Abstract
Cystic fibrosis (CF) is caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, which encodes an anion channel. In the human lung CFTR loss causes abnormal ion transport across airway epithelial cells. As a result CF individuals produce thick mucus, suffer persistent bacterial infections and have a much reduced life expectancy. Trans-epithelial potential difference (Vt) measurements are routinely carried out on nasal epithelia of CF patients in the clinic. CF epithelia exhibit a hyperpolarised basal Vt and a larger Vt change in response to amiloride (a blocker of the epithelial Na+ channel, ENaC). Are these altered bioelectric properties solely a result of electrical coupling between the ENaC and CFTR currents, or are they due to an increased ENaC permeability associated with CFTR loss? To examine these issues we have developed a quantitative mathematical model of human nasal epithelial ion transport. We find that while the loss of CFTR permeability hyperpolarises Vt and also increases amiloride-sensitive Vt, these effects are too small to account for the magnitude of change observed in CF epithelia. Instead, a parallel increase in ENaC permeability is required to adequately fit observed experimental data. Our study provides quantitative predictions for the complex relationships between ionic permeabilities and nasal Vt, giving insights into the physiology of CF disease that have important implications for CF therapy.
Key points
Cystic fibrosis (CF) is a common genetic disease caused by loss-of-function mutations in the cystic fibrosis transmembrane conductance regulator gene, which encodes a channel protein, selective for anions.
In the lungs, the site of the most severe symptoms, CF causes abnormal electrolyte transport in epithelial cells which line the airways.
Airway epithelial ion transport can be assessed by measuring the trans-epithelial potential difference (Vt) which shows characteristic changes in CF individuals. We developed a biophysical model of ion transport in human nasal epithelia, in order to investigate quantitatively which transport parameters underlie these observed bioelectric changes.
We found that loss of apical Cl− permeability alone is insufficient to explain the bioelectric properties of CF epithelia. An increase of apical Na+ permeability must also occur.
This insight has important implications for our understanding of the physiology of CF disease, and hence for potential therapies aimed at correcting the CF ion transport defect.
Introduction
Cystic fibrosis (CF) is a mono-genetic disorder that impairs quality of life and greatly reduces life expectancy (Davies et al. 2007). It is the most common fatal inherited genetic disease found in people of European descent (Dodge et al. 2007). CF is a complex disease, affecting several organs; however, the most frequent cause of death amongst CF sufferers is lung failure resulting from persistent bacterial infections.
It is known that loss-of-function mutations in the CFTR gene product, an anion-selective channel, are the root cause of the disease. Thus, abnormal trans-epithelial electrolyte transport appears to be crucial to the pathogenesis of CF (Rowe et al. 2005). Measurements of the trans-epithelial potential difference (Vt) across nasal epithelia can be used to investigate airway epithelial ion transport and such measurements are often made in vivo to aid diagnosis of CF in the clinic. Vt measurements are also used as outcome measures in clinical trials of drug and gene therapies for the disease (Rowe et al. 2011). CF epithelia show hyperpolarised basal Vt (relative to non-CF epithelia), an increased depolarisation following block of the epithelial Na+ channel (ENaC) with its inhibitor amiloride, a reduced or missing response when the driving force for apical Cl− efflux is increased, and no hyperpolarisation in response to raised intracellular cAMP levels (Knowles et al. 1995).
These bioelectric properties arise as a direct result of mutations in the CFTR gene, but whether or not they are simply a consequence of the loss of apical anion permeability is a matter of debate. It has been suggested that CFTR regulates the activity of other transport processes in epithelial cells, in particular ENaC, with the loss of CFTR resulting in higher basal levels of apical Na+ conductance (Stutts et al. 1995; Donaldson & Boucher, 2007). More recent studies, however, report that Na+ absorption in pig and human CF airway epithelial cultures is not increased (Chen et al. 2010; Itani et al. 2011). These studies suggest that the loss of anion conductance can account for hyperpolarised basal Vt, as well as the increased amiloride-sensitive Vt and altered short-circuit current, because of the way the CFTR currents are electrically coupled to other transport processes. Previous modelling work from a kidney epithelial cell line provides qualitative support for this idea (Horisberger, 2003).
To assess these conflicting views we developed a detailed mathematical model of ion transport in human nasal epithelial (HNE) cells, so as to quantitatively investigate the relationship between individual ionic permeabilities and commonly measured bioelectric properties of the integrated epithelial transport system, such as basal Vt and amiloride-sensitive Vt. Our model differs from most previous studies investigating airway epithelial physiology (Hartmann & Verkman, 1990; Duszyk & French, 1991; Warren et al. 2009; Falkenberg & Jakobsson, 2010) in that it focuses specifically on nasal epithelial cell components and parameter values. A modelling study focused on quantifying ionic permeabilities in non-CF HNE cells has recently been published, but this work did not consider ion transport in CF (Garcia et al. 2013). We use data from primary cultures of both CF and non-CF nasal epithelial cells for model validation, thus allowing us to investigate clinically relevant questions regarding how changes in the underlying transport components give rise to altered nasal Vt measurements in CF.
We found that while the electrical coupling between CFTR and ENaC currents can cause, qualitatively, the type of changes seen in CF, the magnitudes of these effects are not large enough to explain CF abnormalities. Instead, apical Na+ permeability must be increased in CF in order to quantitatively explain the differences observed in bioelectric properties between the non-CF and CF airway epithelium.
Methods
Model overview
Our mathematical model simulates a monolayer of HNE cells placed between two well-perfused compartments containing physiological saline solution (Fig. 1A), thus approximating the environment experienced by HNE cells in vivo during nasal Vt measurements when the airway surface is flooded (or in vitro during an Ussing chamber experiment). In this model Na+, Cl−, K+ and water move between interstitial fluid and airway lumen (paracellular route) and between the cell and external solutions via transport processes in the apical and basolateral plasma membranes (Fig. 1B). The magnitude of the ion flux due to each of these component processes in the model is proportional to a transport parameter that is related to the density of that component in the plasma membrane.
Figure 1. Schematic diagram of epithelial layer (A) and individual epithelial cell (B) separating the airway lumen from the interstitial fluid.
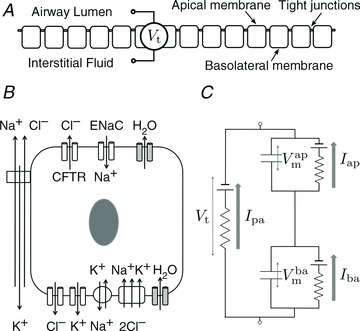
A and B, electrolyte transport occurs across the apical and basolateral membranes, and along the paracellular path through tight junctions. Transport parameters characterise flux through each pathway: CFTR and ENaC channels in the apical membrane are characterised by apical Cl− permeability (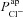 ) and apical Na+ permeability(
) and apical Na+ permeability(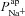 ), respectively; K+ and Cl− channels in the basolateral membrane are characterised by the basolateral K+ (
), respectively; K+ and Cl− channels in the basolateral membrane are characterised by the basolateral K+ (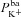 ) and Cl− (
) and Cl− (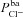 ) permeabilities; the transport parameters for the Na+–K+-ATPase pump proteins and NKCC cotransport proteins in the basolateral membrane are their densities per unit area of the membrane, ρNaK and ρNKCC, respectively. The state of the cell at any time is described by six variables, cell volume (Wi), moles of Na+, Cl− and K+ in the cell (
) permeabilities; the transport parameters for the Na+–K+-ATPase pump proteins and NKCC cotransport proteins in the basolateral membrane are their densities per unit area of the membrane, ρNaK and ρNKCC, respectively. The state of the cell at any time is described by six variables, cell volume (Wi), moles of Na+, Cl− and K+ in the cell (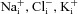 , respectively), and apical (
, respectively), and apical (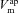 ) and basolateral (
) and basolateral (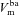 ) membrane potentials. C, equivalent electrical circuit representation of airway epithelium.
) membrane potentials. C, equivalent electrical circuit representation of airway epithelium. 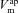 and
and 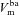 are coupled electrically via the current along the paracellular pathway (Ipa). The trans-epithelial potential difference Vt is given by the difference between lumen and serosal potential (i.e.
are coupled electrically via the current along the paracellular pathway (Ipa). The trans-epithelial potential difference Vt is given by the difference between lumen and serosal potential (i.e. 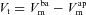 ).
).
We calculate the flux of ions from each individual transport pathway as a function of the driving force and associated transport parameter, and employ an equivalent electrical circuit description of the epithelium to determine membrane (apical, 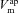 and basal,
and basal, 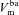 ) and trans-epithelial (Vt) potentials in the open circuit configuration (Fig. 1C). This framework allows us to vary transport parameters or extracellular solution composition, and calculate the resultant changes in membrane potentials and Vt in a quantitative manner. It also allows a quantitative investigation of how these responses to perturbations change when transport processes are varied.
) and trans-epithelial (Vt) potentials in the open circuit configuration (Fig. 1C). This framework allows us to vary transport parameters or extracellular solution composition, and calculate the resultant changes in membrane potentials and Vt in a quantitative manner. It also allows a quantitative investigation of how these responses to perturbations change when transport processes are varied.
Transport pathways included in model
There are four ion channel components included in the model. ENaC and CFTR channels will give rise to apical Na+ (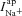 ) and Cl− (
) and Cl− (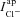 ) currents, respectively, and basolateral K+ and Cl− channels facilitate the basolateral currents
) currents, respectively, and basolateral K+ and Cl− channels facilitate the basolateral currents 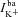 and
and 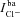 . Apical K+ channels are not included since they do not contribute substantially to Vt (Knowles et al. 1983; Willumsen et al. 1989a). Channel currents were modelled using the Goldman–Hodgkin–Katz (GHK) flux equation (Hille, 2001), which relates the trans-membrane electrochemical driving force (determined by the membrane potential and concentration gradient) to the trans-membrane current, given the permeability of the membrane to a particular ion (see Supplemental material, section S1, available online only). For example, given apical membrane potential (
. Apical K+ channels are not included since they do not contribute substantially to Vt (Knowles et al. 1983; Willumsen et al. 1989a). Channel currents were modelled using the Goldman–Hodgkin–Katz (GHK) flux equation (Hille, 2001), which relates the trans-membrane electrochemical driving force (determined by the membrane potential and concentration gradient) to the trans-membrane current, given the permeability of the membrane to a particular ion (see Supplemental material, section S1, available online only). For example, given apical membrane potential (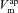 ), lumen and intracellular Na+ concentrations ([Na+]l and [Na+]i), and the permeability of the apical membrane to Na+ (
), lumen and intracellular Na+ concentrations ([Na+]l and [Na+]i), and the permeability of the apical membrane to Na+ (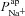 ), we can compute the ENaC current (
), we can compute the ENaC current (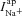 ). Paracellular ion currents (
). Paracellular ion currents (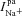 ,
, 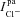 ,
, 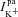 ,
, 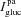 ) are also modelled using the GHK equation (with Vt as electrical driving force, and ion concentrations from the luminal and serosal compartments).
) are also modelled using the GHK equation (with Vt as electrical driving force, and ion concentrations from the luminal and serosal compartments).
We include descriptions of the Na+-K+–2Cl− co-transport protein NKCC1 and Na+–K+-ATPase pump protein in our model, which generate the basolateral ion fluxes JNKCC and JNaK, respectively. We use the model of Benjamin & Johnson to calculate flux from the Na+–K+–2Cl− co-transporter (Benjamin & Johnson, 1997), and the model of Smith & Crampin to describe active transport by the Na+–K+-ATPase (Smith & Crampin, 2004; see Supplemental material, section S1, for full details). The total flux along these transport pathways is proportional to the density of the relevant protein in the basolateral membrane, ρNKCC and ρNaK.
In our model both apical and basolateral membranes are permeable to water. The trans-membrane water flux in both cases (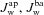 ) is assumed to be proportional to the trans-membrane osmolarity gradient ΔS, where the osmolarity here is given by the total Na+, Cl− and K+ concentrations as well as the concentration of impermeable anions in that given compartment.
) is assumed to be proportional to the trans-membrane osmolarity gradient ΔS, where the osmolarity here is given by the total Na+, Cl− and K+ concentrations as well as the concentration of impermeable anions in that given compartment.
Transport kinetics
Cellular variables evolve in time based on the net influx or efflux of ions and water, and we described these kinetics with a system of coupled, non-linear ordinary differential equations.
Cell volume Wi changes if there is a net influx or efflux of water (water flux is positive in serosal to mucosal direction):
| (1) |
The ionic composition of the intracellular compartment changes due to the net trans-membrane ion fluxes (positive ion currents denote a flux of positive ions out of the cell, positive JNKCC denotes ion flux into the cell, F is the Faraday constant and zn is the valence of the ion n under consideration):
| (2) |
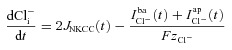 |
(3) |
| (4) |
The equivalent electrical circuit description of the epithelium (Fig. 1C) can be used to calculate how the membrane potentials change due to net apical, basolateral and paracellular currents (Cm is the capacitance per unit area of the plasma membrane):
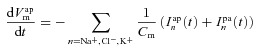 |
(5) |
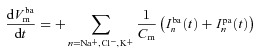 |
(6) |
The trans-epithelial potential difference, with the serosal compartment as the earth, is given by 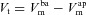 (see Supplemental material, section S1).
(see Supplemental material, section S1).
Baseline transport parameter values
We initially found estimates of transport parameters (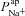 ,
, 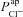 ,
, 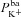 , ρNaK, ρNKCC,
, ρNaK, ρNKCC, 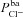 ) from the relevant scientific literature, and refer to these as baseline parameter values (Table 1; note that here we assume that the paracellular permeability, Ppa, is non-selective and does not change in CF; the rationale for this is discussed later; see also Supplemental material, section S4). These were used to give an order of magnitude estimate for each parameter and thus initially identify the region of parameter space on which our parameter estimation should focus.
) from the relevant scientific literature, and refer to these as baseline parameter values (Table 1; note that here we assume that the paracellular permeability, Ppa, is non-selective and does not change in CF; the rationale for this is discussed later; see also Supplemental material, section S4). These were used to give an order of magnitude estimate for each parameter and thus initially identify the region of parameter space on which our parameter estimation should focus.
Table 1.
Baseline values used (column 3) and numerical estimates found for parameter values in non-CF and CF cells (columns 5 and 6) for each free transport parameter (rows 1–6)
| Parameter | Units | Baseline | Reference | Non-CF | CF |
|---|---|---|---|---|---|
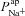 |
μm s−1 | 0.028 | (Willumsen & Boucher, 1991b) | 0.024 | 0.065 |
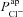 |
μm s−1 | 0.072 | (Willumsen & Boucher, 1991b) | 0.066 | 0.006 |
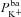 |
μm s−1 | 0.080 | (Falkenberg & Jakobsson, 2010) | 0.103 | 0.400 |
| ρNaK | 10−10 mol cm−2 | 0.400 | * | 0.127 | 0.489 |
| ρNKCC | 10−10 mol cm−2 | 0.400 | * | 0.188 | 2.000 |
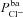 |
μm s−1 | 0.100 | (Falkenberg & Jakobsson, 2010) | 0.097 | 0.144 |
ρNaK and ρNKCC estimated by authors.
Results
CF epithelia have an increased 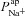
We set out to determine, then to compare, the value of 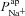 in CF and non-CF nasal epithelial cells. To constrain the model we used extensive data sets obtained from cultured HNE cells, including time course data covering the addition of amiloride or reduction of [ Cl−]l at the apical membrane (Willumsen et al. 1989a,b; Willumsen & Boucher, 1991a,b). We thus formulated an optimisation problem to minimise the residual errors between physiological properties predicted by the mathematical model, and those observed experimentally, by varying transport parameters of interest (see Supplemental material, section S2, for details).
in CF and non-CF nasal epithelial cells. To constrain the model we used extensive data sets obtained from cultured HNE cells, including time course data covering the addition of amiloride or reduction of [ Cl−]l at the apical membrane (Willumsen et al. 1989a,b; Willumsen & Boucher, 1991a,b). We thus formulated an optimisation problem to minimise the residual errors between physiological properties predicted by the mathematical model, and those observed experimentally, by varying transport parameters of interest (see Supplemental material, section S2, for details).
Using simulations made with parameter values optimised for non-CF epithelia, our model accurately fits the observed initial and final steady-state values for membrane and trans-epithelial potentials (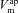 ,
,  and Vt) and concentrations ([ Na+]i, [ Cl−]i) both in amiloride addition and low Cl − experiments (Fig. 2). A similar analysis was carried out to identify parameter values best describing corresponding experimental data obtained on CF epithelia (Supplemental Fig. S3). The optimised parameter values for non-CF and CF epithelia are shown in Table 1.
and Vt) and concentrations ([ Na+]i, [ Cl−]i) both in amiloride addition and low Cl − experiments (Fig. 2). A similar analysis was carried out to identify parameter values best describing corresponding experimental data obtained on CF epithelia (Supplemental Fig. S3). The optimised parameter values for non-CF and CF epithelia are shown in Table 1.
Figure 2. Estimating transport parameters from electrophysiological recordings.
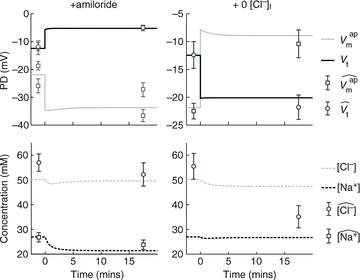
Model predictions for 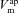 , Vt, [ Na+]i and [ Cl−]i (continuous and dashed lines) are plotted for simulations of ‘+amiloride’ and ‘+ 0[ Cl−]l’ experiments, and compared with their observed values (symbols). Data used are from non-CF HNE cells, and parameter values used for the simulation are those which were found to minimise the residual error between model output and these data (Table 1).
, Vt, [ Na+]i and [ Cl−]i (continuous and dashed lines) are plotted for simulations of ‘+amiloride’ and ‘+ 0[ Cl−]l’ experiments, and compared with their observed values (symbols). Data used are from non-CF HNE cells, and parameter values used for the simulation are those which were found to minimise the residual error between model output and these data (Table 1).
The optimal parameter values obtained for ENaC and CFTR permeability are similar to those estimated experimentally (Table 1). Examining the difference between optimal CF and non-CF parameter values, we found not only that in CF 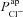 must be reduced (as expected) but also that the value of
must be reduced (as expected) but also that the value of 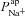 must be significantly increased.
must be significantly increased.
Very little experimental data are available on the magnitude and characteristics of paracellular permeability. In order to determine if increased 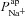 in CF epithelia was dependent on assumptions we had made regarding paracellular ion transport, we repeated the parameter estimation analysis assuming a lower Ppa in CF (Willumsen & Boucher, 1989) and/or a cation-selective paracellular transport (Levin et al. 2006; Flynn et al. 2009). We found that while these differences in paracellular transport do have an influence over the exact value of
in CF epithelia was dependent on assumptions we had made regarding paracellular ion transport, we repeated the parameter estimation analysis assuming a lower Ppa in CF (Willumsen & Boucher, 1989) and/or a cation-selective paracellular transport (Levin et al. 2006; Flynn et al. 2009). We found that while these differences in paracellular transport do have an influence over the exact value of 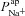 or
or 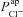 estimated, they do not alter how each of these parameters changes in CF relative to non-CF epithelia (see Supplemental Tables S5 and S6).
estimated, they do not alter how each of these parameters changes in CF relative to non-CF epithelia (see Supplemental Tables S5 and S6).
Feasible ranges of 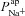 differ between populations of non-CF and CF nasal epithelial cells
differ between populations of non-CF and CF nasal epithelial cells
Although our optimisation results provide good evidence for a change in 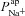 , we were conscious that the data which we used for fitting in the optimisation problem were the mean of several experiments carried out on different primary cultures of HNE cells. Variations in the experimental results obtained from these cells show that a large range of values of, for example, intracellular [ Na+], are physiologically reasonable. We wanted to make sure that by optimising parameter fits to average data we did not exclude parameter sets that could account for both CF and non-CF data given the full range of possible variation (e.g. Willumsen & Boucher, 1991b).
, we were conscious that the data which we used for fitting in the optimisation problem were the mean of several experiments carried out on different primary cultures of HNE cells. Variations in the experimental results obtained from these cells show that a large range of values of, for example, intracellular [ Na+], are physiologically reasonable. We wanted to make sure that by optimising parameter fits to average data we did not exclude parameter sets that could account for both CF and non-CF data given the full range of possible variation (e.g. Willumsen & Boucher, 1991b).
To achieve this, we carried out a large number of simulations with the model, as illustrated schematically in Fig. 3. We first used Monte Carlo sampling to randomly generate 106 parameter sets, sampling values for each transport parameter (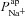 ,
, 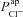 ,
, 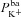 , ρNaK, ρNKCC,
, ρNaK, ρNKCC, 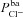 ) from a uniform distribution on a bounded region (from zero to five times) around the relevant baseline parameter value (Fig. 3A). This process provided a population of model parameter sets, each with a unique set of parameter values, steady-state variable values, kinetic properties and so on (Fig. 3B). We next separated the sample population (Fig. 3C) into 1975 parameter sets which predicted observed steady-state and kinetic properties of non-CF HNE cells and 2430 which reproduced the observed steady-state and kinetic behaviour of CF HNE cells (see Table 2 for both non-CF and CF filtering bounds). The other parameter sets which produced non-physiological values or unstable kinetics were discarded (see Supplemental material, section S3, for full details).
) from a uniform distribution on a bounded region (from zero to five times) around the relevant baseline parameter value (Fig. 3A). This process provided a population of model parameter sets, each with a unique set of parameter values, steady-state variable values, kinetic properties and so on (Fig. 3B). We next separated the sample population (Fig. 3C) into 1975 parameter sets which predicted observed steady-state and kinetic properties of non-CF HNE cells and 2430 which reproduced the observed steady-state and kinetic behaviour of CF HNE cells (see Table 2 for both non-CF and CF filtering bounds). The other parameter sets which produced non-physiological values or unstable kinetics were discarded (see Supplemental material, section S3, for full details).
Figure 3. Monte Carlo filtering analysis approach to determine distributions of transport parameter values in CF and non-CF HNE cells.
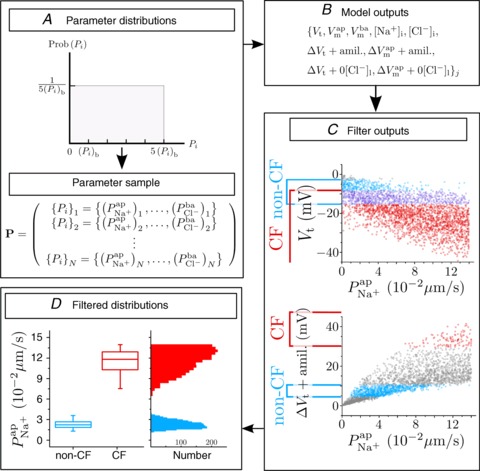
A, a large sample (N= 106) of parameter sets was generated, each set is a vector 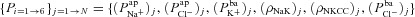 . Each parameter i is sampled from a uniform distribution U(0, 5(Pi)b) around its baseline value (see Table 1). B, for each parameter set {Pi}j, the values of variables Xj at steady state and their value after ‘+amiloride’ and ‘+ 0[ Cl−]l’ perturbations are calculated. C, bounds are placed on the allowed values of these model outputs, for both CF and non-CF states, and parameter sets are classified on this basis. D, filtered parameter distributions can be examined to assess how transport parameters vary between normal and disease states.
. Each parameter i is sampled from a uniform distribution U(0, 5(Pi)b) around its baseline value (see Table 1). B, for each parameter set {Pi}j, the values of variables Xj at steady state and their value after ‘+amiloride’ and ‘+ 0[ Cl−]l’ perturbations are calculated. C, bounds are placed on the allowed values of these model outputs, for both CF and non-CF states, and parameter sets are classified on this basis. D, filtered parameter distributions can be examined to assess how transport parameters vary between normal and disease states.
Table 2.
Constraints on allowed variable values in CF and non-CF cells, based on data from primary cultures of HNE cells
| Non-CF | CF | ||||||
|---|---|---|---|---|---|---|---|
| Property | Units | Lower | Upper | Source | Lower | Upper | Source |
| [Na+]i | mm | 18.0 | 43.2 | (Willumsen & Boucher, 1991b) | 21.0 | 51.3 | (Willumsen & Boucher, 1991a) |
| [Cl−]i | mm | 32.5 | 84.4 | (Willumsen et al. 1989a) | 32.5 | 84.4 | (Willumsen et al. 1989b) |
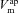 |
mV | −38.6 | −14.9 | (Willumsen & Boucher, 1991b) | −37.7 | 6.7 | (Willumsen & Boucher, 1991a) |
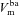 |
mV | −45.1 | −24.2 | (Willumsen & Boucher, 1991b) | −59.3 | −33.6 | (Willumsen & Boucher, 1991a) |
| Vt | mV | −15.5 | −2.7 | (Willumsen & Boucher, 1991b) | −59.2 | −8.2 | (Willumsen & Boucher, 1991a) |
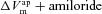 |
mV | −14.0 | −5.5 | (Willumsen et al. 1989a; Willumsen & Boucher, 1991b) | −47.4 | −29.0 | (Willumsen et al. 1989b; Willumsen & Boucher, 1991a) |
| ΔVt+ amiloride | mV | 4.7 | 10.1 | (Willumsen et al. 1989a; Willumsen & Boucher, 1991b) | 30.1 | 47.1 | (Willumsen et al. 1989b; Willumsen & Boucher, 1991a) |
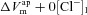 |
mV | 9.2 | 15.0 | (Willumsen et al. 1989a) | −5.3 | 11.1 | (Willumsen et al. 1989b) |
| ΔVt+ 0[ Cl−]l | mV | −12.7 | −6.1 | (Willumsen et al. 1989a) | −16.5 | 9.9 | (Willumsen et al. 1989b) |
Figure 4 illustrates the distributions of transport parameter values which remain after applying the non-CF (blue) and CF (red) filters (see also Supplemental material Figs S4 and S5, and Tables S5 and S6, respectively). While the non-CF and CF distributions are similar for some parameters, the distributions of 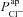 and
and 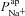 differ markedly, CFTR permeability being decreased and ENaC permeability increased in the disease state. Thus, extending our analysis to take into account the full distribution of allowed cellular variable values, rather than focusing on mean behaviour, confirms that ENaC permeability must be increased in CF relative to non-CF cells, in order to explain the observed quantitative differences in electrophysiological properties.
differ markedly, CFTR permeability being decreased and ENaC permeability increased in the disease state. Thus, extending our analysis to take into account the full distribution of allowed cellular variable values, rather than focusing on mean behaviour, confirms that ENaC permeability must be increased in CF relative to non-CF cells, in order to explain the observed quantitative differences in electrophysiological properties.
Figure 4. Distributions of each transport parameter, found by constraining allowed model behaviour in non-CF (blue) and CF (red) states.
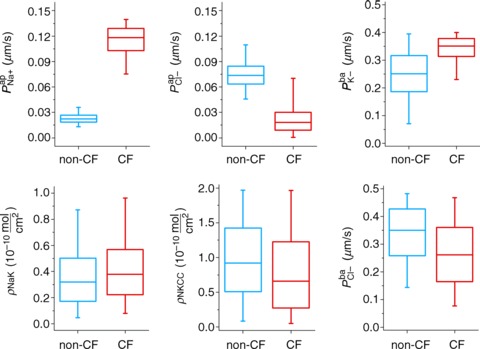
In sequence from low to high, features denoted in each boxplot are: 1st, 25th, 50th (median), 75th and 99th percentiles of the given parameter's distribution.
We repeated this Monte Carlo filtering analysis to determine whether or not decreased paracellular permeability in CF, and/or selectivity of the paracellular pathway, would significantly alter these conclusions. Again we found this was not the case: neither a higher shunt resistance in CF nor a cation-selective paracellular pathway affected our conclusions that permeability distributions were shifted in CF epithelia, with median CFTR permeability decreased and median ENaC permeability increased (see Supplemental material, section S4, Figs S6–S8).
Hyperpolarised Vt in CF can be explained by increased 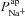 , but not by reduced
, but not by reduced 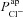
To further investigate the functional relationship between each individual transport parameter, Pi, and the epithelial bioelectric properties in question (i.e. model output variables basal Vt, ΔVt+ amiloride, and ΔVt+ 0[ Cl−]l) we carried out a variance-based sensitivity analysis (Sobie, 2009; Taylor et al. 2009) using the 1975 parameter sets in the non-CF distribution along with their model outputs (see Figs 5 and 6, and Supplemental material, section S4). The coefficients we obtained (Figs 5C and 6C) gave us an objective means of quantifying the relative influence of each transport parameter on these bioelectric properties.
Figure 5. Sensitivity analysis investigating the influence of model parameters on Vt.
A multiple regression of the form 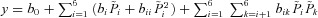 was fitted using the set of normalised parameter values
was fitted using the set of normalised parameter values 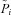 in the non-CF parameter distribution as regressors, and the corresponding model output y={Vt} as the independent variable (see Supplemental material, section S4). Basal Vt is plotted as a function of
in the non-CF parameter distribution as regressors, and the corresponding model output y={Vt} as the independent variable (see Supplemental material, section S4). Basal Vt is plotted as a function of 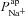 (A) and
(A) and 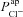 (B), for the 1975 parameter sets belonging to the non-CF distribution. C, strength of linear interaction (bi) between transport parameters
(B), for the 1975 parameter sets belonging to the non-CF distribution. C, strength of linear interaction (bi) between transport parameters 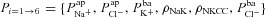 and Vt, found via sensitivity analysis.
and Vt, found via sensitivity analysis. 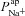 hyperpolarises Vt (b1=−1.49 mV), but changing
hyperpolarises Vt (b1=−1.49 mV), but changing 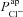 has little influence (b2=−0.02 mV).
has little influence (b2=−0.02 mV).
Figure 6. Sensitivity analysis investigating the influence of model parameters on ΔVt+ amiloride.
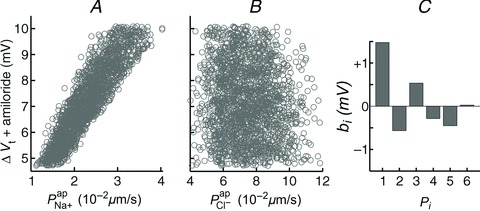
Amiloride-sensitive Vt is plotted against 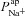 (A) and
(A) and 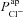 (B), for their non-CF distributions. C, sensitivity analysis results plotting strength of interaction (bi) between ΔVt+ amiloride and transport parameters
(B), for their non-CF distributions. C, sensitivity analysis results plotting strength of interaction (bi) between ΔVt+ amiloride and transport parameters 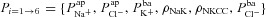 .
. 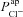 tends to decrease this metric, despite its limited effect on basal Vt.
tends to decrease this metric, despite its limited effect on basal Vt.
Figure 5A and B shows scatter plots of 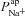 and
and 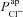 , respectively (from the non-CF parameter value distributions), against basal (steady state) Vt predicted by each. Figure 5C summarises the results of the sensitivity analysis. There is a negative correlation between
, respectively (from the non-CF parameter value distributions), against basal (steady state) Vt predicted by each. Figure 5C summarises the results of the sensitivity analysis. There is a negative correlation between 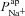 and Vt apparent in panel A, and confirmed by the large negative regression coefficient b1 (−1.49 mV) in panel C. A significant correlation between
and Vt apparent in panel A, and confirmed by the large negative regression coefficient b1 (−1.49 mV) in panel C. A significant correlation between 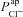 and Vt is not clear in panel B. The sensitivity analysis confirms that while
and Vt is not clear in panel B. The sensitivity analysis confirms that while 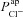 does influence Vt to an extent, on average over this region of parameter space it has a much smaller effect than
does influence Vt to an extent, on average over this region of parameter space it has a much smaller effect than 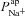 ,
, 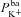 or
or 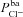 (regression coefficient b2=−0.02 mV, <2%b1). Therefore an increase in
(regression coefficient b2=−0.02 mV, <2%b1). Therefore an increase in 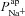 is necessary to hyperpolarise basal Vt to the values seen in CF epithelia, while changes in
is necessary to hyperpolarise basal Vt to the values seen in CF epithelia, while changes in 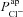 do not influence Vt to the same extent.
do not influence Vt to the same extent.
Amiloride-sensitive Vt is inversely related to 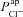 , but is more strongly influenced by
, but is more strongly influenced by 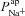
Figure 6A and B illustrates the relationship between the 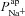 and
and 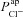 parameter values, respectively, from the non-CF distributions, and the corresponding predicted ΔVt+ amiloride. Not surprisingly, there is a positive correlation between
parameter values, respectively, from the non-CF distributions, and the corresponding predicted ΔVt+ amiloride. Not surprisingly, there is a positive correlation between 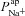 and ΔVt+ amiloride. However, the relationship between ΔVt+ amiloride and
and ΔVt+ amiloride. However, the relationship between ΔVt+ amiloride and 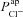 is less obvious. The results of the sensitivity analysis in Fig. 6C show that
is less obvious. The results of the sensitivity analysis in Fig. 6C show that 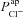 has the second greatest influence on ΔVt+ amiloride. While increasing
has the second greatest influence on ΔVt+ amiloride. While increasing 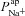 tends to increase the magnitude of ΔVt+ amiloride, increasing
tends to increase the magnitude of ΔVt+ amiloride, increasing 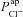 tends to decrease its magnitude.
tends to decrease its magnitude.
Loss of Cl− conductance can hyperpolarise basal Vt, but not to the extent seen in CF
It is clear that 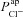 can influence basal Vt, even if it does not do so to the same extent as
can influence basal Vt, even if it does not do so to the same extent as 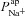 . We wanted to determine, quantitatively, what magnitude of a change in basal Vt the model would predict upon loss of
. We wanted to determine, quantitatively, what magnitude of a change in basal Vt the model would predict upon loss of 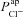 alone, and compare this to the hyperpolarisation of Vt observed in CF. Therefore, for each parameter set producing plausible physiological values in the non-CF distribution, we set
alone, and compare this to the hyperpolarisation of Vt observed in CF. Therefore, for each parameter set producing plausible physiological values in the non-CF distribution, we set 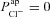 and found the new steady state of the system. We define
and found the new steady state of the system. We define 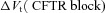 as the difference between this new Vt, and the initial basal Vt when
as the difference between this new Vt, and the initial basal Vt when 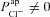 .
.
We can analyse the magnitude of 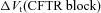 and its relationship to ΔVt+ 0[ Cl−]l (change in Vt induced by reducing [ Cl−]l) which is commonly used as a measure of the underlying Cl− conductance (see Supplemental Fig. S9). For a given ΔVt+ 0[ Cl−]l, CFTR loss can depolarise or hyperpolarise Vt, depending on the magnitudes of the other transport parameter values. The average ΔVt+ 0[ Cl−]l observed experimentally in non-CF HNE cells was not greater than –15mV (Willumsen et al. 1989a), a value close to that reported in vivo, in nasal PD measurements. However, the maximum hyperpolarisation achieved by blocking
and its relationship to ΔVt+ 0[ Cl−]l (change in Vt induced by reducing [ Cl−]l) which is commonly used as a measure of the underlying Cl− conductance (see Supplemental Fig. S9). For a given ΔVt+ 0[ Cl−]l, CFTR loss can depolarise or hyperpolarise Vt, depending on the magnitudes of the other transport parameter values. The average ΔVt+ 0[ Cl−]l observed experimentally in non-CF HNE cells was not greater than –15mV (Willumsen et al. 1989a), a value close to that reported in vivo, in nasal PD measurements. However, the maximum hyperpolarisation achieved by blocking 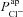 was around −4 mV. This is much smaller in magnitude than the average hyperpolarisation seen in CF patients (and in primary cultures of CF HNE cells; Willumsen & Boucher, 1991b), which is around −20 mV (Knowles et al. 1995).
was around −4 mV. This is much smaller in magnitude than the average hyperpolarisation seen in CF patients (and in primary cultures of CF HNE cells; Willumsen & Boucher, 1991b), which is around −20 mV (Knowles et al. 1995).
Discussion
We have developed a mathematical model of ion transport in human nasal epithelial cells. As the nasal epithelium is the site of in vivo measurements made on patients, it has been well characterised (Willumsen & Boucher, 1989, 1991b; Willumsen et al. 1989b) and is clinically important in the assessment of CF (Simmonds et al. 2011). The advantages of specifically modelling nasal epithelia are thus 2-fold. First, it offers the opportunity to exploit a very large body of existing measurements for validation and parameter estimation purposes. Second, by leading to a better quantitative analysis of nasal potential difference measurements, it improves our understanding of CF disease.
The agreement between our model predictions and the known physiology, in terms of capturing essential changes in membrane potentials and intracellular ion concentrations (Fig. 2), suggests that the model provides a realistic picture of the major epithelial ion transport processes which determine nasal trans-epithelial potential. Fitting the model to experimental data, we observed not only that 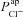 must be reduced, but also that
must be reduced, but also that 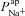 had to increase (Table 1) to account for the bioelectric properties of CF epithelial cells. This prediction also held when we ran multiple simulations to take into account cell variability: parameter sets resulting in steady-state and kinetic characteristics typical of CF cells included not only reduced
had to increase (Table 1) to account for the bioelectric properties of CF epithelial cells. This prediction also held when we ran multiple simulations to take into account cell variability: parameter sets resulting in steady-state and kinetic characteristics typical of CF cells included not only reduced 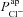 but also increased
but also increased 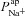 (Fig. 4). The fact that our analysis did not make any initial judgements regarding how parameters should vary in the disease state, and hence did not bias us towards computing these results, gives us confidence in their validity and further demonstrates that the findings regarding ENaC and CFTR permeabilities are robust.
(Fig. 4). The fact that our analysis did not make any initial judgements regarding how parameters should vary in the disease state, and hence did not bias us towards computing these results, gives us confidence in their validity and further demonstrates that the findings regarding ENaC and CFTR permeabilities are robust.
Impact of model assumptions
Inevitably, when describing a complex biological system with a mathematical model, one makes a number of assumptions in order to concentrate on the phenomena of interest, and to keep the analysis tractable. The main assumption we make is that Na+, Cl− and K+ currents largely determine nasal Vt and that the membrane permeabilities of these ions can be estimated from trans-epithelial electrical recordings. In making this simplification we implicitly assume that bicarbonate transport does not substantially impact Vt. In our analysis we saw that 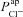 has little effect on basal Vt (Fig. 5), and limited effect on ΔVt+ amiloride (Fig. 6B and C). It is therefore likely that including bicarbonate transport would not alter this picture, as there is significantly less HCO3− transport through CFTR channels than Cl− (Poulsen et al. 1994). Indeed a very recent paper carries out a similar analysis for non-CF nasal epithelia and essentially validates this approach (Garcia et al. 2013).
has little effect on basal Vt (Fig. 5), and limited effect on ΔVt+ amiloride (Fig. 6B and C). It is therefore likely that including bicarbonate transport would not alter this picture, as there is significantly less HCO3− transport through CFTR channels than Cl− (Poulsen et al. 1994). Indeed a very recent paper carries out a similar analysis for non-CF nasal epithelia and essentially validates this approach (Garcia et al. 2013).
Initially, we also made the assumption that changes in paracellular permeability do not drive the bioelectric changes observed in CF. Later, by relaxing this condition, we found that while differences in paracellular permeability or selectivity do influence estimates of 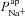 and
and 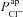 , they do not alter how each of these parameters changes in CF relative to non-CF epithelia (see Supplemental material Tables S5 and S6, Figs S6–S8). The magnitude of the increase in
, they do not alter how each of these parameters changes in CF relative to non-CF epithelia (see Supplemental material Tables S5 and S6, Figs S6–S8). The magnitude of the increase in 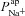 will therefore be influenced by an increased shunt resistance (2-fold rather than 3-fold increase), but the increase of this transport parameter in the disease state is observed consistently.
will therefore be influenced by an increased shunt resistance (2-fold rather than 3-fold increase), but the increase of this transport parameter in the disease state is observed consistently.
Quantifying the influence of CFTR and ENaC currents on nasal Vt
Published modelling work investigating the electrical coupling of CFTR and ENaC fluxes (Horisberger, 2003) showed that increasing 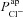 could decrease amiloride-sensitive short-circuit current (Isc) in a kidney epithelial cell model, and Falkenberg and Jakobsson note that Isc is most sensitive to basal apical anion permeability, after the addition of amiloride (Falkenberg & Jakobsson, 2010). More recently, evidence from pig and human airway epithelial cell lines showed that experimentally decreasing apical Cl− conductance can increase ΔVt+ amiloride (Chen et al. 2010; Itani et al. 2011). Our analyis confirms that this relationship exists, qualitatively. However, our modelling approach allows us to quantitatively determine the influence each transport parameter has on the electrical properties of the epithelium (Figs 5 and 6). Thus, we can show that the magnitude of changes in going from non-CF to CF levels of anion permeability were not sufficient to explain the experimentally observed hyperpolarised basal Vt, the increased amiloride-sensitive Vt component, and the decreased ΔVt+ 0[ Cl−]l. In contrast, sensitivity analysis shows that
could decrease amiloride-sensitive short-circuit current (Isc) in a kidney epithelial cell model, and Falkenberg and Jakobsson note that Isc is most sensitive to basal apical anion permeability, after the addition of amiloride (Falkenberg & Jakobsson, 2010). More recently, evidence from pig and human airway epithelial cell lines showed that experimentally decreasing apical Cl− conductance can increase ΔVt+ amiloride (Chen et al. 2010; Itani et al. 2011). Our analyis confirms that this relationship exists, qualitatively. However, our modelling approach allows us to quantitatively determine the influence each transport parameter has on the electrical properties of the epithelium (Figs 5 and 6). Thus, we can show that the magnitude of changes in going from non-CF to CF levels of anion permeability were not sufficient to explain the experimentally observed hyperpolarised basal Vt, the increased amiloride-sensitive Vt component, and the decreased ΔVt+ 0[ Cl−]l. In contrast, sensitivity analysis shows that 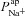 significantly hyperpolarises basal Vt, and is the most important factor in determining the magnitude of ΔVt+ amiloride. Without altering
significantly hyperpolarises basal Vt, and is the most important factor in determining the magnitude of ΔVt+ amiloride. Without altering 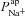 from non-CF levels, the magnitude of the hyperpolarisation of basal Vt and of increased amiloride-sensitive Vt could not be explained.
from non-CF levels, the magnitude of the hyperpolarisation of basal Vt and of increased amiloride-sensitive Vt could not be explained.
One can intuitively understand how the relative influences of ENaC and CFTR permeability on basal and amiloride-sensitive Vt arise, by examining the driving force for movement of Na+ and Cl− ions across the apical membrane. Basal Vt depends implicitly on apical Na+ and Cl− currents, and the changes in these currents with respect to permeability are proportional to driving force. Hence the relative driving force for movement of different ions explains the relative sensitivity of Vt to different permeabilities.
In the representative example of best-fit non-CF parameter values (Table 1), the driving force for Na+ absorption across the apical membrane at steady state is −65.8 mV, as opposed to +1.1 mV for Cl− transport. At these physiological potentials, the Cl− driving force is thus < 2% of that for Na+, consistent with the results of our sensitivity analysis: 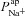 has a much greater influence on Vt than
has a much greater influence on Vt than 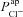 . How then, can we explain the influence of
. How then, can we explain the influence of 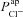 on amiloride-sensitive Vt? After amiloride is added
on amiloride-sensitive Vt? After amiloride is added 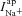 is dramatically reduced and
is dramatically reduced and 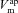 changes, altering the apical Cl− driving force and consquently
changes, altering the apical Cl− driving force and consquently 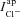 . Again taking these best-fit parameters, this driving force goes from +1.1 mV to −9.5 mV for Cl−, while
. Again taking these best-fit parameters, this driving force goes from +1.1 mV to −9.5 mV for Cl−, while 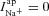 . Therefore
. Therefore 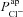 now has a greater relative influence on
now has a greater relative influence on 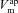 and Vt, while
and Vt, while 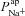 can have no further effect.
can have no further effect.
The results of our sensitivity analysis are in agreement with a range of additional experimental data not used to constrain the model. For example, we observed basal Vt to be strongly dependent on 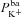 (hyperpolarising). This was found experimentally by Mall et al. (2000) who blocked basolateral K+ channels in human bronchial epithelial (HBE) cells. Modelling studies have also shown that Isc can be increased by stimulating basolateral K+ currents (Falkenberg & Jakobsson, 2010), supporting the hypothesis that increased basolateral K+ conductance is necessary to hyperpolarise the basolateral (and consequently, apical) membrane, providing an increased driving force for Cl− secretion (Cotton, 2000). Further, Vt tends to be depolarised by
(hyperpolarising). This was found experimentally by Mall et al. (2000) who blocked basolateral K+ channels in human bronchial epithelial (HBE) cells. Modelling studies have also shown that Isc can be increased by stimulating basolateral K+ currents (Falkenberg & Jakobsson, 2010), supporting the hypothesis that increased basolateral K+ conductance is necessary to hyperpolarise the basolateral (and consequently, apical) membrane, providing an increased driving force for Cl− secretion (Cotton, 2000). Further, Vt tends to be depolarised by 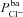 in our model, which agrees with the observations of Fischer and colleagues in human and bovine tracheal primary cultures (Fischer et al. 2007) who also found Vt to be dependent on
in our model, which agrees with the observations of Fischer and colleagues in human and bovine tracheal primary cultures (Fischer et al. 2007) who also found Vt to be dependent on 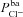 .
.
Finally, it is interesting to note how our model predicts that the density of Na+–K+-ATPase pumps is higher in CF than non-CF cells. This may be necessary in order to deal with the increased rate of Na+ absorption, and higher pump expression has been reported in CF tracheal and nasal epithelia (Stutts et al. 1986).
Implications for clinical nasal potential difference measurements
In Fig. 7 we show the output of simulations of the first three stages of a standard nasal potential difference (nasal Vt) clinical recording: measurement of a basal Vt value, relaxation to a new steady-state value following apical amiloride addition, and transition to a third Vt value upon transfer to Cl−-free conditions (while maintaining amiloride presence). Simulations were run with four different parameterisations: (a) optimal non-CF values (black), (b) optimal non-CF with reduced 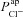 (5% of optimal level, black, dashed), (c) optimal CF values (grey), and (d) optimal CF values with optimal non-CF
(5% of optimal level, black, dashed), (c) optimal CF values (grey), and (d) optimal CF values with optimal non-CF 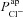 levels (grey, dashed). These simulations illustrate the major findings of our study, and emphasise the potential utility of this mathematical modelling approach.
levels (grey, dashed). These simulations illustrate the major findings of our study, and emphasise the potential utility of this mathematical modelling approach.
Figure 7. Simulations of first stages of a clinical nasal PD test.
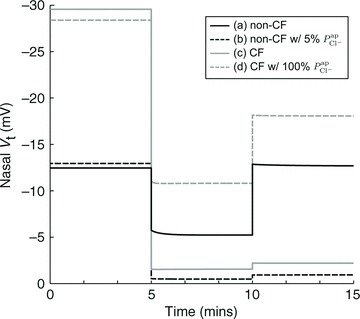
Basal Vt is recorded initially for several minutes, then amiloride is added to the perfusing solution at t = 5 min to block ENaC channels (causing 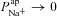 ), and the resultant change in Vt is recorded. At t = 10 min the solution perfusing the luminal surface is changed to a low Cl− solution ([ Cl−]l→ 3 mm) to introduce a diffusion potential for Cl− efflux, and the resultant change in Vt is recorded. Restoring non-CF
), and the resultant change in Vt is recorded. At t = 10 min the solution perfusing the luminal surface is changed to a low Cl− solution ([ Cl−]l→ 3 mm) to introduce a diffusion potential for Cl− efflux, and the resultant change in Vt is recorded. Restoring non-CF 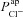 levels in a CF cell does not correct hyperpolarised basal Vt (grey continuous line → grey dashed line). Also, reducing
levels in a CF cell does not correct hyperpolarised basal Vt (grey continuous line → grey dashed line). Also, reducing 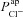 alone to CF levels, in a non-CF cell, does not reproduce a typical CF trace (black continuous line → black dashed line).
alone to CF levels, in a non-CF cell, does not reproduce a typical CF trace (black continuous line → black dashed line).
Traces (a) and (b) illustrate how the loss of apical Cl− permeability (in a non-CF HNE cell) alone cannot account for CF bioelectric properties. With a reduction to 5% of non-CF 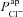 levels, the level of ΔVt+ amiloride increases and ΔVt+ 0[ Cl−]l decreases, but the change of tens of millivolts in basal Vt seen in patients is not observed as only a modest hyperpolarisation occurs.
levels, the level of ΔVt+ amiloride increases and ΔVt+ 0[ Cl−]l decreases, but the change of tens of millivolts in basal Vt seen in patients is not observed as only a modest hyperpolarisation occurs.
Traces (c) and (d) illustrate how our model can be used to investigate strategies aimed at normalising ion transport in CF epithelia. Here we simulate the effect of theoretically increasing 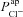 from CF to non-CF levels, in a CF HNE cell. We see that this can ameliorate the ΔVt+ amiloride and ΔVt+ 0[ Cl−]l responses towards non-CF magnitudes, but basal Vt remains hyperpolarised at a typical CF level. This simulation investigates the changes in Vt caused by a hypothetical therapy aimed at increasing Cl− secretion alone. Although the exact pathophysiology of CF lung disease is controversial, the hyperpolarised Vt experienced by CF epithelia will undoubtedly alter driving forces for trans-epithelial ion (and water) movement, a factor which may contribute to the development of CF lung disease. Thus we can see that such a strategy (e.g. stimulating calcium-activated Cl− channels in the apical membrane; Cuthbert, 2011) would not help with restoring basal Vt in native tissues to desired non-CF levels.
from CF to non-CF levels, in a CF HNE cell. We see that this can ameliorate the ΔVt+ amiloride and ΔVt+ 0[ Cl−]l responses towards non-CF magnitudes, but basal Vt remains hyperpolarised at a typical CF level. This simulation investigates the changes in Vt caused by a hypothetical therapy aimed at increasing Cl− secretion alone. Although the exact pathophysiology of CF lung disease is controversial, the hyperpolarised Vt experienced by CF epithelia will undoubtedly alter driving forces for trans-epithelial ion (and water) movement, a factor which may contribute to the development of CF lung disease. Thus we can see that such a strategy (e.g. stimulating calcium-activated Cl− channels in the apical membrane; Cuthbert, 2011) would not help with restoring basal Vt in native tissues to desired non-CF levels.
The measurement of nasal trans-epithelial potentials is widely used as an aid to CF diagnosis and clinical management. Hyperpolarised basal Vt and larger amiloride-sensitive Vt changes are hallmarks of CF disease and have, in recent years, also become central to the debate on the role of sodium hyper-absorption in CF pathology. These same altered bioelectric properties are the foundation of therapeutic approaches aimed at reducing ENaC activity (Hofmann et al. 1998; Coote et al. 2009). The correct interpretation of trans-epithelial potentials therefore carries important implications for both understanding CF and assessing potential therapies. The model presented here therefore can become a highly valuable tool for the interpretation of clinical nasal potential difference measurements and for the development of more effective treatment.
Acknowledgments
None.
Glossary
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- ENaC
epithelial Na+ channel
- PD
potential difference
- Vt
trans-epithelial PD
- HNE cells
human nasal epithelial cells
Additional information
Competing interests
None.
Author contributions
All authors contributed to the conception and design of the mathematical model. D.L.O’D. performed the simulations, data analysis and interpretation of data. All authors contributed to the drafting and revision of the article, and have seen and approved the final version of the manuscript.
Funding
D.L.O’D. was supported by EPSRC grant EP/F500351/1.
Supplementary material
Supplemental material
References
- Benjamin BA, Johnson EA. A quantitative description of the Na-K-2Cl cotransporter and its conformity to experimental data. Am J Physiol Renal Physiol. 1997;273:F473–F482. doi: 10.1152/ajprenal.1997.273.3.F473. [DOI] [PubMed] [Google Scholar]
- Chen J-H, Stoltz DA, Karp PH, Ernst SE, Pezzulo AA, Moninger TO, Rector MV, Reznikov LR, Launspach JL, Chaloner K, Zabner J, Welsh MJ. Loss of anion transport without increased sodium absorption characterizes newborn porcine cystic fibrosis airway epithelia. Cell. 2010;143:911–923. doi: 10.1016/j.cell.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coote K, Atherton-Watson HC, Sugar R, Young A, MacKenzie-Beevor A, Gosling M, Bhalay G, Bloomfield G, Dunstan A, Bridges RJ, Sabater JR, Abraham WM, Tully D, Pacoma R, Schumacher A, Harris J, Danahay H. Camostat attenuates airway epithelial sodium channel function in vivo through the inhibition of a channel-activating protease. J Pharmacol Exp Ther. 2009;329:764–774. doi: 10.1124/jpet.108.148155. [DOI] [PubMed] [Google Scholar]
- Cotton CU. Basolateral potassium channels and epithelial ion transport. Am J Respir Cell Mol Biol. 2000;23:270–272. doi: 10.1165/ajrcmb.23.3.f198. [DOI] [PubMed] [Google Scholar]
- Cuthbert AW. New horizons in the treatment of cystic fibrosis. Br J Pharmacol. 2011;163:173–183. doi: 10.1111/j.1476-5381.2010.01137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JC, Alton EWFW, Bush A. Cystic fibrosis. BMJ. 2007;335:1255–1259. doi: 10.1136/bmj.39391.713229.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge JA, Lewis PA, Stanton M, Wilsher J. Cystic fibrosis mortality and survival in the UK: 1947–2003. Eur Respir J. 2007;29:522–526. doi: 10.1183/09031936.00099506. [DOI] [PubMed] [Google Scholar]
- Donaldson SH, Boucher RC. Sodium channels and cystic fibrosis. Chest. 2007;132:1631–1636. doi: 10.1378/chest.07-0288. [DOI] [PubMed] [Google Scholar]
- Duszyk M, French AS. An analytical model of ionic movements in airway epithelial cells. J Theor Biol. 1991;151:231–247. doi: 10.1016/s0022-5193(05)80362-5. [DOI] [PubMed] [Google Scholar]
- Falkenberg CV, Jakobsson E. A biophysical model for integration of electrical, osmotic, and pH regulation in the human bronchial epithelium. Biophys J. 2010;98:1476–1485. doi: 10.1016/j.bpj.2009.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H, Illek B, Finkbeiner WE, Widdicombe JH. Basolateral Cl channels in primary airway epithelial cultures. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1432–L1443. doi: 10.1152/ajplung.00032.2007. [DOI] [PubMed] [Google Scholar]
- Flynn AN, Itani OA, Moninger TO, Welsh MJ. Acute regulation of tight junction ion selectivity in human airway epithelia. Proc Natl Acad Sci U S A. 2009;106:3591–3596. doi: 10.1073/pnas.0813393106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia GJM, Boucher RC, Elston TC. Biophysical model of ion transport across human respiratory epithelia allows quantification of ion permeabilities. Biophys J. 2013;104:716–726. doi: 10.1016/j.bpj.2012.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann T, Verkman AS. Model of ion transport regulation in chloride-secreting airway epithelial cells. Integrated description of electrical, chemical, and fluorescence measurements. Biophys J. 1990;58:391–401. doi: 10.1016/S0006-3495(90)82385-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. 3rd edn. Sunderland, MA, USA: Sinauer; 2001. [Google Scholar]
- Hofmann T, Stutts MJ, Ziersch A, Rückes C, Weber WM, Knowles MR, Lindemann H, Boucher RC. Effects of topically delivered benzamil and amiloride on nasal potential difference in cystic fibrosis. Am J Respir Crit Care Med. 1998;157:1844–1849. doi: 10.1164/ajrccm.157.6.9709043. [DOI] [PubMed] [Google Scholar]
- Horisberger J-D. ENaC-CFTR interactions: the role of electrical coupling of ion fluxes explored in an epithelial cell model. Pflugers Arch. 2003;445:522–528. doi: 10.1007/s00424-002-0956-0. [DOI] [PubMed] [Google Scholar]
- Itani OA, Chen J-H, Karp PH, Ernst SE, Keshavjee S, Parekh K, Klesney-Tait J, Zabner J, Welsh MJ. Human cystic fibrosis airway epithelia have reduced Cl− conductance but not increased Na+ conductance. Proc Natl Acad Sci U S A. 2011;108:10260–10265. doi: 10.1073/pnas.1106695108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles MR, Gatzy JT, Boucher RC. Relative ion permeability of normal and cystic fibrosis nasal epithelium. J Clin Invest. 1983;71:1410–1417. doi: 10.1172/JCI110894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles MR, Paradiso AM, Boucher RC. In vivo nasal potential difference: techniques and protocols for assessing efficacy of gene transfer in cystic fibrosis. Hum Gene Ther. 1995;6:445–455. doi: 10.1089/hum.1995.6.4-445. [DOI] [PubMed] [Google Scholar]
- Levin MH, Kim JK, Hu J, Verkman AS. Potential difference measurements of ocular surface Na+ absorption analyzed using an electrokinetic model. Invest Ophthalmol Vis Sci. 2006;47:306–316. doi: 10.1167/iovs.05-1082. [DOI] [PubMed] [Google Scholar]
- Mall M, Wissner A, Schreiber R, Kuehr J, Seydewitz HH, Brandis M, Greger R, Kunzelmann K. Role of KVLQT1 in cyclic adenosine monophosphate-mediated Cl− secretion in human airway epithelia. Am J Respir Cell Mol Biol. 2000;23:283–289. doi: 10.1165/ajrcmb.23.3.4060. [DOI] [PubMed] [Google Scholar]
- Poulsen JH, Fischer H, Illek B, Machen TE. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc Natl Acad Sci U S A. 1994;91:5340–5344. doi: 10.1073/pnas.91.12.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe SM, Clancy JP, Wilschanski M. Nasal potential difference measurements to assess CFTR ion channel activity. Methods Mol Biol. 2011;741:69–86. doi: 10.1007/978-1-61779-117-8_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- Simmonds NJ, D'Souza L, Roughton M, Alton EWFW, Davies JC, Hodson ME. Cystic fibrosis and survival to 40 years: a study of cystic fibrosis transmembrane conductance regulator function. Eur Respir J. 2011;37:1076–1082. doi: 10.1183/09031936.00079010. [DOI] [PubMed] [Google Scholar]
- Smith NP, Crampin EJ. Development of models of active ion transport for whole-cell modelling: cardiac sodium-potassium pump as a case study. Prog Biophys Mol Biol. 2004;85:387–405. doi: 10.1016/j.pbiomolbio.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Sobie EA. Parameter sensitivity analysis in electrophysiological models using multivariable regression. Biophys J. 2009;96:1264–1274. doi: 10.1016/j.bpj.2008.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutts MJ, Canessa CM, Olsen JC, Hamrick M, Cohn JA, Rossier BC, Boucher RC. CFTR as a cAMP-dependent regulator of sodium channels. Science. 1995;269:847–850. doi: 10.1126/science.7543698. [DOI] [PubMed] [Google Scholar]
- Stutts MJ, Knowles MR, Gatzy JT, Boucher RC. Oxygen consumption and ouabain binding sites in cystic fibrosis nasal epithelium. Pediatr Res. 1986;20:1316–1320. doi: 10.1203/00006450-198612000-00026. [DOI] [PubMed] [Google Scholar]
- Taylor AL, Goaillard J-M, Marder E. How multiple conductances determine electrophysiological properties in a multicompartment model. J Neurosci. 2009;29:5573–5586. doi: 10.1523/JNEUROSCI.4438-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren NJ, Tawhai MH, Crampin EJ. A mathematical model of calcium-induced fluid secretion in airway epithelium. J Theor Biol. 2009;259:837–849. doi: 10.1016/j.jtbi.2009.04.026. [DOI] [PubMed] [Google Scholar]
- Willumsen NJ, Boucher RC. Shunt resistance and ion permeabilities in normal and cystic fibrosis airway epithelia. Am J Physiol Cell Physiol. 1989;256:C1054–C1063. doi: 10.1152/ajpcell.1989.256.5.C1054. [DOI] [PubMed] [Google Scholar]
- Willumsen NJ, Boucher RC. Transcellular sodium transport in cultured cystic fibrosis human nasal epithelium. Am J Physiol Cell Physiol. 1991a;261:C332–C341. doi: 10.1152/ajpcell.1991.261.2.C332. [DOI] [PubMed] [Google Scholar]
- Willumsen NJ, Boucher RC. Sodium transport and intracellular sodium activity in cultured human nasal epithelium. Am J Physiol Cell Physiol. 1991b;261:C319–C331. doi: 10.1152/ajpcell.1991.261.2.C319. [DOI] [PubMed] [Google Scholar]
- Willumsen NJ, Davis CW, Boucher RC. Intracellular Cl− activity and cellular Cl− pathways in cultured human airway epithelium. Am J Physiol Cell Physiol. 1989a;256:C1033–C1044. doi: 10.1152/ajpcell.1989.256.5.C1033. [DOI] [PubMed] [Google Scholar]
- Willumsen NJ, Davis CW, Boucher RC. Cellular Cl− transport in cultured cystic fibrosis airway epithelium. Am J Physiol Cell Physiol. 1989b;256:C1045–C1053. doi: 10.1152/ajpcell.1989.256.5.C1045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


