Abstract
Background
While several studies report an association between prevalent diabetes mellitus (DM) and cognitive impairment, less is known about incident DM in late life and cognitive decline. Glycemic control among elders with DM may also be associated with cognitive function, but findings are inconsistent.
Objective
To determine if prevalent and incident DM increases risk of cognitive decline, and if, among elders with DM, poor glucose control is related to worse cognitive performance.
Design
Prospective cohort study.
Setting
Health Aging and Body Composition Study at two community clinics.
Participants
A total of 3,069 elders (mean age 74.2 years; 42% black; 52% female).
Main Outcome Measures
Participants completed the Modified Mini-Mental State Examination (3MS) and Digit Symbol Substitution Test (DSST) at baseline and selected intervals over 10 years. DM status was determined at baseline and during follow-up visits. Glycosylated hemoglobin A1c (HbA1c) was measured at year 1 (baseline), 4, 6, and 10 from fasting whole blood.
Results
At baseline 717 (23.4%) participants had prevalent DM and 2352 (76.6%) were without diabetes, 159 of whom developed incident DM during follow-up. Participants with prevalent DM had lower baseline test scores than participants without DM (3MS: 88.8 vs. 90.9; DSST: 32.5 vs 36.3, respectively; |t|=6.09, p=0.001 for both tests). Results from mixed-effects models showed a similar pattern for 9-year decline (3MS: −6.0 vs. −4.5 point decline; |t|=2.66, p=0.008; DSST: −7.9 vs. −5.7, point decline; |t|=3.69, p=0.001, respectively). Participants with incident DM tended to have baseline and 9-year decline scores between the other two groups but were not statistically different from the group without diabetes. Multivariate adjustment for demographics and medical co-morbidities produced similar results. Among participants with prevalent DM, HbA1c level was associated with lower average mean cognitive scores (3MS p for overall=0.003; DSST p for overall=0.04), even after multivariate adjustment.
Conclusion
Among well-functioning older adults, DM and poor glucose control among those with DM are associated with worse cognitive function and greater decline. This suggests severity of DM may contribute to accelerated cognitive aging.
Introduction
In the United States, approximately 27% (10.9 million) of adults aged 65 years and older have diabetes mellitus (DM) 1. The risk of both DM and cognitive impairment increases with age. Findings from several studies suggest an association between DM and increased risk of cognitive impairment and dementia, including Alzheimer’s disease (AD) 2–5. However, the association between DM and cognitive function in older adults continues to be debated, and less is known regarding incident DM in late-life and cognitive function over time.
Many studies report a link between DM and decreased cognitive function, with a stronger association found in older (> 60 years) adults compared to younger groups 6–8. However, most studies investigating DM and cognitive function have either been case-control or prospective studies that focused on prevalent DM determined only at baseline 2, 5. Little is known about cognitive function in older adults with newly diagnosed DM, limiting our understanding of the association between emergent DM and cognitive performance. In addition, poor glucose control, measured by glycosylated hemoglobin A1C (HbA1c), has emerged as a possible risk factor for cognitive decline among elders with DM; results, however, have been inconsistent 9–14.
We sought to evaluate the association between prevalent and incident DM and cognitive function at baseline and over time in a diverse group of well-functioning older adults. Our secondary aim was to determine if glycemic control, as measured by HbA1c, among those with DM is associated with cognitive function. Our hypothesis was that participants with prevalent DM would have worse cognitive function over 9-years compared to those without DM, and that participants with incident DM would have a decline in cognitive function intermediate between those with prevalent DM and those remaining free of DM. Among those with prevalent DM, we hypothesized that higher HbA1C level would correspond to lower cognitive scores.
Methods
Study Population
Participants were enrolled in the Health Aging and Body Composition (Health ABC) Study, a prospective cohort study beginning in 1997 of 3,075 community-dwelling white and black older adults then aged 70–79 years living in Memphis, TN or Pittsburgh, PA. Participants were recruited from a random sample of white Medicare-eligible elders within the designated zip codes and all age-and function eligible blacks. Exclusion criteria included reported difficulties performing activities of daily living, walking a quarter of a mile or climbing 10 steps without resting. Participants also had to be free of life threatening cancers and planning to remain within the study area for at least 3 years.
This study was approved by the institutional review boards of the University of Pittsburgh and the University of Tennessee, Memphis, and that of the coordinating center, the University of California, San Francisco. All participants signed an informed written consent, approved by the institutional review boards at the clinical sites.
Measurements
Prevalent diabetes mellitus (DM) was defined at baseline by self-report, use of hypoglycemic medication, a fasting glucose of ≥126 mg/dl, or a 2-hour glucose tolerance test >200mg/dl, in accordance with the American Diabetes Association (ADA) criteria in place near the start of the Health ABC study (ADA 2002). DM status was assessed at each follow-up visit by self-reported diabetes status or hypoglycemic medication use, or by elevated fasting glucose taken at years 2, 4 and 6. Those who developed DM during follow-up were defined as having incident DM.
Glycosylated hemoglobin A1c (HbA1c) was measured at year 1 (baseline), 4, 6, and 10 from fasting whole blood using fully automated analyzers that utilize nonporous ion-exchange high performance liquid chromatography (HPLC) for separation of HbA1c. HbA1c level was analyzed by approximate tertile as low (< 7%), mid (7–8%) and high (≥8%).
The Modified Mini-Mental State Examination (3MS) was administered at baseline visit (Year 1) and repeated at Years 3, 5, 8, and 10 follow-up visits. The 3MS is a brief, general cognitive battery with components for orientation, concentration, language, praxis, and immediate and delayed memory 15. Scores range from 0 to 100 points, with lower scores indicating poorer performance. The Digit Symbol Substitution Test (DSST), administered in Years 1, 5, 8, and 10, measures attention, psychomotor speed, and executive function 16. The DSST score was calculated as the total number of test items correctly coded in 90 seconds with a maximum (best) score possible of 90.
Covariates
Possible covariates included the baseline self-reported age, race, sex, level of education (categorized as less than high school, some high school, high school or more education), and number of alcoholic drinks per day (categorized as less than one vs. one or more drinks per day). Depressive symptoms were assessed using the 20-item Center for Epidemiologic Studies-Depression Scale (CES-D)17. Body mass index (BMI) (kg/m2) was calculated from direct height and weight measurements at baseline. Hypertension was determined using self-report, medication use, and clinical measurements taken at the baseline examination. Stroke and MI were based on self-report, clinic data, and medication use. Apolipoprotein E (APOE) genotype was determined by the 5'-nuclease assay18 in the Human Genetics laboratory at the University of Pittsburgh and participants were coded as e4 carriers or non-carriers.
Statistical Analyses
We first performed bivariate analyses to test for associations between DM group and baseline characteristics. We used chi-square analysis for categorical variables and F-test for continuous variables.
We used mixed effects linear regression models to determine the association between DM group and baseline test scores as well as change in scores over 9-years. The mixed effects linear regression models estimated the change in cognitive scores over time allowing person-specific differences in the cognitive score at baseline and rate of cognitive decline. Incident DM was treated as a time-varying covariate, categorized according to DM determined during any of the follow-up visits. We then created a multivariate mixed effects model adjusting for characteristics (time-dependent when possible) that significantly differed across DM group at baseline (p<0.05) (Table 1) or that have been previously shown to be associated with cognitive function.
Table 1.
Baseline characteristics of the 3,069 participants by diabetes mellitus (DM) status
| Baseline Characteristics Mean (SD) or % |
Non-DM N=2193 |
Incident N=159 |
Prevalent N=717 |
p-value* |
|---|---|---|---|---|
| Age, years | 74.1 (2.9) | 73.7 (3.1) | 74.2 (2.8) | .18 |
| Black (%) | 828 (37.7) | 75 (47.2) | 374 (52.2) | <.001 |
| Female (%) | 1180 (53.8) | 78 (49.1) | 326 (45.5) | <.001 |
| Education | <.001 | |||
| Less than high school | 501 (22.9) | 38 (23.9) | 231 (32.4) | |
| High school | 710 (32.5) | 53 (33.5) | 236 (33.3) | |
| Current smoker | 241 (11) | 15 (8.7) | 62 (9.4) | .19 |
| Alcohol use (≥ 1 drink/day) | 172 (7.9) | 13 (8.2) | 42 (5.9) | .19 |
| Stroke (%) | 160 (7.4) | 17 (10.8) | 69 (9.8) | .06 |
| Myocardial Infarction (%) | 228 (10.4) | 24 (15.2) | 105 (14.6) | <.001 |
| Hypertension (%) | 1244 (56.7) | 115 (72.3) | 510 (71.1) | <.001 |
| Body Mass Index (kg/m2) | 26.7 (4.6) | 29.6 (4.9) | 28.9 (4.9) | <.001 |
| Depression score ≥16 (%) | 88 (4.0) | 7 (4.4) | 26 (3.6) | .86 |
| Apolipoprotein E e4 | 596 (28.7) | 49 (32.9) | 191 (28.2) | 51 |
We next examined the association between HbA1c level and cognitive test scores among participants with prevalent DM using unadjusted and adjusted mixed effects linear regression models, similar to those used to test for associations between DM group and cognitive scores. HbA1c status was treated as a time-varying covariate, updated with values obtained during the follow-up visit proximal to cognitive testing. Since HbA1c level was analyzed using slightly different assays in the Health ABC study during separate years and the interaction between time and HbA1c was not significant, we assessed cognitive score at mean time of follow-up for HbA1c values among those with prevalent DM
All analyses were conducted using SAS statistical software, version 9.2 (SAS Institute Inc, Cary, North Carolina), and were 2-tailed with the statistical significance level set at p<0.05.
Results
Of the 3,069 participants in our study, 717 (23.4 %) had prevalent diabetes mellitus (DM) and 159 (5.2%) developed DM over follow-up. The mean (SD) age of participants at baseline was 74.2 (2.9). Forty-two percent were black and 52% were female. Prevalent DM was associated with black race, being male, and having less than a high school education, a history of MI or hypertension, and a higher BMI (Table 1).
At baseline, persons with prevalent DM had lower unadjusted 3MS and DSST scores compared to those without DM (|t|=6.09; p=0.001 for both tests) (Table 2). Adjusting for age, race, sex and education produced similar results. Compared to those without DM, participants with prevalent DM had slightly lower mean 3MS baseline scores (89.7 vs 90.5, |t|=2.5; p=0.01) and DSST scores (34.3 vs. 35.5, |t|=2.24; p=0.03) (Figure 1). Baseline cognitive scores were similar for those with incident DM compared to participants without DM although scores tended to be between those for prevalent DM and without DM. Additional adjustment for time-dependent MI and hypertension and baseline BMI did not significantly alter the relationship between DM and cognitive scores. There was no interaction with race, sex, education, or APOE e4 and DM on cognitive decline (p>0.05 for all).
Table 2.
Unadjusted mean (SE) cognitive test scores by diabetes mellitus (DM) status
| Cognitive test | Diabetes status | ||
|---|---|---|---|
| Non-DM N=2193 |
Incident N=159 |
Prevalent N=717 |
|
| Modified Mini-Mental State Examination | |||
| Baseline | 90.9 (0.2) | 90.2 (0.5) | 88.8 (0.3)* |
| 9-year change score | −4.5 (0.3) | −5.8 (1.4) | −6.0 (0.5)* |
| Digit Symbol Substitution Test | |||
| Baseline | 36.3 (0.3) | 35.3 (0.7) | 32.5 (0.5)* |
| 9-year change score | −5.7 (0.3) | −4.4 (1.8) | −7.9 (0.5)* |
p-value <0.05 compared to participants without diabetes
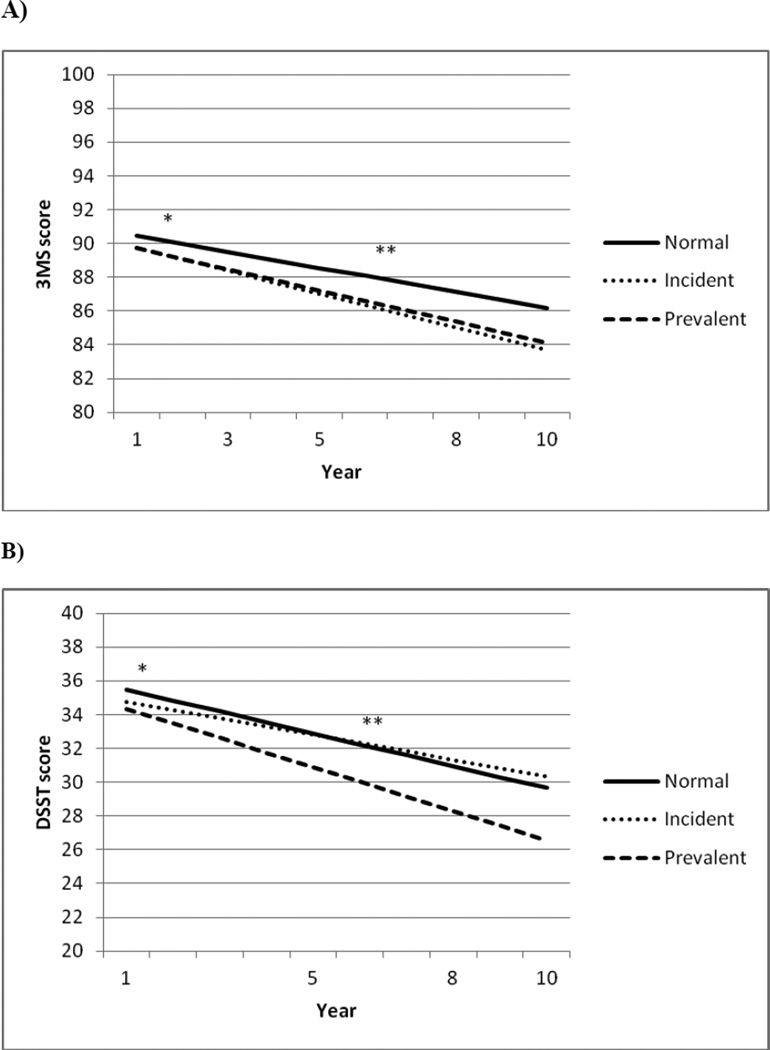
Figure 1.
Baseline and 9-year cognitive decline scores by diabetes status, adjusting for age, race, sex, and education.
a)* Baseline Modified Mini Mental State Examination (3MS) score: prevalent vs. normal, |t|=2.5; p-value=0.01; incident vs. normal, |t|=1.69; p value=0.09; **3MS 9-year decline slope: prevalent vs. normal, |t|=2.37; p value=0.02
b)* Baseline Digit Symbol Substitution Test (DSST) score: prevalent vs. normal, |t|=2.24p-value=0.03;**DSST 9-year decline slope: prevalent vs. normal, |t|=3.25p-value=0.001
After an average of 9-years, participants with prevalent DM had significantly greater decline on both the 3MS (|t|=2.66; p=0.008) and the DSST (|t|=3.69; p=0.001) compared to those without DM (Table 2). After adjusting for age, race, sex, and education, these differences remained significant (prevalent DM: mean (SE) 3MS: −5.6 (0.5) point decline; p=0.02; DSST: −7.8 (0.5) point decline; |t|=2.37; p=0.001) compared to participants without DM (3MS:−4.3 (0.3) point decline; DSST:−5.8 (0.3) point decline) (Figure 1). Compared to participants without DM, those with incident DM did not have significantly greater decline in scores but demonstrated mean score decline between the other groups.
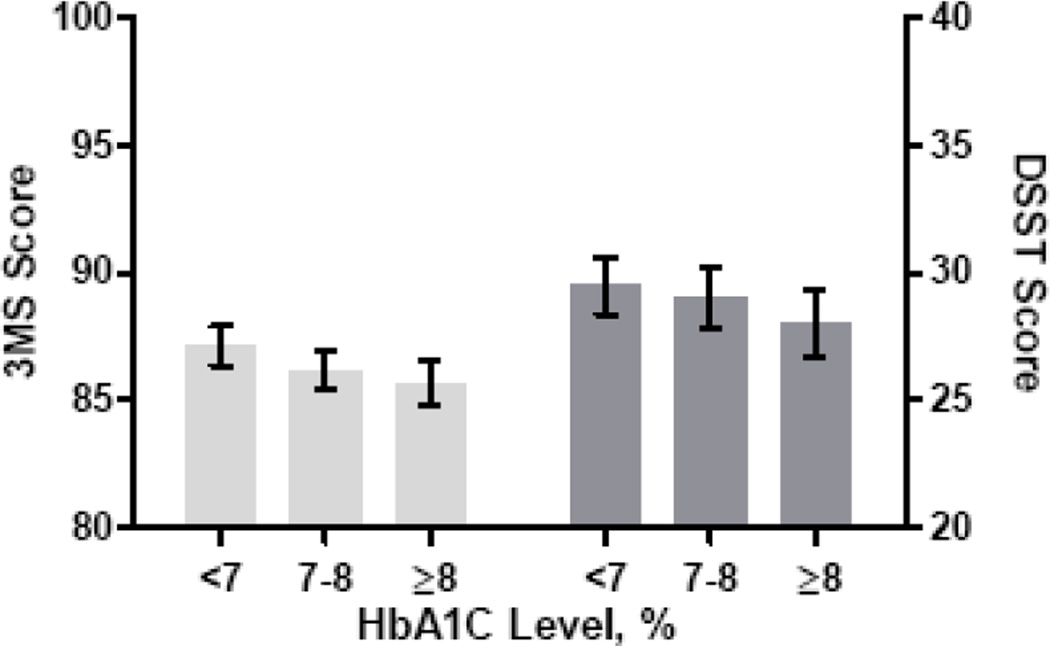
Among participants with prevalent DM, higher levels of HbA1c were associated with lower 3MS and DSST test scores. Among participants with prevalent DM with a HbA1c value, at an average mean time of follow-up of3.5 years, mid- (7–8%; n=219) and high (≥8%; n=227) HbA1c level had significantly lower average mean scores than those with a low level (≤7%; n=269) on both the 3MS (3MS: low: 87.1, (0.4); mid: 86.2, (0.4), high: 85.7, (0.5); F=3.36; p for overall= 0.003) and the DSST (low: 29.5, (0.6); mid: 29.0, (0.6); high: 28.0, (0.7); p for overall=0.04) (Figure 2). After adjusting for age, race, sex, and education, scores remained significantly lower for the mid- and high tertile on the 3MS but were no longer significant for the DSST (F=0.87; p for overall=0.42). Additional adjustment for MI, hypertension and BMI did not appreciably change results.
Figure 2.
Unadjusted  Modified Mini Mental State Examination (3MS) and
Modified Mini Mental State Examination (3MS) and  Digit Symbol Substitution Test (DSST) scores by glycosylated hemoglobin A1C (HbA1C) level at mean time of follow-up.
Digit Symbol Substitution Test (DSST) scores by glycosylated hemoglobin A1C (HbA1C) level at mean time of follow-up.
Discussion
In this prospective study of well-functioning older adults, persons with prevalent diabetes mellitus (DM) had lower baseline and greater 9-year decline in cognitive scores compared to participants who remained free of DM over the follow-up. Participants who developed DM during follow-up tended to have scores between those without DM and those with prevalent DM but scores were not statistically different from the non-DM group. Decline scores on the 3MS among those with incident DM were also similar to those with prevalent DM. In addition, participants with DM who had higher glycosylated hemoglobin A1c (HbA1c) level performed more poorly on cognitive tests suggesting that glucose control is related to cognitive function.
Our results are consistent with prior studies reporting an association between DM and an increased risk of cognitive impairment2, 5. A few studies have also investigated cognitive function in the early stages of DM and report a trend toward reduced cognitive function in adults with recently diagnosed DM19, 20. For example, a prospective study of a group of middle-aged adults (aged 43–70 years) found that, in participants over age 60 years, participants who developed DM during follow-up had greater cognitive decline than those without DM, and that those with prevalent DM at the study’s baseline had the greatest cognitive decline 20. Another study in older adults reported small reductions in cognitive function in participants recently diagnosed with DM compared to a non-DM control group 19. Results from the current study showed a similar trend toward intermediate cognitive decline in older adults with incident DM. This suggests that delaying or preventing the onset of DM may prove beneficial for maintaining cognitive function in older adults, especially considering a longer duration of DM has been linked to worse cognitive function, including mild cognitive impairment 21
Hyperglycemia has been proposed as a mechanism that may contribute to the association between DM and reduced cognitive function 22. The ADA recommends maintaining a HbA1c level of <7% to help prevent microvascular complications23 and our results add to a body of literature that suggests maintaining a HbA1c at this level may also help with cognitive health. Higher HbA1c level has been associated with worse cognitive outcomes in both cross-sectional 9, 11 and prospective studies 10, 12, but results have been inconsistent 13, 14. For example, in the Rancho Bernardo Study, glycemic control was found to mediate the relationship between DM and cognitive decline12 but this association was not found in the Atherosclerosis Risk in Communities (ARIC) study13. However, participants in the ARIC study were younger (mean age 56 years) and may have been less likely to experience cognitive decline. In our study, participants with higher levels of HbA1c had lower cognitive scores, strengthening support for an increased risk of cognitive impairment with poor glucose control among older adults with DM. Results from the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Memory in Diabetes (MIND) trial also found an association between HbA1C levels and reduced cognitive performance, however an intensive glycemic control intervention was not shown to benefit cognitive function 24. Hyperglycemia may contribute to cognitive impairment through such mechanisms as the formation of advanced glycation endproducts 25, inflammation 26 and microvascular disease 27. However, glycemic control needs to be considered in light of other studies suggesting that hypoglycemia episodes may also be linked to dementia 28. This suggests elderly individuals with DM should be carefully monitored for optimal care.
There are several other mechanisms which may underlie the association of DM and glucose control and reduced cognitive function. Individuals with DM are at an increased risk for renal disease, depression, stroke, hypertension, hyperlipidemia, and cardiovascular disease, each of which may impair cognitive performance 29–31. In our study, adjustment for several co-morbidities did not significantly alter the associations between DM and glucose control and reduced cognitive function, however we cannot rule out residual confounding.
Strengths of this study include a prospective design with a long follow-up period and a relatively large and diverse sample. For incident DM, cognitive function was measured at baseline before the onset of disease. For HbA1c, we used a cut-off of less than 7% for glycemic control, which has been recommended by the ADA to reduce microvascular disease23.We were also able to adjust for several potential confounders. The study also had several limitations. The Health ABC study enrolled only well-functioning elders at baseline and results may not be generalized to elderly individuals with functional disabilities. The cognitive test battery was limited to general cognitive function and processing domains. In addition the differences in cognitive scores between the groups were relatively small and the clinical significance is unclear. Analysis of HbA1c changes over time was restricted due to inconsistency in assays used during different years of Health ABC study follow-up. We did not assess insulin resistance or levels, which may also be related to cognitive function 32. In addition, due to the small sample size of those with incident DM, our power to detect group differences was restricted. We also did not have information on duration or severity of DM for those with prevalent diabetes at study baseline.
This study supports the hypothesis that older adults with DM have reduced cognitive function and that poor glycemic control may contribute to this association. Future studies should determine if early diagnosis and treatment of DM lessens the risk of developing cognitive impairment and if maintaining optimal glucose control helps mitigate the effect of DM on cognition.
Acknowledgement
Study funding: NIA contract #s: N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant #s: R01-AG028050, NINR grant R01-NR012459. This research was supported in part by the Intramural Research Program of the NIH, National Institute of Aging and by a grant from the American Health Assistance Foundation, grant number A201-0029. Dr. Yaffe is supported in part by a National Institute of Aging Grant (K24AG031155). Dr. Yaffe has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Author Contributions:
Dr. Yaffe: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, study supervision, obtaining funding.
Ms. Falvey: drafting/revising the manuscript.
Mr. Hamilton: drafting/revising the manuscript, analysis or interpretation of data, statistical analysis.
Dr. Schwartz: drafting/revising the manuscript, analysis or interpretation of data.
Dr. Simonsick: analysis or interpretation of data, acquisition of data.
Dr. Satterfield: drafting/revising the manuscript, acquisition of data, study supervision.
Dr. Cauley: drafting/revising the manuscript, acquisition of data.
Dr. Rosano: drafting/revising the manuscript.
Dr. Launer: drafting/revising the manuscript.
Dr. Strotmeyer: drafting/revising the manuscript.
Dr. Harris: drafting/revising the manuscript, acquisition of data, study supervision.
Author Disclosures:
Dr. Yaffe has served on data safety monitoring boards for Pfizer Inc., Medivation, Inc. and the NIH (NIMH and NIA trials); and as received research support from the NIH (NIA, NIDDK, NIMH), the Department of Defense, American Health Assistance Foundation, Anonymous Foundation, and the Alzheimer Association.
Ms. Falvey has no disclosures to report.
Mr. Hamilton has no disclosures to report.
Dr. Schwartz serves on a scientific advisory board for GlaxoSmithKline; has received speaker honoraria from Amgen and Merck Serono; has received funding for travel from Amgen; and receives research support from Merck Serono, GlaxoSmithKline, the NIH (NIDDK, NIA).
Dr. Simonsick serves as an Associate Editor for the Journal of Gerontology Medical Sciences
Dr. Satterfield receives research support from the NIH/NIA.
Dr. Cauley receives research support from Novartis.
Dr. Rosano reports no disclosures.
Dr. Launer receives research support from the NIH/NIA Intramural Research Program.
Dr. Strotmeyer reports no disclosures.
Dr. Harris receives research support from the NIH.
References
- 1.Centers for Disease Control and Prevention. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 2.Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006;5:64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- 3.Luchsinger JA, Reitz C, Patel B, Tang MX, Manly JJ, Mayeux R. Relation of diabetes to mild cognitive impairment. Arch Neurol. 2007;64:570–575. doi: 10.1001/archneur.64.4.570. [DOI] [PubMed] [Google Scholar]
- 4.Yaffe K, Blackwell TL, Kanaya AM, Davidowitz N, Barrett-Connor E, Krueger K. Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology. 2004;63:658–663. doi: 10.1212/01.wnl.0000134666.64593.ba. [DOI] [PubMed] [Google Scholar]
- 5.Profenno L, Porsteinsson A, Faraone S. Meta-analysis of Alzheimer's Disease Risk with Obesity, Diabetes, and Related Disorders. Biol Psychiatry. 2010;67:505–512. doi: 10.1016/j.biopsych.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Ryan CM, Geckle M. Why is learning and memory dysfunction in Type 2 diabetes limited to older adults? Diabetes Metab Res Rev. 2000;16:308–315. doi: 10.1002/1520-7560(2000)9999:9999<::aid-dmrr141>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 7.Xu W, Qiu C, Gatz M, Pedersen N, Johansson B, Fratiglioni L. Mid- and Late-Life Diabetes in Relation to the Risk of Dementia: A Population-Based Twin Study. Diabetes. 2009;58:71–77. doi: 10.2337/db08-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biessels GJ, Deary IJ, Ryan CM. Cognition and diabetes:a lifespan perspective. Lancet Neurol. 2008;7:184–190. doi: 10.1016/S1474-4422(08)70021-8. [DOI] [PubMed] [Google Scholar]
- 9.Cukierman-Yaffe T, Gerstein HC, Williamson JD, et al. Relationship between baseline glycemic control and cognitive function in individuals with type 2 diabetes and other cardiovascular risk factors. Diabetes Care. 2009;32:221–226. doi: 10.2337/dc08-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yaffe K, Blackwell T, Whitmer RA, Krueger K, Barrett-Connor E. Glycosylated hemoglobin level and development of mild cognitive impairment or dementia in older women. J Nutr Health Aging. 2006;10:293–295. [PubMed] [Google Scholar]
- 11.Nguyen HT, Grzywacz JG, Arcury TA, et al. Linking glycemic control and executive function in rural older adults with diabetes mellitus. J Am Geriatr Soc. 2010;58:1123–1127. doi: 10.1111/j.1532-5415.2010.02857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanaya AM, Barrett-Connor E, Gildengorin G, Yaffe K. Change in cognitive function by glucose tolerance status in older adults: a 4-year prospective study of the Rancho Bernardo Study Cohort. Arch Intern Med. 2004;164:1327–1333. doi: 10.1001/archinte.164.12.1327. [DOI] [PubMed] [Google Scholar]
- 13.Christman AL, Matsushita K, Gottesman RF, et al. Glycated haemoglobin and cognitive decline: the Atherosclerosis Risks in Communities (ARIC) study. Diabetologia. 2011 doi: 10.1007/s00125-011-2095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saczynski JS, Jónsdóttir MK, Garcia ME, et al. Cognitive impairment:an increasing important complication of type 2 diabetes: The Age, Gene/Environment Susceptibility-Reykjavik Study. Am J Epidemiol. 2008;168:1132–1139. doi: 10.1093/aje/kwn228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teng E, Chui H. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 16.Wechsler D. Wechsler Adult Intelligence Scale-Third Edition (WAIS-III) Third ed. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- 17.Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 18.Livak K. SNP genotyping by the 5'-nuclease reaction. Methods Mol Biol. 2003;212:129–147. doi: 10.1385/1-59259-327-5:129. [DOI] [PubMed] [Google Scholar]
- 19.Ruis C, Biessels GJ, Gorter KJ, Van Den Donk M, Kappelle LJ, Rutten GEHM. Cognition in the Early Stages of Type 2 Diabetes. Diabetes Care. 2009;32:1261–1265. doi: 10.2337/dc08-2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nooyens A, Baan C, Spijkerman A, Verschuren W. Type 2 Diabetes and Cognitive Decline in Middle-Aged Men and Women: The Doetinchem Cohort Study. Diabetes Care. 2010;33:1964–1969. doi: 10.2337/dc09-2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts RO, Geda YE, Knopman DS, et al. Association of duration and severity of diabetes mellitus with mild cognitive impairment. Arch Neurol. 2008;65:1066–1073. doi: 10.1001/archneur.65.8.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cox DJ, Kovatchev BP, Gonder-Frederick LA, et al. Relationships between hyperglycemia and cognitive performance among adults with type1 and type 2 diabetes. Diabetes Care. 2005;28:71–77. doi: 10.2337/diacare.28.1.71. [DOI] [PubMed] [Google Scholar]
- 23.The American Diabetes Association. Executive Summary: Standards of Medical Care in Diabetes-2012. Diabetes Care. 2012;35:s4–s10. doi: 10.2337/dc12-s004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Launer L, Miller ME, Williamson JD, et al. Effects of intensive glucose lowering on brain structure and function in people with type 2 diabetes (ACCORD MIND): a randomised open-label study. Lancet Neurol. 2011;10:969–977. doi: 10.1016/S1474-4422(11)70188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vlassara H, Palace MR. Diabetes and advanced glycation endproducts. J Intern Med. 2002;251:87–101. doi: 10.1046/j.1365-2796.2002.00932.x. [DOI] [PubMed] [Google Scholar]
- 26.Yaffe K, Kanaya A, Lindquist K, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA. 2004;292:2237–2242. doi: 10.1001/jama.292.18.2237. [DOI] [PubMed] [Google Scholar]
- 27.Launer LJ. Demonstrating the case that AD is a vascular disease: epidemiologic evidence. Ageing Res Rev. 2002;1:61–77. doi: 10.1016/s0047-6374(01)00364-5. [DOI] [PubMed] [Google Scholar]
- 28.Whitmer RA, Karter AJ, Yaffe K, Quesenberry CPJ, Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA. 2009;301:1565–1572. doi: 10.1001/jama.2009.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strachan MW, Reynolds RM, Marioni RE, Price JF. Cognitive function, dementia and type 2 diabetes mellitus in the elderly. Nat Rev Endocrinol. 2011;7:108–114. doi: 10.1038/nrendo.2010.228. [DOI] [PubMed] [Google Scholar]
- 30.Yaffe K, Ackerson L, Kurella Tamura M, et al. Chronic kidney disease and cognitive function in older adults: findings from the Chronic Renal Insufficiency Cohort Cognitive Study. Journal of the American Geriatrics Society. 2010;58:338–345. doi: 10.1111/j.1532-5415.2009.02670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knopman D, Boland LL, Mosley T, et al. Cardiovascular risk factors and cognitve decline in middle-aged adults. Neurology. 2001;56:42–48. doi: 10.1212/wnl.56.1.42. [DOI] [PubMed] [Google Scholar]
- 32.Tan ZS, Beiser AS, Fox CS, et al. Association of metabolic dysregulation with volumetric brain magnetic resonance imaging and cognitive markers of subclinical brain aging in middle-aged adults: The Framingham offspring study. Diabetes Care. 2011;34:1766–1770. doi: 10.2337/dc11-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]