Abstract
Strategies to expand regulatory T cells hold therapeutic potential for ameliorating T cell-mediated autoimmunity. Recently, we reported that the requirements for T cell receptor signaling in conventional T cell and regulatory T cell proliferation are different. Using mutant mice that display defective T cell receptor-mediated phospholipase Cγ (PLCγ) activation, we hereby demonstrate that PLCγ activation is required for antigen-specific conventional T cell proliferation but not for IL-2-induced regulatory T cell proliferation. This led us to hypothesize that in conjunction with IL-2, pharmacological inhibition of T cell receptor-mediated PLCγ activation might offer a novel therapeutic strategy to expand regulatory T cells while simultaneously inhibiting conventional T cell proliferation. Indeed, using the calcineurin inhibitor Cyclosporine A to inhibit signaling downstream of PLCγ, we found that Cyclosporine A attenuated antigen-specific Tconv proliferation but permitted IL-2-induced regulatory T cell expansion in vitro and in vivo. Furthermore, the combination of Cyclosporine A and IL-2 was superior over either Cyclosporine A or IL-2 monotherapy in protection against the T cell-mediated demyelinating autoimmune disease mouse model, experimental autoimmune encephalomyelitis. Thus, a combination of TCR signaling inhibition and IL-2 might be a beneficial strategy in expanding regulatory T cells and inhibiting conventional T cell proliferation in autoimmune settings.
Keywords: Regulatory T cells, conventional T cells, T cell receptor, IL-2, proliferation, autoimmunity
1. Introduction
Regulatory T cells (Treg)s are a subset of T cells with suppressive properties. They are crucial for protecting the host from autoimmunity by inhibiting the response of self-reactive T cells and for preventing immunopathology in overexuberant immune responses directed against foreign antigens (1, 2). Because of their potent inhibitory function, therapies aimed at the selective expansion of Tregs hold therapeutic potential for ameliorating conventional T cell (Tconv)-mediated diseases (3, 4). Indeed, previous studies in mice have demonstrated that Tregs can be selectively increased in vivo by pharmacologic intervention and that this expansion of Tregs leads to positive outcomes in a variety of Tconv-mediated diseases (5–9).
In order to devise strategies to increase Treg numbers in vivo, it is critical to understand the signaling mechanisms that lead to Treg proliferation. Several signaling pathways implicated in Treg proliferation include those driven by IL-2, co-stimulatory molecules, and the TCR (10–13). IL-2 is essential for the maintenance and proliferation of Tregs, as the acute neutralization of IL-2 collapses the homeostasis of Tregs (14) and the administration of IL-2 promotes Treg proliferation (5). In addition to IL-2, Treg proliferation was believed to require the interaction of the TCR with MHC class II (MHCII) expressed on dendritic cells (DC)s. In support of this argument, adoptively transferred Tregs do not proliferate in MHCII-deficient hosts and the deletion of TCR signaling proteins in T cells leads to the decrease in Treg division and survival (10, 11, 15, 16). However, we have recently shown that the provision of exogenous IL-2 induces the proliferation of Tregs adoptively transferred in MHCII-deficient hosts or when the Tregs lacked TCR signaling capacity (17). This suggests that TCR signaling is dispensable for IL-2-induced Treg proliferation. As antigen-specific Tconv proliferation is entirely dependent on TCR stimulation, we sought to take advantage of the differential requirement of the TCR in the proliferation of these two T cell subsets. Thus, we tested whether the pharmacological inhibition of TCR signaling in combination with IL-2 could allow Tregs to selectively expand while simultaneously inhibiting the antigen-specific proliferation of Tconvs.
In the present manuscript, we demonstrate that costimulation but not TCR-activated phospholipase Cγ (PLCγ) is required for IL-2-induced Treg proliferation. Using the calcineurin inhibitor Cyclosporine A (CSA) to inhibit signaling pathways downstream of PLCγ, we show that in combination with IL-2, CSA increases Tregs while preventing antigen-specific Tconv proliferation. Moreover, CSA and IL-2 displayed an additive effect to protect against experimental allergic encephalomyelitis. Thus, a combination of TCR signaling inhibition and IL-2 might be beneficial to increase the Treg:Tconv ratio in treatment of autoimmunity.
2. Materials and Methods
2.1 Mice
C57BL/6 (B6), B6.SJL (CD45.1 congenic), and OT-II TCR transgenic mice were purchased from the National Cancer Institute or Taconic Farms (Germantown, NY). Mice expressing one loxp-flanked allele of Src homology 2 domain-containing leukocyte phosphoprotein of 76 kD (SLP-76) with either one allele of wild-type (WT) SLP-76 (cHet mice) or SLP-76 with a Y145F mutation (Y145F mice) were previously described (18, 19). A Tamoxifen-inducible Cre was used for deletion of the loxp-flanked SLP-76 and a ROSA26-yellow fluorescent protein (YFP) was used as a Cre reporter. Foxp3.GFP-reporter mice were generously provided by Dr. Vijay Kuchroo. Mice were housed in specific pathogen-free conditions and treated in strict compliance with Institutional Animal Care and Use Committee regulations of the University of Pennsylvania.
2.2 Flow cytometry, cell sorting, and data analysis
Antibodies for flow cytometry were purchased from BD Biosciences (San Jose, CA), eBioscience (San Diego, CA), Biolegend (San Diego, CA), and Molecular Probes, Invitrogen (Carlsbad, CA). Cells were stained as reported (17, 20) and analyzed by LSR II or FACSCanto (BD Biosciences). For cell sorting, CD4 and CD8 magnetic bead sorting (Miltenyi Biotec, Auburn, CA) was followed by FACSAria cell sorting (BD Biosciences). FACS-sorted populations were typically of >95% purity. Data were analyzed with FlowJo software (TreeStar, Ashland, OR). Dead cells were excluded from analysis with LIVE/DEAD Fixable Aqua Dead Cell staining. Cell division data from CFSE dilution profiles were transformed into “Division Index” data using FlowJo’s “Proliferation” function whenever possible. The fixation and permeabilization used for Foxp3 staining rids of GFP and YFP fluorescence allowing analysis of CFSE dilution in GFP+ and YFP+ cells. Statistical analysis was performed with Prism software (GraphPad, San Diego, CA).
2.3 In vitro T cell proliferation assays
For Treg proliferation assays, FACS-sorted Foxp3.GFP+CD4+ Tregs or YFP+CD4+CD25+ Tregs were labeled with CFSE. To obtain YFP+CD4+CD25+ T cells, cHet or Y145F mice were orally administered 200 μg/g body weight of Tamoxifen in corn oil for 5 days and rested 5 days. CFSE-labeled Tregs (1×104 cells/well) and MACS-sorted DCs (1×105 cells/well) were plated in 200 μl T cell media (MEM-α with 10% FBS, 1% penicillin/ streptomycin, 10 mM HEPES, and 1 × 10−5 M 2-mercaptoethanol) with mouse GM-CSF (10 ng/ml; PeproTech, Rocky Hill, NJ) and human IL-2 (50 U/ml; PeproTech) with or without anti-CD3 (0.1 μg/ml; BD Biosciences) in 96-well flat bottom plates. CD11c+ DCs were obtained from spleens of mice subcutaneously injected 8-10 days prior with FLT3L-expressing EL4 cells. Cells were cultured with or without the indicated factors at 37 °C and analyzed by flow cytometry 4 days later. For Tconv proliferation assays, FACS-sorted Foxp3.GFP−CD4+ cells or YFP+CD4+CD25− T cells were labeled with CFSE. CFSE-labeled Tconvs (5×104 cells/well) and MACS-sorted DCs (1×105 cells/well) were co-cultured in the presence of GM-CSF (10 ng/ml) and anti-CD3 antibody (0.1 μg/ml) with or without human IL-2 (50 U/ml), and analyzed by flow cytometry 3 days later. CTLA-4 Ig fusion protein (CTLA-4-Ig; R&D Systems) and/or anti-OX40L (clone RM134L; Biolegend) antibody were used at 40 μg/ml. Of note, the fixation and permeabilization used for Foxp3 staining rids of GFP and YFP fluorescence allowing analysis of CFSE dilution in GFP+ and YFP+ cells.
2.4 Adoptive transfer of T cells and ovalbumin (OVA) immunization
FACS-sorted CFSE-labeled CD4+CD25−CD45RBhigh T cells from OT-II mice (CD45.1+) were injected i.v. into B6 mice (CD45.2+). One day later, the mice were injected intraperitoneally with 100 μg OVA (Sigma-Aldrich, St. Louis, MO) in 200 μl PBS/alum (2.25 mg; Thermo scientific) suspension. The mice were then treated with or without different combinations of IL-2 immune complex (IL-2IC)s and CSA (Bedford Laboratories, Bedford, OH). IL-2ICs were prepared by mixing 5 μg of anti-IL-2 antibody (clone JES6-1D; BioXCell, West Lebanon, NH) with 1 μg of mouse IL-2 (eBioscience) for 30 minutes on ice in 200 μl PBS. CSA (50 mg/kg) and IL-2ICs (6 μg/mouse) were injected intraperitoneally as indicated.
2.5 Induction of EAE and stimulation of T cells with MOG peptide
EAE was induced as described previously (21, 22). Briefly, mice were injected with a total of 200 μg of myelin oligodendrocyte glycoprotein (MOG) peptide (MOG35–55; MEVGWYRSPFSRVVHLYRNGK; CSBio, CA) emulsified in 500 μg CFA. Mice received 200 ng Pertussis toxin (List Biological Laboratories, Campbell, CA) intraperitoneally at the time of immunization and 48 h later. The mice were also treated with or without IL-2ICs and/or CSA as indicated. Mice were clinically scored on a five-point scale for signs of disease (0 = no weakness, 1 = limp tail, 2 = mild hindlimb paresis, 3 = moderate to severe hindlimb paresis, 4 = hindlimb paralysis, 5 = moribund or dead). For the combined analysis of three different experiments (n = 7–8 mice/experiment), the clinical scores were normalized to the maximum disease score observed in each experiment and represented as % maximal disease score. Ex vivo quantification of antigen-specific, IFN-γ and IL-17-producing CD4+ T cells was performed as described previously (22). Briefly, draining lymph nodes were isolated from mice and re-stimulated with MOG peptide or media alone for 18 hours. For the final four hours, cells were cultured in the presence of monensin. Intracellular staining for IFN-γ and IL-17 was performed using the BD Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer’s instructions.
3. Results
3.1 Treg proliferation in vitro requires costimulation but not TCR signaling
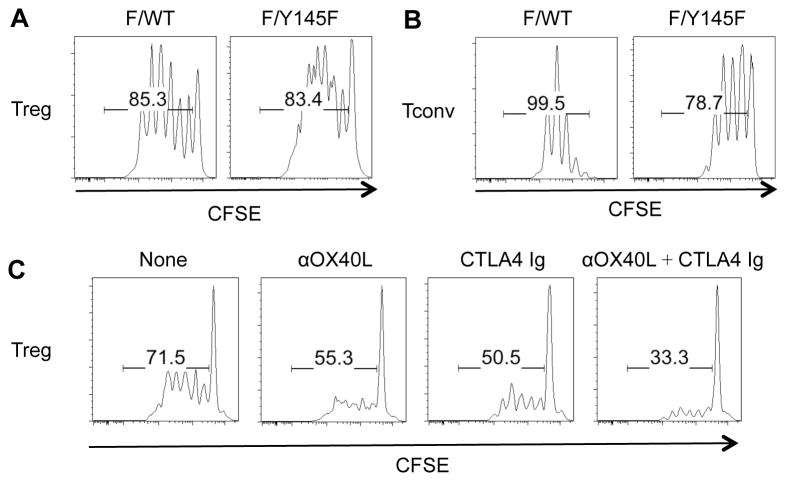
One major signaling pathway downstream of the TCR occurs through PLCγ, which leads to Ca2+ flux and diacylglycerol-mediated signaling (23). To test whether this TCR-activated pathway was required for Treg proliferation, we used Y145F mice that express a Tamoxifen-inducible Cre and one floxed and one Y145F mutant allele of SLP-76. T cells from Tamoxifen-treated Y145F mice exhibit defective PLCγ phosphorylation and Ca2+ flux (24). Despite this defect, Tregs from Tamoxifen-treated cHet mice (one floxed and one WT allele of SLP-76) and Y145F mice proliferated equally well in response to IL-2 and DCs (Fig. 1A). In contrast, anti-CD3-induced proliferation of Y145F Tconvs was significantly attenuated compared to cHet Tconvs (Fig. 1B), suggesting that TCR signaling in Y145F T cells is impaired. These data suggest that while anti-CD3-mediated Tconv proliferation is dependent on TCR-mediated PLCγ activation, IL-2-induced Treg proliferation does not require this pathway.
Fig. 1.
IL-2-induced cHet and Y145F Treg but not anti-CD3-induced Tconv proliferation is similar and requires costimulation. YFP+ cHet and Y145F Tregs and Tconvs were FACS-sorted and labeled with CFSE. Tregs were co-cultured with syngeneic DCs and IL-2. Tconvs were additionally stimulated with anti-CD3. CFSE dilution of (A) Tregs and (B) Tconvs was analyzed by flow cytometry 4 days and 3 days later, respectively. (C) CFSE-labeled FACS-sorted YFP+ Y145F Tregs were cultured with B6-derived DCs and IL-2 in the presence or absence of CTLA-4 Ig (40 μg/ml) and/or anti-OX40L (40 μg/ml). Treg division was analyzed by flow cytometry 4 days later. One representative of 2 independent experiments is shown.
We previously reported that DCs are absolutely required for Treg proliferation (20). To test whether DC-derived co-stimulatory signals were essential for Treg proliferation, Tregs were co-cultured with DCs and IL-2 in the presence or absence of CTLA-4-Ig and/or anti-OX40L antibody (Fig. 1C). The combination of CTLA-4-Ig and anti-OX40L antibody markedly reduced IL-2-induced Y145F Treg proliferation, suggesting that Tregs depend on co-stimulatory molecule stimulation rather than TCR stimulation in IL-2-induced proliferation.
3.2 CSA exhibits differential effects on Treg and Tconv proliferation
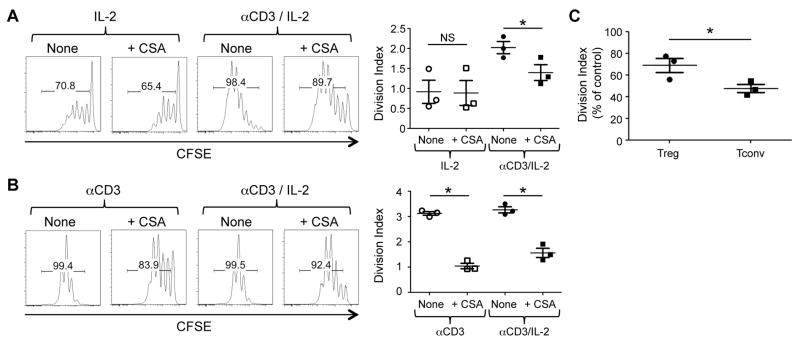
Based on results from the Y145F Tregs, we hypothesized that the combination of pharmacological TCR signal inhibition and IL-2 receptor activation might promote Treg proliferation while inhibiting antigen-specific Tconv expansion. To test this hypothesis, we examined the effect of the calcineurin inhibitor CSA on Treg and Tconv proliferation. As predicted, the addition of CSA minimally affected IL-2-induced Treg proliferation (Fig. 2A). In contrast, anti-CD3-induced Tconv proliferation was attenuated by CSA both in the absence and presence of IL-2 (Fig. 2B). Of note, the addition of anti-CD3 increased IL-2-induced proliferation of Tregs (Fig. 2A). Although CSA inhibited the anti-CD3-augmented portion of Treg proliferation, the impact of CSA on proliferation was still significantly higher on Tconv compared to Treg proliferation (Fig 2C). These results suggest that CSA preferentially attenuates Tconv over Treg proliferation, even when both T cell subsets are stimulated through their TCR.
FIGURE 2.
CSA permits IL-2-induced Treg division while inhibiting Tconv proliferation in vitro. (A) CFSE-labeled FACS-sorted Tregs were co-cultured with syngeneic DCs and IL-2 with or without CSA (100 ng/ml) in the absence or presence or of anti-CD3 antibody. Representative histograms gated on Tregs treated with or without CSA (100 ng/ml) are shown. (B) CFSE-labeled FACS-sorted Tconvs were co-cultured with syngeneic DCs and anti-CD3 with or without CSA (100 ng/ml) in the absence or presence of IL-2. Representative histograms gated on Tconvs treated with or without CSA (100 ng/ml) are shown. One representative of 3 independent experiments is shown. The plots to the right show the division index plotted as mean ± SEM of n = 3/group from 3 independent experiments. * p < 0.01 by paired, two-tailed Student t test. NS = not significant. (C) The division index of Tregs and Tconvs cultured in the presence of anti-CD3 antibody and IL-2 was normalized to the control and is plotted as mean ± SEM of n = 3 independent experiments. * p < 0.01 by unpaired, two-tailed Student t test.
To investigate the effect of CSA in combination with IL-2 on antigen-specific Tconv proliferation and Treg expansion in vivo, we used OT-II TCR transgenic mice that are specific for an OVA-derived epitope. Mice that were adoptively transferred with OT-II Tconvs were immunized with OVA/Alum and treated with PBS, IL-2ICs alone, CSA alone, or CSA+IL-2ICs for 3 consecutive days. Four days after adoptive transfer, OT-II Tconv division and expansion were seen in PBS-treated and IL-2IC-treated mice, whereas those of CSA- or CSA+IL-2IC-treated mice were significantly attenuated (Fig. 3A, 3C, 3D). In contrast to antigen-specific Tconv expansion, the combination of IL-2 with CSA resulted in an increased fraction and absolute number of host Tregs (Fig. 3B, 3E, 3F). These results suggest that the concurrent injection of IL-2ICs with CSA increases the Treg:Tconv ratio while preventing antigen-specific Tconv expansion in vivo.
Fig. 3.
CSA permits IL-2-induced Treg expansion and function while preventing antigen-specific Tconv proliferation in vivo. CFSE-labeled FACS-sorted OT-II Tconvs (CD45.1+) were adoptively transferred into WT B6 mice (CD45.2+) and immunized with OVA/Alum on Day 3 after transfer. The mice were also injected with either vehicle (PBS), CSA, IL-2ICs, or CSA+IL-2ICs on Days 3–5. On Day 6 after transfer, CFSE dilution of OT-II Tconvs and host-derived T cells in the spleen were analyzed by flow cytometry. (A) Representative plots of OT-II-derived Tconv CFSE dilution and (B) host-derived CD4+ Treg percentages are shown. (C) The division index and (D) absolute numbers of OT-II Tconvs is represented as mean ± SEM of n = 6 mice/group from two independent experiments. (E) Percentages and (F) absolute numbers of host CD4+ Tregs is represented as mean ± SEM of n = 6 mice/group from two independent experiments. * p < 0.05; ** p < 0.001 by two-tailed Student t test compared to the PBS-treated group. (G) Foxp3.GFP-reporter mice were treated with PBS, CSA, IL-2ICs, or CSA+IL-2ICs. FACS-sorted Foxp3.GFP+ Tregs from these mice were co-cultured with CFSE-labeled FACS-sorted Tconvs (CD45.2+) and irradiated feeder cells (CD45.1+) at various Treg:Tconv ratios in the presence of anti-CD3 antibody. CFSE dilution of CD45.2+ Tconvs was analyzed by flow cytometry 4 days later. The division index of Tconvs was plotted against various Treg:Tconv ratios. One representative of 2 independent experiments is shown.
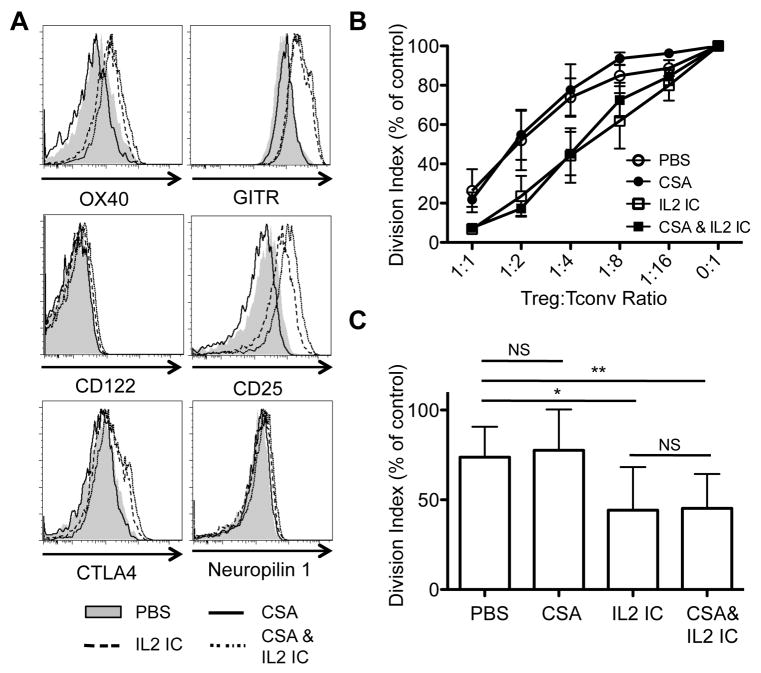
CSA treatment has been reported to suppress Foxp3 expression and could potentially inhibit Treg function (25–27). Indeed, Tregs from CSA-treated mice exhibited lower Foxp3 levels, but the addition of IL-2ICs to CSA rescued the decreased Foxp3 expression in Tregs (Fig. 3B). Moreover, Tregs from IL-2IC- and CSA+IL-2IC-treated mice expressed comparable levels of the high affinity IL-2 receptor (CD122 and CD25) and other co-receptors (OX-40, GITR, CTLA-4) important for Treg function (Fig. 4A). Importantly, Tregs from mice treated with IL-2ICs displayed significantly enhanced suppressive function, which was sustained even when the mice were additionally treated with CSA (Fig. 4B, C). In contrast, the suppressive function of Tregs from mice treated with CSA alone was not significantly different compared to Tregs from mice treated with PBS (Fig. 4B, C). Together, these results demonstrate that CSA+IL-2IC treatment can selectively expand Tregs in vivo while preventing antigen-specific Tconv proliferation and that these expanded Tregs display enhanced suppressive function with retention of phenotypic markers.
Fig. 4.
Tregs from mice treated with CSA plus IL-2 ICs retain phenotypic markers and display enhanced suppressive function. (A) Foxp3 GFP-reporter mice were treated with vehicle (PBS), CSA, IL-2 ICs, or CSA plus IL-2 ICs for 3 days and Tregs were analyzed for expression of OX40, GITR, CTLA4, CD122, CD25, and Neuropilin 1, 1 day after the last treatment. (B) FACS-sorted GFP+ Tregs from these mice were co-cultured with CFSE-labeled FACS-sorted Tconvs (CD45.2+) and irradiated feeder cells (CD45.1+) at various Treg:Tconv ratios in the presence of anti-CD3 antibody. The division index was normalized to cultures with no Tregs (0:1 Treg:Tconv ratio) in each experiment. The data are represented as mean ± SD of 3 independent experiments. (C) Statistical analysis was performed on the division index at the 1:4 Treg:Tconv ratio. * p < 0.05 and ** p < 0.01 by paired t test. NS = not significant.
3.3 The combination of CSA and IL-2ICs is more effective than CSA or IL-2IC monotherapy in attenuating EAE disease severity
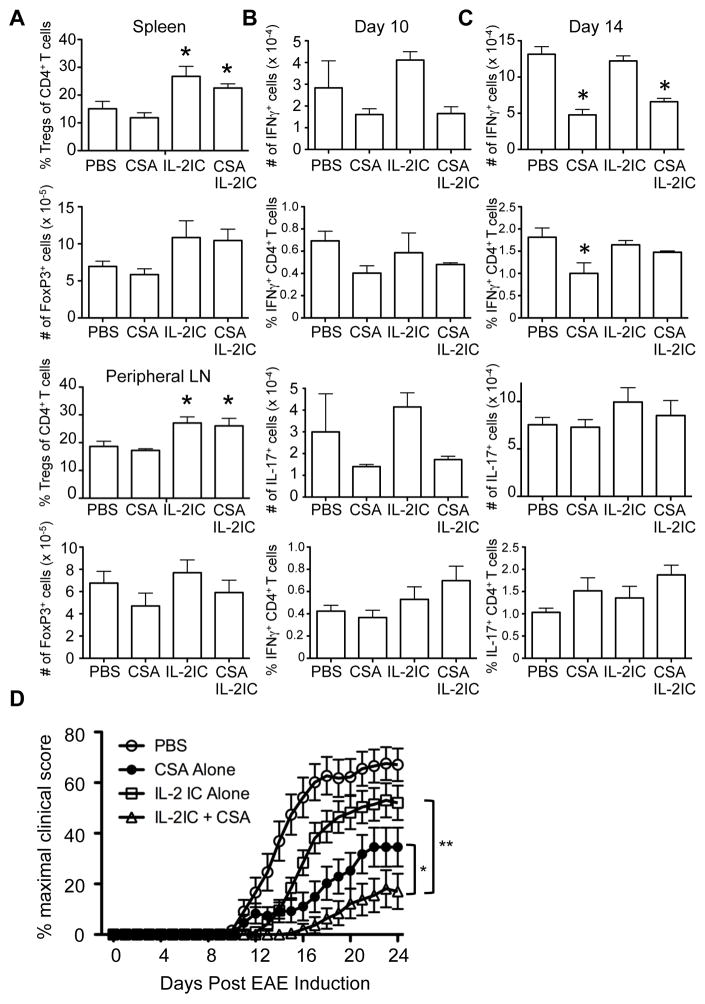
It has been previously shown that Tregs play an important role in modulating EAE disease severity in vivo (28, 29). Given that CSA inhibited antigen-specific Tconv proliferation while maintaining the potential for IL-2-induced Treg expansion, we hypothesized that the combination of CSA and IL-2ICs would be optimal for attenuating EAE disease severity. To this end, we induced EAE in mice and treated them with PBS alone, CSA alone, IL-2ICs alone, or CSA+IL-2ICs for 3 consecutive days 2 days after EAE induction. We first examined whether an increase in the Treg:Tconv ratio was observed in the secondary lymphoid organs of CSA+IL-2IC-treated EAE-induced mice. As expected, a significant elevation in Treg percentages was seen in the spleen and peripheral lymph nodes of mice treated with IL-2ICs alone or CSA+IL-2ICs (Fig. 5A). Total Treg numbers also trended to be increased in the spleen but not in the lymph nodes of IL-2IC- and CSA+IL-2IC-treated mice. Next, to gauge the effect of CSA and IL-2ICs on antigen-specific Tconvs during EAE, we examined the number of MOG-specific CD4+ T cells expressing IFNγ and IL-17 in these mice, as these cytokines are important for EAE pathogenesis (30). A trend towards decreased numbers and percentages of MOG-specific CD4+ T cells producing IFNγ was seen with CSA treatment with or without IL-2ICs on Day 10 (Fig. 5B). Compared to Day 10, an increase in the number and fraction of MOG-specific IFNγ+ CD4+ T cells was seen on Day 14. On Day 14, the number of MOG-specific IFNγ+ CD4+ T cells was significantly reduced in CSA-treated mice, even in conjunction with IL-2IC treatment (Fig. 5C). However, CSA had little effect on IL-17+ CD4+ T cells on Days 10 and 14, except for a trend towards decreased total numbers of IL-17-producing cells on Day 10. Consistent with previous data by others (5), IL-2IC treatment alone did not suppress cytokine-producing T cells during EAE.
Fig 5.
The combination of CSA and IL-2ICs is more effective in protection against EAE than CSA or IL-2ICs alone. EAE was induced on Day 0 by immunization of MOG35–55 in CFA. Mice were treated with vehicle (PBS), CSA, IL-2ICs, or CSA+IL-2ICs from days 1–3. (A)Spleen and peripheral lymph nodes were harvested 7 days after immunization and analyzed for %Tregs of all CD4+ T cells and the absolute number of Tregs by flow cytometry. (B) 10 or (C) 14 days after EAE induction, peripheral lymph nodes were harvested and restimulated with MOG35–55 peptide. CD4+ T cells were analyzed for expression of IFNγ (top) and IL-17 (bottom) by flow cytometry. The total number and percentage of CD4+ T cells producing each cytokine are shown. Data are represented as mean ± SD of n = 3 mice/group. * p < 0.05 by two-tailed Student t test compared to the PBS-treated group. (D) The clinical scores of mice from 3 independent experiments were combined and represented as percent of mean maximal disease score ± SEM of n = 22–24 mice. * p < 0.05 and ** p < 0.01 by ANOVA with Tukey’s post hoc test.
We next quantified disease severity based on the clinical presentation of the EAE-induced mice. Compared to PBS-treated control mice, treatment with IL-2ICs or CSA alone significantly diminished the clinical score and delayed disease onset (Fig. 5D, Table 1). Importantly, mice that were treated CSA+IL-2ICs showed significantly reduced disease severity and disease incidence compared to mice treated with either IL-2ICs or CSA alone (Fig. 5D, Table 1). These results demonstrate that CSA exhibits an additive effect with IL-2ICs and that the short-term injection of CSA+IL-2ICs might be effective in treatment of Tconv-mediated autoimmune disorders such as EAE.
Table 1.
The effect of CSA, IL-2IC and combination therapy on EAE
| Treatment | Incidence | Day of onseta (mean ± SEM) | Maximal score (mean ± SEM) |
|---|---|---|---|
| PBS | 22/24 | 14.1 ± 0.7 | 2.8 ± 0.3 |
| IL-2IC | 19/23 | 15.4 ± 0.5 | 2.1 ± 0.3 |
| CSA | 16/23 | 20.2 ± 1.4b | 1.7 ± 0.4c |
| CSA + IL-2IC | 6/22d | 19.7 ± 1.4e | 0.6 ± 0.5f |
The day of onset is inclusive of mice exhibiting clinical disease only.
p < 0.001 vs. PBS group, p < 0.005 vs. IL-2IC group by two-tailed Student t test.
p < 0.05 vs. PBS group by two-tailed Student t test.
p < 0.001 vs. all groups by Fisher exact test.
p < 0.01 vs. PBS group, p < 0.05 vs. IL-2IC group by two-tailed Student t test;
p < 0.001 vs. IL-2IC and PBS groups, p < 0.05 vs. CSA group by two-tailed Student t test.
4. Discussion
A deep understanding of the mechanisms that control Treg proliferation in vivo is necessary for enhancing clinical applications involving Tregs. TCR stimulation of Tregs has been considered necessary for Treg expansion, since Tregs adoptively transferred into MHCII-deficient hosts do not proliferate (15). However, more recent studies have challenged this view by demonstrating that Tregs proliferate in the absence of MHCII in vitro if IL-2 is provided (20, 31). This raised the possibility that Tregs cannot proliferate in MHCII-deficient mice because of the lack of IL-2 production by Tconvs. This notion is supported by our recent findings showing that the provision of exogenous IL-2 partially restores the proliferation defect of Tregs adoptively transferred into MHCII-deficient hosts or of Tregs lacking TCR signaling capacity (17).
In this study, we demonstrated that among the downstream TCR signaling pathways, those leading from PLCγ activation are not necessary for IL-2-induced Treg expansion. Based on these data, we hypothesized that when given in conjunction with IL-2, pharmacological inhibition of TCR signaling pathways downstream of PLCγ would inhibit Tconv proliferation while allowing Tregs to expand. Indeed, calcineurin inhibition by CSA did not inhibit IL-2-induced Treg expansion but blocked antigen-specific Tconv proliferation in vitro and in vivo. Even in the presence of IL-2 and overt TCR stimulation by anti-CD3 antibody, CSA had a stronger effect on Tconv proliferation than Treg proliferation. These effects translated to a beneficial effect of CSA+IL-2 in EAE disease progression. Thus, the combination of IL-2 and TCR signaling inhibitors could be potentially used as a strategy to increase the Treg:Tconv ratio for treatment of autoimmunity.
Although CSA permits IL-2-induced Treg expansion, Treg function could be inhibited by CSA as the interaction of NFAT and Foxp3 is important for Treg function (32). Consistent with this notion, detrimental effects of CSA on Tregs including Foxp3 downregulation have been reported (25, 26, 33). However, as IL-2 induces Foxp3 expression (34, 35), we found that CSA-mediated downregulation of Foxp3 expression was reversed by co-administration of IL-2. Moreover, Treg phenotypic markers were maintained and Treg ex vivo function was enhanced in CSA+IL-2IC-treated mice. Although Treg function can potentially occur in a TCR-independent manner (36), it is still likely that continuous inhibition of TCR signaling would lead to diminished Treg function. Thus, CSA+IL-2 might still be best used as short-term treatment so that the full suppressive function of Tregs can be maintained.
The key factor driving Treg expansion and survival is IL-2. IL-2 receptor ligation can lead to the activation of three main signaling pathways: PI3K, ERK, and JAK-STAT. Interestingly, it has previously been shown that compared to Tconvs, Tregs display a distinct IL-2 receptor signaling pattern, where the PI3K signaling pathway fails to be activated (37). This has been attributed to high expression of phosphatase and tensin homolog (PTEN) in Tregs, which antagonizes the PI3K pathway. Hence, IL-2 signaling alone is insufficient to induce Treg proliferation, unless PTEN is suppressed by TCR stimulation (38). Alternatively, the PI3K pathway can be engaged by co-stimulatory signals provided by DCs. Our data suggest that in the presence of syngeneic DCs, IL-2 and costimulatory signals by play a dominant role in Treg proliferation. Thus, blockade of TCR signals by CSA has a minimal effect on Treg expansion.
Our proposal that Treg expansion by IL-2IC treatment is responsible for the additive effect on the reduction in severity of EAE in combination with CSA administration is consistent with numerous reports on the role of Tregs in EAE. IL-2ICs have previously been shown to expand Tregs, which correlates with a reduction in EAE clinical score (5). Furthermore, the adoptive transfer of Tregs is effective in attenuating disease severity in EAE (39, 40). Treg depletion would be necessary to test the precise role of Tregs after CSA and IL-2IC combination therapy during EAE. Unfortunately, this was not feasible, as the available methods for Treg depletion are untenable for our system. Foxp3-DTR mice treated with diphtheria toxin are prone to mortality, particularly in disease models such as EAE (41, 42), which has led to severe limitations in interpretations of Foxp3-DTR experimental use in EAE (43). Anti-CD25 monoclonal antibody administration is also inappropriate for our studies since anti-CD25 monoclonal antibody treatment in vivo functions to either shed or internalize the CD25 molecule (44), which would confound our central objective of determining the effects of IL-2 administration during EAE. Nonetheless, strong evidence suggests that the balance between Treg and Tconvs is crucial to the outcome in EAE (45). This is consistent with our finding that the number of MOG-specific CD4+ T cells producing IFN-γ is unchanged with IL-2 administration alone but reduced by CSA treatment with or without IL-2. Hence, the ratio of Treg to antigen-specific Tconvs is most elevated in the CSA and IL-2IC treatment group, correlating with a greater reduction in EAE disease severity.
5. Conclusions
In summary, we took advantage of the differential requirement of TCR signaling in Tconv vs. Treg proliferation and showed that the combination of CSA and IL-2 expands Tregs, while simultaneously inhibiting antigen-specific Tconv proliferation. These effects translated to a beneficial and additive effect of IL-2 and CSA in EAE disease progression. Thus, a combination of TCR signaling inhibition and IL-2 might be a beneficial approach for expanding Tregs while simultaneously inhibiting Tconv proliferation in autoimmune settings.
Acknowledgments
We thank the Kambayashi, Wu, Behrens, Nichols, and Koretzky lab members for helpful discussions. This work was supported by grants from the National Blood Foundation, the University of Pennsylvania internal funds, and the National Institutes of Health (K08HL086503, K08NS062138, R01HL111501).
Abbreviations used in this article
- CSA
Cyclosporine A
- DC
dendritic cell
- EAE
experimental autoimmune encephalomyelitis
- IC
immune complex
- MHCII
major histocompatability complex class II
- PLCγ
phospholipase Cγ
- PTEN
phosphatase and tensin homolog
- SLP-76
Src homology 2 domain-containing leukocyte phosphoprotein of 76 kD
- Tconv
conventional T cell
- Treg
regulatory T cell
- WT
wild-type
- YFP
yellow fluorescent protein
Footnotes
Disclosures
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30(5):616–25. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buckner JH. Mechanisms of impaired regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat Rev Immunol. 2010;10(12):849–59. doi: 10.1038/nri2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat Rev Immunol. 2003;3(3):199–210. doi: 10.1038/nri1027. [DOI] [PubMed] [Google Scholar]
- 5.Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD, et al. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med. 2009;206(4):751–60. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steiner D, Brunicki N, Bachar-Lustig E, Taylor PA, Blazar BR, Reisner Y. Overcoming T cell-mediated rejection of bone marrow allografts by T-regulatory cells: synergism with veto cells and rapamycin. Exp Hematol. 2006;34(6):802–8. doi: 10.1016/j.exphem.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 7.Zeiser R, Leveson-Gower DB, Zambricki EA, Kambham N, Beilhack A, Loh J, et al. Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells compared with conventional CD4+ T cells. Blood. 2008;111(1):453–62. doi: 10.1182/blood-2007-06-094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao W, Lu Y, El Essawy B, Oukka M, Kuchroo VK, Strom TB. Contrasting effects of cyclosporine and rapamycin in de novo generation of alloantigen-specific regulatory T cells. Am J Transplant. 2007;7(7):1722–32. doi: 10.1111/j.1600-6143.2007.01842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruggenenti P, Perico N, Gotti E, Cravedi P, D’Agati V, Gagliardini E, et al. Sirolimus versus cyclosporine therapy increases circulating regulatory T cells, but does not protect renal transplant patients given alemtuzumab induction from chronic allograft injury. Transplantation. 2007;84(8):956–64. doi: 10.1097/01.tp.0000284808.28353.2c. [DOI] [PubMed] [Google Scholar]
- 10.Kim JK, Klinger M, Benjamin J, Xiao Y, Erle DJ, Littman DR, et al. Impact of the TCR signal on regulatory T cell homeostasis, function, and trafficking. PLoS One. 2009;4(8):e6580. doi: 10.1371/journal.pone.0006580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen S, Chuck MI, Zhu M, Fuller DM, Yang CW, Zhang W. The importance of LAT in the activation, homeostasis, and regulatory function of T cells. J Biol Chem. 2010;285(46):35393–405. doi: 10.1074/jbc.M110.145052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burchill MA, Yang J, Vang KB, Moon JJ, Chu HH, Lio CW, et al. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity. 2008;28(1):112–21. doi: 10.1016/j.immuni.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang Q, Henriksen KJ, Boden EK, Tooley AJ, Ye J, Subudhi SK, et al. Cutting edge: CD28 controls peripheral homeostasis of CD4+CD25+ regulatory T cells. J Immunol. 2003;171(7):3348–52. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 14.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201(5):723–35. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhandoola A, Tai X, Eckhaus M, Auchincloss H, Mason K, Rubin SA, et al. Peripheral expression of self-MHC-II influences the reactivity and self-tolerance of mature CD4(+) T cells: evidence from a lymphopenic T cell model. Immunity. 2002;17(4):425–36. doi: 10.1016/s1074-7613(02)00417-x. [DOI] [PubMed] [Google Scholar]
- 16.Gavin MA, Clarke SR, Negrou E, Gallegos A, Rudensky A. Homeostasis and anergy of CD4(+)CD25(+) suppressor T cells in vivo. Nat Immunol. 2002;3(1):33–41. doi: 10.1038/ni743. [DOI] [PubMed] [Google Scholar]
- 17.Zou T, Satake A, Corbo-Rodgers E, Schmidt AM, Farrar MA, Maltzman JS, et al. Cutting edge: IL-2 signals determine the degree of TCR signaling necessary to support regulatory T cell proliferation in vivo. J Immunol. 2012;189(1):28–32. doi: 10.4049/jimmunol.1200507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu GF, Corbo E, Schmidt M, Smith-Garvin JE, Riese MJ, Jordan MS, et al. Conditional deletion of SLP-76 in mature T cells abrogates peripheral immune responses. Eur J Immunol. 2011;41(7):2064–73. doi: 10.1002/eji.201040809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jordan MS, Smith JE, Burns JC, Austin JE, Nichols KE, Aschenbrenner AC, et al. Complementation in trans of altered thymocyte development in mice expressing mutant forms of the adaptor molecule SLP76. Immunity. 2008;28(3):359–69. doi: 10.1016/j.immuni.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zou T, Caton AJ, Koretzky GA, Kambayashi T. Dendritic cells induce regulatory T cell proliferation through antigen-dependent and -independent interactions. J Immunol. 2010;185(5):2790–9. doi: 10.4049/jimmunol.0903740. [DOI] [PubMed] [Google Scholar]
- 21.Racke MK. Experimental autoimmune encephalomyelitis (EAE) Curr Protoc Neurosci. 2001;Chapter 9(Unit 9):7. doi: 10.1002/0471142301.ns0907s14. [DOI] [PubMed] [Google Scholar]
- 22.Wu GF, Shindler KS, Allenspach EJ, Stephen TL, Thomas HL, Mikesell RJ, et al. Limited sufficiency of antigen presentation by dendritic cells in models of central nervous system autoimmunity. J Autoimmun. 2011;36(1):56–64. doi: 10.1016/j.jaut.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith-Garvin JE, Koretzky GA, Jordan MS. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jordan MS, Sadler J, Austin JE, Finkelstein LD, Singer AL, Schwartzberg PL, et al. Functional hierarchy of the N-terminal tyrosines of SLP-76. J Immunol. 2006;176(4):2430–8. doi: 10.4049/jimmunol.176.4.2430. [DOI] [PubMed] [Google Scholar]
- 25.Coenen JJ, Koenen HJ, van Rijssen E, Kasran A, Boon L, Hilbrands LB, et al. Rapamycin, not cyclosporine, permits thymic generation and peripheral preservation of CD4+ CD25+ FoxP3+ T cells. Bone Marrow Transplant. 2007;39(9):537–45. doi: 10.1038/sj.bmt.1705628. [DOI] [PubMed] [Google Scholar]
- 26.Zeiser R, Nguyen VH, Beilhack A, Buess M, Schulz S, Baker J, et al. Inhibition of CD4+CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood. 2006;108(1):390–9. doi: 10.1182/blood-2006-01-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mantel PY, Ouaked N, Ruckert B, Karagiannidis C, Welz R, Blaser K, et al. Molecular mechanisms underlying FOXP3 induction in human T cells. J Immunol. 2006;176(6):3593–602. doi: 10.4049/jimmunol.176.6.3593. [DOI] [PubMed] [Google Scholar]
- 28.Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol. 2002;169(9):4712–6. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- 29.O’Connor RA, Anderton SM. Foxp3+ regulatory T cells in the control of experimental CNS autoimmune disease. J Neuroimmunol. 2008;193(1–2):1–11. doi: 10.1016/j.jneuroim.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 30.Schreiner B, Heppner FL, Becher B. Modeling multiple sclerosis in laboratory animals. Semin Immunopathol. 2009;31(4):479–95. doi: 10.1007/s00281-009-0181-4. [DOI] [PubMed] [Google Scholar]
- 31.Swee LK, Bosco N, Malissen B, Ceredig R, Rolink A. Expansion of peripheral naturally occurring T regulatory cells by Fms-like tyrosine kinase 3 ligand treatment. Blood. 2009;113(25):6277–87. doi: 10.1182/blood-2008-06-161026. [DOI] [PubMed] [Google Scholar]
- 32.Wu Y, Borde M, Heissmeyer V, Feuerer M, Lapan AD, Stroud JC, et al. FOXP3 controls regulatory T cell function through cooperation with NFAT. Cell. 2006;126(2):375–87. doi: 10.1016/j.cell.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 33.Segundo DS, Ruiz JC, Izquierdo M, Fernandez-Fresnedo G, Gomez-Alamillo C, Merino R, et al. Calcineurin inhibitors, but not rapamycin, reduce percentages of CD4+CD25+FOXP3+ regulatory T cells in renal transplant recipients. Transplantation. 2006;82(4):550–7. doi: 10.1097/01.tp.0000229473.95202.50. [DOI] [PubMed] [Google Scholar]
- 34.Zorn E, Nelson EA, Mohseni M, Porcheray F, Kim H, Litsa D, et al. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108(5):1571–9. doi: 10.1182/blood-2006-02-004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao Z, Kanno Y, Kerenyi M, Stephens G, Durant L, Watford WT, et al. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 2007;109(10):4368–75. doi: 10.1182/blood-2006-11-055756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szymczak-Workman AL, Workman CJ, Vignali DA. Cutting edge: regulatory T cells do not require stimulation through their TCR to suppress. J Immunol. 2009;182(9):5188–92. doi: 10.4049/jimmunol.0803123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bensinger SJ, Walsh PT, Zhang J, Carroll M, Parsons R, Rathmell JC, et al. Distinct IL-2 receptor signaling pattern in CD4+CD25+ regulatory T cells. J Immunol. 2004;172(9):5287–96. doi: 10.4049/jimmunol.172.9.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walsh PT, Buckler JL, Zhang J, Gelman AE, Dalton NM, Taylor DK, et al. PTEN inhibits IL-2 receptor-mediated expansion of CD4+ CD25+ Tregs. J Clin Invest. 2006;116(9):2521–31. doi: 10.1172/JCI28057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGeachy MJ, Stephens LA, Anderton SM. Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4+CD25+ regulatory cells within the central nervous system. J Immunol. 2005;175(5):3025–32. doi: 10.4049/jimmunol.175.5.3025. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, Koldzic DN, Izikson L, Reddy J, Nazareno RF, Sakaguchi S, et al. IL-10 is involved in the suppression of experimental autoimmune encephalomyelitis by CD25+CD4+ regulatory T cells. Int Immunol. 2004;16(2):249–56. doi: 10.1093/intimm/dxh029. [DOI] [PubMed] [Google Scholar]
- 41.Zammit DJ, Cauley LS, Pham QM, Lefrancois L. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity. 2005;22(5):561–70. doi: 10.1016/j.immuni.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Isaksson M, Lundgren BA, Ahlgren KM, Kampe O, Lobell A. Conditional DC depletion does not affect priming of encephalitogenic Th cells in EAE. Eur J Immunol. 2012;42(10):2555–63. doi: 10.1002/eji.201142239. [DOI] [PubMed] [Google Scholar]
- 43.Subramanian S, Yates M, Vandenbark AA, Offner H. Oestrogen-mediated protection of experimental autoimmune encephalomyelitis in the absence of Foxp3+ regulatory T cells implicates compensatory pathways including regulatory B cells. Immunology. 2011;132(3):340–7. doi: 10.1111/j.1365-2567.2010.03380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kohm AP, McMahon JS, Podojil JR, Begolka WS, DeGutes M, Kasprowicz DJ, et al. Cutting Edge: Anti-CD25 monoclonal antibody injection results in the functional inactivation, not depletion, of CD4+CD25+ T regulatory cells. J Immunol. 2006;176(6):3301–5. doi: 10.4049/jimmunol.176.6.3301. [DOI] [PubMed] [Google Scholar]
- 45.Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13(4):423–31. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]