Abstract
Hemopexin provides neuroprotection in mouse models of stroke and intracerebral hemorrhage and protects neurons in vitro against heme or reactive oxygen species (ROS) toxicity via heme oxygenase-1 (HO1) activity. To model human brain neurons experiencing hemorrhages and inflammation, we used human neuroblastoma cells, heme–hemopexin complexes, and physiologically relevant ROS, for example, H2O2 and HOCl, to provide novel insights into the underlying mechanism whereby hemopexin safely maintains heme and iron homeostasis. Human amyloid precursor protein (hAPP), needed for iron export from neurons, is induced ~twofold after heme–hemopexin endocytosis by iron from heme catabolism via the iron-regulatory element of hAPP mRNA. Heme– hemopexin is relatively resistant to damage by ROS and retains its ability to induce the cytoprotective HO1 after exposure to tert-butylhydroperoxide, although induction is impaired, but not eliminated, by exposure to high concentrations of H2O2 in vitro. Apo-hemopexin, which predominates in non-hemolytic states, resists damage by H2O2 and HOCl, except for the highest concentrations likely in vivo. Heme– albumin and albumin are preferential targets for ROS; thus, albumin protects hemopexin in biological fluids like CSF and plasma where it is abundant. These observations provide strong evidence that hemopexin will be neuroprotective after traumatic brain injury, with heme release in the CNS, and during the ensuing inflammation. Hemopexin sequesters heme, thus preventing unregulated heme uptake that leads to toxicity; it safely delivers heme to neuronal cells; and it activates the induction of proteins including HO1 and hAPP that keep heme and iron at safe levels in neurons.
Keywords: APP, heme, hemopexin, iron, neuroprotection, stroke
Iron metabolism is altered in cerebrovascular disease and elevated brain iron is associated with the pathogenesis of the aging brain, Alzheimer’s disease [AD (Crapper McLachlan et al. 1991; Bartzokis et al. 2000)], and neurodegeneration in stroke and intracerebral hemorrhage. The incidence of brain microhemorrhages that release heme increases with age (Takahashi et al. 2000; Qureshi et al. 2001) and contributes to AD pathology (Tong et al. 2005; Brenner 2008; Cordonnier and van der Flier 2011) probably by heme-induced oxidative stress via lipid peroxidation (Gutteridge and Smith 1988; Timmins et al. 1995) and release of redox-active ferrous iron from heme catabolism by heme oxygenases. Iron toxicity from ferrous iron, produced within the reducing environment of cells, generates the highly reactive hydroxyl radical (Hershko 2007). Normally, iron storage, use in biochemical and regulatory purposes, or export together minimize levels of reactive ‘free’ iron. The human amyloid precursor protein (hAPP), whose expression and processing is dysregulated in AD leading to toxic amyloid burden, acts in neurons as a ferroxidase for iron export (Duce et al. 2010). Ferroxidases prevent oxidative stress by converting ferrous ions to ferric. In human neuroblastoma SH-SY5Y cells, considered good models of human neurons, iron levels are controlled in part by hAPP as high levels promote iron export, whereas hAPP ablation increases iron retention (Zheng et al. 2009). Furthermore, hAPP is induced by iron in SH-SY5Y cells, and iron influx induces APP (ferroxidase-II) translational up-regulation mediated by the release of Iron-regulatory protein repressor from iron-responsive element sequences (IRE) in the 5′ untranslated region of the precursor transcript (Cho et al. 2010).
Inflammation rapidly follows brain damage from stroke, hemorrhage, and trauma. Oxidative stress from reactive oxygen species (ROS) generated by neutrophils and other phagocytic cells is exacerbated by heme from hemoglobin. Heme, free or bound to globin, reacts with hydroperoxides generated in vivo from lipid peroxides during oxidative stress or normal metabolism to produce ROS, including highly reactive radical species. This reactivity of heme that destroys biomolecules is prevented when it is bound to hemopexin because access of ROS to the heme is then restricted (Gutteridge and Smith 1988; Timmins et al. 1995). Neuroprotection by hemopexin has been documented in mouse models of stroke (Li et al. 2009) and intracerebral hemorrhage (Chen et al. 2010) implicating heme as a toxic molecule. Cytoprotection of neurons by hemopexin against damage by heme and ROS in part requires active heme oxygenase-1 [HO1 (Li et al. 2009)]. Numerous published studies show that raising HO1 levels is protective (Morse and Choi 2002). This occurs using carbon monoxide released from heme catabolism to activate the ataxia telangiectasia mutated (ATM) protein needed for double-stranded DNA repair (Otterbein et al. 2011). However, whether the inducible HO1 and the constitutively expressed HO2, whose basal levels are high in brain cells, protects neurons in brain injury is not clear (Chen and Regan 2004; Schipper 2004; Song et al. 2006), and there may be cell-type-specific differences, for example, astrocytes may differ from neurons (Schipper et al. 2009a,b; Song et al. 2012). Therefore, it is important to characterize the mechanistic details of iron and heme metabolism in brain cells and the role in this of neuroprotective hemopexin.
Under in vitro conditions designed to mimic brain damage after bleeding and oxidative stress from inflammation, we here address whether hemopexin binds heme and regulates HO1 and hAPP in models of human neurons, the SH-SY5Y neuroblastoma cells. Our observations support that hemopexin protects brain neurons immediately after such damage and the ensuing inflammatory conditions, by heme sequestration, safe heme delivery, and induction of HO1 and hAPP. While the extracellular antioxidant role of hemopexin (Gutteridge and Smith 1988) is maintained, the intracellular free heme and iron are kept at safe levels in neurons. Thus, protection by hemopexin is proposed to be important in stroke, hemorrhage, and traumatic brain injury. Such protection may be progressively lost in neurodegenerative conditions like AD in which redox-active metals and oxidative stress contribute to the pathology.
Methods
Chemicals and reagents
Ham’s F12 Nutrient Mixture, Dulbecco’s Modified Eagle Medium (DMEM), Penicillin-Streptomycin, and sodium pyruvate were purchased from Invitrogen Corporation (Carlsbad, CA, USA). Eagle’s Minimum Essential Medium (EMEM) and fetal bovine serum (FBS) were purchased from the American Type Culture Collection (Manassas, VA, USA). Iron-protoporphyrin IX (heme) was obtained from Frontier Scientific (Logan, UT, USA), dissolved in dimethyl sulfoxide (DMSO) and the concentration determined spectrophotometrically as described previously (Eskew et al. 1999) (Flaherty et al. 2008). All other chemicals were purchased from Sigma Aldrich Chemical Company (St. Louis, MO, USA).
Preparation of heme–hemopexin complexes
Apo-hemopexin was isolated and characterized as described (Eskew et al. 1999). Stoichiometric iron-protoporphyrin IX (heme)–HPX complexes (> 90–95% saturated) were prepared, characterized and quantified following published procedures and using published extinction coefficients at 414 nm (Eskew et al. 1999). Heme–hemopexin complexes (15 and 25 µM) were prepared for tissue culture experiments at a molar ratio of 0.9 : 1 (heme : protein) in phosphate-buffered saline (PBS), exposed to ROS or solvent as described, dialyzed to remove ROS, then filter sterilized (Millex-GV Syringe-Driven Filter Unit, Hydrophilic Durapore, 0.22 µm, Millipore, Cork, Ireland) before addition to the incubation medium. Throughout this work, the concentrations of apo-hemopexin and heme–hemopexin complexes were determined from their absorbance spectra using published extinction coefficients [εmM = 110/ mM/cm at 280 nm and εmm= 120/mM/cm at 414 nm (Morgan and Muller-Eberhard 1972; Morgan and Smith 2001; Flaherty et al. 2008)].
Equimolar heme–hemopexin complexes (10 µM) in PBS were incubated with either H2O2 or tert-butyl hydroperoxide (tBuOOH) at molar ratios of 1 : 2.5 to 1 : 15 at 37°C for between 2 min and 2 h as noted in the figure legends. The concentration of H2O2 (Sigma-Aldrich) was determined spectrophotometrically [εM = 43.6/ M/cm at 240 nm (Jiang et al. 1990)]; and H2O2 was also used as the standard to determine the concentration of tBuOOH (MP Biomedicals, Solon, OH, USA) using the PeroXOquant Quantitative Peroxide Assay Kit (Thermo Scientific, Rockford, IL, USA). The stability to peroxides of heme bound to human serum albumin (HSA; purchased from Sigma-Aldrich) was similarly assessed [εmM = 36.6/mM/cm at 280 nm (Hunter and McDuffie 1959; Wallevik 1973)]. In all cases, the concentration of hemopexin protein and heme-protein complex was determined from their absorbance spectra (Morgan et al. 1976). The extent of heme binding was assessed from the Soret absorbance, that is, 414 nm for heme–HPX and 402 nm for heme–HSA. In a second series of experiments, apo-hemopexin solutions were treated with either H2O2 or tBuOOH by incubation for 15 min at 37°C followed by subsequent measurement of heme binding following addition of equimolar amounts of heme/DMSO (2 µL). The effect of the peroxides on hemopexin or HSA was assessed by comparison of the absorbance spectra of proteins exposed to equal volumes of water. The extent of heme binding was determined by subtracting baseline absorbance at 650 nm (absorbance at this wavelength remained constant under experiment conditions) from the Soret absorbance. In a third series, to model ROS derived from H2O2 in the respiratory burst of neutrophils, both heme–hemopexin and apo-hemopexin were similarly treated, as described above for peroxides, with hypochlorous acid (HOCl) at molar ratios of 1 : 1 to 1 : 500 and 1 : 1 to 1 : 40, respectively. The stability of albumin and heme–albumin complexes to HOCl was also assessed as described above for peroxide treatments. HOCl was prepared by diluting sodium hypochlorite (NaOCl, Sigma-Aldrich) in PBS. The concentration of NaOCl was determined by dilution in 1 M NaOH and measurement of absorbance [εM = 350/M/cm at 292 nm, pH ≥ 11 (Morris 1966)].
Tissue culture, cell lysis, immunoblot, and IRE regulation analyses
Neuroblastoma cells, SH-SY5Y, were purchased from ATCC (Manassas, VA, USA) and grown in 50:50 EMEM (Eagles Minimal Essential Medium): Ham’s F12 medium supplemented with 10% fetal bovine serum and 1% penicillin–streptomycin. Cells were seeded (5.0 × 105 −1.0 × 106 cells/well in six-well plates) until nearly confluent. The cells were rinsed with warm, equilibrated growth medium and incubated with heme–hemopexin or PBS as described in the figure legends. For assessment of the ability of peroxide-exposed heme–hemopexin complexes to induce HO1, the protein complexes in PBS were treated with either H2O2 or tBuOOH at 1 : 1 or 1 : 10 molar ratio for 10 min at 24°C followed by dialysis for 1 h at 4°C. The solution was then sterile filtered before addition to the cell incubation medium (25 µM) followed by incubation for 6–17 h before preparation of cell lysates as described in figure legends.
Whole-cell extracts were prepared by the addition of Mammalian Protein Extraction Buffer (M-PER, 100 µL; Pierce Biotechnology, Rockford, IL, USA) and centrifugation (3000 g for 10 min). The protein concentration of the cell extracts was determined using the Bicinchoninic acid assay (BCA; Pierce Biotechnology) with bovine serum albumin as standard. Proteins in samples of whole-cell extracts (15 µg) were resolved on SDS–PAGE gradient gels (4–20% acrylamide gels, Bio-Rad, Hercules, CA, USA) under reducing conditions, and the target proteins detected by western immunoblotting using polyvinylidene difluoride membranes. The primary antibodies used were anti-HO1 (rabbit polyclonal raised against a synthetic peptide 1–30 amino acid residues: SPA-896, Assay Designs, Ann Arbor, MI, USA; 1 : 5000 dilution); anti-HO2 (rabbit polyclonal raised against a synthetic peptide specific to an N-terminal sequence: SPA-897, Assay Designs; 1 : 1000) anti-glyceraldehyde 3-phosphate dehydrogenase, GAPDH, (mouse monoclonal anti-GAPDH: sc-47724, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA; 1 : 5000 dilution), anti-actin (mouse monoclonal: sc-8432, Santa Cruz Biotechnology Inc.; 1 : 1000)’ and anti-C-terminal APP (rabbit polyclonal antibody raised against a synthetic peptide 676–695 amino acid residues: A8717, Sigma Aldrich; 1 : 1000 dilution). The secondary antibodies used (both at 1 : 10 000 dilution) were goat anti-mouse IgG–HRP and goat anti-rabbit IgG–HRP (Santa Cruz Biotechnology Inc.). The ECL Western Blot Detection reagent (GE Healthcare Life Sciences, Piscataway, NJ, USA) was used as instructed by the manufacturer. The blots were scanned with a Typhoon 9400 Variable Mode Imager (GE Healthcare Life Sciences) and the signals quantified by segment analysis using UN-SCAN-IT gel digitizing software (Silk Scientific, Orem, UT, USA) as previously published (Flaherty et al. 2008). Neuroblastoma SH-SY5Y cells were stably transfected with the pIRES (APP 5′UTR) construct as previously described (Bandyopadhyay et al. 2006) and, following published procedures, incubated with heme–hemopexin, apo-hemopexin or PBS at the concentration and times indicated in the text.
Statistical analyses
Statistical analyses were conducted using GraphPad Prism 6 (GraphPad Software Inc., La Jolla, CA, USA) as previously (Rish et al. 2007) and details are presented in the figure legends.
Results
Heme–hemopexin increases the endogenous levels of holo-APP in human neuroblastoma SH-SY5Y cells in part via iron from heme catabolism
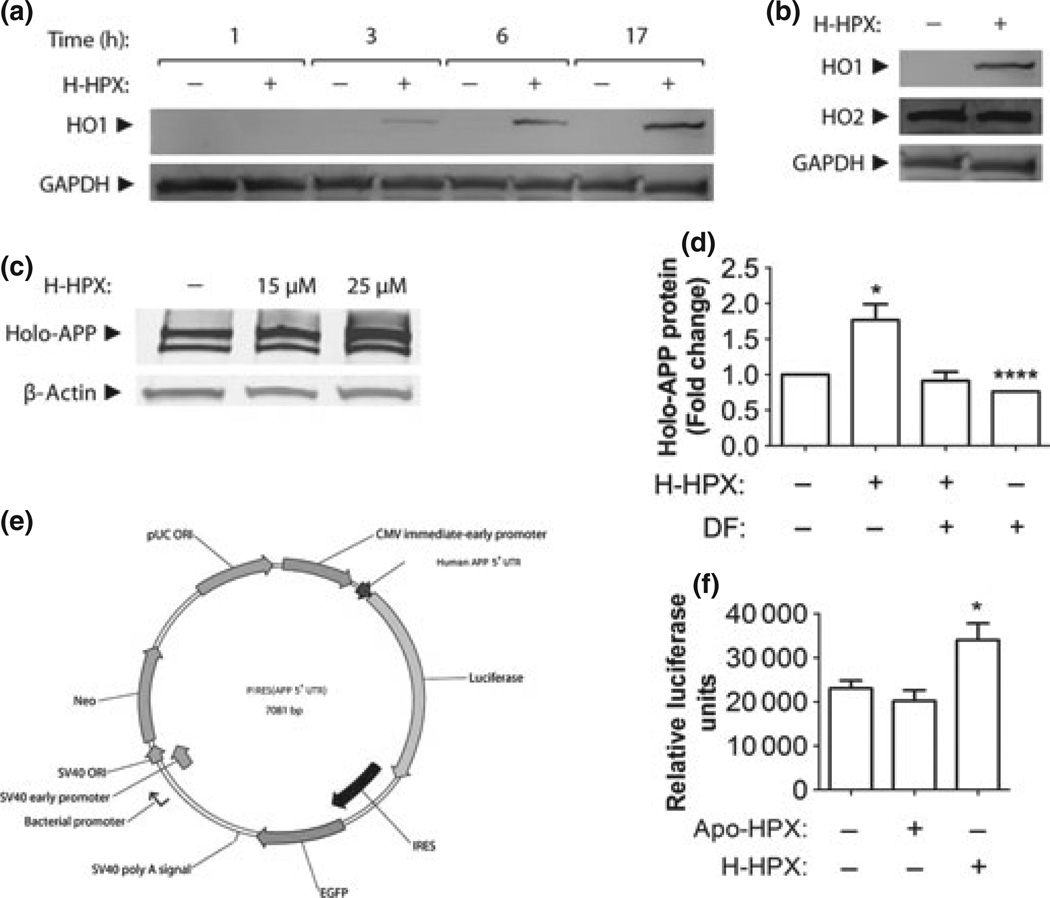
To determine how heme delivery to human neuronal cells by hemopexin affects heme and iron metabolism, we first established that human neuroblastoma SH-SY5Y cells respond to heme–hemopexin in a manner similar to primary cultures of mouse neurons (Li et al. 2009), that is, via heme delivery for heme oxygenase-1 (HO1) induction. We used 10 –25 µM heme–hemopexin as a model of heme load as previously published (Li et al. 2009). HO1 is readily induced by heme–hemopexin and remains elevated for at least 17 h (Fig. 1a and Fig. 6); whereas HO2, which is constitutively expressed at high levels in neurons does not change, as expected.
Fig. 1.
Heme–hemopexin induces amyloid precursor protein in an iron-dependent manner in human neuroblastoma cells. Human SH-SY5Y cells growing in normal culture medium that contains 10% fetal bovine serum (FBS) were incubated for up to 17 h with heme–hemopexin (H-HPX) or an equivalent volume of phosphate-buffered saline (PBS) as indicated. In (a), HO1 protein induction by 25 µM heme–hemopexin is apparent within 3 h and continues to increase over 17 h using immunoblots of whole-cell extracts (15 µg protein/lane) with GAPDH as the loading control. In (b), while HO1 is induced, the high basal levels of HO2 are unchanged at 17 h after heme–hemopexin. The induction of amyloid precursor protein (APP) following 17-h incubation with heme–hemopexin (c) is prevented by the iron chelator, desferri-oxamine [100 µM DF, (d)]. Holo-APP levels (Mr ca. 100 kDa) were increased ~1.8-fold by 25 µM H-HPX (d). A one-tailed, unpaired Welch’s t test between the control and treated samples gave p = 0.0204 and p < 0.0001. The data shown are the mean ± SEM values from four independent experiments. One set of error bars is so small they are not visible on this scale. A higher molecular weight form of hAPP detected in these blots after cells are incubated with H-HPX is an intracellular precursor of hAPP. In (f), 25 µM heme–hemopexin is a more effective inducer than 25 µM apo-hemopexin of luciferase activity from a reporter gene construct, pIRES(APP 5’UTR) in (e), containing the APP 5’UTR with its IRE stably transfected in SH-SY5Y neuroblastoma cells. PBS is the solvent control. The data shown in (f) are from a representative experiment (n = 6 each set), of three independent experiments, using 25 µM apo-hemopexin and heme–hemopexin with PBS as solvent control. A two-sided, Dunnett-t test gave *p = 0.0260 between the control and H-HPX treatments. Induction of luciferase activity by heme–hemopexin was also apparent at 10-µM concentrations (data not shown).
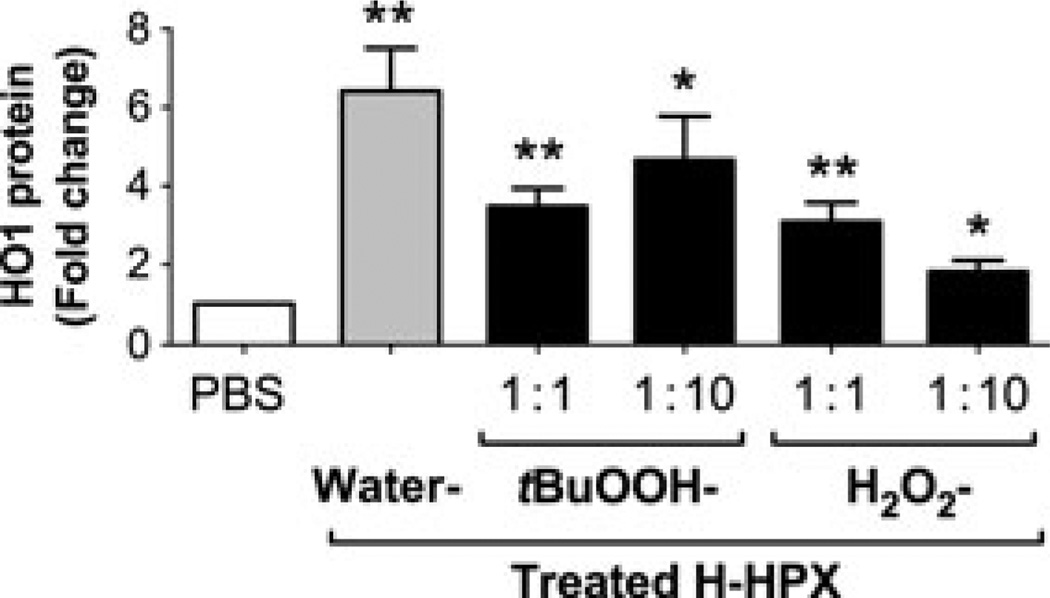
Fig. 6.
Heme–hemopexin exposed to physiologically relevant concentrations of peroxides still induces the cytoprotective heme oxygenase-1 (HO1). Comparison of the levels of HO1 after 6-h incubation of human SH-SY5Y cells with either phosphate-buffered saline (PBS) or 25 µM heme–hemopexin complexes (H-HPX) exposed to either H2O2 or tert-butyl hydroperoxide at 1 : 1 or 1 : 10 molar ratios or with an equivalent volume of water as indicated in the figure (further details are presented in the Materials and Methods section). The levels of HO1 were assessed by immunoblotting as described above in the legend for Fig. 1 and the data shown are the mean and SEM from three independent experiments with GAPDH as the loading control. The figure is composed of the pairwise comparisons using a one-tailed, unpaired Student’s t-test between the PBS control and each H–HPX incubation (significance levels are *p < 0.05 and **p < 0.01). Individual p values from left to right are p = 0.0038, p = 0.0029, p = 0.0151, p = 0.0067, and p = 0.0227, respectively.
Heme delivered to cells by hemopexin is rapidly catabolized and the iron released is stored on ferritin (Davies et al. 1979; Smith and Morgan 1979) and ferritin protein is induced (Hunt et al. 1996b; Sung et al. 2000). Because iron induces hAPP (Rogers et al. 2002; Schobel et al. 2006; Cho et al. 2010) at the translational level via IRE/IRP analogous to ferritin, the APP–IRE is a strong candidate site for translational regulation by heme–hemopexin. APP protein levels are increased ~twofold when neuroblastoma cells are incubated with heme–hemopexin for 17 h (Fig. 1c), and iron is required as the induction is reduced significantly by an iron chelator, desferrioxamine (DF, Fig. 1d). To determine the role of the IRE in the 5′UTR of human APP mRNA in hAPP regulation by heme–hemopexin, we utilized SH-SY5Y neuroblastoma cells stably transfected with a construct comprising the APP 5′UTR with a luciferase reporter gene. After incubation with heme–hemopexin (10–25 µM), luciferase activity was significantly higher in these cells than in those with apo-hemopexin or the PBS solvent control (Fig. 1f).
Heme–hemopexin complexes are relatively resistant to damage by reactive oxygen species in vitro compared with the apo-protein
Many different types of brain injury are thought to be because of toxicity from heme released from hemoglobin, as recently shown for stroke (Li et al. 2009); and brain damage is rapidly followed by activation of the inflammatory cascade generating ROS. For hemopexin to protect brain cells, including neurons, under these conditions, it needs to be stable in the presence of ROS. Therefore, we next tested the hypothesis that heme–hemopexin complexes and apo-hemopexin would be resistant to damage by representative ROS known to be generated in vivo during the inflammatory response. In addition to the tert-butylhydroperoxide (tBu-OOH) used previously (Timmins et al. 1995; Li et al. 2009), we investigated the effects of hydrogen peroxide (H2O2). Also, because H2O2 is a substrate for myeloperoxidase (MPO) in the respiratory burst of neutrophils and macrophages to generate hypochlorous acid (HOCl) at sites of inflammation and MPO is also released into biological fluids, we then assessed whether HOCl impairs the ability of hemopexin to bind heme. The concentration ranges of ROS investigated here are based on published studies of levels that are likely in vivo.
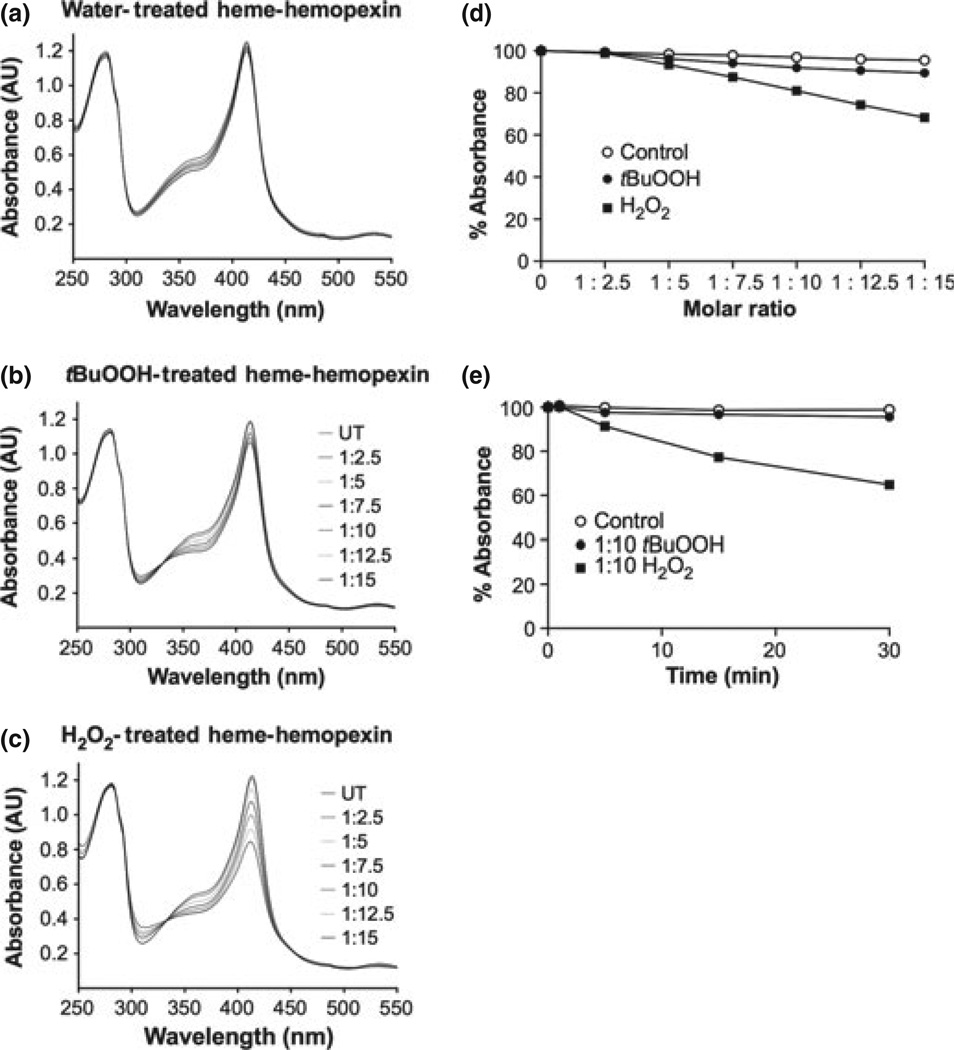
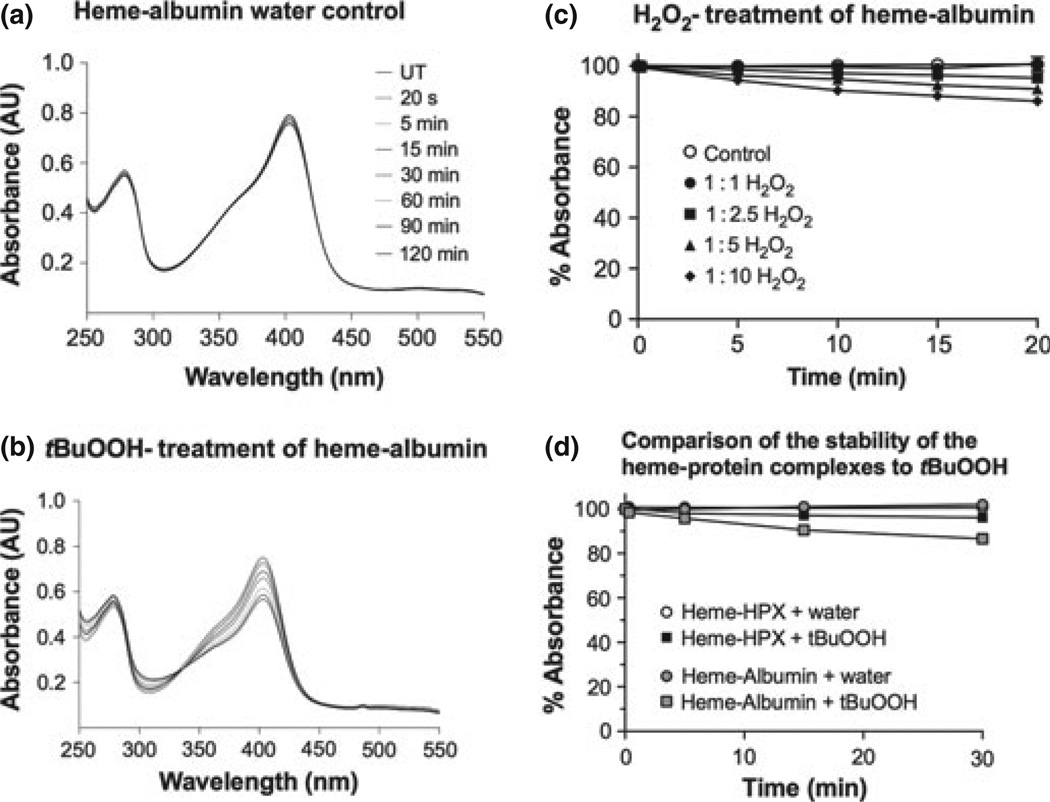
To model hemolysis followed by inflammation, as occurs in stroke with ischemia reperfusion injury and intracerebral hemorrhage, we first determined whether heme–hemopexin complexes were stable in the presence of H2O2 tBuOOH and then compared with HOCl. Characteristic absorbance spectra reveal that heme–hemopexin complexes (10 µM) were essentially completely stable in the presence of up to a 10-fold molar excess of tBuOOH at 37°C (Fig. 2b and d). These complexes were also unaffected by up to 2.5-fold molar excess of H2O2, which is well within the 5–15 µM H2O2 physiological range of production by human neutrophils (Test and Weiss 1984; Liu and Zweier 2001); but there was a 20% loss of heme binding after 10-min exposure to supraphysiological levels of H2O2 at 37°C (indicated by the decreased absorbance at the Soret region 414 nm; see Fig. 2c and d), which occurred within a few minutes (Fig. 2e). Nevertheless, there appears to be no gross denaturation of the protein by H2O2.
Fig. 2.
The heme–hemopexin complex (H–HPX) is resistant to damage by tert-butyl hydroperoxide and to physiological amounts of H2O2. Shown are the characteristic absorbance spectra (a–c) after heme– hemopexin (10 µM) was incubated at 37°C with (a) the solvent control, water, (b) tBuOOH, or (c) H2O2. The peroxide concentration was increased in the sample by the sequential addition of stock peroxide (1.5 µL) over a range of 1 : 2.5–1 : 15 molar ratios, and the controls received an equivalent volume of water. The absorbance spectra were recorded 2 min after each addition of peroxide. A summary of these absorbance data as percent absorbance at 414 nm of the untreated sample is shown in (d). In both titrations, an isosbestic point was apparent [at 328 and 330 nm in (b and c), respectively], which is evidence for two species of heme. Heme–hemopexin complexes were incubated at 37°C with either tBuOOH or H2O2 at a molar ratio of 1 : 10 and the absorbance spectra recorded at 1, 5, 15, and 30 min after addition of peroxide or an equivalent volume of water as control (e). At these supraphysiological concentrations of H2O2, some heme binding loss is detected by a decrease in absorbance at 414 nm over 30 min (and less than 10% more over the next 90 min). However, this is a relatively slow process compared with the rate that the complex is cleared from the circulation, which occurs within a few minutes (Smith and Morgan 1979). Data shown in panels a–d are from one representative experiment of two to three independent experiments. Data in panel e are the mean ± SEM values from two independent experiments.
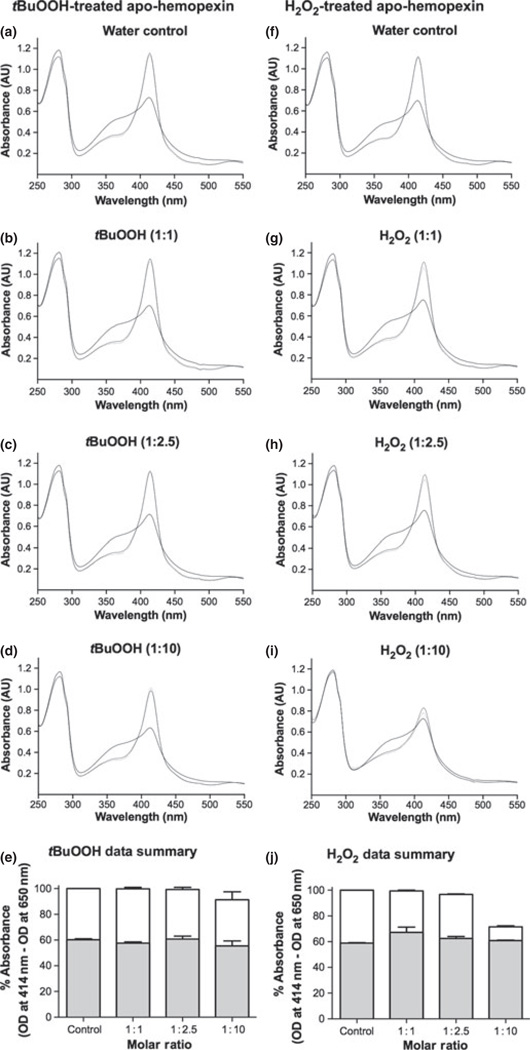
Normally, only minor amounts of heme–hemopexin are expected from the wear and tear of red blood cells in capillary beds in the plasma and other biological fluids including cerebrospinal fluid. Heme levels in normal volunteers is ~0.2 µM (Reiter et al 2002) and haptoglobin levels range from 2–23 µM (Gupta et al. 2011; Vermeulen Wind-sant et al. 2012). Thus, apo-hemopexin (at 10–25 µM) often predominates in biological fluids. Heme binds to hemopexin quickly [Kd less than 1 pM (Hrkal et al. 1974)]. Here, we have shown that at physiological temperature of 37°C, stoichiometric complexes are formed completely within seconds and are cleared by the liver from the circulation within 1–5 min (Smith and Morgan 1979). Both tBuOOH (Fig. 3a – e) and H2O2 (Fig. 3f – j) appeared to have little effect on the apo-protein; and supraphysiological levels caused only a ~10% and 25% loss, respectively, in its ability to bind heme (Fig. 3e and j).
Fig. 3.
Apo-hemopexin is stable to peroxides and can bind heme under in vitro conditions designed to mimic inflammation. Apo-hemopexin (10 µM) was incubated at 37°C with either (b–d) tert-butyl hydroperoxide (tBuOOH) or (g–i) H2O2 at 1 : 1, 1: 2.5, and 1 : 10 molar ratios for 15 min or water as solvent control (a and f, respectively). A stoichiometric amount of heme was then added and the absorbance spectra were recorded immediately (solid line) and at 15 (dashed line) and 30 min (dotted line) showing maximum heme binding. The OD at 414 nm is a measure of the amount of heme bound to hem-opexin. Spectra shown are from one of two independent experiments for each set. Absorbance data (e and j) are the mean ± SEM from two independent experiments for each set. Gray and white bars represent the initial heme binding and maximum heme binding (after 15 min.), respectively. While the initial rapid heme binding is unaffected by tBuOOH or H2O2, apo-hemopexin is damaged only by supraphysiological levels of H2O2 [i.e., 100 µM, (j)], which decreased total heme binding by ~25% compared with 8% by 100 µM tBuOOH. The heme binding has been normalized by subtracting the absorbance at 650 nm, which is identical in all spectra, from the absorbance at the Soret maximum.
In contrast to these in vitro conditions where hemopexin is the sole protein present, in vivo hemopexin would be in an environment surrounded by many other proteins, all of which are potential ROS targets. In human plasma, albumin is present in a 70-fold molar excess over hemopexin (Morgan et al. 1976) and provides a potential reservoir of two heme-binding sites per molecule in hemolytic states (Beaven et al. 1974). However, the 5-coordination state of the heme and low affinity binding do not protect the heme effectively (Grinberg et al. 1999). These published studies were carried out at 22°C with a 40-fold molar excess of peroxides. Using more physiologically relevant conditions, the absorbance data in Fig. 4 reveal that heme–albumin complexes (1 : 1– 1 : 10 molar ratio) are more susceptible to damage by tBuOOH (Fig. 4d) and H2O2 (Fig. 4c) than are heme– hemopexin complexes (Fig. 2e).
Fig. 4.
Heme–albumin is more sensitive to damage by peroxides than heme–hemopexin. Human serum albumin (10 µM) was incubated with an equimolar amount of heme for 30 min at 37°C followed by addition of 100 µM tBuOOH (b) at a 1 : 10 molar ratio or equivalent volumes of water as control (a). Absorbance spectra were recorded prior to treatment and after treatment at 20 s, 5, 15, 30, 60, 90, and 120 min. The heme–albumin complex was not stable in the presence of either peroxide as shown by the rapid decrease in the absorbance at 402 nm (within 5–10 min.) indicating loss of bound heme. The isosbestic point at 330 nm is evidence for two species of heme. (c) shows the rapid decrease in heme bound to albumin on exposure to H2O2. Heme-albumin complexes (10 µM) were prepared (30-min binding at 37°C), incubated with 10, 25, 50, or 100 µM H2O2, and spectra recorded after 5, 10, 15, and 20 min. Absorbance spectra were normalized relative to untreated heme-albumin sample. Data shown are the mean ± SEM from two independent experiments. The data in (d) show that heme-albumin (grey circle and square) was significantly more sensitive to damage by 100 µM tBuOOH than heme–hemopexin (open circle, closed square). Data shown are the mean ± SEM from two independent time courses for each protein complex.
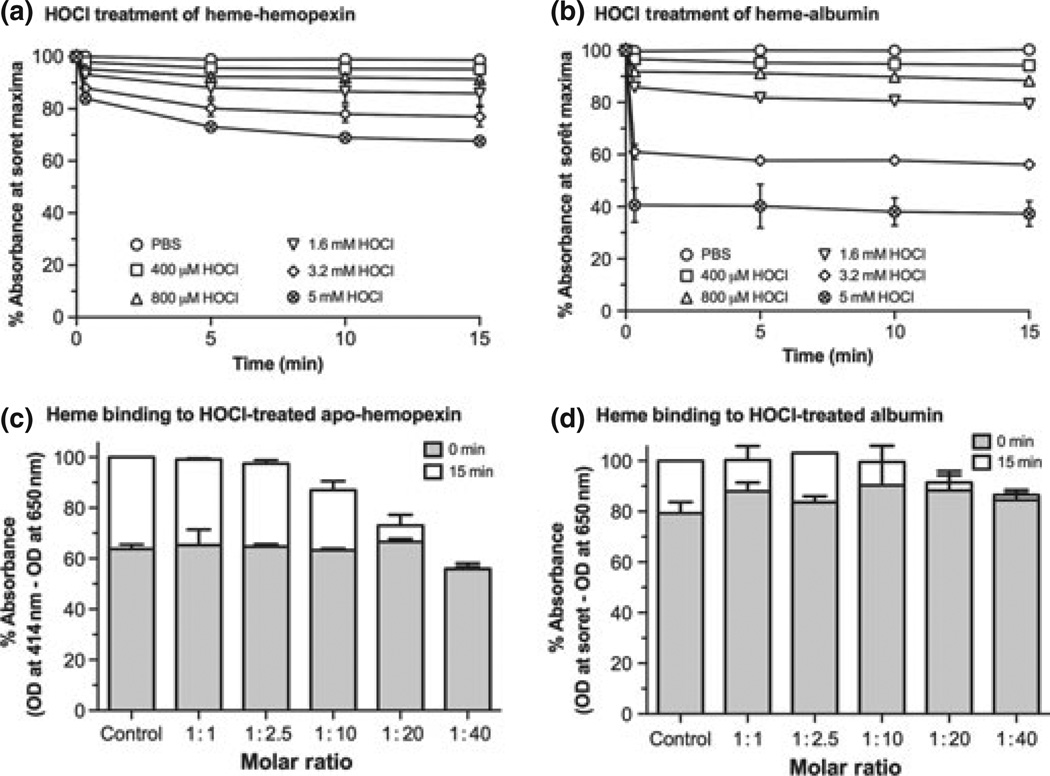
Hypochlorous acid (HOCl), a potent bactericidal and viricidal agent, is considered one of the most physiologically relevant ROS/oxidizing agents in vivo. The concentrations of HOCl are estimated to be 12.5–250 µM (McCall et al. 2001), although significantly higher levels (~3–8 mM) may occur in diseased lungs including in cystic fibrosis (Guo et al. 1995). Heme–hemopexin complexes are essentially unaffected by HOCl even at molar ratios of 1 : 40, that is, 400 µM (Fig. 5a). However, 1.6–5 mM HOCl cause a small dose-dependent loss of heme binding (~10–25%), which occurs almost instantaneously (i.e., within 10–20 s). The heme-binding site appears to remain intact because there is no change in the Soret maximum (414 nm). While heme binding to hemopexin protects the heme and stabilizes the protein, damage to heme–albumin complexes by HOCl is more extensive, especially at concentrations above 400 µM (Fig. 5b). Heme loss, indicated by a decrease in absorbance in the Soret region with a concomitant increase at 370– 390 nm, and denaturation of albumin, indicated by an increase in absorbance at 250 nm (data not shown), occurs at a 40- to 320-fold molar ratio of albumin to HOCl. In addition, the maximum Soret absorbance of albumin-bound heme becomes red shifted from 402 nm to 404/406 nm with increasing concentrations of HOCl (starting at 100 µM) and with longer exposure times. This indicates that there are changes in the environment of the heme.
Fig. 5.
Heme–hemopexin is more resistant to damage by hypochlo-rous acid than heme–albumin, whereas albumin appears initially more resistant to this reactive oxygen species (ROS) than apo-hemopexin. Panels a and b: Heme–hemopexin or heme–albumin complexes, respectively, at 10 µM in phosphate-buffered saline (PBS) were incubated for up to 15 min at 37°C with increasing concentrations of hypochlorous acid (HOCl) at 1 : 40–1 : 500 molar ratios. The absor-bance spectra were recorded from 250 to 700 nm at the times indicated (~10 s to 15 min). Heme–hemopexin was more resistant to damage than heme–albumin, retaining ~90% heme-binding capacity in the presence of 800 µM HOCl; and even at 5 mM HOCl there was only ~25% loss of heme binding. Any damage to heme–hemopexin was rapid and increased slowly over 10 min; whereas heme–albumin was damaged almost instantaneously, especially at millimolar levels of HOCl (1.6–5 mM). Panels c and d: apo-hemopexin (10 µM) or albumin were incubated for 15 min at 37°C with HOCl at 1 : 1–1 : 40 molar ratios as indicated. A stoichiometric amount of heme was then added and the absorbance spectra were recorded immediately (i.e., ~10– 20 s, gray bars) and after 15 min (white bars) for maximum heme binding (414 nm for heme bound to hemopexin; and Soret maximum for heme–albumin). Heme binding was normalized by subtracting baseline absorbance at 650 nm from these absorbances. Data shown are the mean ± SEM of two independent experiments. The initial very rapid binding of heme to hemopexin was not affected even by the highest physiological levels of HOCl (up to 400 µM). Total heme binding (white bars) was decreased by ~12, 24, and 45% by 100, 200, and 400 µM HOCl, respectively, but the heme-binding site remains intact (i.e., the Soret maximum, 414 nm, did not change significantly). The rapid heme binding to albumin was also essentially unaffected by HOCl (up to 400 µM), but total binding was decreased after exposure of albumin to 200 µM HOCl. Damage that affected heme binding, at concentrations greater than 100 µM, was revealed by decreased absorbance in the Soret region with a gradual red shift in the absorbance maximum from 402 to 406 nm that developed with time and increasing HOCl concentration (data not shown). Because of these changes in the heme environment, the data have been normalized and heme binding is calculated as the percent change in absorbance at the Soret maximum minus that at 650 nm as described in the legend to Fig. 3. There was no apparent change in the absorbance spectra of heme at the concentrations of HOCl used (data not shown).
The initial essentially instantaneous heme-binding ability of apo-hemopexin is not greatly affected by HOCl at concentrations less than or equal to 200 µM (Fig. 5c); however, higher concentrations do begin to decrease its ability to bind heme. Above 200 µM, there are small increases in absorbance at 250 nm of apo-hemopexin after exposure to HOCl, consistent with direct effects on the protein; although, there are only very small decreases in absorbance at 280 nm but no aggregation (data not shown). Because of these changes in the protein spectra, we normalized the total heme binding by subtracting the absorbance at 650 nm, which does not change, from the absorbance at the Soret maximum. After heme addition to the HOCl-treated hemopexin, the decreased absorbance in the Soret region accompanied by increased absorbance at 370– 390 nm with time is consistent with a loss of heme binding (data not shown) compared with the untreated hemopexin. There is a 25–40% loss in total heme binding by hemopexin at concentrations of HOCl above 200 µM (see data summary, Fig. 5c).
In the presence of HOCl, there are small incremental changes in the albumin spectrum at 250 nm that are apparent at 100 µM and increase dose dependently with HOCl concentrations (data not shown). The total heme binding by albumin, normalized as described above (Fig. 1d), appears to be retained, but damage to albumin that impairs heme binding starts to occur at 100–200 µM HOCl. Furthermore, total heme binding is significantly decreased by 200–400 µM HOCl (see Fig. 5d). Although albumin maintains its ability to quickly bind heme after exposure to 400 µM HOCl, this binding per se is not protective as the heme–albumin complex is very susceptible to damage by HOCl as described above. Overall, these observations support that heme binding to albumin does not protect the heme. Also, in vivo hemopexin will be protected in biological fluids by albumin, which is a preferential target of ROS.
Peroxide-exposed heme–hemopexin induces heme oxygenase-1 in human neuroblastoma cells
As expected, the human neuroblastoma cells, like primary neurons, normally express high basal levels of the HO2 isozyme and low levels of the inducible HO1 isozyme, which is increased by heme–hemopexin (see Fig. 1). Tert-butyl hydroperoxide-treated heme–hemopexin induced HO1 ~40– 60% compared with the untreated complexes. Exposure to equimolar H2O2 reduced induction by heme–hemopexin to about half that seen with the water-treated complex and higher peroxide concentrations caused further impairment of this ability, but did not prevent induction (Fig. 6). Because of the high concentrations of protein targets more susceptible to H2O2, including albumin (as shown in Fig. 4) in biological fluids, which will limit damage to hemopexin, we conclude that heme–hemopexin complexes formed in vivo would remain effective inducers of the HO1 enzyme under most inflammatory conditions.
Discussion
Our basic premise is that characterization of the natural protective action of hemopexin against heme toxicity in stroke, intracerebral hemorrhage and other brain trauma, including the regulatory consequences of uptake of heme– hemopexin by brain cells, will provide a window to understand events that normally maximize defense against neurodegeneration. Our first goal was to help further define the changes in iron metabolism in neurons after endocytosis of heme–hemopexin. We show that HO1 and hAPP protein levels are induced in human neuroblastoma cells that are considered to be good models of human neurons. Human APP induction by heme–hemopexin requires iron and the IRE of APP mRNA as expected as the APP–IRE is 75% homologous with the canonical ferritin IRE and heme– hemopexin induces ferritin (Hunt et al. 1996b). We conclude that hAPP is induced by iron derived from the breakdown of heme after delivery by hemopexin because: (i) hAPP induction by heme–hemopexin is decreased by simultaneous incubation of cells with the iron chelator, desferrioxamine; and (ii) heme–hemopexin increases luciferase activity in hAPP–IRE constructs stably transfected in SH-SY5Y cells,more than apo-hemopexin. The induction of HO1, known to occur at the transcriptional level, is detectable before the translational regulation of hAPP by heme–hemopexin. By inducing the expression of proteins that control iron homeo-stasis in addition to those controlling heme levels, heme delivery by hemopexin ensures that intracellular concentrations of heme and also iron from heme breakdown are kept at a safe level. Our second goal was to model human neurons experiencing microhemorrhages and inflammation in the brain. We used human neuroblastoma cells, heme–hemopexin complexes, and physiologically relevant levels of H2O2 and HOCl. The effects of these two ROS were compared with that of tert-butyl hydroperoxide, another model ROS. Our principal findings from these experiments are that heme– hemopexin complexes and apo-hemopexin are essentially stable to the levels of H2O2 and HOCl generated from the respiratory burst and that hemopexin can still bind heme and induce HO1 in models of human neurons. In vivo, other proteins (e.g., albumin) present in plasma and cerebrospinal fluid are expected to provide preferential targets for ROS and thus ameliorate the damage to apo-hemopexin seen here at the supraphysiological levels of H2O2 in vitro. Levels of H2O2 at 100 µM or higher have been recorded in various disease states (Schroder and Eaton 2008). Thus, our observations provide strong evidence that hemopexin will remain protective in the brain as an extracellular antioxidant (i.e., heme binder) and HO1 inducer both for normal metabolism of neurons and after stroke, trauma, and intracerebral hemorrhage. Nevertheless, the potential for damage to apo-hemopexin in vivo by localized high concentrations of H2O2 and HOCl certainly exists, for example, at the neutrophil surface as the respiratory burst is stimulated and ROS are released from the cell. Any damaging effects of HOCl to circulating plasma proteins including hemopexin and albumin may be tempered by dietary flavonoids, as shown for hemoglobin (Gebicka and Banasiak 2012).
In the context of neuroprotection by the hemopexin system, hAPP induction, whose ferroxidase activity is needed for iron export and transferrin iron loading in neurons (Duce et al. 2010), helps to maintain intracellular free iron concentrations at a safe level by facilitating iron release to transferrin. Neurons express ferritin for iron storage. In addition, hemopexin delivers heme safely to primary neurons, in a controlled and regulated manner that is needed for HO1 gene transcription (Alam and Smith 1989) via Nrf2 (Alam et al. 1999; Smith 2011) and a regulatory soluble domain of HO1 (Lin et al. 2007). In fact, not only does hemopexin induce HO1 and ferritin (Hunt et al. 1996b), but it also provides cells with the heme substrate for this enzyme. Thus, neuroprotection by hemopexin will also come from HO1 activity releasing CO from heme, which has recently been shown to activate double-stranded DNA repair via the actions of the ATM protein (Otterbein et al. 2011). Consistent with this model, HO1 activity is needed for the protection by heme–hemopexin against heme toxicity and oxidative stress in primary neurons (Li et al. 2009).
In ischemia, neuroprotection by HO1 would be attenuated by a limitation of substrates such as oxygen for HOs and NADPH for the obligatory NADPH-cytochrome P-450 reductase needed for both HO1 and HO2 enzymatic activity. NADPH is central to the enzymes that are needed for cellular defense against ROS, including GSH and thioredoxin reductases. Insufficient NADPH generation may become critical when glucose availability is compromised in neurons and when there is hypoxia because the major source for NADPH is the oxidative phase of the pentose phosphate pathway. The retina is an isolated part of the brain and, in fact, a coordinated balance of glycolysis with heme catabolism in liver and retinal pigment epithelial cells has recently been proposed (Li et al. 2012).
Another potential mechanism that might negatively impact neuroprotection within cells, involves the observation that HOs are inactivated by binding to APP (Takahashi et al. 2000). This protein–protein interaction involves the catalytic domain of HO and the zinc-binding site of APP (Takahashi et al. 2000). In APP, the HO1-binding domain (amino acid residues 190–288) and neighboring zinc-binding site (residues 181–188) are N-terminal to a ferroxidase-1 (FD-1) homology domain (residues 401–417, Duce et al. 2010). The FD-1 domain and other relevant ferroxidase catalytic sites are thought to aid in APP-dependent iron export via ferroportin. While the APP domain with the zinc-binding site faces the ER lumen (Takahashi et al. 2000), there is evidence from two groups that the catalytic site of HO1 faces the cytosol: fluorescent protein protection assays after expression of GFP-tagged HO1 constructs in macrophages (Gottlieb et al. 2012); and complex formation between HO1, its obligatory P-450 reductase, and the enzyme next in the heme catabolic pathway—the cytosolic protein, biliverdin reductase (Yoshinaga et al. 1982). Furthermore, there is no apparent toxicity when primary neurons (Li et al. 2009) or neuroblastoma cells are incubated with high concentrations of heme– hemopexin to mimic a hemolytic event, as might be expected if intracellular heme or iron levels rise uncontrollably because of inactive HO and/or APP. Thus, after heme delivery by hemopexin, an HO–APP interaction that inhibits their function is not supported by experimental evidence.
Given that brain injury rapidly leads to the activation of inflammatory processes, it is important that we here provide evidence that hemopexin protects neurons and potentially other brain cells during inflammation because it continues to bind heme and induce HO1 after exposure to three types of ROS. In inflammation, ROS including HOCl are released into plasma and other biological fluids principally from polymorphonuclear neutrophils, but also by some monocytes and tissue macrophages. HOCl production requires the combined action of plasma membrane NADPH oxidase, which generates superoxide that dismutates to H2O2, and myeloperoxidase, which converts H2O2 to hypochlorous acid by oxidizing chloride in plasma (Fliss 1988; Weiss 1989; Kettle and Winterbourn 1994). The damage from heme comes from intermediates generated during the two-electron reduction by heme of hydroperoxides, for example, peroxyl radicals and a high-energy oxidized state of heme (Timmins et al. 1995). Although heme is ultimately regenerated, the intermediates produced react with, and thus damage, many biological substrates. Tert-butyl hydroperoxide is one model for such peroxides. On the basis of a comparison of the effects of cumene hydroperoxide and t- and n-BuOOH, Timmins and colleagues (Timmins et al. 1995) concluded that the ability to access heme in the heme-binding site of hemopexin varies depending upon the size, reactivity, and hydrophobicity of the peroxides, and the steric hindrance of the individual peroxide functional group. The carbohydrate chains of hemopexin were also considered protective. Consistent with this, we found that there were differences in the effects of the three ROS investigated on hemopexin. In general, heme–hemopexin complexes were stable to peroxides. Access of tBuOOH to hemopexin, compared with H2O2, may be because of limited access as tBuOOH is larger than H2O2. Even greater steric hindrance effects are likely for the types of ROS that hemopexin would encounter in vivo because they will be non-solvated, membrane-bound lipid hydroperoxides or protein hydroperoxide species (Timmins et al. 1995). Only the very highest physiological concentrations of HOCl (i.e., > 200 µM) slightly damaged apo-hemopexin with some impairment of heme binding, whereas the heme–hemopexin complex was essentially stable in the presence of this ROS. Thus, in vivo, the extent of any damage to hemopexin will depend upon the type of ROS (molecular size and reactivity) as well as its concentration and where hemopexin is located in the body.
Nevertheless, some potential for damage to apo-hemopexin especially by the highest concentrations of H2O2 and HOCl that might occur in vivo exists. Such damage in severe and prolonged inflammatory states as in sepsis may be one means that leads to a hemopexin deficiency if the oxidized protein is recognized as non-native, removed from the circulation, and catabolized, instead of being recycled after endocytosis (Smith and Morgan 1979; Hunt et al. 1996a). This is relevant because while local inflammation in the brain contributes to neurodegeneration, systemic infection also leads to poorer outcomes in stroke and neurodegenerative conditions like AD with neurovascular alterations (McColl et al. 2008). Also, hemopexin supplementation protects mice with severe sepsis from death (Larsen et al. 2010).
Consistent with its role in defending against heme toxicity even under inflammatory conditions, peroxide-exposed heme –hemopexin was still able to induce HO1 in human neuroblastoma cells. Nevertheless, our studies reveal that depending on the type of peroxide and its concentration there is a potential for impairment in vivo. The induction levels were less (40–60%) than those seen with the control heme– hemopexin complexes and there were differences: the effects of tBuOOH, which is a larger molecule than H2O2, were less than those of H2O2. Furthermore, receptor-mediated delivery of heme by hemopexin activates the expression of certain protective proteins in addition to HO1 that heme does not (Eskew et al. 1999; Vanacore et al. 2000). Although haptoglobin protects hemoglobin from H2O2, exposure of haptoglobin–hemoglobin complexes to 100 µM H2O2 causes heme to become covalently attached to protein. This completely prevents HO1 induction, although the complexes are recognized by the receptor CD163 and taken up into CD163-expressing human embryonic kidney cells in vitro (Buehler et al. 2009).
Several studies (Gutteridge and Smith 1988; Vincent et al. 1988; Timmins et al. 1995) have shown that when heme is bound to hemopexin, its chemical reactivity is dampened much more than when bound to albumin. For example, hemin-catalyzed peroxidation of linoleic acid micelles was inhibited ~90% by hemopexin, but only ~50% by albumin (Gutteridge and Smith 1988). The susceptibility of heme– albumin complexes to oxidative damage is considered to be because of the high spin, 5-coordinated heme (Pasternack et al. 1983) and the far lower affinity for heme [Kds of 10 nM and 1 µM, (Beaven et al. 1974)] than hemopexin [Kd less than pM, (Hrkal et al. 1974)]. We have shown here that albumin, present in ~100-fold molar excess over hemopexin in plasma, is also susceptible to ROS-mediated damage and thus provides an ROS ‘sink’ in vivo.
In hemopexin deficiency states, heme (from methemoglobin or heme-albumin) readily enters cells and is toxic. The mechanism(s) are not defined, but probably involve diffusion across the plasma membrane lipid bilayer and possibly diffusion-limited passage through channels, both of which would be unregulated rapidly raising intracellular heme levels to where they are toxic. Also, although not addressed directly here, hemopexin is expected to bind heme (Morgan and Muller-Eberhard, 1972; Smith 2011) even when the extracellular pH at nerve junctions decreases from 7.4 to 6.5 after ischemic stroke (Siesjo et al. 1996). Thus, neuroprotection by hemopexin when hemoglobin is released comes initially from heme sequestration and then from regulatory events on heme–hemopexin receptor binding followed by endocytosis and heme release within the cell. The extracellular antioxidant activity of hemopexin not only protects surface molecules and those in biological fluids from damage by heme and the activation by heme of immune system cells that secrete pro-inflammatory cytokines (Figueiredo et al. 2007; Liang et al. 2009; Lin et al. 2012) but also prevents the uncontrolled uptake of heme that leads to toxicity.
Acknowledgements
The authors state that there is no conflict of interest. We wish to thank Dr. Clare Hawkins (Heart Research Institute, Newtown, Australia) and Dr. John Dawson (Univ. of South Carolina) for helpful discussions, and Mr. Lee Likins and Dr. S. Simons (UMKC) for their advice with the statistical analyses used. Vinusha Tummala (Department of Psychiatry-Neuroscience, Massachusetts General Hospital) is thanked for her technical help in the initial stages of the APP-IRE regulation studies. The illustration for the “in this issue” is by Joe Moran (UMKC-School of Medicine) and the neuron image is courtesy of the National Institute on Aging/ National Institutes of Health. This work was partially supported by the National Institute of Health Grant NIH R21DK64363, the University of Missouri Research Board, and University of Missouri Kansas City RIF (all to A.S.) and an Alzheimer’s Association Zenith Award (J.T.R.).
Abbreviations used
- APP
amyloid precursor protein
- BCA
bicinchoninic acid
- CNS
central nervous system
- DF
desferrioxamine
- DMEM
Dulbecco’s Modified Eagle Medium
- DMSO
dimethyl sulfoxide
- EMEM
Eagle’s Minimum Essential Medium
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- hAPP
human amyloid precursor protein
- HO1
heme oxygenase-1
- HOCl
hypochlorous acid
- MPO
myeloperoxidase
- NaOCl
sodium hypochlorite
- PBS
phosphate-buffered saline
- ROS
reactive oxygen species
- tBuOOH
tert-butyl hydroperoxide
References
- Alam J, Smith A. Receptor-mediated transport of heme by hemopexin regulates gene expression in mammalian cells. J. Biol. Chem. 1989;264:17637–17640. [PubMed] [Google Scholar]
- Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL. Nrf2, a Cap’n’Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J. Biol. Chem. 1999;274:26071–26078. doi: 10.1074/jbc.274.37.26071. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay S, Ni J, Ruggiero A, Walshe K, Rogers MS, Chattopadhyay N, Glicksman MA, Rogers JT. A high-throughput drug screen targeted to the 5’untranslated region of Alzheimer amyloid precursor protein mRNA. J. Biomol. Screen. 2006;11:469–480. doi: 10.1177/1087057106287271. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Sultzer D, Cummings J, Holt LE, Hance DB, Henderson VW, Mintz J. In vivo evaluation of brain iron in Alzheimer disease using magnetic resonance imaging. Arch. Gen. Psychiatry. 2000;57:47–53. doi: 10.1001/archpsyc.57.1.47. [DOI] [PubMed] [Google Scholar]
- Beaven GH, Chen S-H, D’Albis A, Gratzer WB. A spectroscopic study of the haemin-human-serum-albumin system. Eur. J. Biochem. 1974;41:539–546. doi: 10.1111/j.1432-1033.1974.tb03295.x. [DOI] [PubMed] [Google Scholar]
- Brenner SR. Neurovascular unit dysfunction: a vascular component of Alzheimer disease? Neurology. 2008;70:243–244. doi: 10.1212/01.wnl.0000299721.10189.fe. [DOI] [PubMed] [Google Scholar]
- Buehler PW, Abraham B, Vallelian F, et al. Haptoglobin preserves the CD163 hemoglobin scavenger pathway by shielding hemoglobin from peroxidative modification. Blood. 2009;113:2578–2586. doi: 10.1182/blood-2008-08-174466. [DOI] [PubMed] [Google Scholar]
- Chen J, Regan RF. Heme oxygenase-2 gene deletion increases astrocyte vulnerability to hemin. Biochem. Biophys. Res. Commun. 2004;318:88–94. doi: 10.1016/j.bbrc.2004.03.187. [DOI] [PubMed] [Google Scholar]
- Chen L, Zhang X, Chen-Roetling J, Regan RF. Increased striatal injury and behavioral deficits after intracerebral hemorrhage in hemopexin knockout mice. J. Neurosurg. 2010;114:1159–1167. doi: 10.3171/2010.10.JNS10861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HH, Cahill CM, Vanderburg CR, Scherzer CR, Wang B, Huang X, Rogers JT. Selective translational control of the Alzheimer amyloid precursor protein transcript by iron regulatory protein-1. J. Biol. Chem. 2010;285:31217–31232. doi: 10.1074/jbc.M110.149161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordonnier C, van der Flier WM. Brain microbleeds and Alzheimer’s disease: innocent observation or key player? Brain. 2011;134:335–344. doi: 10.1093/brain/awq321. [DOI] [PubMed] [Google Scholar]
- Crapper McLachlan DR, Dalton AJ, Kruck TP, Bell MY, Smith WL, Kalow W, Andrews DF. Intramuscular desferrioxamine in patients with Alzheimer’s disease. Lancet. 1991;337:1304–1308. doi: 10.1016/0140-6736(91)92978-b. [DOI] [PubMed] [Google Scholar]
- Davies DM, Smith A, Muller-Eberhard U, Morgan WT. Hepatic subcellular metabolism of heme from heme-hemopexin: incorporation of iron into ferritin. Biochem. Biophys. Res. Commun. 1979;91:1504–1511. doi: 10.1016/0006-291x(79)91235-x. [DOI] [PubMed] [Google Scholar]
- Duce JA, Tsatsanis A, Cater MA, et al. Iron-export ferroxidase activity of beta-amyloid precursor protein is inhibited by zinc in Alzheimer’s disease. Cell. 2010;142:857–867. doi: 10.1016/j.cell.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskew JD, Vanacore RM, Sung L, Morales PJ, Smith A. Cellular protection mechanisms against extracellular heme: heme- hemopexin, but not free heme, activates the N-terminal c-Jun kinase. J. Biol. Chem. 1999;274:638–648. doi: 10.1074/jbc.274.2.638. [DOI] [PubMed] [Google Scholar]
- Figueiredo RT, Fernandez PL, Mourao-Sa DS, Porto BN, Dutra FF, Alves LS, Oliveira MF, Oliveira PL, Graca-Souza AV, Bozza MT. Characterization of heme as activator of Toll-like receptor 4. J. Biol. Chem. 2007;282:20221–20229. doi: 10.1074/jbc.M610737200. [DOI] [PubMed] [Google Scholar]
- Flaherty MM, Rish KR, Smith A, Crumbliss AL. An investigation of hemopexin redox properties by spectro-electrochemistry: biological relevance for heme uptake. Biometals. 2008;21:239–248. doi: 10.1007/s10534-007-9112-9. [DOI] [PubMed] [Google Scholar]
- Fliss H. Oxidation of proteins in rat heart and lungs by polymorphonuclear leukocyte oxidants. Mol. Cell. Biochem. 1988;84:177–188. doi: 10.1007/BF00421053. [DOI] [PubMed] [Google Scholar]
- Gebicka L, Banasiak E. Hypochlorous acid-induced heme damage of hemoglobin and its inhibition by flavonoids. Toxicol. In Vitro. 2012;26:924–929. doi: 10.1016/j.tiv.2012.04.010. [DOI] [PubMed] [Google Scholar]
- Gottlieb Y, Truman M, Cohen LA, Leichtmann-Bardoogo Y, Meyron-Holtz EG. Endoplasmic reticulum anchored heme-oxygenase-1 faces the cytosol. Haematologica. 2012;97:1489–1493. doi: 10.3324/haematol.2012.063651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinberg LN, O’Brien PJ, Hrkal Z. The effects of heme-binding proteins on the peroxidative and catalatic activities of hemin. Free Radic. Biol. Med. 1999;27:214–219. doi: 10.1016/s0891-5849(99)00082-9. [DOI] [PubMed] [Google Scholar]
- Guo Y, Schneider LA, Wangensteen OD. HOCl effects on tracheal epithelium: conductance and permeability measurements. J. Appl. Physiol. 1995;78:1330–1338. doi: 10.1152/jappl.1995.78.4.1330. [DOI] [PubMed] [Google Scholar]
- Gupta S, Ahern K, Nakhl F, Forte F. Clinical usefulness of haptoglobin levels to evaluate hemolysis in recently transfused patients. Adv. Hematol. 2011;2011:389854. doi: 10.1155/2011/389854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge JM, Smith A. Antioxidant protection by haemopexin of haem-stimulated lipid peroxidation. Biochem. J. 1988;256:861–865. doi: 10.1042/bj2560861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko C. Mechanism of iron toxicity. Food Nutr. Bull. 2007;28:S500–S509. doi: 10.1177/15648265070284S403. [DOI] [PubMed] [Google Scholar]
- Hrkal Z, Vodrazka Z, Kalousek I. Transfer of heme from ferrihemoglobin and ferrihemoglobin isolated chains to hemopexin. Eur. J. Biochem. 1974;43:73–78. doi: 10.1111/j.1432-1033.1974.tb03386.x. [DOI] [PubMed] [Google Scholar]
- Hunt RC, Handy I, Smith A. Haem exacerbates reactive oxygen species toxicity to human retinal pigment epithelial cells while the haem-binding protein, haemopexin, is protective. J. Cell. Physiol. 1996a;168:81–86. doi: 10.1002/(SICI)1097-4652(199607)168:1<81::AID-JCP10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Hunt RC, Hunt DM, Gaur N, Smith A. Hemopexin in the human retina: protection of the retina against heme- mediated toxicity. J. Cell. Physiol. 1996b;168:71–80. doi: 10.1002/(SICI)1097-4652(199607)168:1<71::AID-JCP9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Hunter MJ, McDuffie FC. Molecular weight studies on human serum albumin after reduction and alkylation of disulfide bonds. J. Am. Chem. Soc. 1959;81:1400–1406. [Google Scholar]
- Jiang Z-Y, Woollard ACS, Wolff SP. Hydrogen peroxide production during experimental protein glycation. FEBS Lett. 1990;268:69–71. doi: 10.1016/0014-5793(90)80974-n. [DOI] [PubMed] [Google Scholar]
- Kettle AJ, Winterbourn CC. Assays for the chlorination activity of myeloperoxidase. Methods Enzymol. 1994;233:502–512. doi: 10.1016/s0076-6879(94)33056-5. [DOI] [PubMed] [Google Scholar]
- Larsen R, Gozzelino R, Jeney V, et al. A central role for free heme in the pathogenesis of severe sepsis. Sci. Transl. Med. 2010;2:51ra, 71ra. doi: 10.1126/scitranslmed.3001118. [DOI] [PubMed] [Google Scholar]
- Li RC, Saleem S, Zhen G, Cao W, Zhuang H, Lee J, Smith A, Altruda F, Tolosano E, Dore S. Heme-hemopexin complex attenuates neuronal cell death and stroke damage. J. Cereb. Blood Flow Metab. 2009;29:953–964. doi: 10.1038/jcbfm.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Takeda K, Ishikawa K, Yoshizawa M, Sato M, Shibahara S, Furuyama K. Coordinated expression of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 4 and heme oxygenase 2: evidence for a regulatory link between glycolysis and heme catabolism. Tohoku J. Exp. Med. 2012;228:27–41. doi: 10.1620/tjem.228.27. [DOI] [PubMed] [Google Scholar]
- Liang X, Lin T, Sun G, Beasley-Topliffe L, Cavaillon JM, Warren HS. Hemopexin down-regulates LPS-induced proinflammatory cytokines from macrophages. J. Leukoc. Biol. 2009;86:229–235. doi: 10.1189/jlb.1208742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q, Weis S, Yang G, et al. Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress. J. Biol. Chem. 2007;282:20621–20633. doi: 10.1074/jbc.M607954200. [DOI] [PubMed] [Google Scholar]
- Lin T, Sammy F, Yang H, Thundivalappil S, Hellman J, Tracey KJ, Warren HS. Identification of hemopexin as an anti-inflammatory factor that inhibits synergy of hemoglobin with HMGB1 in sterile and infectious inflammation. J. Immunol. 2012;189:2017–2022. doi: 10.4049/jimmunol.1103623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zweier JL. A real-time electrochemical technique for measurement of cellular hydrogen peroxide generation and consumption: evaluation in human polymorphonuclear leukocytes. Free Radic. Biol. Med. 2001;31:894–901. doi: 10.1016/s0891-5849(01)00665-7. [DOI] [PubMed] [Google Scholar]
- McCall MR, Carr AC, Forte TM, Frei B. Ldl modified by hypochlorous acid is a potent inhibitor of lecithin-cholesterol acyltransferase activity. Arterioscler. Thromb. Vasc. Biol. 2001;21:1040–1045. doi: 10.1161/01.atv.21.6.1040. [DOI] [PubMed] [Google Scholar]
- McColl BW, Rothwell NJ, Allan SM. Systemic inflammation alters the kinetics of cerebrovascular tight junction disruption after experimental stroke in mice. J. Neurosci. 2008;28:9451–9462. doi: 10.1523/JNEUROSCI.2674-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan WT, Muller-Eberhard U. Interactions of porphyrins with rabbit hemopexin. J. Biol. Chem. 1972;247:7181–7187. [PubMed] [Google Scholar]
- Morgan W, Smith A. Binding and transport of iron-porphyrins by hemopexin. In: Mauk G, Sykes AG, editors. Advances in Inorganic Chemistry. London, UK & San Diego, CA, USA: Academic Press; 2001. pp. 205–241. [Google Scholar]
- Morgan WT, Liem HH, Sutor RP, Muller-Ebergard U. Transfer of heme from heme-albumin to hemopexin. Biochim Biophys. Acta. 1976;444:435–445. doi: 10.1016/0304-4165(76)90387-1. [DOI] [PubMed] [Google Scholar]
- Morris JC. The Acid Ionization Constant of HOCl from 5 to 35°. J. Phys. Chem. 1966;70:3798–3805. [Google Scholar]
- Morse D, Choi AM. Heme oxygenase-1: the “emerging molecule” has arrived. Am. J. Respir. Cell Mol. Biol. 2002;27:8–16. doi: 10.1165/ajrcmb.27.1.4862. [DOI] [PubMed] [Google Scholar]
- Otterbein LE, Hedblom A, Harris C, Csizmadia E, Gallo D, Wegiel B. Heme oxygenase-1 and carbon monoxide modulate DNA repair through ataxia-telangiectasia mutated (ATM) protein. Proc. Natl Acad. Sci. USA. 2011;108:14491–14496. doi: 10.1073/pnas.1102295108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternack RF, Gibbs EJ, Hoeflin E, Kosar WP, Kubera G, Skowronek CA, Wong NM, Muller-Eberhard U. Hemin binding to serum proteins and the catalysis of interprotein transfer. Biochemistry. 1983;22:1753–1758. doi: 10.1021/bi00277a002. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N. Engl. J. Med. 2001;344:1450–1460. doi: 10.1056/NEJM200105103441907. [DOI] [PubMed] [Google Scholar]
- Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, III, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat. Med. 2002;8:1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- Rish KR, Swartzlander R, Sadikot TN, Berridge MV, Smith A. Evidence that heme and heme-hemopexin interact with cell growth-associated plasma membrane electron transport. Biochem. Biophys. Acta Bioenerg. 2007;1767:1107–1117. doi: 10.1016/j.bbabio.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Rogers JT, Randall JD, Cahill CM, et al. An iron-responsive element type II in the 5’-untranslated region of the Alzheimer’s amyloid precursor protein transcript. J. Biol. Chem. 2002;277:45518–45528. doi: 10.1074/jbc.M207435200. [DOI] [PubMed] [Google Scholar]
- Schipper HM. Heme oxygenase-1: transducer of pathological brain iron sequestration under oxidative stress. Ann. N. Y. Acad. Sci. 2004;1012:84–93. doi: 10.1196/annals.1306.007. [DOI] [PubMed] [Google Scholar]
- Schipper HM, Gupta A, Szarek WA. Suppression of glial HO-1 activity as a potential neurotherapeutic intervention in AD. Curr. Alzheimer Res. 2009a;6:424–430. doi: 10.2174/156720509789207985. [DOI] [PubMed] [Google Scholar]
- Schipper HM, Song W, Zukor H, Hascalovici JR, Zeligman D. Heme oxygenase-1 and neurodegeneration: expanding frontiers of engagement. J. Neurochem. 2009b;110:469–485. doi: 10.1111/j.1471-4159.2009.06160.x. [DOI] [PubMed] [Google Scholar]
- Schobel S, Neumann S, Seed B, Lichtenthaler SF. Expression cloning screen for modifiers of amyloid precursor protein shedding. Int. J. Dev. Neurosci. 2006;24:141–148. doi: 10.1016/j.ijdevneu.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Schroder E, Eaton P. Hydrogen peroxide as an endogenous mediator and exogenous tool in cardiovascular research: issues and considerations. Curr. Opin. Pharmacol. 2008;8:153–159. doi: 10.1016/j.coph.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Siesjo BK, Katsura K, Kristian T. Acidosis-related damage. Adv. Neurol. 1996;71:209–236. [PubMed] [Google Scholar]
- Smith A. Mechanisms of Cytoprotection by Hemopexin. In: Kadish KM, Smith KM, Guilard R, editors. Handbook of Porphyrin Science. Biochemistry of Tetrapyrroles. Singapore: World Scientific Publishing Co. Pte. Ltd.; 2011. pp. 217–356. [Google Scholar]
- Smith A, Morgan WT. Haem transport to the liver by haemopexin. Receptor-mediated uptake with recycling of the protein. Biochem. J. 1979;182:47–54. doi: 10.1042/bj1820047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Su H, Song S, Paudel HK, Schipper HM. Over-expression of heme oxygenase-1 promotes oxidative mitochondrial damage in rat astroglia. J. Cell. Physiol. 2006;206:655–663. doi: 10.1002/jcp.20509. [DOI] [PubMed] [Google Scholar]
- Song W, Zukor H, Lin SH, et al. Schizophrenia-like features in transgenic mice overexpressing human HO-1 in the astrocytic compartment. J. Neurosci. 2012;32:10841–10853. doi: 10.1523/JNEUROSCI.6469-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung L, Morales P, Shibata M, Shipulina N, Smith A. Defenses against extracellular heme-mediated oxidative damage: use of iron and copper chelators to investigate the role of redox active iron, copper and heme in the hemopexin heme transport system. In: Badman DG, Bergeron RJ, Brittenham GM, editors. Iron Chelators: New Development Strategies. Sarotoga, FL: Saratoga Publishing Group; 2000. pp. 67–86. [Google Scholar]
- Takahashi M, Dore S, Ferris CD, et al. Amyloid precursor proteins inhibit heme oxygenase activity and augment neurotoxicity in Alzheimer’s disease. Neuron. 2000;28:461–473. doi: 10.1016/s0896-6273(00)00125-2. [DOI] [PubMed] [Google Scholar]
- Test ST, Weiss SJ. Quantitative and temporal characterization of the extracellular H2O2 pool generated by human neutrophils. J. Biol. Chem. 1984;259:399–405. [PubMed] [Google Scholar]
- Timmins GS, Davies MJ, Song DX, Muller-Eberhard U. EPR studies on the effects of complexation of heme by hemopexin upon its reactions with organic peroxides. Free Radic. Res. 1995;23:559–569. doi: 10.3109/10715769509065277. [DOI] [PubMed] [Google Scholar]
- Tong XK, Nicolakakis N, Kocharyan A, Hamel E. Vascular remodeling versus amyloid beta-induced oxidative stress in the cerebrovascular dysfunctions associated with Alzheimer’s disease. J. Neurosci. 2005;25:11165–11174. doi: 10.1523/JNEUROSCI.4031-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanacore R, Eskew J, Morales P, Sung L, Smith A. Role for copper in transient oxidation and nuclear translocation of MTF-1, but not of NFkB, by the hemopexin heme transport system. Antioxid. Redox Signal. 2000;2:739–752. doi: 10.1089/ars.2000.2.4-739. [DOI] [PubMed] [Google Scholar]
- Vermeulen Windsant IC, de Wit NC, Sertorio JT, Beckers EA, Tanus-Santos JE, Jacobs MJ, Buurman WA. Blood transfusions increase circulating plasma free hemoglobin levels and plasma nitric oxide consumption: a prospective observational pilot study. Crit. Care. 2012;16:R95. doi: 10.1186/cc11359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent SH, Grady RW, Shaklai N, Snider JM, Muller-Eberhard U. The influence of heme-binding proteins in heme-catalyzed oxidations. Arch. Biochem. Biophys. 1988;265:539–550. doi: 10.1016/0003-9861(88)90159-2. [DOI] [PubMed] [Google Scholar]
- Wallevik K. Reversible denaturation of human serum albumin by pH, temperature, and guanidine hydrochloride followed by optical rotation. J. Biol. Chem. 1973;248:2650–2655. [PubMed] [Google Scholar]
- Weiss SJ. Tissue destruction by neutrophils. N. Engl. J. Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- Yoshinaga T, Sassa S, Kappas A. The occurrence of molecular interactions among NADPH-cytochrome c reductase, heme oxygenase, and biliverdin reductase in heme degradation. J. Biol. Chem. 1982;257:7786–7793. [PubMed] [Google Scholar]
- Zheng W, Xin N, Chi ZH, Zhao BL, Zhang J, Li JY, Wang ZY. Divalent metal transporter 1 is involved in amyloid precursor protein processing and Abeta generation. FASEB J. 2009;23:4207–4217. doi: 10.1096/fj.09-135749. [DOI] [PubMed] [Google Scholar]