Abstract
This study was designed to determine whether deficits in adult serial pattern learning caused by adolescent nicotine exposure persist as impairments in asymptotic performance, whether adolescent nicotine exposure differentially retards learning about pattern elements that are inconsistent with “perfect” pattern structure, and whether there are sex differences in rats’ response to adolescent nicotine exposure as assessed by a serial multiple choice task. The current study replicated the results of our initial report (Fountain, Rowan, Kelley, Willey, & Nolley, 2008) using this task by showing that adolescent nicotine exposure (1.0 mg/kg/day nicotine for 35 days) produced a specific cognitive impairment in male rats that persisted into adulthood at least a month after adolescent nicotine exposure ended. In addition, sex differences were observed even in controls, with additional evidence that adolescent nicotine exposure significantly impaired learning relative to same-sex controls for chunk boundary elements in males and for violation elements in females. All nicotine-induced impairments were overcome by additional training so that groups did not differ at asymptote. An examination of the types of errors rats made indicated that adolescent nicotine exposure slowed learning without affecting rats’ cognitive strategy in the task. This data pattern suggests that exposure to nicotine in adolescence may have impaired different aspects of adult stimulus-response discrimination learning processes in males and females, but left abstract rule learning processes relatively spared in both sexes. These effects converge with other findings in the field and reinforce the concern that adolescent nicotine exposure poses an important threat to cognitive capacity in adulthood.
1. Introduction
Recent research has shown that exposing rats to nicotine during adolescence can cause deficits in adult cognition that are revealed as impairments of the ability to learn to produce complex sequences of behavior (Fountain et al., 2008). The fact that adolescent nicotine exposure produces adult cognitive deficits in sequential learning of this type is perhaps not surprising given that adolescent nicotine exposure has already been shown to produce long-lasting neurophysiological changes in the brains of adult rats and mice (Abreu-Villaca et al., 2003; Abreu-Villaca, Seidler, & Slotkin, 2003; Abreu-Villaca, Seidler, Tate, & Slotkin, 2003; Adriani et al., 2003; Counotte et al., 2011; Marco et al., 2006; Marco et al., 2007; C. G. McDonald et al., 2007; C. G. McDonald et al., 2005; Slotkin, Bodwell, Ryde, & Seidler, 2008; Trauth, Seidler, McCook, & Slotkin, 1999; Trauth, McCook, Seidler, & Slotkin, 2000; Trauth, Seidler, & Slotkin, 2000a; Trauth, Seidler, Ali, & Slotkin, 2001). Other research also indicates that adolescent nicotine exposure can produce persistent behavioral changes long after exposure ends. These changes include deficits in locomotor behavior, rearing, and grooming activity (Schochet, Kelley, & Landry, 2004; Slawecki & Ehlers, 2002; Slawecki, Gilder, Roth, & Ehlers, 2003; Trauth, Seidler, & Slotkin, 2000b), enhanced passive avoidance behavior (Trauth, Seidler, & Slotkin, 2000b), heightened fear conditioning and anxiety-like behavior (Iniguez et al., 2009; Slawecki et al., 2003; Smith et al., 2006) increased susceptibility to later addiction (Adriani et al., 2003; Adriani, Deroche-Gamonet, Le Moal, Laviola, & Piazza, 2006; Hutchison & Riley, 2008; Kelley & Middaugh, 1999; Kelley & Rowan, 2004; Klein, 2001). Specifically cognitive impairments caused by adolescent nicotine exposure include deficits in visuospatial attentional performance (Counotte et al., 2009), impaired context conditioning (Spaeth, Barnet, Hunt, & Burk, 2010), and impaired rat serial pattern learning (Fountain et al., 2008). Of the foregoing behavioral studies, most were conducted with males only, but sex differences have been observed in increased consumption of opioids in adult male rats compared to females (Klein, 2001) and in decreased grooming activity in adult female rats compared to males (Trauth, Seidler, & Slotkin, 2000b) after adolescent nicotine exposure, whereas sex differences were not observed in impairments of context learning after adolescent nicotine exposure (Spaeth et al., 2010). Interestingly, some observed changes in adult behavior after adolescent nicotine exposure are opposite the effects of nicotine in adult rats not previously exposed to nicotine in adolescence. For example, while adolescent nicotine exposure causes deficits in grooming activity in female rats (Trauth, Seidler, & Slotkin, 2000b), both acute and chronic nicotine exposure in adult rats increase grooming behaviors without sex-selectivity (Booze et al., 1999; Iwamoto, 1984). Taken together, this previous research suggests that adolescence is a period of vulnerability for the effects of nicotine on neurophysiological development and behavior. The focus of the research reported in this paper is to further characterize the extent and nature of the adult cognitive deficits caused by adolescent nicotine exposure that are observed in a complex cognitive paradigm for rats, namely, sequential learning.
Whereas many of the foregoing animal models are typically used to examine the effects of adolescent nicotine exposure on neurophysiological development and simple adult behaviors, few animal models of adolescent nicotine exposure focus on adult complex learning and memory. One exception is a serial pattern learning paradigm for adult rats called the serial multiple choice (SMC) task (Fountain et al., 2006). Serial pattern learning can be defined as a process of learning to arrange behaviors sequentially through time. The SMC task for rats is analogous to nonverbal pattern-learning tasks requiring human subjects to make responses in a particular sequential order according to a fixed and highly structured pattern (Restle & Brown, 1970a; Restle & Brown, 1970b). Rats in this task learn to perform a highly structured pattern of nose poke responses in a circular array of nose poke receptacles for water reinforcement (Fountain & Rowan, 1995a; Fountain & Rowan, 1995b; Fountain, Benson, & Wallace, 2000; Fountain, 2008). The measure of greatest interest on each element (trial) of the pattern is whether or not the first choice rats make is correct. Which receptacle will be the correct choice on any trial is predetermined by the programmed serial pattern designated for each group of rats to learn. In the study by Fountain et al. (2008), a 24-element serial pattern composed of eight 3-element chunks was used:
123-234-345-456-567-678-781-812-…repeat pattern
Digits indicate the clockwise position of correct receptacles in the circular array on each trial, dashes indicate a 3-second pause that served as phrasing cues (Muller & Fountain, 2010), and all other intertrial intervals were 1 second. The first element of each 3-element chunk is termed the chunk-boundary element. In this pattern, chunk-boundary elements occurred every 3 elements at serial positions 1, 4, 7, 10, 13, 16, 19, and 22. Each chunk-boundary element followed a phrasing cue. The second and third elements in each chunk are designated within-chunk elements.
The SMC task is potentially useful for assessing neurotoxic effects on cognition because it appears to recruit multiple concurrent cognitive systems including associative stimulus-response learning, serial position learning involving timing or counting processes, and rule abstraction processes (Fountain & Benson, 2006; Fountain et al., 2012; Kundey & Fountain, 2010; Muller & Fountain, 2010). Learning to anticipate chunk-boundary elements has been shown to depend on both associative stimulus-response learning and serial-position learning concurrently, whereas learning to anticipate within-chunk elements depends on learning a motor program or abstract rules that are independent of external stimuli (Muller & Fountain, 2010). Psychobiological studies have also been conducted to examine the pattern of learning deficits in this pattern when rats were trained under systemically administered MK-801, an N-methyl-D-aspartate receptor (NMDAr) antagonist that blocks learning via long-term potentiation in hippocampus and other brain areas (Fountain & Rowan, 2000). MK-801 blocked learning to anticipate chunk-boundary elements with minimal disruption of acquisition for within-chunk elements (Fountain & Rowan, 2000). Thus, behavioral studies show that learning to anticipate these two types of elements depends on different underlying cognitive systems and that these dissociable cognitive systems likely depend on dissociable neural systems (Fountain & Rowan, 2000; Fountain et al., 2012).
Fountain et al. (2008) used this paradigm to examine the effects of adolescent nicotine exposure on adult rat cognitive capacities. The effects of adolescent nicotine exposure (1.0 mg/kg/day, 5 days/week for 5 weeks) on adult cognitive capacity were assessed by training nicotine-exposed and control rats in the SMC task during adulthood beginning on postnatal day 95 (P95). Adolescent nicotine exposure produced differential learning impairments in adulthood for different pattern element types. Adolescent exposure to nicotine caused a transient retardation of learning for within-chunk elements. For chunk-boundary elements, the learning impairment caused by adolescent nicotine exposure was more pronounced, longer-lasting, and still evident at the end of the 21-day experiment. Thus, the fact that adolescent nicotine exposure caused differential adult learning deficits for chunk-boundary and within-chunk elements in combination with the foregoing behavioral analysis of the multiple cognitive systems recruited in serial pattern learning (cf. Fountain et al., 2012; Muller & Fountain, 2010) suggest that multiple cognitive systems may have been affected by adolescent nicotine exposure and that this paradigm can provide potential hypotheses regarding the nature of adolescent nicotine-induced dysfunction in multiple adult cognitive systems.
Several questions remain regarding the nature of the deficits caused by adolescent nicotine exposure in this serial pattern learning paradigm. Fountain et al. (2008) demonstrated that adolescent nicotine exposure retarded adult rat serial pattern learning for both within-chunk and chunk-boundary elements. Retardation for within-chunk elements was transient, whereas the deficit for chunk-boundary elements lasted until the end of the 21-day experiment. Because acquisition was not followed to asymptotic levels of performance for chunk-boundary elements, these data do not speak to whether chunk-boundary deficits represented a transient impairment of learning that would have been overcome by extended training (as observed in within-chunk elements) or instead reflected a more profound deficit that would have resulted in poorer asymptotic levels of acquisition in nicotine-exposed rats. In the current study, the number of patterns per day was doubled and the length of the experiment was extended from the 3 weeks of training in the earlier study to 7 weeks of training in the present study to answer this question.
Fountain et al. (2008) used a perfect pattern whose elements by definition could be predicted completely by two hierarchically arranged rules (Fountain & Rowan, 1995b). The pattern had no elements that violated the simple structure, that is, the pattern had no violation elements. However, earlier work has shown that both rats and mice find violation elements unusually difficult to learn and that they may employ different cognitive strategies for learning violation elements compared to those employed for learning chunk-boundary and within-chunk elements. One piece of evidence supporting this latter claim is that on violation element trials, rats persist in making intrusion errors (errors of commission) consistent with pattern structure long after the rest of the pattern is learned (Fountain & Rowan, 1995a; Fountain, Krauchunas, & Rowan, 1999; 2000). That is, in a violation pattern (i.e., a pattern containing at least 1 violation element) such as 123–234–345–456–567–678–781–818, where the final element is the violation element (underlined), rats persist in producing a rule-consistent “2” response instead of the required violation “8” response for the violation element until the rest of the pattern is virtually mastered (Fountain & Rowan, 1995a; Muller & Fountain, 2010). Thus, rats and mice treat violation elements as “exceptions-to-the-rule” and typically learn to anticipate violation elements by associative learning involving multiple item cues from several preceding trials that signal the impending violation trial (Kundey & Fountain, 2010). Learning to anticipate a violation element is completely prevented by NMDAr blockade by MK-801 exposure which results in nearly 100% error rates on the violation trial consisting almost exclusively of rule-consistent intrusion responses (Fountain & Rowan, 2000). Thus, a second question of interest is whether adolescent nicotine exposure would affect rats’ ability to learn to anticipate a violation element that is inconsistent with overall pattern structure. The current study employed the violation pattern described above to examine the effects of adolescent nicotine exposure on rats’ acquisition of this very difficult type of pattern element. It should be noted that this pattern is identical to that used by Fountain et al. (2008) with the exception of the addition of a violation as the final element, which allows for an assessment of the ability to replicate results obtained earlier with chunk-boundary and within-chunk element types.
Finally, it is not known whether or not adolescent nicotine exposure has a sex-selective effect on acquisition of this task. Fountain et al. (2008) reported effects of adolescent nicotine exposure on serial pattern learning for male rats only. Given the extant literature showing differences in behavioral and neural effects of nicotine in adolescent female versus male rats (e.g., Mateos et al., 2010), there are good reasons to expect sex-specific effects. Accordingly, the current study examined the effects of adolescent nicotine exposure on rats’ serial pattern learning in male and female rats.
2. Materials and methods
2.1. Animal care and nicotine treatment
The subjects were 48 Long Evans rats (Rattus norvegicus), 24 male and 24 female, bred in-house. On P21, rats of the same sex were caged together in groups of 3 without littermates. Rats were distinguished from their cagemates by colored marks applied to each rat’s tail. Colored marks were reapplied as necessary until P60 when all rats were separated into individual housing. Each group of rats was randomly assigned to a treatment group. Beginning on P25, rats received daily intraperitoneal injections of 1.0 mg/kg nicotine bitartrate (Sigma Chemical, Saint Louis, MO; expressed as the weight of the free base) or saline vehicle as 1.0 ml/kg body weight. These injections were never observed to cause seizure or proconvulsive activity. Rats were weighed and injected daily for 35 consecutive days through P59 and were then given 35 days drug free prior to testing as adults. During the 35-day period of daily drug injections from P21-59, nicotine-injected rats grew at a slower rate than rats receiving control injections, with males and females weighing 92.1% and 88.8% of controls, respectively, by the last day of injections. An ANOVA conducted on rats’ daily percentage of control body weight data found no significant main effects or interactions, indicating no differences in percentage of control body weight between males and females for any day of the injection period (p > 0.05). All rats were given free access to food in their home cage throughout the experiment and free access to water until water restriction began on P91. After nicotine injections ended, male and female nicotine-injected rats recovered to control levels of body weight by P91. Once water restriction was implemented beginning on P91, rats were weighed daily to ensure they remained at 80% of their free-feeding weight throughout the experiment. Throughout the experiment rats were given 3–6 minutes of water and occasional supplemental water as needed outside the conditioning chambers after testing each day to keep them at 80% of their free-feeding weight. All rats were kept on a 15:9-h light-dark cycle, with testing occurring during the light portion of the cycle.
2.2. Apparatus
Four shaping chambers constructed of clear Plexiglas (15 × 30 × 30 cm) had stainless steel wire mesh flooring and a single nose-poke receptacle (2.5-cm diameter PVC pipe end caps painted flat black) centered on one end wall 5.0 cm above the floor. The nose-poke receptacle was equipped with an infrared emitter-detector pair to detect nose-poke responses and a white LED cue light centered in the back of the receptacle. Six octagonal testing chambers were also constructed of clear Plexiglas (15 cm wide × 30 cm tall walls with 40 cm between opposite walls) and had stainless steel wire mesh flooring and a nose-poke receptacle described above centered 5.0 cm above the floor on each chamber wall.
Both types of chambers were housed in sound-attenuating enclosures with 10-ml syringes that served as water reservoirs affixed to an internal wall of the enclosure. These syringes were connected by Tygon tubing (VWR Scientific, Performance Plastics 1/32-inch, #R-3603) to solenoids (General Valve Corp. Vac. 20 psig. 24 volts) and then to the receptacles. The solenoids thus controlled delivery of water droplets to the nose-poke receptacles. Background white noise masked extraneous noise. Shaping and testing chambers were controlled by a computer and an interface (Med Associates interface; Grayson Stadler power supply Model E 783 DA) from an adjoining room. Rats in the shaping and testing chambers were monitored from the computer room via closed circuit cameras mounted inside the sound attenuating enclosures.
2.3. Procedure
2.3.1. Shaping procedure
Rats were water deprived beginning on P91 for 48 h before initial nose-poke shaping. Two consecutive days of nose-poke shaping sessions occurred on P93 and P94. At the beginning of each trial, the receptacle light was illuminated. When the rat made a nose-poke response, the receptacle light was turned off and a 0.025 ml droplet of water was delivered through the bottom of the receptacle. A 1-s intertrial interval separated trials on P93, and a 2-s intertrial interval separated trials on P94. Criterion for being included in the study was set at 240 responses within one hour on each of these two consecutive days. All rats met criterion on P94.
2.3.2. Testing procedure
Testing in the SMC task began on P95, the day after rats completed nose poke shaping. At the beginning of each trial, all 8 nose poke receptacles of the octagonal chamber were illuminated and the rat was free to respond at any of the 8 receptacles. If the rat’s first response was correct, the rat received a water droplet reward. If an incorrect response was produced, the rat entered a correction procedure where only the correct receptacle light was illuminated and the rat received a water droplet reward only after choosing the correct receptacle. All rats were required to learn the same 24-element (trial) serial pattern composed of eight 3-element “chunks”:
123-234-345-456-567-678-781-818- …repeat pattern
where digits indicate the clockwise position of the correct receptacles within the circular array of the octagonal chamber on successive trials. Dashes indicate 3-s pauses that served as phrasing cues preceding chunk-boundary elements and as the inter-pattern interval; other intertrial intervals 1 s. Rats were tested on 10 patterns per day for 49 consecutive days.
3. Results
Generally, the results show that adolescent nicotine exposure impaired some aspects of serial pattern learning but not others, and that the observed effects of nicotine exposure on later cognitive dysfunction were sex-selective. As shown in Fig. 1, correct choice data showed that adolescent exposure to 1.0 mg/kg of nicotine from P25-59 produced a transient learning impairment for chunk-boundary elements for males and for the violation element for females. An analysis of variance (ANOVA) was conducted on rats’ daily mean correct response rates for each element type across the 49 days of the experiment. Main effects and interactions were considered significant if p <0.05.
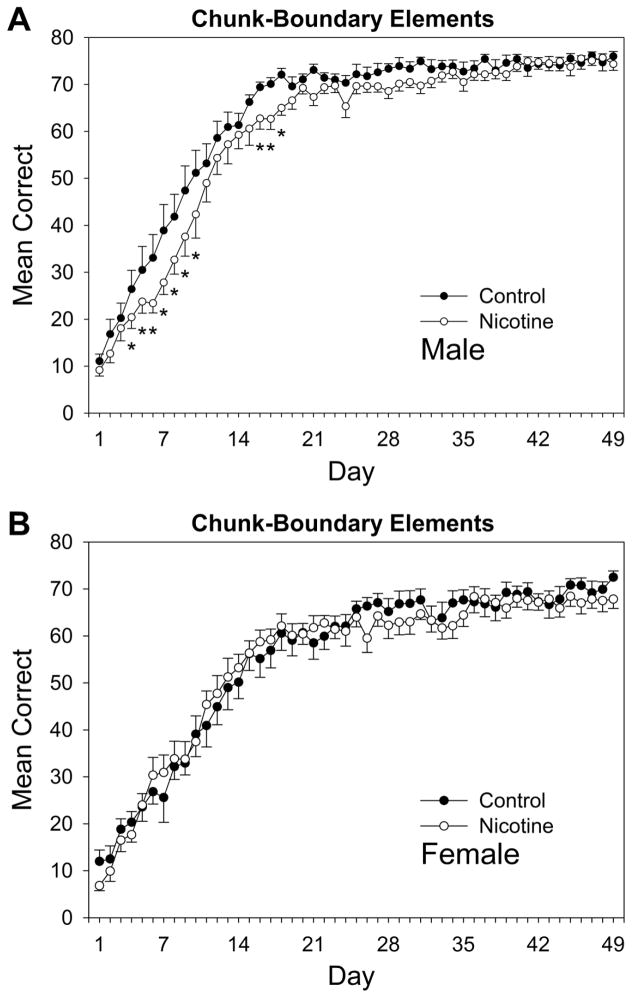
Fig. 1.
Acquisition curves for chunk-boundary elements of the pattern for (A) male and (B) female adult rats over 49 days of training beginning on P95. Rats received prior adolescent exposure to either 1.0 mg/kg nicotine or an equivalent volume of saline from P25-59. Error bars: ±SEM. *p < 0.05 vs. same-sex controls.
Acquisition curves for chunk-boundary elements, that is, the first element of chunks that always immediately followed phrasing cues, are shown in Fig. 1. For male and female rats’ performance (Fig. 1A and 1B, respectively), a drug × sex × day repeated measures ANOVA conducted on rats’ daily mean correct-response rates on chunk-boundary elements revealed a significant main effect for day of the experiment, F(48,2112) = 279.88, p < 0.001, and sex, F(1,44) = 21.88, p < 0.001. The ANOVA also revealed a drug × sex × day interaction that approached significance, F(48,2112) = 1.35, p = 0.054. Planned comparisons based on the appropriate error term from the ANOVA showed that male adult rats previously receiving 1.0 mg/kg nicotine during adolescence displayed a learning impairment for this element type compared to controls on days 4–10 and 16–18 of the experiment. These results demonstrate that adolescent nicotine exposure retarded adult serial pattern learning for chunk-boundary elements in male rats, a finding that replicates Fountain et al. (2008). Females did not display the same impairment for this element type, indicating that adolescent nicotine exposure effects on adult serial pattern learning were sex-selective.
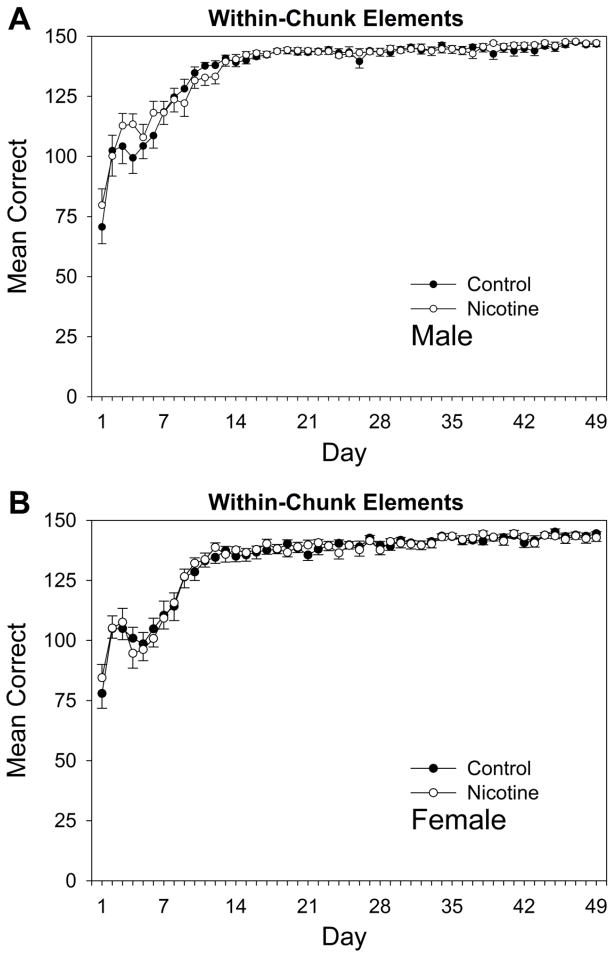
Acquisition curves for within-chunk elements, namely the second and third elements of each chunk, are shown in Fig. 2. For male and female rats’ performance on within-chunk elements (Fig. 2A and 2B, respectively), a drug × sex × day repeated measures ANOVA conducted on rats’ daily mean correct-response rates revealed significant main effects for sex, F(1,44) = 11.44, p = 0.002, and day of the experiment, F(48,2112) = 139.00, p < 0.001. All other main effects and interactions for the within-chunk elements for males and females were not significant. Thus, female rats without regard to drug condition made significantly more errors on within-chunk elements than males, though this difference was not detectable on individual days, as indicated by a nonsignificant sex × day interaction (p > 0.05). However, exposure to nicotine in adolescence did not cause impaired learning for within-chunk elements for either sex.
Fig. 2.
Acquisition curves for within-chunk elements of the pattern for (A) male and (B) female adult rats over 49 days of training beginning on P95. Rats received prior adolescent exposure to either 1.0 mg/kg nicotine or an equivalent volume of saline from P25-59.
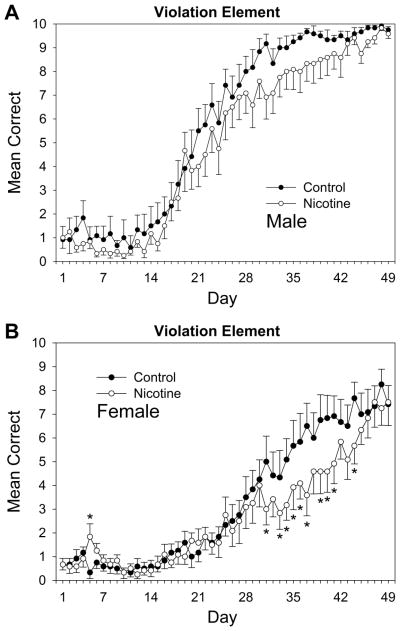
Finally, acquisition curves for the violation element are shown in Fig. 3. For male and female rats’ performance on violation elements (Fig. 3A and 3B, respectively), a drug × sex × day repeated measures ANOVA conducted on rats’ daily mean correct-response rates revealed significant main effects for day, F(48,2112) = 124.36, p < 0.001, and sex, F(1,44) = 37.77, p < 0.001, and an interaction of sex × day, F(48,2112) = 9.88, p < 0.001. A drug × day interaction approached significance, F(48,2112) = 1.34, p = 0.060. Planned comparisons revealed that female adult rats previously receiving 1.0 mg/kg nicotine during adolescence displayed a learning impairment for this element type compared to controls on days 5, 31, 33–37, 39–41 and 44 of the experiment. These results demonstrate that adolescent nicotine exposure impaired later adult learning for the violation element in females. Males did not display the same impairment for this element type, though consistent group mean differences that did not reach significance were observed.
Fig. 3.
Acquisition curves for the violation element of the pattern for (A) male and (B) female adult rats over 49 days of training beginning on P95. Rats received prior adolescent exposure to either 1.0 mg/kg nicotine or an equivalent volume of saline from P25-59. Error bars: ±SEM. *p < 0.05 vs. same-sex controls.
In the foregoing analyses, a significant main effect for sex was observed for each of the aforementioned element types. This indicated that male and female rats differed in the way they acquired the pattern independent of adolescent nicotine exposure. To examine this notable finding separately, sex × day repeated measures ANOVAs were conducted on data from control rats only of both sexes in separate analyses for each element type. For chunk-boundary elements, the ANOVA revealed significant main effects for day of the experiment, F(48,1056) = 124.41, p < 0.001, and sex, F(1,22) = 12.9, p = 0.002. For within-chunk elements, the ANOVA revealed a significant main effect of day of the experiment, F(48,1056) = 75.94, p < 0.001. For the violation element, the ANOVA revealed significant main effects of day of the experiment, F(48,1056) = 69.23, p < 0.001, and sex, F(1,22) = 19.77, p < 0.001, and a significant Sex × Day interaction, F(48,1056) = 4.50, p < 0.001. Taken together, these results indicate that the two sexes of rats acquired some aspects of the pattern at different rates even in control conditions. Specifically, female control rats showed slower acquisition of chunk-boundary and violation elements than male control rats, but control groups of the two sexes did not differ in acquisition of within-chunk elements. That is, sex differences were observed in control rats’ acquisition for chunk-boundary and violation elements, but not for within-chunk elements. Whether these acquisition rate differences between sexes in control conditions reflect differences in choice of behavioral strategy or “cognitive style” is not clear without additional evidence, such as the analysis of error data provided below.
When intrusion data were collapsed across the entire experiment and examined, clear patterns were observed in the types of intrusion errors rats produced for the different types of elements of their pattern. For chunk-boundary elements, the most frequent type of intrusion error for rats in all conditions (male and female, control and nicotine-exposed) was a perseveration response, that is, a repetition of the last correct response. An example of such an error after a 2-3-4 chunk would be a “4” response on the chunk boundary trial following the chunk when a “3” response was correct. Across all groups, this type of error accounted for 37–41% of all errors made on chunk-boundary trials. Rats in all conditions also made 17–19% “overextension” errors cite consisting of an extrapolation of the “+1” rule of the preceding chunk. An example of such an error would be extrapolating a 2-3-4 chunk by producing a “5” response on the chunk-boundary trial following the chunk when a “3” response was the correct response. Finally, rats in all conditions also made 17–21% “back 2” errors on chunk-boundary trials. This “back 2” error can be characterized as an “inaccuracy” error produced by moving one location too far in the correct direction in the array on the chunk-boundary element. An example of such an error after a 2-3-4 chunk would be moving 2 receptacles counterclockwise in the array to produce a “2” response on the chunk-boundary trial following the chunk when moving 1 receptacle counterclockwise in the array, a “3” response, was correct. Similarly for the violation element, rats in all conditions responded essentially the same, with all groups producing more than 75% of their errors as extrapolations of the within-chunk “+1” rule; that is, on the third element of the violation chunk, 8-1-8, all rats tended to respond with a rule-consistent but incorrect “2”--rather than “8--to produce an 8-1-2 chunk that was structurally consistent with the rest of the chunks of the pattern. Thus, although acquisition rates for chunk-boundary and violation element types varied by drug-exposure group and by sex, the proportion of error types observed that is, the relative distribution of errors in the array as a percentage of errors committed did not appear to differ between groups. These results suggest that differences in acquisition related to rats’ sex or caused by prior adolescent nicotine exposure are not reflective of fundamental changes in behavioral strategy, cognitive processes employed, or differences in “cognitive style.”
4. Discussion
The current study replicated the results of our initial report which showed that adolescent nicotine exposure produced a specific cognitive impairment in male rats that persisted into adulthood at least a month after adolescent nicotine exposure ended (Fountain et al., 2008). In that earlier study, male rats exposed to nicotine in adolescence, like the male rats in the current study, had more difficulty than control rats in learning to anticipate the point of transition between chunks in the serial pattern. In the present study, this was observed as retarded learning for chunk-boundary elements, that is, the first element of chunks. Examining the types of intrusion errors nicotine-exposed rats produced on chunk-boundary trials revealed that the observed learning impairment could be described as a retardation of learning rather than a shift in behavioral or cognitive strategy. This conclusion that the observed impairment in learning chunk-boundary elements represented a slowing of the learning process rather than a change to a different learning mechanism was supported by the fact that nicotine-exposed male rats’ pattern of intrusion errors did not differ from the rather specific and unique pattern observed in controls. Finally, in Fountain et al. (2008), acquisition was not followed to asymptotic levels of performance for chunk-boundary elements and the nicotine-induced impairment was still observed at the end of the study. In that study, even controls had reached only approximately 60% correct on chunk-boundary elements when the study was terminated. To determine whether the previously-reported chunk-boundary deficit represented a transient effect, the length of the current study was increased from a total of 105 training patterns in the earlier study (5 patterns per day for 21 days) to a total of 490 training patterns in the present study (10 patterns per day for 49 days). The results of the current study showed that the learning impairment for chunk-boundary elements did not result ultimately in a lower asymptotic level of acquisition in adult males following earlier adolescent nicotine exposure. Our current results showed that nicotine-exposed male rats did eventually reach the same asymptotic level of performance as control rats, albeit more slowly. Thus, the nicotine-induced learning impairment for chunk-boundary elements we observed in male rats in both studies could be overcome by additional training; rats exposed to nicotine in adolescence were able to perform as well as controls on chunk-boundary elements by the last day of training.
One methodological change in the current study compared to Fountain et al. (2008) was the addition of a violation element to the pattern that all rats were required to learn. As stated earlier, violation elements are by definition elements of the pattern that do not fit the structure of the rest of the pattern. In the current study, rats learned the same pattern as in Fountain et al. (2008) with the exception that the final element of the pattern was replaced with a violation element. In the pattern used here, 123-234-345-456-567-678-781-818, the second and third element of every 3-element chunk followed a “+1” rule with the exception of the last element of the pattern which was an “8” rather than the rule-consistent “2” used in Fountain et al. (2008). Whereas rats in Fountain et al. (2008) had no more difficulty learning to make a “2” response as the last element of the pattern than they did learning any other within-chunk element of the pattern, rats in the current study found learning to make an “8” response the violation element as the last element of the pattern to be the hardest element in the pattern by far, as is evident when the slow acquisition for the violation element (Fig. 3a) is compared to the faster acquisition for within-chunk elements (Fig. 2a) and chunk-boundary elements (Fig. 1a). This outcome has been observed in a number of other studies which provide evidence for cognitive rule-learning in rats because (a) violations elements, wherever they are located in a pattern, are always unusually difficult to learn relative to other types of pattern elements and (b) errors that rats commit on violations elements tend overwhelmingly to be consistent with pattern structure (Fountain & Rowan, 1995a; Muller & Fountain, 2010). This latter effect was also observed in the current study; rats in all groups made high rates of rule-consistent “2” errors on the violation element when the “8” response was correct.
Fountain et al. (2008) tested male rats only, thus another novel aspect of the current study was our examination of the effects of adolescent nicotine exposure on female rats’ acquisition of serial patterns in the SMC task. Prior to the present study, the performance of female rats in the SMC task had not been assessed, and with several studies in the literature demonstrating sex differences in common learning tasks using rats (Jonasson, 2005; Maren, De Oca, & Fanselow, 1994; Williams & Meck, 1991), it was unknown prior to this study how control females would perform in comparison to males in this serial pattern learning paradigm. In the current study, sex differences were observed even in controls; female rats learned chunk-boundary elements and the violation element slower than males. Adolescent nicotine effects on serial pattern learning in adulthood were also sex-specific. Females learned chunk-boundary elements at an overall slower rate than males, but females did not show a learning impairment in adulthood that was caused by earlier nicotine exposure during adolescence. In contrast, for females, learning about the violation element was significantly slowed by earlier adolescent nicotine exposure relative to controls, whereas adolescent nicotine effects on violation element learning did not reach significance for males. Taken together, these results indicate that adolescent nicotine exposure causes impairment in the associative process utilized in acquiring the chunk-boundary and violation element types, but leaves the rule learning process employed for the acquisition of the within-chunk elements unaffected. Moreover, these results indicate that exposure to nicotine in adolescence differentially affects learning and cognitive systems in male and female rats. Importantly, the SMC task revealed sex differences in adolescent nicotine effects on adult cognitive capacity, thus demonstrating its potential utility for studying sex differences in adolescent nicotine exposure effects on adult cognitive capacity in a cognitive task that recruits both associative learning and rule learning processes.
The principal conclusion from this replication and extension of our earlier study (Fountain et al., 2008) is that the adolescent brain is vulnerable to damage caused by nicotine exposure that produces learning deficits measureable in adulthood well after nicotine exposure ends. Moreover, evidence from this study suggests that male and female rats are differentially affected by adolescent nicotine exposure, that is, that there are sex-specific effects of adolescent nicotine exposure. The nature of the adult learning impairments observed in male and female rats caused by adolescent nicotine exposure suggests a failure in the ability to use discriminative cues in working memory that has been described in the animal literature as hippocampal-dependent “recollection-like” memory (Agster, Fortin, & Eichenbaum, 2002; Fortin, Agster, & Eichenbaum, 2002; Fortin, Wright, & Eichenbaum, 2004; Heise, Hrabrich, Lilie, & Martin, 1975) or, in other paradigms, as “episodic-like” memory (Clayton & Dickinson, 1998; Clayton, Salwiczek, & Dickinson, 2007; Correia, Dickinson, & Clayton, 2007; DeVito & Eichenbaum, 2010; Zhou & Crystal, 2011). The relevant associative memory mechanisms appear to depend on intact cholinergic function (Heise et al., 1975), intact NMDA-receptor-system function (Fountain & Rowan, 2000), and intact hippocampus and medial prefrontal cortex in rats (Agster et al., 2002; DeVito & Eichenbaum, 2010; Fortin et al., 2002), thus implicating hippocampal and cortical systems that underlie working memory and executive function. The latter idea fits particularly well with other evidence that adolescent nicotine exposure causes impairments in adult hippocampal-dependent context conditioning but spares simpler forms of cue learning (Spaeth et al., 2010), and with evidence that nicotine exposure during adolescence adversely affects nicotinic cholinergic receptor expression and concomitant brain development preferentially targeting hippocampus and related cortical structures during this period of development (Dwyer, McQuown, & Leslie, 2009). Whereas, little is known regarding the neural basis of rule learning in the SMC task, the results of the current study suggest that future studies with the SMC task can be designed to identify the specific adult neural learning and memory systems impaired by adolescent nicotine exposure. Though it is premature to extrapolate our findings to humans, if impairments similar to those caused by adolescent nicotine exposure in rats are also produced by real-world adolescent nicotine exposures in humans, the fact that the effects we have observed in rats are transient that is, they may be overcome by additional training does not reduce our concern from a public health perspective.
Highlights.
We examined deficits in adult sequential learning after adolescent nicotine exposure.
Adolescent nicotine exposure produced sex-specific cognitive impairment.
Impairments persisted into adulthood at least a month after nicotine exposure ended.
All nicotine-induced impairments were overcome by additional training.
Adolescent nicotine exposure poses a threat to cognitive capacity in adulthood.
Acknowledgments
The project described was supported by Award Number R15DA023349 from the National Institute on Drug Abuse to S. B. Fountain. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health. We thank Kristen Kolar, Jeremy Meduri, and Elizabeth Soehngen for assistance in collecting data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Laura R. G. Pickens, Email: lglass2@kent.edu.
James D. Rowan, Email: jrowan@wesleyancollege.edu.
Rick A. Bevins, Email: rbevins@unlserve.unl.edu.
Stephen B. Fountain, Email: sfountai@kent.edu.
References
- Abreu-Villaca Y, Seidler FJ, Qiao D, Tate CA, Cousins MM, Thillai I, Slotkin TA. Short-term adolescent nicotine exposure has immediate and persistent effects on cholinergic systems: Critical periods, patterns of exposure, dose thresholds. Neuropsychopharmacology. 2003;28(11):1935–1949. doi: 10.1038/sj.npp.1300221. [DOI] [PubMed] [Google Scholar]
- Abreu-Villaca Y, Seidler FJ, Slotkin TA. Impact of adolescent nicotine exposure on adenylyl cyclase-mediated cell signaling: Enzyme induction, neurotransmitter-specific effects, regional selectivities, and the role of withdrawal. Brain Research. 2003;988(1–2):164–172. doi: 10.1016/s0006-8993(03)03368-7. [DOI] [PubMed] [Google Scholar]
- Abreu-Villaca Y, Seidler FJ, Tate CA, Slotkin TA. Nicotine is a neurotoxin in the adolescent brain: Critical periods, patterns of exposure, regional selectivity, and dose thresholds for macromolecular alterations. Brain Research. 2003;979(1–2):114–128. doi: 10.1016/s0006-8993(03)02885-3. [DOI] [PubMed] [Google Scholar]
- Adriani W, Deroche-Gamonet V, Le Moal M, Laviola G, Piazza PV. Preexposure during or following adolescence differently affects nicotine-rewarding properties in adult rats. Psychopharmacology. 2006;184(3–4):382–390. doi: 10.1007/s00213-005-0125-1. [DOI] [PubMed] [Google Scholar]
- Adriani W, Spijker S, Deroche-Gamonet V, Laviola G, Le Moal M, Smit AB, Piazza PV. Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats. The Journal of Neuroscience. 2003;23(11):4712–4716. doi: 10.1523/JNEUROSCI.23-11-04712.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agster KL, Fortin NJ, Eichenbaum H. The hippocampus and disambiguation of overlapping sequences. Journal of Neuroscience. 2002;22(1529–2401; 13):5760–5768. doi: 10.1523/JNEUROSCI.22-13-05760.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booze RM, Welch MA, Wood ML, Billings KA, Apple SR, Mactutus CF. Behavioral sensitization following repeated intravenous nicotine administration: Gender differences and gonadal hormones. Pharmacology, Biochemistry, and Behavior. 1999;64(4):827–839. doi: 10.1016/s0091-3057(99)00169-0. [DOI] [PubMed] [Google Scholar]
- Clayton NS, Dickinson A. Episodic-like memory during cache recovery by scrub jays. Nature. 1998;395(6699):272–274. doi: 10.1038/26216. [DOI] [PubMed] [Google Scholar]
- Clayton NS, Salwiczek LH, Dickinson A. Episodic memory. Current Biology. 2007;17(6):R189–R191. doi: 10.1016/j.cub.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Correia SPC, Dickinson A, Clayton NS. Western scrub-jays anticipate future needs independently of their current motivational state. Current Biology. 2007;17(10):856–861. doi: 10.1016/j.cub.2007.03.063. [DOI] [PubMed] [Google Scholar]
- Counotte DS, Goriounova NA, Li KW, Loos M, van der Schors RC, Schetters D, Spijker S. Lasting synaptic changes underlie attention deficits caused by nicotine exposure during adolescence. Nature Neuroscience. 2011;14(4):417–419. doi: 10.1038/nn.2770. [DOI] [PubMed] [Google Scholar]
- Counotte DS, Spijker S, Van de Burgwal LH, Hogenboom F, Schoffelmeer AN, De Vries TJ, Pattij T. Long-lasting cognitive deficits resulting from adolescent nicotine exposure in rats. Neuropsychopharmacology. 2009;34(2):299–306. doi: 10.1038/npp.2008.96. [DOI] [PubMed] [Google Scholar]
- DeVito LM, Eichenbaum H. Distinct contributions of the hippocampus and medial prefrontal cortex to the “what-where-when” components of episodic-like memory in mice. Behavioural Brain Research. 2010;215(2):318–325. doi: 10.1016/j.bbr.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer JB, McQuown SC, Leslie FM. The dynamic effects of nicotine on the developing brain. Pharmacology & Therapeutics. 2009;122(2):125–139. doi: 10.1016/j.pharmthera.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin NJ, Agster KL, Eichenbaum HB. Critical role of the hippocampus in memory for sequences of events. Nat Neurosci. 2002;5(1097–6256; 5):458–462. doi: 10.1038/nn834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin NJ, Wright SP, Eichenbaum H. Recollection-like memory retrieval in rats is dependent on the hippocampus. Nature. 2004;431(1476–4687; 7005):188–191. doi: 10.1038/nature02853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fountain SB. Pattern structure and rule induction in sequential learning. Comparative Cognition & Behavior Review. 2008;3:66–85. [Google Scholar]
- Fountain SB, Benson AM, Wallace DG. Number, but not rhythmicity, of temporal cues determines phrasing effects in rat serial-pattern learning. Learning and Motivation. 2000;31(4):301–322. [Google Scholar]
- Fountain SB, Benson DM. Chunking, rule learning, and multiple item memory in rat interleaved serial pattern learning. Learning and Motivation. 2006;37(2):95–112. [Google Scholar]
- Fountain SB, Krauchunas SM, Rowan JD. Serial-pattern learning in mice: Pattern structure and phrasing. The Psychological Record. 1999;49(2):173–192. [Google Scholar]
- Fountain SB, Rowan JD. aSensitivity to violations of “run” and “trill” structures in rat serial-pattern learning. Journal of Experimental Psychology: Animal Behavior Processes. 1995a;21(1):78–81. [PubMed] [Google Scholar]
- Fountain SB, Rowan JD. Coding of hierarchical versus linear pattern structure in rats and humans. Journal of Experimental Psychology: Animal Behavior Processes. 1995b;21(3):187–202. doi: 10.1037//0097-7403.21.3.187. [DOI] [PubMed] [Google Scholar]
- Fountain SB, Rowan JD, Kelley BM, Willey AR, Nolley EP. Adolescent exposure to nicotine impairs adult serial pattern learning in rats. Experimental Brain Research. 2008;187(4):651–656. doi: 10.1007/s00221-008-1346-4. [DOI] [PubMed] [Google Scholar]
- Fountain SB, Rowan JD, Muller MD, Kundey SMA, Pickens LRG, Doyle KE. The organization of sequential behavior: Conditioning, memory, and abstraction. In: Wasserman EA, Zentall TR, editors. Handbook of comparative cognition. Oxford: Oxford University Press; 2012. pp. 594–614. [Google Scholar]
- Fountain SB, Rowan JD, Muller MD, Smith DPA, Chenoweth AM, Wallace DG. Sequence production paradigms for exploring the organization of sequential behavior. In: Anderson MJ, editor. Tasks and techniques: A sampling of methodologies for the investigation of animal learning, behavior, and cognition. Hauppauge, NY: Nova Science; 2006. pp. 245–260. [Google Scholar]
- Fountain SB, Rowan JD. Differential impairments of rat serial pattern learning and retention induced by MK-801, and NMDA receptor antagonist. Psychobiology. 2000;28(1):32–44. [Google Scholar]
- Heise GA, Hrabrich B, Lilie NL, Martin RA. Scopolamine effects on delayed spatial alternation in the rat. Pharmacology, Biochemistry and Behavior. 1975;3:993–1002. doi: 10.1016/0091-3057(75)90007-6. [DOI] [PubMed] [Google Scholar]
- Hutchison MA, Riley AL. Adolescent exposure to nicotine alters the aversive effects of cocaine in adult rats. Neurotoxicology and Teratology. 2008;30(5):404–411. doi: 10.1016/j.ntt.2008.04.004. [DOI] [PubMed] [Google Scholar]
- Iniguez SD, Warren BL, Parise EM, Alcantara LF, Schuh B, Maffeo ML, Bolanos-Guzman CA. Nicotine exposure during adolescence induces a depression-like state in adulthood. Neuropsychopharmacology. 2009;34(6):1609–1624. doi: 10.1038/npp.2008.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto ET. An assessment of the spontaneous activity of rats administered morphine, phencyclidine, or nicotine using automated and observational methods. Psychopharmacology. 1984;84(3):374–382. doi: 10.1007/BF00555216. [DOI] [PubMed] [Google Scholar]
- Jonasson Z. Meta-analysis of sex differences in rodent models of learning and memory: A review of behavioral and biological data. Neuroscience and Biobehavioral Reviews. 2005;28(8):811–825. doi: 10.1016/j.neubiorev.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Kelley BM, Middaugh LD. Periadolescent nicotine exposure reduces cocaine reward in adult mice. Journal of Addictive Diseases : The Official Journal of the ASAM, American Society of Addiction Medicine. 1999;18(1055-0887; 3):27–39. doi: 10.1300/J069v18n03_04. [DOI] [PubMed] [Google Scholar]
- Kelley BM, Rowan JD. Long-term, low-level adolescent nicotine exposure produces dose-dependent changes in cocaine sensitivity and reward in adult mice. International Journal of Developmental Neuroscience : The Official Journal of the International Society for Developmental Neuroscience. 2004;22(0736–5748; 5–6):339–348. doi: 10.1016/j.ijdevneu.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Klein LC. Effects of adolescent nicotine exposure on opioid consumption and neuroendocrine responses in adult male and female rats. Experimental and Clinical Psychopharmacology. 2001;9(3):251–261. doi: 10.1037//1064-1297.9.3.251. [DOI] [PubMed] [Google Scholar]
- Kundey SMA, Fountain SB. Blocking in rat serial pattern learning. Journal of Experimental Psychology: Animal Behavior Processes. 2010;36(2):307–312. doi: 10.1037/a0016523. [DOI] [PubMed] [Google Scholar]
- Marco EM, Granstrem O, Moreno E, Llorente R, Adriani W, Laviola G, Viveros MP. Subchronic nicotine exposure in adolescence induces long-term effects on hippocampal and striatal cannabinoid-CB1 and mu-opioid receptors in rats. European Journal of Pharmacology. 2007;557(1):37–43. doi: 10.1016/j.ejphar.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Marco EM, Llorente R, Moreno E, Biscaia JM, Guaza C, Viveros MP. Adolescent exposure to nicotine modifies acute functional responses to cannabinoid agonists in rats. Behavioural Brain Research. 2006;172(1):46–53. doi: 10.1016/j.bbr.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Maren S, De Oca B, Fanselow MS. Sex differences in hippocampal long-term potentiation (LTP) and pavlovian fear conditioning in rats: Positive correlation between LTP and contextual learning. Brain Research. 1994;661(1–2):25–34. doi: 10.1016/0006-8993(94)91176-2. [DOI] [PubMed] [Google Scholar]
- Mateos B, Borcel E, Loriga R, Luesu W, Bini V, Llorente R, Viveros MP. Adolescent exposure to nicotine and/or the cannabinoid agonist CP 55,940 induces gender-dependent long-lasting memory impairments and changes in brain nicotinic and CB1 cannabinoid receptors. Journal of Psychopharmacology (Oxford, England) 2010 doi: 10.1177/0269881110370503. [DOI] [PubMed] [Google Scholar]
- McDonald CG, Eppolito AK, Brielmaier JM, Smith LN, Bergstrom HC, Lawhead MR, Smith RF. Evidence for elevated nicotine-induced structural plasticity in nucleus accumbens of adolescent rats. Brain Research. 2007;1151:211–218. doi: 10.1016/j.brainres.2007.03.019. [DOI] [PubMed] [Google Scholar]
- McDonald CG, Dailey VK, Bergstrom HC, Wheeler TL, Eppolito AK, Smith LN, Smith RF. Periadolescent nicotine administration produces enduring changes in dendritic morphology of medium spiny neurons from nucleus accumbens. Neuroscience Letters. 2005;385(2):163–167. doi: 10.1016/j.neulet.2005.05.041. [DOI] [PubMed] [Google Scholar]
- Muller MD, Fountain SB. Concurrent cognitive processes in rat serial pattern learning: Item memory, serial position, and pattern structure. Learning and Motivation. 2010;41:252–272. doi: 10.1016/j.lmot.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restle F, Brown ER. Organization of serial pattern learning. In: Bower GH, editor. Psychology of learning and motivation. New York: Academic Press; 1970a. [Google Scholar]
- Restle F, Brown ER. Serial pattern learning. Journal of Experimental Psychology. 1970b;83:120–125. [Google Scholar]
- Schochet TL, Kelley AE, Landry CF. Differential behavioral effects of nicotine exposure in adolescent and adult rats. Psychopharmacology. 2004;175(3):265–273. doi: 10.1007/s00213-004-1831-9. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Ehlers CL. Lasting effects of adolescent nicotine exposure on the electroencephalogram, event related potentials, and locomotor activity in the rat. Developmental Brain Research. 2002;138(1):15–25. doi: 10.1016/s0165-3806(02)00455-8. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Gilder A, Roth J, Ehlers CL. Increased anxiety-like behavior in adult rats exposed to nicotine as adolescents. Pharmacology, Biochemistry, and Behavior. 2003;75(2):355–361. doi: 10.1016/s0091-3057(03)00093-5. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Bodwell BE, Ryde IT, Seidler FJ. Adolescent nicotine treatment changes the response of acetylcholine systems to subsequent nicotine administration in adulthood. Brain Research Bulletin. 2008;76(1–2):152–165. doi: 10.1016/j.brainresbull.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Smith LN, McDonald CG, Bergstrom HC, Brielmaier JM, Eppolito AK, Wheeler TL, Smith RF. Long-term changes in fear conditioning and anxiety-like behavior following nicotine exposure in adult versus adolescent rats. Pharmacology, Biochemistry and Behavior. 2006;85(1):91–97. doi: 10.1016/j.pbb.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Spaeth AM, Barnet RC, Hunt PS, Burk JA. Adolescent nicotine exposure disrupts context conditioning in adulthood in rats. Pharmacology, Biochemistry, and Behavior. 2010;96(4):501–506. doi: 10.1016/j.pbb.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trauth JA, McCook EC, Seidler FJ, Slotkin TA. Modeling adolescent nicotine exposure: Effects on cholinergic systems in rat brain regions. Brain Research. 2000;873(1):18–25. doi: 10.1016/s0006-8993(00)02465-3. [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, Ali SF, Slotkin TA. Adolescent nicotine exposure produces immediate and long-term changes in CNS noradrenergic and dopaminergic function. Brain Research. 2001;892(2):269–280. doi: 10.1016/s0006-8993(00)03227-3. [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, McCook EC, Slotkin TA. Adolescent nicotine exposure causes persistent upregulation of nicotinic cholinergic receptors in rat brain regions. Brain Research. 1999;851(1–2):9–19. doi: 10.1016/s0006-8993(99)01994-0. [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, Slotkin TA. An animal model of adolescent nicotine exposure: Effects on gene expression and macromolecular constituents in rat brain regions. Brain Research. 2000a;867(1–2):29–39. doi: 10.1016/s0006-8993(00)02208-3. [DOI] [PubMed] [Google Scholar]
- Trauth JA, Seidler FJ, Slotkin TA. Persistent and delayed behavioral changes after nicotine treatment in adolescent rats. Brain Research. 2000b;880(1–2):167–172. doi: 10.1016/s0006-8993(00)02823-7. [DOI] [PubMed] [Google Scholar]
- Williams CL, Meck WH. The organizational effects of gonadal steroids on sexually dimorphic spatial ability. Psychoneuroendocrinology. 1991;16(1–3):155–176. doi: 10.1016/0306-4530(91)90076-6. [DOI] [PubMed] [Google Scholar]
- Zhou WY, Crystal JD. Validation of a rodent model of episodic memory. Animal Cognition. 2011;14(3):325–340. doi: 10.1007/s10071-010-0367-0. [DOI] [PMC free article] [PubMed] [Google Scholar]