS, Vega S, Nieto MA, Cano A, Portillo F. A molecular role for lysyl oxidase-like 2 enzyme in snail regulation and tumor progression. EMBO J. 2005;24:3446–3458. doi: 10.1038/sj.emboj.7600781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Peinado H, Moreno-Bueno G, Hardisson D, Perez-Gomez E, Santos V, Mendiola M, de Diego JI, Nistal M, Quintanilla M, Portillo F, Cano A. Lysyl oxidase-like 2 as a new poor prognosis marker of squamous cell carcinomas. Cancer Res. 2008;68:4541–4550. doi: 10.1158/0008-5472.CAN-07-6345. [DOI] [PubMed] [Google Scholar]
- 76.Moreno-Bueno G, Salvador F, Martin A, Floristan A, Cuevas EP, Santos V, Montes A, Morales S, Castilla MA, Rojo-Sebastian A, Martinez A, Hardisson D, Csiszar K, Portillo F, Peinado H, Palacios J, Cano A. Lysyl oxidase-like 2 (LOXL2), a new regulator of cell polarity required for metastatic dissemination of basal-like breast carcinomas. EMBO Mol Med. 2011;3:528–544. doi: 10.1002/emmm.201100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jo M, Lester RD, Montel V, Eastman B, Takimoto S, Gonias SL. Reversibility of epithelial-mesenchymal transition (EMT) induced in breast cancer cells by activation of urokinase receptor-dependent cell signaling. J Biol Chem. 2009;284:22825–22833. doi: 10.1074/jbc.M109.023960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lester RD, Jo M, Montel V, Takimoto S, Gonias SL. uPAR induces epithelial-mesenchymal transition in hypoxic breast cancer cells. J Cell Biol. 2007;178:425–436. doi: 10.1083/jcb.200701092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cannito S, Novo E, Compagnone A, Valfre di Bonzo L, Busletta C, Zamara E, Paternostro C, Povero D, Bandino A, Bozzo F, Cravanzola C, Bravoco V, Colombatto S, Parola M. Redox mechanisms switch on hypoxia-dependent epithelial-mesenchymal transition in cancer cells. Carcinogenesis. 2008;29:2267–2278. doi: 10.1093/carcin/bgn216. [DOI] [PubMed] [Google Scholar]
- 80.Louie E, Nik S, Chen JS, Schmidt M, Song B, Pacson C, Chen XF, Park S, Ju J, Chen EI. Identification of a stem-like cell population by exposing metastatic breast cancer cell lines to repetitive cycles of hypoxia and reoxygenation. Breast Cancer Res. 2010;12:R94. doi: 10.1186/bcr2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xing F, Okuda H, Watabe M, Kobayashi A, Pai SK, Liu W, Pandey PR, Fukuda K, Hirota S, Sugai T, Wakabayshi G, Koeda K, Kashiwaba M, Suzuki K, Chiba T, Endo M, Mo YY, Watabe K. Hypoxia-induced Jagged2 promotes breast cancer metastasis and self-renewal of cancer stem-like cells. Oncogene. 2011;30:4075–4086. doi: 10.1038/onc.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bendinelli P, Matteucci E, Maroni P, Desiderio MA. NF-κB activation, dependent on acetylation/deacetylation, contributes to HIF-1α activity and migration of bone metastatic breast carcinoma cells. Mol Cancer Res. 2009;7:1328–1341. doi: 10.1158/1541-7786.MCR-08-0548. [DOI] [PubMed] [Google Scholar]
- 83.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nature Rev Cancer. 2010;10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Galliher AJ, Schiemann WP. 3 integrin and Src facilitate transforming growth factor-mediated induction of epithelial-mesenchymal transition in mammary epithelial cells. Breast Cancer Res. 2006;8:R42. doi: 10.1186/bcr1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Galliher AJ, Schiemann WP. Src phosphorylates Tyr284 in TGF-β type II receptor and regulates TGF-β stimulation of p38 MAPK during breast cancer cell proliferation and invasion. Cancer Res. 2007;67:3752–3758. doi: 10.1158/0008-5472.CAN-06-3851. [DOI] [PubMed] [Google Scholar]
- 86.Galliher-Beckley AJ, Schiemann WP. Grb2 binding to Tyr284 in TβR-II is essential for mammary tumor growth and metastasis stimulated by TGF-β. Carcinogenesis. 2008;29:244–251. doi: 10.1093/carcin/bgm245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tian M, Schiemann WP. PGE2 receptor EP2 mediates the antagonistic effect of COX-2 on TGF-β signaling during mammary tumorigenesis. FASEB J. 2010;24:1105–1116. doi: 10.1096/fj.09-141341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wendt MK, Schiemann WP. Therapeutic targeting of the focal adhesion complex prevents oncogenic TGF-β signaling and metastasis. Breast Cancer Res. 2009;11:R68. doi: 10.1186/bcr2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wendt MK, Smith JA, Schiemann WP. p130Cas is required for mammary tumor growth and transforming growth factor-β-mediated metastasis through regulation of Smad2/3 activity. J Biol Chem. 2009;284:34145–34156. doi: 10.1074/jbc.M109.023614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bhowmick NA, Zent R, Ghiassi M, McDonnell M, Moses HL. Integrin β1 signaling is necessary for transforming growth factor-β activation of p38MAPK and epithelial plasticity. J Biol Chem. 2001;276:46707–46713. doi: 10.1074/jbc.M106176200. [DOI] [PubMed] [Google Scholar]
- 91.Wendt MK, Schiemann BJ, Parvani JG, Lee YH, Kang Y, Schiemann WP. TGF-β stimulates Pyk2 expression as part of an epithelial-mesenchymal transition program required for metastatic outgrowth of breast cancer. Oncogene. 2012 doi: 10.1038/onc.2012.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Korpal M, Yan J, Lu X, Xu S, Lerit DA, Kang Y. Imaging transforming growth factor-beta signaling dynamics and therapeutic response in breast cancer bone metastasis. Nature Med. 2009;15:960–966. doi: 10.1038/nm.1943. [DOI] [PubMed] [Google Scholar]
- 93.Shibue T, Weinberg RA. Integrin β1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proc Natl Acad Sci USA. 2009;106:10290–10295. doi: 10.1073/pnas.0904227106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Barkan D, El Touny LH, Michalowski AM, Smith JA, Chu I, Davis AS, Webster JD, Hoover S, Simpson RM, Gauldie J, Green JE. Metastatic growth from dormant cells induced by a Col-I-enriched fibrotic environment. Cancer Res. 2010;70:5706–5716. doi: 10.1158/0008-5472.CAN-09-2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Whittard JD, Craig SE, Mould AP, Koch A, Pertz O, Engel Jr, Humphries MJ. E-cadherin is a ligand for integrin 2 1. Matrix Biol. 2002;21:525–532. doi: 10.1016/s0945-053x(02)00037-9. [DOI] [PubMed] [Google Scholar]
- 96.Mizejewski GJ. Role of integrins in cancer: survey of expression patterns. Proc Soc Exp Biol Med. 1999;222:124–138. doi: 10.1177/153537029922200203. [DOI] [PubMed] [Google Scholar]
- 97.Weaver VM, Lelievre S, Lakins JN, Chrenek MA, Jones JC, Giancotti F, Werb Z, Bissell MJ. 4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2002;2:205–216. doi: 10.1016/s1535-6108(02)00125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Watabe T, Miyazono K. Roles of TGF-β family signaling in stem cell renewal and differentiation. Cell Res. 2009;19:103–115. doi: 10.1038/cr.2008.323. [DOI] [PubMed] [Google Scholar]
- 99.Creighton CJ, Chang JC, Rosen JM. Epithelial-mesenchymal transition (EMT) in tumor-initiating cells and its clinical implications in breast cancer. J Mammary Gland Biol Neoplasia. 2010;15:253–260. doi: 10.1007/s10911-010-9173-1. [DOI] [PubMed] [Google Scholar]
- 100.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Battula VL, Evans KW, Hollier BG, Shi Y, Marini FC, Ayyanan A, Wang RY, Brisken C, Guerra R, Andreeff M, Mani SA. Epithelial-mesenchymal transition-derived cells exhibit multilineage differentiation potential similar to mesenchymal stem cells. Stem Cells. 2010;28:1435–1445. doi: 10.1002/stem.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shipitsin M, Campbell LL, Argani P, Weremowicz S, Bloushtain-Qimron N, Yao J, Nikolskaya T, Serebryiskaya T, Beroukhim R, Hu M, Halushka MK, Sukumar S, Parker LM, Anderson KS, Harris LN, Garber JE, Richardson AL, Schnitt SJ, Nikolsky Y, Gelman RS, Polyak K. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11:259–273. doi: 10.1016/j.ccr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 103.Ben-Porath I, Thomson MW, Carey VJ, Ge R, Bell GW, Regev A, Weinberg RA. An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 2008;40:499–507. doi: 10.1038/ng.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Morel AP, Lievre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS ONE. 2008;3:e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, Rimm DL, Wong H, Rodriguez A, Herschkowitz JI, Fan C, Zhang X, He X, Pavlick A, Gutierrez MC, Renshaw L, Larionov AA, Faratian D, Hilsenbeck SG, Perou CM, Lewis MT, Rosen JM, Chang JC. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci USA. 2009;106:13820–13825. doi: 10.1073/pnas.0905718106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Penheiter SG, Singh RD, Repellin CE, Wilkes MC, Edens M, Howe PH, Pagano RE, Leof EB. Type II transforming growth factor-β receptor recycling is dependent upon the clathrin adaptor protein Dab2. Mol Biol Cell. 2010;21:4009–4019. doi: 10.1091/mbc.E09-12-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Turley EA, Veiseh M, Radisky DC, Bissell MJ. Mechanisms of disease: epithelial-mesenchymal transition--does cellular plasticity fuel neoplastic progression? Nat Clin Pract Oncol. 2008;5:280–290. doi: 10.1038/ncponc1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Asiedu MK, Ingle JN, Behrens MD, Radisky DC, Knutson KL. TGFβ/TNFα-mediated epithelial-mesenchymal transition generates breast cancer stem cells with a claudin-low phenotype. Cancer Res. 2011;71:4707–4719. doi: 10.1158/0008-5472.CAN-10-4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hennessy BT, Gonzalez-Angulo AM, Stemke-Hale K, Gilcrease MZ, Krishnamurthy S, Lee JS, Fridlyand J, Sahin A, Agarwal R, Joy C, Liu W, Stivers D, Baggerly K, Carey M, Lluch A, Monteagudo C, He X, Weigman V, Fan C, Palazzo J, Hortobagyi GN, Nolden LK, Wang NJ, Valero V, Gray JW, Perou CM, Mills GB. Characterization of a naturally occurring breast cancer subset enriched in epithelial-to-mesenchymal transition and stem cell characteristics. Cancer Res. 2009;69:4116–4124. doi: 10.1158/0008-5472.CAN-08-3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Prat A, Parker JS, Karginova O, Fan C, Livasy C, Herschkowitz JI, He X, Perou CM. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010;12:R68. doi: 10.1186/bcr2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Taube JH, Herschkowitz JI, Komurov K, Zhou AY, Gupta S, Yang J, Hartwell K, Onder TT, Gupta PB, Evans KW, Hollier BG, Ram PT, Lander ES, Rosen JM, Weinberg RA, Mani SA. Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proc Natl Acad Sci USA. 2010;107:15449–15454. doi: 10.1073/pnas.1004900107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Taylor RA, Toivanen R, Frydenberg M, Pedersen J, Harewood L, Collins AT, Maitland NJ, Risbridger GP B. Australian Prostate Cancer. Human epithelial basal cells are cells of origin of prostate cancer, independent of CD133 status. Stem Cells. 2012;30:1087–1096. doi: 10.1002/stem.1094. [DOI] [PubMed] [Google Scholar]
- 113.Floor S, van Staveren WC, Larsimont D, Dumont JE, Maenhaut C. Cancer cells in epithelial-to-mesenchymal transition and tumor-propagating-cancer stem cells: distinct, overlapping or same populations. Oncogene. 2011;30:4609–4621. doi: 10.1038/onc.2011.184. [DOI] [PubMed] [Google Scholar]
- 114.Tsuji T, Ibaragi S, Shima K, Hu MG, Katsurano M, Sasaki A, Hu GF. Epithelial-mesenchymal transition induced by growth suppressor p12CDK2-AP1 promotes tumor cell local invasion but suppresses distant colony growth. Cancer Res. 2008;68:10377–10386. doi: 10.1158/0008-5472.CAN-08-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Celia-Terrassa T, Meca-Cortes O, Mateo F, de Paz AM, Rubio N, Arnal-Estape A, Ell BJ, Bermudo R, Diaz A, Guerra-Rebollo M, Lozano JJ, Estaras C, Ulloa C, Alvarez-Simon D, Mila J, Vilella R, Paciucci R, Martinez-Balbas M, de Herreros AG, Gomis RR, Kang Y, Blanco J, Fernandez PL, Thomson TM. Epithelial-mesenchymal transition can suppress major attributes of human epithelial tumor-initiating cells. J Clin Invest. 2012;122:1849–1868. doi: 10.1172/JCI59218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yu Z, Pestell TG, Lisanti MP, Pestell RG. Cancer stem cells. Int J Biochem Cell Biol. 2012;44:2144–2151. doi: 10.1016/j.biocel.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Itoh M, Bissell MJ. The organization of tight junctions in epithelia: implications for mammary gland biology and breast tumorigenesis. J Mammary Gland Biol Neoplasia. 2003;8:449–462. doi: 10.1023/B:JOMG.0000017431.45314.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Agiostratidou G, Hulit J, Phillips GR, Hazan RB. Differential cadherin expression: potential markers for epithelial to mesenchymal transformation during tumor progression. J Mammary Gland Biol Neoplasia. 2007;12:127–133. doi: 10.1007/s10911-007-9044-6. [DOI] [PubMed] [Google Scholar]
- 119.Chaffer CL, Thompson EW, Williams ED. Mesenchymal to epithelial transition in development and disease. Cells Tissues Organs. 2007;185:7–19. doi: 10.1159/000101298. [DOI] [PubMed] [Google Scholar]
- 120.Brabletz T. To differentiate or not - routes towards metastasis. Nature Rev Cancer. 2012 doi: 10.1038/nrc3265. [DOI] [PubMed] [Google Scholar]
- 121.The TGF-β Family. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2008. [Google Scholar]
- 122.Buijs JT, Henriquez NV, van Overveld PG, van der Horst G, Que I, Schwaninger R, Rentsch C, Ten Dijke P, Cleton-Jansen AM, Driouch K, Lidereau R, Bachelier R, Vukicevic S, Clezardin P, Papapoulos SE, Cecchini MG, Lowik CW, van der Pluijm G. Bone morphogenetic protein 7 in the development and treatment of bone metastases from breast cancer. Cancer Res. 2007;67:8742–8751. doi: 10.1158/0008-5472.CAN-06-2490. [DOI] [PubMed] [Google Scholar]
- 123.Buijs JT, van der Horst G, van den Hoogen C, Cheung H, de Rooij B, Kroon J, Petersen M, van Overveld PG, Pelger RC, van der Pluijm G. The BMP2/7 heterodimer inhibits the human breast cancer stem cell subpopulation and bone metastases formation. Oncogene. 2012;31:2164–2174. doi: 10.1038/onc.2011.400. [DOI] [PubMed] [Google Scholar]
- 124.Owens P, Pickup MW, Novitskiy SV, Chytil A, Gorska AE, Aakre ME, West J, Moses HL. Disruption of bone morphogenetic protein receptor 2 (BMPR2) in mammary tumors promotes metastases through cell autonomous and paracrine mediators. Proc Natl Acad Sci USA. 2012;109:2814–2819. doi: 10.1073/pnas.1101139108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Giampieri S, Manning C, Hooper S, Jones L, Hill CS, Sahai E. Localized and reversible TGF-β signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol. 2009;11:1287–1296. doi: 10.1038/ncb1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li R, Liang J, Ni S, Zhou T, Qing X, Li H, He W, Chen J, Li F, Zhuang Q, Qin B, Xu J, Li W, Yang J, Gan Y, Qin D, Feng S, Song H, Yang D, Zhang B, Zeng L, Lai L, Esteban MA, Pei D. A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem Cell. 2010;7:51–63. doi: 10.1016/j.stem.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 127.Howe EN, Cochrane DR, Richer JK. The miR-200 and miR-221/222 microRNA families: opposing effects on epithelial identity. J Mammary Gland Biol Neoplasia. 2012;17:65–77. doi: 10.1007/s10911-012-9244-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chang CJ, Chao CH, Xia W, Yang JY, Xiong Y, Li CW, Yu WH, Rehman SK, Hsu JL, Lee HH, Liu M, Chen CT, Yu D, Hung MC. p53 regulates epithelial-mesenchymal transition and stem cell properties through modulating miRNAs. Nat Cell Biol. 2011;13:317–323. doi: 10.1038/ncb2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cieply B, Riley Pt, Pifer PM, Widmeyer J, Addison JB, Ivanov AV, Denvir J, Frisch SM. Suppression of the epithelial-mesenchymal transition by grainyhead-like-2. Cancer Res. 2012;72:2440–2453. doi: 10.1158/0008-5472.CAN-11-4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang D, Richardson DR. Endoplasmic reticulum protein 29 (ERp29): An emerging role in cancer. Int J Biochem Cell Biol. 2011;43:33–36. doi: 10.1016/j.biocel.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 131.Bambang IF, Lee YK, Richardson DR, Zhang D. Endoplasmic reticulum protein 29 regulates epithelial cell integrity during the mesenchymal-epithelial transition in breast cancer cells. Oncogene. 2012 doi: 10.1038/onc.2012.149. [DOI] [PubMed] [Google Scholar]
- 132.Allington TM, Galliher-Beckley AJ, Schiemann WP. Activated Abl kinase inhibits oncogenic transforming growth factor-β signaling and tumorigenesis in mammary tumors. FASEB J. 2009;23:4231–4243. doi: 10.1096/fj.09-138412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hunter T. Treatment for chronic myelogenous leukemia: the long road to imatinib. J Clin Invest. 2007;117:2036–2043. doi: 10.1172/JCI31691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sherbenou DW, Druker BJ. Applying the discovery of the Philadelphia chromosome. J Clin Invest. 2007;117:2067–2074. doi: 10.1172/JCI31988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chew HK, Barlow WE, Albain K, Lew D, Gown A, Hayes DF, Gralow J, Hortobagyi GN, Livingston R. A phase II study of imatinib mesylate and capecitabine in metastatic breast cancer: Southwest Oncology Group Study 0338. Clin Breast Cancer. 2008;8:511–515. doi: 10.3816/CBC.2008.n.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cristofanilli M, Morandi P, Krishnamurthy S, Reuben JM, Lee BN, Francis D, Booser DJ, Green MC, Arun BK, Pusztai L, Lopez A, Islam R, Valero V, Hortobagyi GN. Imatinib mesylate (Gleevec) in advanced breast cancer-expressing C-Kit or PDGFR-α: clinical activity and biological correlations. Ann Oncol. 2008;19:1713–1719. doi: 10.1093/annonc/mdn352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Modi S, Seidman AD, Dickler M, Moasser M, D'Andrea G, Moynahan ME, Menell J, Panageas KS, Tan LK, Norton L, Hudis CA. A phase II trial of imatinib mesylate monotherapy in patients with metastatic breast cancer. Breast Cancer Res Treat. 2005;90:157–163. doi: 10.1007/s10549-004-3974-0. [DOI] [PubMed] [Google Scholar]