Abstract
Chronic mountain sickness (CMS) or lack of adaptation to live in high altitudes is related to environmental hypoxia and excessive erythrocytosis (EE) (Hemoglobin>21 and >19g/dl for men and women, respectively). Diagnosis of CMS (“Qinghai CMS Score”) is based on seven signs/symptoms (breathlessness and/or palpitations, sleep disturbance, cyanosis, dilatation of veins, paresthesia, headache, tinnitus) and the score for EE. The present study was designed to determine the association between hemoglobin, Qinghai CMS score, CMS clinical score (7 signs/symptoms) and Health Status using a health survey composed of 20 items.
The rate of CMS (32.6%) was higher than the rate of EE (9.7%; P<0.002). A significant inverse relationship was observed between CMS clinical score and health status score (r=−0.56 for men, and r=−0.55 for women, P<0.01). However, CMS clinical score was not different in groups with different Hb levels.
Health status score was significantly higher in subjects without CMS. In conclusion, elevated hemoglobin levels were not associated with elevated CMS clinical score.
Keywords: Chronic mountain sickness, chronic hypoxia, excessive erythrocytosis, Peru, gender
1. Introduction
Chronic mountain sickness (CMS) is a condition characterized by a lack of adaptation to live in high altitudes (Xing et al., 2008). This condition was first described by Carlos Monge in the Peruvian Andes in 1925 (Monge, 1942) and it is related to lower oxygen availability (hypoxia) in the environment resulting in lower arterial oxygen saturation or pulse oxygen saturation (SpO2) (hypoxemia).
Hypoventilation is the major contributor to the hypoxemia resulting in excessive erythrocytosis (Peñaloza and Arias Stella, 2007). Hypoventilation is accentuated by a severe hypoxemia mainly during sleep at high altitude (Spicuzza et al., 2004). However, recently, it has been suggested the possibility that excessive erythrocytosis itself begets hypoventilation, which results in greater hypoxemia, further erythropoietic response, and more hemoglobin levels (Swenson, 2012).
Excessive erythrocytosis was defined as hemoglobin (Hb) value ≥21 g/dL in men or ≥19 g/dL in women (Kong et al., 2011) based on the fact that distribution of Hb levels in men shifts to the right in relation to Hb distribution in women. However, there are not clinical parameters that justify these different cut-off Hb values to define EE in men and women at high altitudes. This is an important issue since EE is the main parameter in the diagnosis of chronic mountain sickness. Moreover, limits of normal hemoglobin as a function of altitude and antiquity of life at high altitudes may vary considerably around the word (Beall et al., 2002; Wu et al., 2005; Gonzales, 2007; Gonzales, 2013).
CMS is a clinical syndrome that occurs to native or long-life residents above 2500 m., and characterized by excessive erythrocytosis, severe hypoxemia, and in some cases moderate or severe pulmonary hypertension, which may evolve to cor pulmonale, leading to right ventricular failure (Leon-Velarde et al., 2005). The clinical picture of CMS gradually disappears after descending to low altitude and reappears when returning to high altitude. The prevalence of CMS increases with altitude and age, and it is more frequent in men (Peñaloza and Arias Stella, 2007).
The diagnosis of CMS, is based on a test defined by a consensus statement and named “The Qinghai CMS score” (Leon-Velarde et al., 2005). The test includes assessments of 7 signs or symptoms of CMS and the score assigned for EE (score=0 if Hb level is below the cut-off point for EE and score=3 if Hb level is defined as EE). The signs/symptoms included in the test are breathlessness and/or palpitations, sleep disturbance, cyanosis, dilatation of veins, paresthesia, headache, tinnitus and excessive erythrocytosis. It is diagnosed as CMS when score is over 5 (Leon-Velarde et al., 2005).
According to definition of EE, the values of Hb ≥19–21 g/dL are defined as EE in women but not in men. It is unknown if men and women within this range of Hb values have differences in the CMS clinical score based on 7 signs/symptoms of CMS.
It is also important to know if this test also reflects a health status at high altitudes. In the literature, there are different generic health-related quality of life (HRQL) surveys for assessment of health status. In Peru, we have used and validated in populations at sea level and at high altitude a shortened version consisting in 20 questions (SF-20) (Gonzales, 2010) of the Short Form 36 Health Survey (SF-36) (Vilagut et al., 2008; Banegas et al., 2007; Vilagut et al., 2005) to score health status.
High hemoglobin levels are associated with high systolic and diastolic blood pressure at low (Kawamoto et al., 2011) and at high altitudes (Gonzales and Tapia, 2013; Jefferson et al., 2002). However, it is unknown if the Qinghai CMS score correlates with arterial blood pressure.
For such reason, the present study has been designed to determine the rate of EE and the rate of CMS in men and women at high altitude. In addition, the study aimed to determine the association between hemoglobin and both CMS clinical score and Qinghai CMS score, and the association between both CMS clinical score and Qinghai CMS score with the score for health status (SF-20). The role of menopausal status on Hb, SpO2, CMS score and Health status score were also assessed.
2. Material and Methods
2.1. Study area
A cross-sectional study was performed during 2010 in two districts of the province of Junin (Carhuamayo and Junin) located at 4100 m (13,450 ft) in the Peruvian central Andes. The districts were divided into sectors using existing maps and random samples of houses were selected from each sector. In each house, the subjects aged 35–75 years old were recruited. A time of residence over 3000 m for more than 30 years was considered as criteria for inclusion. Subjects were asked to voluntarily participate in the study after carefully reviewing and signing an informed consent form. This study was approved by the Institutional Review Board (IRB) of the Universidad Peruana Cayetano Heredia.
2.2. Data collection
The participation of the volunteers included, among other things the filling out of a questionnaire and a venous blood sample was requested. The questionnaire explored sociodemographic characteristics, health status, personal and family medical history and seven signs and symptoms of chronic mountain sickness. During the home visit, the pulse oxygen saturation (SpO2) and the arterial blood pressure of each recruited subject were taken in sitting position. After the interview, subjects were asked to get their blood drawn the next morning in the laboratory of the local medical center in fasting condition. A total of 506 subjects (157 men and 349 women) were recruited for the study.
In the medical center, the body weight, height and waist circumference were obtained. Body mass index (BMI) was calculated as Kg/m2. A blood sample was drawn by venipuncture between 08:00 and 11:00 h.
2.3. Hemoglobin measurement
Hemoglobin concentration was measured in situ using the HemoCue system (Anglholm, Sweden). Hemoglobin values were consistent with hematocrit at maximum. Hematocrit measurements were done using the microhematocrit method. In this study, the hemoglobin values were used for analysis. Data were expressed as g/dL. In cases of discordant values, the hematocrit value was used to calculate the hemoglobin concentration.
2.4. Pulse oxygen saturation (SpO2)
The arterial oxygen saturation was measured in the second left finger using a Nellcor N-20 pulse oximeter (Pleasanton, Ca, USA). This equipment simultaneously gives the heart rate value.
2.5. Chronic mountain sickness score (CMS score)
All participants completed a test for assessment of signs and symptoms of chronic mountain sickness. The test included 7 signs/symptoms: 1) breathlessness and/or palpitations; 2) sleep disturbance; 3) presence of cyanosis, 4) dilatation of veins, 5) paresthesias, 6) headaches, and 7) tinnitus. A value of 0 was assigned to negative answers and values from 1, 2 and 3 to positive answers (mild, moderate and severe, respectively) (Table 1).
Table 1.
List of the 20 items of the SF-20 Health Survey
| Item | Survey | Answers |
|---|---|---|
| 1 | In general, would you say your health is: | Poor, fair, good, excellent |
| 2 | Compared to one year ago, how would you rate your health in general now: | Worse, about the same, better |
| 3 | In a typical day. Does your health now limit you in vigorous activities? If so, how much? | Limited a lot, limited a little, not limited at all |
| 4 | In a typical day. Does your health now limit you in moderate activities? If so, how much? | Limited a lot, limited a little, not limited at all |
| 5 | In a typical day. Does your health now limit you in lifting or carrying groceries? If so, how much? | Limited a lot, limited a little, not limited at all |
| 6 | In a typical day. Does your health now limit you in climbing stairs? If so, how much? | Limited a lot, limited a little, not limited at all |
| 7 | In a typical day. Does your health now limit you in bending, kneeling, stooping, bathing or dressing? If so, how much? | Limited a lot, limited a little, not limited at all |
| 8 | During the past 4 weeks, have you had to reduce the time you spent on work or other activities as a result of your physical health? | Yes, Not |
| 9 | During the past 4 weeks, have you had to reduce the time you spent on work or other activities as a result of any emotional problem (such as feeling depressed and anxious)? | Yes, Not |
| 10 | Have you had bodily pain during the past 4 weeks? | Yes, Not |
| 11 | During the past 4 weeks, how much did pain interfere with your normal work (including both work outside the home and homework? | Extremely, moderately. Not at all. |
| 12 | During the past 4 weeks, did you have a lot of energy? | None of the time, some of the time, most of the time, all of the time. |
| 13 | During the past 4 weeks, did you have very nervous? | All of the time. Most of the time, some of the time, none of the time. |
| 14 | During the past 4 weeks, have you felt downhearted and blue? | All of the time. Most of the time, some of the time, none of the time. |
| 15 | During the past 4 weeks, have you felt calm and peaceful? | None of the time, some of the time, most of the time, all of the time. |
| 16 | During the past 4 weeks, did you feel worn out and tired? | All of the time. Most of the time, some of the time, none of the time. |
| 17 | During the past 4 weeks, have you been a happy person? | None of the time, some of the time, most of the time, all of the time. |
| 18 | I seem to get sick a little easier than other people | Yes, No |
| 19 | I am as healthy as anybody I know | No, yes |
| 20 | I expect my health to get worse | Yes, No |
The score of clinical signs and symptoms was defined according to the sum of points obtained for each of the 7 signs/symptoms of CMS and named CMS clinical score. For diagnosis of chronic mountain sickness, a value of 3 was added if hemoglobin value was ≥21 g/dL in men or ≥19 g/dL in women (León Velarde et al., 2005) and the total score is considered the “Qinghai CMS Score”.
For the present study the diagnosis of CMS was defined when Qinghai CMS Score was >5 (León Velarde et al., 2005). In summary, when the CMS test included Hb measurement, the final score was defined as Qinghai chronic mountain sickness score. When we used the 7-item score, without Hb, the value was defined as Chronic mountain sickness clinical score (7 items without Hb).
2.6. Health status score
A generic health-related quality of life (HRQL) questionnaire was used for assessment of their health status. An adaptation of this questionnaire has been previously validated in populations at low and high altitudes (Gonzales, 2010).
The health questionnaire based on 20 questions or items about health (SF-20) included 7 dimensions or domains: Physical functioning scale, Role Physical scale, Bodily pain scale, general health, Vitality scale, Role emotional scale and mental health scale (Vilagut et al., 2005). Five items were related to general health, 5 items to physical activities in relation to current health status, two items related to limitation on work or other regular daily activities as a result of physical health, two items on bodily pain, one item about vitality and 5 items on mental health (Table 2).
Table 2.
List of signs and symptoms of chronic mountain sickness
| Item | Signs or symptoms | Score |
|---|---|---|
| 1 | Breathlessness and/or palpitations | 0=no 1= mild 2=moderate 3=severe |
| 2 | Sleep disturbance | 0= Slept as well as usual 1= Did not sleep as well as usual 2= Woke many times, poor night’s sleep 3= Could not sleep at all |
| 3 | Cyanosis | 0=no 1= mild 2=moderate 3=severe |
| 4 | Dilatation of veins | 0=no 1= mild 2=moderate 3=severe |
| 5 | Paresthesia | 0=no 1= mild 2=moderate 3=severe |
| 6 | Headache | 0=no 1= mild 2=moderate 3=severe |
| 7 | Tinnitus | 0=no 1= mild 2=moderate 3=severe |
| 8 | Hemoglobin (Hb) | No EE=0 EE=3 |
EE=excessive erythrocytosis in men Hb≥21 g/dl and in females Hb≥19 g/dl
All items are scored on a scale of 0 to 100, with higher scores indicating better health-related quality of life. The number of possible responses per item ranges from two to four.
The items in the SF-20 score are not common with items from the CMS score test (Tables 1 and 2). The SF-20 scale scores address the perceived general health status in the daily life, whereas CMS score is centered on specific signs and symptoms of the disease.
2.7. Blood pressure
Sitting BP was measured in the left arm using an aneroid sphygmomanometer. Systolic (SBP) and diastolic (DBP) blood pressure were obtained from each subject and results were expressed in mm Hg.
2.8. Statistical Analyses
Analyses were performed using STATA 10.0 (Stata Corp, College Station, TX). Data are expressed as mean ± standard deviation (SD). The homogeneity of variances was determined by applying the Bartlett test. If homogeneous, the analysis of variance (ANOVA) test or the Students’ “t” test was used to determine differences among groups. If there were differences, the mean comparisons between each one of the groups were determined using the Scheffé test.
The variables with no homogenous distribution were analyzed using the Kruskal-Wallis non-parametric test or they were transformed. The comparisons between pairs of medians were determined using the Mann-Whitney U test. Chi-square test or Fisher’s exact test were carried out to determine differences among frequencies or proportions. Bivariate regression analyses were also performed to assess the association between hemoglobin and each sign or symptom used in the Chronic Mountain Sickness Score. In the case of women, data were also assessed according presence or absence of menopause. Correlations between each sign/symptom of CMS score test and health status score or the domains Physical activity or Role Physical of the SF-20 were also calculated. Multivariate regression analyses were assessed to determine the association of different independent variables (i.e. age, body mass index, pulse oxygen saturation, hemoglobin, smoking, drinking alcohol, and health status score) with chronic mountain sickness clinical score in men and women. In women, menopause and use of biofuel for cooking were controlled in the analysis. Health status score was calculated as the sum of the SF-20 but also according to each dimension or domain (Physical functioning scale, Role Physical scale, Bodily pain scale, general health, Vitality scale, Role emotional scale and mental health scale). For each health dimension was calculated the mean value.
For comparisons, a P value <0.05 was considered as significant.
3. Results
In Table 3 is observed that men at high altitude have higher age, Hb, SBP, DBP and health status score than women. Women have higher BMI than men, whereas SpO2, CMS clinical score (7 items) and “Qinghai CMS Score” (7 items+Hb) were similar between men and women (P>0.05).
Table 3.
Physiological parameters in men and women from Junin (4100 m)
| Variable | Males (n=157) | Females (n=349) | P |
|---|---|---|---|
| Age (years) | 55.18±11.78 | 50.29±11.02 | <0.01 |
| SpO2 (%) | 87.97±3.76 | 88.04±3.74 | >0.05 |
| Hemoglobin (g/dl) | 19.37±2.00 | 16.37±1.68 | <0.001 |
| BMI (Kg/m2) | 25.93±3.00 | 27.32±4.48 | <0.01 |
| Heart rate/min | 68.23±10.27 | 69.94±8.97 | >0.05 |
| SBP (mm Hg) | 117.4±12.02 | 114.20±12.14 | <0.05 |
| DBP (mm Hg) | 74.7±11.03 | 72.38±10.83 | <0.05 |
| Health Status score | 1358.5±347.1 | 1224.9±396.76 | <0.05 |
| CMS score (7 items) | 4.23±3.51 | 4.21±3.17 | >0.05 |
| CMS score (7 items+Hb) | 4.77±3.88* | 4.40±3.36* | >0.05 |
Data are mean±standard deviation.
P>0.05 with respect to CMS score (7 items).
SpO2: Pulse oxygen saturation; BMI: Body mass index; SBP: Systolic blood pressure; DBP: diastolic blood pressure. CM: Chronic mountain sickness.
Bivariate analysis showed significant correlations between pulse oxygen saturation and hemoglobin levels in both sexes (P<0.05). In men, Hb values were related only with sleep disturbances, cyanosis and dilatation of veins. In women, Hb values were associated with cyanosis. All other associations between Hb and signs/symptoms of CMS were not significant (P>0.05). Hb levels were directly related with CMS clinical score (7 items) in men (r=0.17; P<0.05) but not in women (r=0.1, P>.05). Pulse oxygen saturation values were inversely related with CMS clinical score (7 items) in men (r= −0.17; P<0.05) but not in women (r= −0.1, P>.05). Hb was related to arterial blood pressure in women (P<0.05) but not in men. Finally, hemoglobin levels were not related with health status score in both sexes (Table 4).
Table 4.
Bivariate regression analysis between hemoglobin, pulse oxygen saturation, CMS score, blood pressure and health status score in men and women from Junin, Peru (4100 m).
| X,Y | Males (n=157) Coefficient of Correlation (r) | Females (n=349) Coefficient of Correlation (r) |
|---|---|---|
| SpO2, Hb | r=−0.32** | r=−0.20** |
| Hb, Breathlessness | r=0.09 | r=0.05 |
| Hb, Sleep disturbance | r=0.16** | r=0.10 |
| Hb, cyanosis | r=0.16** | r=0.13** |
| Hb, dilatation of veins | r=0.21* | r=0.03 |
| Hb, paresthesias | r=−0.04 | r=−0.03 |
| Hb, headaches | r=0.11 | r=0.09 |
| Hb, tinnitus | r=0.10 | r=0.08 |
| Hb, CMS (7 items) | r=0.18** | r=0.11 |
| SpO2, CMS (7 items) | r=−0.17** | r=−0.1 |
| Hb, SBP | r=0.1 | r=0.17** |
| Hb, DBP | r=0.05 | r=0.12** |
| Hb, Health Status | r=−0.1 | r=−0.05 |
P<0.01;
P<0.05
In the population studied, 17.2% of men and 6.3% of women showed excessive erythrocytosis with differences between gender (P=0.0003). However, there is not a gender difference in the “Qinghai CMS Score”. In fact, in the same population, a “Qinghai CMS Score”>5 was observed in 28.6% of men and in 29.5% of women (P>0.05). The rate of CMS (32.6%) was higher than the rate of EE (9.7%) (chi square=9.97; P<0.002). The rate of CMS moderate/severe (CMS Qinghai score ≥11)(Leon-Velarde et al., 2005) was observed in 7.9% of the subjects without differences between men (9.5%) and women (5.3%)(Chi square test: 0.25, P=0.61).
Regressions between chronic mountain sickness clinical scores (7 items) (Figure 1) or Qinghai CMS score (Figure 2) and health status scores in men and women were observed. A significant inverse relationship between both scores was observed (r=−0.55 for CMS clinical score, P<0.001 and r=−0.52 for Qinghai CMS score, P<0.001). In Figures 3–5 are observed regression only in the group with Qinghai CMS score >5 (r=0.35, P<0.001), >5–<11 (r=0.24, P<0.01) and >10 (r=0.24, P>0.1), respectively. In Table 5, subjects were grouped according category of Qinghai CMS score: 0–5, >5–10 and >10–22 and data related to Health Status Score and the 7 domains of the SF-20 survey were compared in these groups. Total Health Status Score or each domain (Physical function, Role Physical, Role Emotional, Bodily Pain, Vitality, Mental health and General Health were significantly reduced as Qinghai CMS score increased. Only vitality score in the group with CMS score >10–22 was not different with values in the group with CMS score >5–10 (P>0.05).
Figure 1.
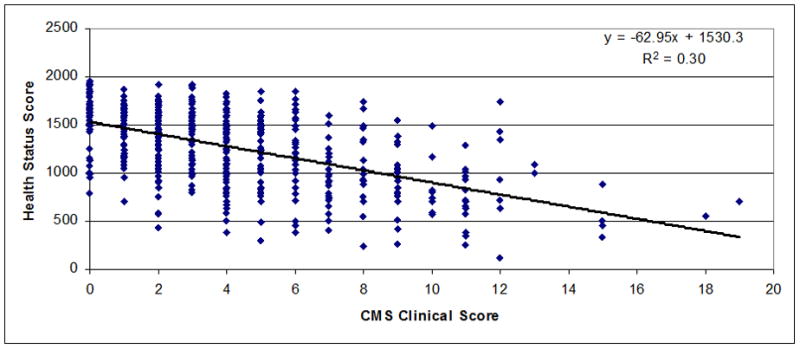
Association between CMS Clinical Score (7 signs and symptoms) and Health Status Score in men and women from Junin, Peru (4100 m) (n=502; r=−0.55; P<0.001).
Figure 2.
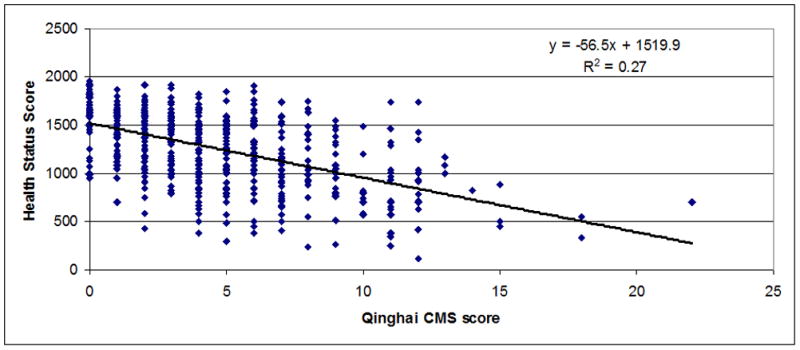
Association between Qinghai CMS Score and Health Status Score in men and women from Junin, Peru (4100 m) (n=502; r=−0.52; P<0.001).
Figure 3.
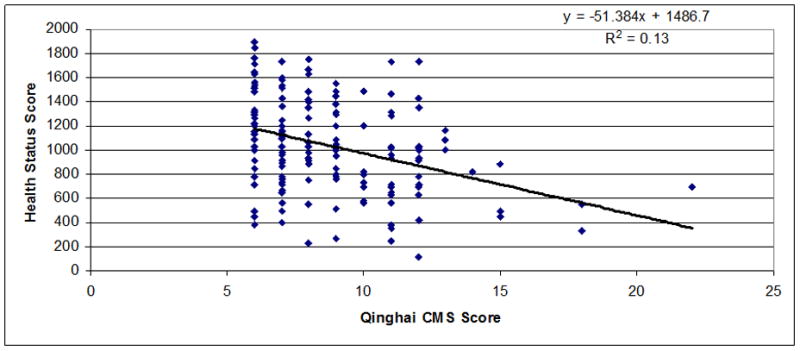
Association between Qinghai CMS Score and Health Status Score in men and women from Junin, Peru (4100 m) with Qinghai CMS Score >5 (n=164; r=−0.35; P<0.001).
Figure 5.
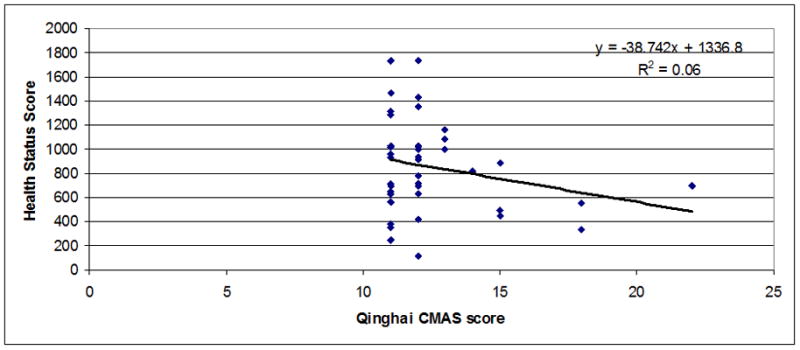
Association between Qinghai CMS Score and Health Status Score in men and women from Junin, Peru (4100 m) with Qinghai CMS Score >10–22 (n=41; r=−0.24; P<0.1).
Table 5.
Health Status Score of SF-20 survey and score of the seven domains of the SF-20 survey according to different Qinghai CMS Score in subjects from Junin at 4100 m.
| Qinghai CMS score | 0–5 (338) | >5–10 (123) | >10–22 (41) |
|---|---|---|---|
| Health Status Score | 1376±18 | 1102±34* | 846±57*,a |
| Physical Function | 78.6±1.4 | 63.4±2.9* | 51.5±5.1*,c |
| Role Physical | 75.3±1.9 | 54.7±3.8* | 37.6±6.5*,c |
| Role Emotional | 75.9±1.9 | 57.9±3.8* | 39.3±6.7*,c |
| Bodily Pain | 70.1±1.7 | 46.7±2.6* | 29.9±4.1*,a |
| Vitality | 62.7±1.1 | 50.1±1.7* | 43.6±3.2* |
| Mental Health | 66.8±0.9 | 56.8±1.6* | 49.3±2.1*,b |
| General Health | 63.5±1.3 | 55.1±2.3** | 37.6±4.1*,a |
Data are mean ± standard error of the mean (SEM). Between parentheses are number of subjects.
P<0.0001;
P=0.0016 with respect to the group with Qinghai CMS score =0–5.
P<0.001;
P<0.005;
P<0.05 with respect to the group with Qinghai CMS score >5–10.
Correlation between Health Status Score and items of signs/symptoms of CMS score showed better correlation with headache (r=−0.43; P<0.0001). Correlations with others signs and symptoms of CMS were also significant but in a less magnitude. No correlation was observed between Health status Score and Score of Hb (Data do not shown). At all the best correlation was observed with CMS clinical score (R=−0.56; P<0.0001) and less with the Qinghai CMS score (R=−0.53; P<0.0001). When the domain Physical activity was correlated with items of CMS, it was observed the better correlation with headache (r=−0.24; P<0.0001). However, when Role Physical was used to correlate with items of CMS, it was observed that breathlessness/palpitation correlated better with Role Physical (R=−0.36; P<0.0001). Once again, the overall CMS clinical score correlated better with Role Physical domain of the SF.20 survey (r=−0.40; P<0.0001) (Data do not shown).
According to the definition of EE, the Hb range of ≥19–21 g/dl is considered as EE in women but not in men. In Table 6, subjects were grouped according to this Hb range. According to the results, to obtain this Hb range (≥19–21 g/dl) women required lower pulse oxygen saturation than men (86.2% and 88.2%, respectively; p<0.05). However, the CMS clinical score (7 items) was not different between sexes. Similarly, SBP and DBP were not significantly different between sexes (P>0.05). Women with Hb >21 g/dl were excluded from analysis since only two belonged to this Hb category.
Table 6.
SpO2, CMS score (7 items), SBP and DBP in men and women from Junin, Peru (4100 m) according different ranges of hemoglobin from ≥19 g/dl – ≤21 g/dl and Hb>21 g/dl
| Hb range (g/dl) | Sex | Number | SpO2 | CMS score (7 items) | Systolic Blood Pressure (mm Hg) | Diastolic blood pressure (mm Hg) |
|---|---|---|---|---|---|---|
| >21 | Males | 27 | 86.1±5.19 | 4.25±2.49 | 119.4±11.58 | 76.5±8.83 |
| ≥19–≤21 | Males | 52 | 88.2±3.32 | 3.77±3.53 | 117.4±11.03 | 74.7±12.98 |
| ≥19–≤21 | Females | 20 | 86.2±3.89b | 5.10±3.58 | 118.0±6.93 | 73.4±9.39 |
Data are mean ±standard deviation (SD). Group pf women with Hb>21 g/dl were excluded from analysis due to small number of subjects (n=2).
P<0.05 with respect to males with Hb>21 g/dl;
P<0.05 with respect to males. Two females with Hb>21 g/dl were excluded from analysis.
Taken into account the study of Reeves and Leon-Velarde (2004) that suggests that optimal Hb should be 17.5 g/dl at high altitudes, two groups were formed, one with Hb >17.5 g/dl and the other one with Hb ≤17.5 g/dl. Data related to SpO2, CMS score, and arterial blood pressure compared among subjects with Hb >15–17.5 and those with Hb ≤15 g/dl were not different (P>0.05) (Data not shown). For such reason, data in these groups were also joined in one group (Hb≤17.5 g/dl). Results can be observed in Table 7. Subjects at high altitudes with Hb ≤17.5 g/dl showed higher SpO2 and lower systolic and diastolic blood pressure than subjects with Hb >17.5 g/dl (P<0.01). However, CMS score based on 7 items of signs and symptoms was not different in groups with different Hb levels (P>0.05).
Table 7.
SpO2, CMS score (7 items), SBP and DBP in subjects from Junin, Peru (4100 m) according to levels of hemoglobin from >17.5 g/dl ≤17.5 g/dl
| Hb range (g/dl) | Number | SpO2 | CMS score (7 items) | Systolic Blood Pressure (mm Hg) | Diastolic blood pressure (mm Hg) |
|---|---|---|---|---|---|
| >17.5 | 211 | 87.47±3.77 | 4.53±3.49 | 118.1±11.91 | 74.98±10.17 |
| ≤17.5 | 295 | 88.44±3.61 | 3.99±3.09 | 113.0±12.02 | 71.76±10.99 |
| P | <0.01 | >0.05 | <0.01 | <0.01 |
Data are mean ±standard deviation (SD). P= Statistical significance.
When subjects were grouped according to presence (Score>5) or absence of CMS (Score ≤ 5) based in the “Qinghai CMS Score”, it was observed that BMI, SpO2, hemoglobin levels, SBP and DBP were similar in men or women with CMS score ≤ 5 (Absent CMS) or with CMS score >5 (P>0.05). However, health status score was significantly higher in men or women without CMS (CMS score ≤ 5) (Data not shown).
Pulse oxygen saturation (SpO2), and health status score were significantly higher whereas Hb concentration, the CMS clinical score and the Qinghai CMS score were significantly lower in premenopausal women than in those with menopause (Table 8). No differences were observed between CMS clinical score and Qinghai CMS score in any of the studied groups (menopausal and pre-menopausal women) (P>0.05).
Table 8.
Pulse oxygen saturation, hemoglobin, health status score, CMS clinical score and Qinghai CMS score in pre-menopausal and post-menopausal women from Junin (4100 m).
| Variable | Pre-menopausal (n=151) | Post-menopausal (n=198) | P |
|---|---|---|---|
| Pulse oxygen saturation (%) | 89.51±3.24 | 86.93±3.74 | <0.01 |
| Hemoglobin (g/dl) | 15.74±1.76 | 16.86±1.74 | <0.01 |
| Health status score | 1310±365 | 1160±405 | <0.01 |
| CMS clinical score | 3.63±3.03* | 4.66±3.30* | <0.01 |
| Ginghai CMS score | 3.69±3.12 | 4.95±3.44 | <0.01 |
Data are mean ±standard deviation (SD). P= Statistical significance. CMS= Chronic mountain sickness.
P<0.05 between CMS clinical score and Qinghai CMS score.
In the multivariate analysis, health status score was the only variable associated with CMS clinical score in men and women (P<0.000). No associations were observed with age, hemoglobin levels, BMI, smoking, drinking alcohol in both sexes, and with menopause and use of biofuel for cooking in women (Tables 9 and 10).
Table 9.
Multiple regression analyses to determine the association of CMS clinical scores (7 item) with age, hemoglobin levels, body mass index, smoking, drinking alcohol, menopause, used of biofuel for cooking and score of health status in women living at 4100 m in Junin.
| Model | Coefficient β | Standard Error | P |
|---|---|---|---|
| Constant | 7.217 | 2.065 | .001 |
| Age (years) | .008 | .021 | .683 |
| Hemoglobin (g/dl) | .076 | .084 | .366 |
| Body mass index (Kg/m2) | .036 | .032 | .260 |
| Smoke | .017 | .921 | .985 |
| Drinking alcohol | −.232 | .457 | .613 |
| Menopause | .119 | .449 | .791 |
| Use of biofuel for cooking | −.083 | .261 | .750 |
| Health status score | −.004 | .000 | .000 |
Menopause: 0=Not, 1=Yes; Fuel for cooking: 1=biofuel; 2=gas.
Table 10.
Multiple regression analysis to determine the association of CMS clinical scores (7 item) with age, hemoglobin levels, body mass index, smoking, drinking alcohol, and score of health status in men living at 4100 m in Junin.
| Model | Coefficient β | Standard Error | P |
|---|---|---|---|
| Constant | 6.047 | 3.773 | .111 |
| Age (years) | .013 | .021 | .541 |
| Hemoglobin (g/dl) | .228 | .122 | .063 |
| Body mass index (Kg/m2) | −.013 | .078 | .870 |
| Smoke | .583 | .693 | .402 |
| Drinking alcohol | .372 | .492 | .451 |
| Health status score | −.006 | .001 | .000 |
4. Discussion
EE based on the Gaussian distribution of Hb in healthy highlanders who reside in Cerro de Pasco at 4340 m in Peru was defined as men with Hb value over 21.3 g/dL (Monge et al., 1992). However, Reeves and Leon-Velarde showed that optimal Hb and SpO2 levels at High altitude, which allows greater oxygen extraction (a cardiac output sparing effect), were 87% and 17.5 g/dl respectively (Reeves and Leon-Velarde, 2004). Since, cut-off Hb value to define EE are different between men and women and both are based on mathematical criteria, it is necessary to know if these cut-offs to define EE are also related with clinical signs and symptoms.
4.1. CMS clinical score and hemoglobin
The present study, which uses assessments of SpO2, Hb levels, CMS score, and health status score, shows that the CMS clinical score (7 items) was not related with Hb levels. This means that men and women with EE are not necessarily associated with signs and symptoms of CMS. Moreover, EE rates (17.2% in men and 6.3% in women) were lower than the CMS rates according to “The Qinghai CMS score” (28.6% in men and 29.5% in women). This means that there are men and women with signs and symptoms of CMS but without EE. This is an interesting finding since CMS was related to EE and it was presumed this elevated Hb levels as responsible of the signs and symptoms of CMS.
The EE rate found in men in the present study (17.2%) at 4100 m was similar to that observed in Cerro de Pasco at 4340 m (15.6%) (Monge et al., 1992). This suggests that the Hb pattern of the population in Junin (4100 m) was not different from that pattern in Cerro de Pasco (4340 m).
Hb was associated with SpO2 similar to the findings in almost all studies performed at high altitude (see Peñaloza and Arias-Stella, 2007) suggesting that in Junin at 4100 m the response to low environmental blood pressure measured in terms of SpO2 and hemoglobin values were similar to other studies in Cerro de Pasco at 4340 m. However, SpO2 and Hb levels were not different in men or women with or without CMS diagnosed through “The Qinghai CMS score”. This raises the question if the items used for the assessment of signs and symptoms of CMS are unspecific.
4.2. The Qinghai CMS score and Health status score
“The Qinghai CMS score” (Leon-Velarde et al., 2005) actually reflects a health status since it was correlated with values of health status score using a validated generic health-related quality of life (HRQL) questionnaire (Gonzales, 2010). In fact, men or women with low score of CMS clinical score (7 items) have a higher health status score. However, no differences in the health status score were observed in men or women with different Hb levels. This finding is interesting since data from CMS score test address specific clinical signs or symptoms of the chronic mountain sickness, whereas the SF-20 assess perceived general health status in the daily life.
These data suggest that CMS defined by high Hb levels or excessive erythrocytosis does not reflect a lower health status at all. This is in accordance with a previous finding, which establishes that the aerobic exercise capacity of patients with EE is preserved in spite of severe pulmonary hypertension and relative hypoventilation (Groepenhoff et al., 2012). Previously, it was demonstrated in Cerro de Pasco, Peru (4340 m) that paresthesia and sleep disorders, two of the symptoms included in the “Qinghai CMS score”, were not related with hemoglobin levels (Gonzales et al., 2011).
In another study carried out at the same altitude (4340 m), rates of four of the seven signs/symptoms included in the CMS score (sleep disorders, venous dilatation, paresthesia and tinnitus) were not different among men with or without EE (Gonzales et al., 2011a). Only breathing/palpitations, cyanosis and headache were significantly higher in men with EE (Gonzales et al., 2011a). The present study showed that sleep disturbances, cyanosis and dilatation of vein in men were associated with hemoglobin values, whereas in women only cyanosis was related with Hb values.
It is interesting to note that similar Hb values in men and women at high altitudes are attained with lower SpO2 in women. This is because women have Hb levels ranged between 19 and 21 g/dl due to the lower SpO2 values and men have the same Hb range due to the erythropoietic activity of higher androgens levels than women (Shahani et al., 2009).
Whereas a significant correlation was observed between Qinghai CMS score and Health Status Score in the SF-20 survey, the correlation was reduced when correlations are calculated in three different groups of Qinghai CMS score (scores=0–5, >5–10, and >10–22). The correlation of Pearson was not significant with the score >10–22. This is probably due to the fact that sample size was lower in this group and that range of CMS values was small. In fact, 76% of cases had Qinghai CMS score values between 11 and 12.
The finding that slope of the line in the regression analysis of the group with Qinghai CMS >10 is lower than in the other groups indicates that predicted Health Status Scores are lower in this group than in the groups with lower CMS score. Moreover, when mean values of Health Status Score using the SF-20 survey were calculated, the group with moderate/severe CMS showed the lowest value for Health Status Score. The same was observed when the 7 scales of the SF-20 were assessed in relation to Qinghai CMS score.
The observation that scores on the general score of the SF-20 or scores of the 7 domains of the SF-20 showed a clear gradient according to the level of clinical severity of CMS is in accordance with the finding of similar relationship between the SF-36 survey and the level of clinical severity in patients with chronic obstructive pulmonary disease (Alonso et al., 1998).
4.3. CMS score and cut-off point for hemoglobin to define excessive erythrocytosis
Another question raised in relation to sex is the criteria used for CMS score. In fact, different cutoff Hb values were used to define EE in men and women (i.e. 21 and 19 g/dl, respectively). The cut-off points are based on the Gaussian distribution of Hb in men and women. This criterion suggests that women but not men with Hb >19–<21 g/dl have more clinical signs/symptoms of CMS. However, our results showed that the CMS clinical score based on the 7 signs/symptoms studied and arterial blood pressure was not different between men and women with these Hb values. We previously found, at high altitudes, that elevated Hb levels were associated with elevated values of arterial blood pressure (Gonzales and Tapia, 2013).
If we used a cutoff Hb value of 17.5 g/dL suggested as optimal at high altitude (Reeves and Leon-Velarde, 2004), it was observed that subjects with Hb>17.5 g/dl had lower SpO2, and higher values of systolic and diastolic blood pressure than those with Hb ≤17.5 g/dl. However, the CMS score based on 7 signs and symptoms was not different among groups. Higher blood pressure has been observed in men with EE in Cerro de Pasco (4340 m) and this was associated with increased uric acid levels, which appears to be caused by increased urate generation secondary to systemic hypoxia (Jefferson et al., 2002). The association between high hemoglobin levels and higher arterial blood pressure has also been observed in populations living at low altitudes (Kawamoto et al., 2011).
These data also suggest that Hb cut-off to define excessive erythrocytosis should be assessed using clinical marker and that signs and symptoms of CMS should be re-assessed.
According to the items included in the Qinghai CMS score, all of these are associated with CMS. For instance, sleep related hypoxemia is associated with EE and with CMS (Spicuzza et al., 2004). For such reason sleep disturbances is considered as an item of assessment in the Qinghai CMS score. Headache and acral paresthesias are also associated with CMS (Appenzeller et al., 2004; 2005). These symptoms seem to be associated with low ATPase activity (Appenzeller et al., 2002) and low ATP1A1 subunit of the ATPase gene mRNA expression (Appenzeller et al., 2005). Low ATPase activity has been related with oxidative stress (Gokulakrishnan and Alim 2010). Oxidative stress is increased in subjects with chronic residence at high altitude (Jefferson et al., 2004).
It is suggested that susceptibility to headache at high altitude might depend in part of gene expression (Appenzeller et al., 2004). For such reason, as observed in the present study, no relationship between hemoglobin levels and headache at high altitudes was observed.
It is clear that CMS is associated with different health problems. In fact, men with CMS in Cerro de Pasco (4340 m) showed elevated mean pulmonary pressure and right ventricular dilation but did not display impaired systolic ventricular function (Maignan et al., 2009).
4.4. Menopause and CMS
As previously demonstrated, menopause is a risk factor for CMS and EE (Gonzales and Villena, 2000; Gonzales, 2004). The present study confirms that post menopausal women reduces SpO2 and increase Hb levels and this was associated with high CMS clinical score and Qinghai CMS score but lower health status score. In addition, SpO2 in premenopausal women (89.51%) was higher than in men (87.97%, P<0.01).
This is in accordance with the finding that at high altitude, nocturnal periodic breathing affects males more than females (Lombardi et al, 2013). We have previously demonstrated that low pulse oxygen saturation in post-menopausal women at high altitude is related to high serum testosterone/estradiol ratio (Gonzales and Villena, 2000).
4.5. Limitation of the study
A limitation of the study is the paucity of subjects with more clinically relevant and severe CMS.
4.6. Perspectives and conclusions
In conclusion, the present study showed that elevated Hb levels were not associated with elevated scores of signs and symptoms of CMS whereas CMS score was associated with Health Status Score. The fact that Qinghai CMS score was not different with CMS clinical score in men and women suggests that subjects with high CMS score has not necessarily associated elevated hemoglobin levels. Data suggest the need to reassessment of the items to be included in the diagnosis of CMS.
Figure 4.
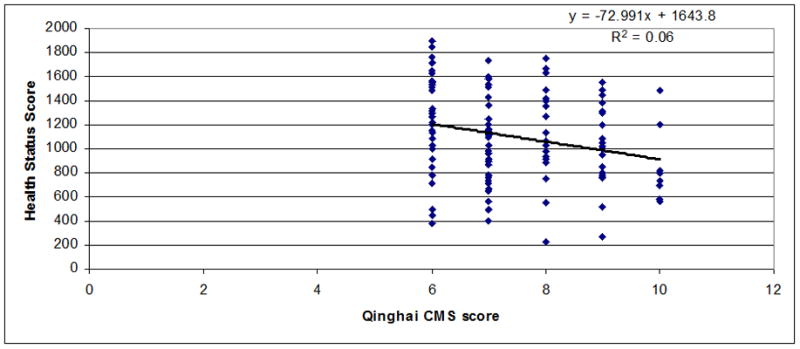
Association between Qinghai CMS Score and Health Status Score in men and women from Junin, Peru (4100 m) with Qinghai CMS Score >5–10 (n=123; r=−0.24; P<0.01).
Highlights.
Chronic mountain sickness score was related with health status score but not with hemoglobin levels at high altitudes.
A significant inverse relationship was observed between chronic mountain sickness clinical score and health status score.
The rate of chronic mountain sickness was higher than the rate of excessive erythrocytosis.
Acknowledgments
This study was supported by a Grant from the Fogarty Program of The National Institutes of Health of the United States (NIH Research Grant # 5-D43TW005746-04 funded by the Fogarty International Center, National Institutes on Environmental Health Services, National Institute for Occupational Safety and Health, and the Agency for Toxic Substances and Disease Registry). We acknowledge to MSc. Ana Huambachano, Bach. Carmen Maldonado, Bach. Ana Lucía Chirinos, Bach. Narda Malpartida, and MSc. Jessica Nieto for their support in the work of field.
Footnotes
Disclosures of Conflict of Interest
The author has no conflicts of interest or financial ties to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alonso J, Prieto L, Ferrer M, Vilagut G, Broquetas JM, Roca J, Batlle JS, Antó JM. Testing the measurement properties of the Spanish version of the SF-36 health survey among male patienets with chronic obstructive pulmonary disease Quality of life in COPD Study Group. J Clin Epidemiol. 1998;51(11):1087–1094. doi: 10.1016/s0895-4356(98)00100-0. [DOI] [PubMed] [Google Scholar]
- Appenzeller O, Thomas PK, Ponsford S, Gamboa JL, Cáceda R, Milner P. Acral paresthesias in the Andes and neurology at sea level. Neurology. 2002;59 (10):1532–1535. doi: 10.1212/01.wnl.0000034761.86543.a1. [DOI] [PubMed] [Google Scholar]
- Appenzeller O, Passino C, Roach R, Gamboa J, Gamboa A, Bernardi L, Bonfichi M, Malcovati L. Cerebral vasoreactivity in Andeans and headache at sea level. J Neurol Sci. 2004;219(1–2):101–106. doi: 10.1016/j.jns.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Appenzeller O, Minko T, Qualls C, Pozharo V, Gamboa J, Gamboa A, Wang Y. Migraine in the Andes and headache at sea level. Cephalalgia. 2005;25(12):1117–1121. doi: 10.1111/j.1468-2982.2005.00973.x. [DOI] [PubMed] [Google Scholar]
- Banegas JR, López-García E, Graciani A, Guallar-Castillón P, Gutierrez-Fisac JL, Alonso J, Rodriguez-Artalejo F. Relationship between obesity, hypertension and diabetes, and health-related quality of life among the elderly. Eur J Cardiovasc Prev Rehabil. 2007;14:456–462. doi: 10.1097/HJR.0b013e3280803f29. [DOI] [PubMed] [Google Scholar]
- Beall CM, Decker MJ, Brittenham GM, Kushner I, Gebremedhin A, Strohl KP. An Ethiopian pattern of human adaptation to high-altitude hypoxia. Proc Natl Acad Sci U S A. 2002;99(26):17215–8. doi: 10.1073/pnas.252649199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokulakrishnan A, Ali AR. Cigarette smoke-induced biochemical perturbations in human erythrocytes and attenuation by epigallocatechin-3-gallate--tea catechin. Pharmacol Rep. 2010;62(5):891–899. doi: 10.1016/s1734-1140(10)70349-2. [DOI] [PubMed] [Google Scholar]
- Gonzales GF. Peruvian contributions to the study on human reproduction at high altitude: From the chronicles of the Spanish conquest to the present. Resp Physiol Neurobiol. 2007;158:172–179. doi: 10.1016/j.resp.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Gonzales GF. Maca: Del alimento perdido de los Incas al milagro de los Andes: Estudio de seguridad alimentaria y nutricional. Segurança Alimentar e Nutricional, Campinas. 2010;16–17:16–36. [Google Scholar]
- Gonzales GF. Hematocrit values in women at high altitude and its relationship with sex hormone levels. J Qinghai Med, Coll. 2004;25:267–272. [Google Scholar]
- Gonzales GF. Serum testosterone levels and excessive erythrocytosis at high altitudes during the process of adaptation to high altitude. Asian J Androl. 2013 doi: 10.1038/aja.2012.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales GF, Villena AE. Low pulse oxygen saturation in post-menopausal women at high altitude is related to high serum testosterone/estradiol ratio. Int J Gynec Obst. 2000;71:147–154. doi: 10.1016/s0020-7292(00)00270-8. [DOI] [PubMed] [Google Scholar]
- Gonzales GF, Tapia V. Association of high altitude-induced hypoxemia to lipid profile and glycemia in men and women living at 4,100m in the Peruvian Central Andes. Endocrinol Nutr. 2013;60(2):79–86. doi: 10.1016/j.endonu.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Gonzales GF, Tapia V, Gasco M, Gonzales-Castañeda C. Serum testosterone levels and score of chronic mountain sickness in Peruvian men natives at 4340 m. Andrologia. 2011;43(3):189–195. doi: 10.1111/j.1439-0272.2010.01046.x. [DOI] [PubMed] [Google Scholar]
- Gonzales GF, Tapia V, Gasco M, Rubio J, Gonzales-Castañeda C. High serum zinc and serum testosterone levels were associated with excessive erythrocytosis in men at high altitudes. Endocrine. 2011a;40(3):472–480. doi: 10.1007/s12020-011-9482-1. [DOI] [PubMed] [Google Scholar]
- Groepenhoff H, Overbeek MJ, Mulè M, van der Plas M, Argiento P, Villafuerte FC, Beloka S, Faoro V, Macarlupu JL, Guenard H, de Bisschop C, Martinot JB, Vanderpool R, Penaloza D, Naeije R. Exercise pathophysiology in patients with chronic mountain sickness exercise in chronic mountain sickness. Chest. 2012;142(4):877–884. doi: 10.1378/chest.11-2845. [DOI] [PubMed] [Google Scholar]
- Jefferson JA, Escudero E, Hurtado ME, Kelly JP, Swenson ER, Wener MH, Burnier M, Maillard M, Schreiner GF, Schoene RB, Hurtado A, Johnson RJ. Hyperuricemia, hypertension, and proteinuria associated with high-altitude polycythemia. Am J Kidney Dis. 2002;39(6):1135–1142. doi: 10.1053/ajkd.2002.33380. [DOI] [PubMed] [Google Scholar]
- Jefferson JA, Simoni J, Escudero E, Hurtado ME, Swenson ER, Wesson DE, Schreiner GF, Schoene RB, Johnson RJ, Hurtado A. Increased oxidative stress following acute and chronic high altitude exposure. High Alt Med Biol. 2004;5(1):61–69. doi: 10.1089/152702904322963690. [DOI] [PubMed] [Google Scholar]
- Kawamoto R, Tabara Y, Kohara K, Miki T, Kusunoki T, Takayama S, Abe M. Hemoglobin is Associated with Serum High Molecular Weight Adiponectin in Japanese Community-Dwelling Persons. J Atheroscler Thromb. 2011;18:182–189. doi: 10.5551/jat.6379. [DOI] [PubMed] [Google Scholar]
- Kong FY, Li Q, Liu SX. Poor sleep quality predicts decreased cognitive function independently of chronic mountain sickness score in young soldiers with polycythemia stationed in Tibet. High Alt Med Biol. 2011;12(3):237–242. doi: 10.1089/ham.2010.1079. [DOI] [PubMed] [Google Scholar]
- León-Velarde F, Maggiorini M, Reeves JT, Aldashev A, Asmus I, Bernardi L, Ge RL, Hackett P, Kobayashi T, Moore LG, Penaloza D, Richalet JP, Roach R, Wu T, Vargas E, Zubieta-Castillo G, Zubieta-Calleja G. Consensus statement on chronic and subacute high altitude diseases. High Alt Med Biol. 2005;6(2):147–157. doi: 10.1089/ham.2005.6.147. [DOI] [PubMed] [Google Scholar]
- Lombardi C, Meriggi P, Agostoni P, Faini A, Bilo G, Revera M, Caldara G, Di Rinzo M, Castiglioni P, Maurizio B, Gregorini F, Mancia G, Parati G Highcare Investigators. High-altitude hypoxia and periodic breathing during sleep:gender-related differences. J Sleep Res. 2013;22(3):322–330. doi: 10.1111/jsr.12012. [DOI] [PubMed] [Google Scholar]
- Maignan M, Rivera-Ch M, Privat C, Leon-Velarde F, Richalet JP, Pham I. Pulmonary pressure and cardiac function in chronic muntain sickness patients. Chest. 2009;135(2):499–504. doi: 10.1378/chest.08-1094. [DOI] [PubMed] [Google Scholar]
- Monge CC, Arregui A, Leon-Velarde F. Pathophysiology and epidemiology of chronic mountain sickness. Int J Sports Med. 1992;13:S79–S81. doi: 10.1055/s-2007-1024603. [DOI] [PubMed] [Google Scholar]
- Monge C. Life in the Andes and chronic mountain sickness. Science. 1942;95(2456):79–84. doi: 10.1126/science.95.2456.79. [DOI] [PubMed] [Google Scholar]
- Peñaloza D, Arias-Stella J. The heart and pulmonary circulation at high altitudes. Healthy highlanders and chronic mountain sickness. Circulation. 2007;115:1132–1146. doi: 10.1161/CIRCULATIONAHA.106.624544. [DOI] [PubMed] [Google Scholar]
- Reeves JT, Leon-Velarde F. Chronic mountain sickness: recent studies of the relationship between hemoglobin concentration and oxygen transport. High Alt Med Biol. 2004;5(2):147–155. doi: 10.1089/1527029041352090. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Vilagut G, Garin O, Cunillera O, Tresserras R, Brugulat P, Mompart A, Medina A, Ferrer M, Alonso J. Reference guidelines for the 12-Item Short-Form Health Survey version 2 based on the Catalan general population. Med Clin (Barc) 2012;139(14):613–625. doi: 10.1016/j.medcli.2011.10.024. [DOI] [PubMed] [Google Scholar]
- Shahani S, Braga-Basaria M, Maggio M, Basaria S. Androgens and eryhtropoiesis: Past and present. J Endocrinol Invest. 2009;32:704–716. doi: 10.1007/BF03345745. [DOI] [PubMed] [Google Scholar]
- Spicuzza L, Casiraghi N, Gamboa A, Keyl C, Schneider A, Mori A, Leon-Velarde F, Di Maria GU, Bernardi L. Sleep-related hypoxaemia and excessive erythrocytosis in Andean high-altitude natives. Eur Respir J. 2004;23:41–46. doi: 10.1183/09031936.03.00000703. [DOI] [PubMed] [Google Scholar]
- Swenson ER. Normal exercise capacity in chronic mountain sickness. Chest. 2012;142(4):823–825. doi: 10.1378/chest.12-0933. [DOI] [PubMed] [Google Scholar]
- Vilagut G, Valderas JM, Ferrer M, Garin O, López-García E, Alonso J. Interpretation of SF-36 and SF-12 questionnaires in Spain: physical and mental components. Med Clin (Barc) 2008;130:726–735. doi: 10.1157/13121076. [DOI] [PubMed] [Google Scholar]
- Vilagut G, Ferrer M, Rajmil L, Rebollo P, Permanyer-Miralda G, Quintana JM, Santed R, Valderas JM, Ribera A, Domingo-Salvany A, Alonso J. The Spanish version of the Short Form 36 Health Survey: a decade of experience and new developments. Gac Sanit. 2005;19:135–150. doi: 10.1157/13074369. [DOI] [PubMed] [Google Scholar]
- Wu T, Wang X, Wei C, Cheng H, Wang X, Li Y, Ge-Dong, Zhao H, Young P, Li G, Wang Z. Hemoglobin levels in Qinghai-Tibet: different effects of gender for Tibetans vs. Han. J Appl Physiol. 2005;98:598–604. doi: 10.1152/japplphysiol.01034.2002. [DOI] [PubMed] [Google Scholar]
- Xing G, Qualls C, Huicho L, Rivera-Ch M, Stobdan T, Slessarev M, Prisman E, Ito S, Wu H, Norboo A, Dolma D, Kunzang M, Norboo T, Gamboa JL, Claydon VE, Fisher J, Zenebe G, Gebremedhin A, Hainsworth R, Verma A, Appenzeller O. Adaptation and mal-adaptation to ambient hypoxia; Andean, Ethiopian and Himalayan patterns. PLoS One. 2008;3(6):e2342. doi: 10.1371/journal.pone.0002342. [DOI] [PMC free article] [PubMed] [Google Scholar]


